Abstract
Objectives:
To compare the frequency of anxiety/depressive symptoms and use of anxiolytic-hypnotics/antidepressants in smokers with and without COPD and to identify characteristics associated with having unmedicated symptoms.
Methods:
Cross-sectional analysis of ambulatory, current/former smokers ≥10 pack years enrolled in the COPDGene study. We measured anxiety/depressive symptoms using the Hospital Anxiety and Depression Scale (subscales ≥8), recorded anxiolytic-hypnotic/antidepressant use, and defined unmedicated symptoms as elevated anxiety/depressive symptoms and not on medications. Regression analysis identified characteristics associated with having unmedicated symptoms.
Key Results:
Of 5331 current/former smokers (45% with and 55% without COPD), 1332 (25.0%) had anxiety/depressive symptoms. Anxiety symptoms were similar in frequency in smokers with and without COPD (19.7% overall), while depressive symptoms were most frequent in severe-very severe COPD at 20.7% (13.1% overall). In the entire cohort, 1135 (21.2%) were on medications. Anxiolytic-hypnotic use was highest in severe-very severe COPD (range 7.6%−12.0%), while antidepressant use showed no significant variation in smokers with and without COPD (range 14.7%−17.1%). Overall, 881 (66% of those with symptoms) had unmedicated symptoms, which was associated with African American race (adjusted OR 2.95, 95% CI 2.25–3.87), male gender (adjusted OR 1.93, 95% CI 1.57–2.36), no health insurance (adjusted OR 2.38, 95% CI 1.30–4.35), severe-very severe COPD (adjusted OR 1.48, 95% CI 1.04–2.11), and higher respiratory symptoms/exacerbation history (adjusted OR 2.21, 95% CI 1.62–3.02).
Conclusions:
Significant unmet mental health care needs exist in current and former smokers with and without COPD. One in five have unmedicated symptoms, identified by key demographic and clinical characteristics.
Keywords: clinical epidemiology, pulmonary diseases, mental health, access to care, population health, smoking, chronic obstructive pulmonary disease, anxiety, depression, antidepressive agents, antianxiety agents
INTRODUCTION
Anxiety and depression affect nearly one in five adults and are the leading cause of disease burden in the United States(1). Anxiety and depressive symptoms are elevated in chronic obstructive pulmonary disease (COPD)(2, 3) and are also believed to go unrecognized in smokers at risk for COPD(4–7). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging system categorizes smokers into those with and without COPD and provides an ideal framework to compare anxiety and depressive symptoms in the context of anxiolytic-hypnotic/antidepressant use, which has not previously been examined. Characteristics associated with having anxiety or depressive symptoms but not using medications have also not been explored in this population.
Half of adults with anxiety and depression do not receive pharmacologic treatment, and racial disparities exist in access to mental health services nationwide(8–12). Smokers are especially subject to biological and socioeconomic factors that contribute to a high frequency of anxiety and depressive symptoms compared to non-smokers(5, 13, 14), and it is likely that unmedicated symptoms are also a significant problem in smokers. Anxiolytic-hypnotics and antidepressants in conjunction with psychotherapy are the cornerstone of therapy for anxiety and depression in the general population(15), and antidepressants are