Abstract
Purpose:
Nitazoxanide was recently reported as having in vitro effectiveness against the rubella virus. Immunodeficiency-related vaccine-derived rubella occurs in some patients who have an inherited immunodeficiency and who received the MMR vaccine. This study investigated the in vivo effectiveness of nitazoxanide therapy.
Methods:
This is a retrospective analysis of seven patients treated with nitazoxanide as salvage therapy for immunodeficiency-related vaccine-derived rubella infection. The patients were recruited from an ongoing rubella detection surveillance project.
Results:
Seven patients with persistent rubella were treated with nitazoxanide and one demonstrated significant clinical improvement. Two additional patients exhibited diminished viral capsid production with one patient having transient slowing of progression. The cohort overall generally had low T cell counts and had a high burden of co-morbidities. There were three deaths. Two deaths were from PML and one was related to hematopoietic stem cell transplantation.
Conclusions:
Nitazoxanide has limited in vivo anti-viral effects for immunodeficiency-related vaccine-derived rubella. Most patients did not exhibit clinical improvement.
Keywords: Granulomas, chronic inflammation, vaccine, nitazoxanide, MMR
Introduction
Rubella virus is a single-stranded RNA virus and is an important human pathogen because of its ability to cause fetal malformations during in utero infection [1]. It is not thought to represent a dangerous pathogen for immunocompetent adults, although persistent infections in immune privileged body sites have been described. Indeed, rubella-associated arthritis/arthralgia can develop in adults due to persistent vaccine-strain infection [2]. Fuch’s uveitis is also associated with persistent infection with rubella virus and a few cases of uveitis have been observed with vaccine-strain rubella [3; 4]. These cases demonstrate the ability of the rubella virus to evade immune eradication/control even in immunocompetent individuals although the reasons for this are not defined. Recently, patients with primary immunodeficiency have been identified with persistence of vaccine-strain rubella associated with chronic inflammation [5; 6]. Immunodeficiency-related vaccine-derived rubella virus (iVDRV) was initially identified in patients with cutaneous granulomas. More recently, iVDRV has been detected in other sites of chronic inflammation [5; 6].
To date, there are no known treatments that are highly effective. In most cases, the inflammation has progressed relentlessly unless hematopoietic stem cell transplantation (HSCT) has been performed with resolution of the immunodeficiency, although one patient has been reported to have responded to topical steroid treatment [7]. Rubella virus is insensitive to type I interferons and no other antiviral therapy is of proven benefit. The inability of specific maternal and fetal antibody to clear rubella virus infection in the fetus also suggests that humoral immunity alone is not sufficient in eliminating the persisting virus [8]. Thus, most of the tools utilized for chronic viral infections in immunodeficient patients are not thought to be effective for rubella virus.
Rubella virus replication can be inhibited in vitro with the drug nitazoxanide [9]. Nitazoxanide is approved for the treatment of parasites, protozoa, and anaerobic bacteria. It has been demonstrated to have antiviral activities and is being tested as a therapeutic intervention for hepatitis C, influenza, rotavirus, and norovirus. Nitazoxanide has a favorable adverse event profile and is used frequently in children for the treatment of parasitic disease. The mechanism of its anti-viral action is not understood but it may act in a cell-intrinsic fashion to limit replication. We recently demonstrated that nitazoxanide inhibited rubella virus replication in human umbilical vein endothelial, Vero, and A549 cells [9]. The inhibitory concentration was consistent with serum levels obtained during therapeutic use. Analysis of the effect of nitazoxanide revealed multiple mechanisms of inhibition at both early and late stages of infection. We therefore retrospectively analyzed the effect of nitazoxanide treatment in a case series of immunodeficient patients with persistent iVDRV at our institutions.
Methods
For this study, seven patients with a primary immunodeficiency and persistent iVDRV were identified. Ongoing surveillance for iVDRV in the USA at the CDC and in France at the Institut Pasteur for rubella capsid and genome [5; 6] led to definition of the initial iVDRV cohort and submitting physicians identified nitazoxanide use. Based on in vitro efficacy, limited adverse events, poor prognosis, and absence of other proven interventions, clinicians at each site decided to institute therapy with nitazoxanide. This is a retrospective study of outcomes of the seven patients. A structured case report form was used to collect standardized data from each physician. No statistical analysis was performed.
Results
All patients had a significant T cell-oriented immunodeficiency and most had low T cell counts in peripheral blood (Table 1). Only patients 3 and 4 had normal T cell counts. T cell function was not systematically assessed. Natural killer cell counts (NK cells) were generally normal. The age of onset of inflammatory lesions ranged from 15 months of age to 16 years and all patients had previously received the MMR vaccine months to years prior to symptoms. All patients had skin involvement that was described as initially psoriasiform or resembling pyoderma gangrenosum (Figure 1). The cutaneous granulomas spread relentlessly and in one case led to amputation and in another case led to surgical excision in an effort to control the spread. Neither effort at surgical control of the lesions was successful. Empiric treatments directed at mycobacteria or fungi were not successful. Immune suppression appeared to help in two cases but did not lead to resolution. In the most severe cases, multiple organs were ultimately affected. Rubella capsid protein was detected in all patients and virus RNA was recovered from patients 2, 4 and 5. Different iVDRV strains with multiple non-overlapping mutations were identified in the skin biopsies by sequencing viral genomes. Survival was generally dismal and progressive multifocal leukoencephalopathy (PML) was the final cause of death in two patients. HSCT was curative in one patient with resolution of skin lesions. Immunoglobulin was used uniformly without benefit.
Table 1.
Clinical summary of cases
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|
| Diagnosis | DNA Ligase IV | Ataxia Telangiectasia | Ataxia telangiectasia | Ataxia Telangiectasia | CID (unknown genetics) | Coronin1A | Cartilage hair hypoplasia |
| MMR vaccine | 12m | 24m | 12m and 17m | 18m | 5y and 9y | 3y and 5.7y | 1y |
| Age of onset of inflammatory process | 15m | 30m | 39m | 10 years | 13 years | 13 years | 15 years |
| Sites of chronic inflammation | Skin Liver Intestine |
Skin | Skin | Skin | Skin | Skin and underlying bone | Skin Liver Kidney Larynx Palate Bone marrow |
| Sites where rubella was detected by immunofluorescence or PCR | Skin Liver |
Skin | Skin | Skin | Skin | Skin | Skin Liver Kidney |
| CD3 count | 380 cells/uL | 240 cells/uL | 3074 cells/uL | 3085 cells/uL | 453 cells/uL | 464 cells/uL | 550 cells/uL |
| CD4 count | 73 cells/uL | 153 cells/uL | 2450 cells/uL | 1091 cells/uL | 189 cells/uL | 224 cells/uL | 450 cells/uL |
| CD19 count | 21 cells/uL | 77 cells/uL | 178 cells/uL | 0 cells/uL (postrituximab) | 312 cells/uL | 24 cells/uL | 120 cells/uL |
| NK cell count | 432 cells/uL | 184 cells/uL | 1158 cells/uL | 560 cells/uL | 105 cells/uL | 144 cells/uL | 120 cells/uL |
| Previous treatments | Immune modulatory: Topical steroids No improvement | Antimicrobial: Cryotherapy Erythromycin Imiquimod No improvement Immune modulatory: Tacrolimus cream Hydroxycholorquine Topical steroids No improvement Oral prednisone led to some improvement |
Immune modulatory: Topical steroids NSAID No improvement | Immune modulatory: Abatacept No improvement | Antimicrobial: Linezolid Clarithromycin Rifampin Isoniazid Ethambutal Voriconazole Posaconazole Interferon No improvement Amputation of finger- continued local spread |
Antimicrobial: Cotrimoxazole, Azithromycin, Fluconazole, Amoxicillin-clavulanic acid, Ciprofloxacin No improvement Immune modulatory: Topical steroids No improvement Surgical excision- local recurrence |
Antimicrobial:Azathioprine Azithromycin No improvement Immune modulatory; Mycophenolic acid No improvement Cyclosporine and steroids- partial response |
| Treated with IVIG | + | + | + | + | + | + | + |
| Response to nitazoxanide | Dose: 32mg/kg/d Given for norovirus- for 5 weeks with no improvement in skin lesions |
Dose: 7mg/kg/d Tried for one year without improvement |
Dose: 11mg/kg/d Tried for five months without improvement |
Dose: 22mg/kg/d Slowing in progression and loss of rubella in skin on biopsy but subsequent involvement of bone |
Dose: 16mg/kg/d Tried for 5 months without clinical improvement although rubella became undetectable by immunohistochemistry |
Dose: 25mg/kg/d Tried for 4 months, no improvement |
Dose: 20mg/kg/d After two months, lesions in skin, liver and kidney improved and improvement was sustained until death |
| Outcome | HSCT with ultimate death at d+ 135 although with improvement in lesions | Progression of granulomas with persistent rubella on repeat biopsy | HSCT with improvement in lesions (currently d+140) | Alive | Deceased from PML | Alive without HSCT (age at last F/U 18 years) | Deceased from PML |
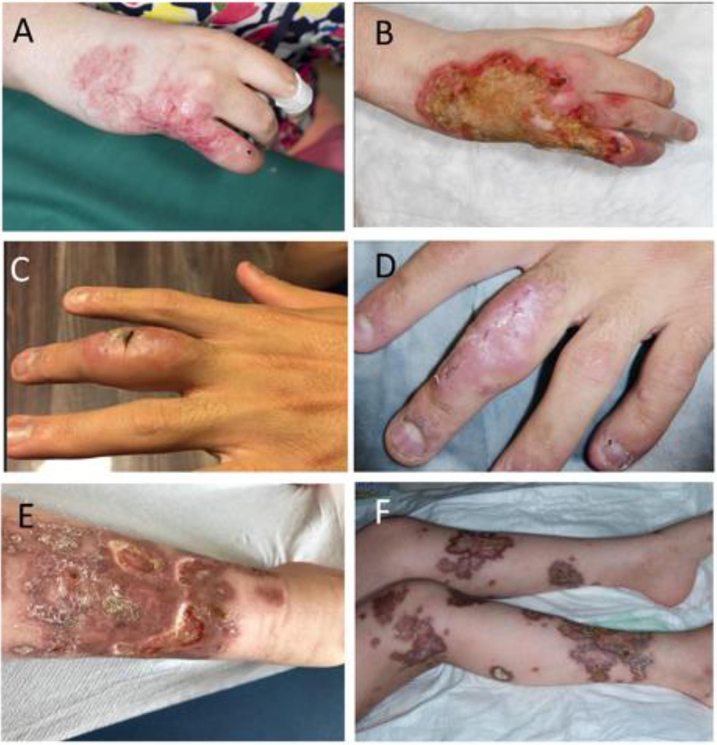
Figure 1. Immunodeficiency-related vaccine-derived rubella virus (iVDRV) clinical features.
A) Patient 5 with typical granulomatous inflammation. B) Patient 5 demonstrating relentless progression over one year. C) Patient 6 in 2016 after surgical excision. D) Patient 6 after 2.5 months of nitazoxanide. E) Patient 3 right leg prior to stem cell transplant. F) Legs of Patient 3 legs 20 days after transplantation.
Nitazoxanide was used largely as salvage therapy in these extremely ill patients. Rubella capsid protein became undetectable (Figure 2) in two patients (Patients 4 and 5) but without clinical improvement although progression may have stabilized. In one case, clear clinical improvement coincided with the institution of nitazoxanide (Patient 7). In the other four cases, nitazoxanide had no obvious benefit (Table 1).
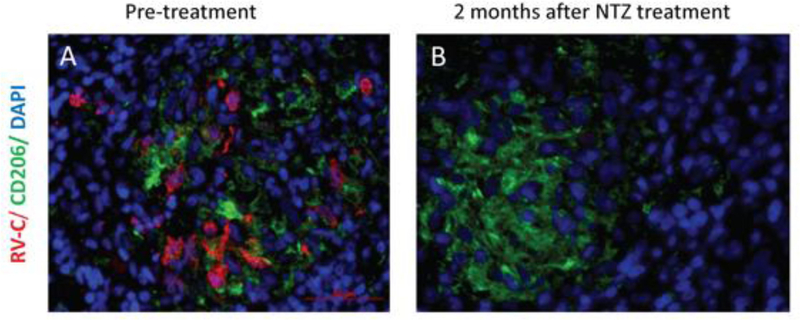
Figure 2. Regression of viral capsid expression after nitazoxanide.
A) Patient 5 immunohistochemistry rubella capsid detection (red) in granuloma (CD206 in green is an M2 macrophage marker). DAPI is provided as a nuclear stain. B) Patient 5 after two months of nitazoxanide treatment (NTZ). There is no detectable rubella capsid.
Discussion
This study provides critical information on treatment approaches for clinicians caring for immunodeficient patients with this challenging complication. The recent identification of iVDRV in granulomatous inflammation has led to increasing recognition, however, there no known therapeutic approach. Nitazoxanide may have provided some limited benefit in our study. One patient had dramatic resolution, only to die later of PML (Patient 7). Similarly, one patient may have stabilized with diminished rubella capsid detection, but this patient also succumbed to PML (Patient 5). Patient 4 was thought have slowed in the progression of lesions but did not resolve any existing lesions. Our model of this condition describes an interplay between acquired mutations that allow escape from T cell control and compromised T cell function. We hypothesize that the administration of live attenuated vaccine rubella in patients with T cell compromise allows prolonged persistence. The additional time for replication allows the acquisition of mutations that favor persistence in M2 macrophages. The acquired mutations further facilitate escape from T cell control. If nitazoxanide is beneficial, it may require a threshold of competent T cells, may require longer treatments, or it may be effective against iVDRV strains that have specific replication characteristics. These data do not provide strong support for efficacy of nitazoxanide as salvage therapy, however, the drug has limited adverse events and may provide limited benefit. The question of whether it might provide more clinical effectiveness earlier in the infection is unanswered. This study also highlights a second finding. The patients who are susceptible to iVDRV are extremely immunodeficient. Definitive treatment with HSCT had not been performed in these patients for a variety of reasons, but is underway for patient 3 (currently d+140) and may provide the only curative strategy. The poor outcomes in this study document the uphill battle faced by patients with combined immunodeficiency who are not transplanted [10]. Out of two long-term survivors in this small study, one had an HSCT and the second one is 18 years old at the time of this report and is clinically stable but with ongoing inflammatory iVDRV disease.
Conclusions
In summary, nitazoxanide may have provided some benefit in 3/7 patients with persistent iVDRV. Additional study may define the variables related to responsiveness.
Acknowledgments
Funding: This study was supported by the Wallace Chair of Pediatrics and the CDC.
Footnotes
Conflict of interest:
The authors declare they have ho conflicts of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, US Department of Health and Human Services.
References
- [1].Tondury G, and Smith DW: Fetal rubella pathology. J Pediatr 68: 867–79, 1966 [DOI] [PubMed] [Google Scholar]
- [2].Fraser JR, Cunningham AL, Hayes K, Leach R, and Lunt R: Rubella arthritis in adults. Isolation of virus, cytology and other aspects of the synovial reaction. Clin Exp Rheumatol 1: 287–93, 1983 [PubMed] [Google Scholar]
- [3].Ferrini W, Aubert V, Balmer A, Munier FL, and Abouzeid H: Anterior uveitis and cataract after rubella vaccination: a case report of a 12-month-old girl. Pediatrics 132: e1035–8, 2013 [DOI] [PubMed] [Google Scholar]
- [4].Abernathy E, Peairs RR, Chen MH, Icenogle J, and Namdari H: Genomic characterization of a persistent rubella virus from a case of Fuch’ uveitis syndrome in a 73 year old man. J Clin Virol 69: 104–9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bodemer C, Sauvage V, Mahlaoui N, Cheval J, Couderc T, Leclerc-Mercier S, Debre M, Pellier I, Gagnieur L, Fraitag S, Fischer A, Blanche S, Lecuit M, and Eloit M: Live rubella virus vaccine long-term persistence as an antigenic trigger of cutaneous granulomas in patients with primary immunodeficiency. Clin Microbiol Infect 20: O656–63, 2014 [DOI] [PubMed] [Google Scholar]
- [6].Perelygina L, Plotkin S, Russo P, Hautala T, Bonilla F, Ochs HD, Joshi A, Routes J, Patel K, Wehr C, Icenogle J, and Sullivan KE: Rubella persistence in epidermal keratinocytes and granuloma M2 macrophages in patients with primary immunodeficiencies. J Allergy Clin Immunol 138: 1436–1439 e11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Privette ED, Ram G, Treat JR, Yan AC, and Heimall JR: Healing of granulomatous skin changes in ataxia-telangiectasia after treatment with intravenous immunoglobulin and topical mometasone 0.1% ointment. Pediatr Dermatol 31: 703–7, 2014 [DOI] [PubMed] [Google Scholar]
- [8].Dudgeon JA, Marshall WC, and Peckham CS: Humoral immune responses in congenital rubella. Lancet 2: 480–1, 1972 [DOI] [PubMed] [Google Scholar]
- [9].Perelygina L, Hautala T, Seppanen M, Adebayo A, Sullivan KE, and Icenogle J: Inhibition of rubella virus replication by the broad-spectrum drug nitazoxanide in cell culture and in a patient with a primary immune deficiency. Antiviral Res 147: 58–66, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Speckmann C, Doerken S, Aiuti A, Albert MH, Al-Herz W, Allende LM, Scarselli A, Avcin T, Perez-Becker R, Cancrini C, Cant A, Di Cesare S, Finocchi A, Fischer A, Gaspar HB, Ghosh S, Gennery A, Gilmour K, Gonzalez-Granado LI, Martinez-Gallo M, Hambleton S, Hauck F, Hoenig M, Moshous D, Neven B, Niehues T, Notarangelo L, Picard C, Rieber N, Schulz A, Schwarz K, Seidel MG, Soler-Palacin P, Stepensky P, Strahm B, Vraetz T, Warnatz K, Winterhalter C, Worth A, Fuchs S, Uhlmann A, Ehl S, and EBMT PCsotIEWPot: A prospective study on the natural history of patients with profound combined immunodeficiency: An interim analysis. J Allergy Clin Immunol 139: 1302–1310 e4, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]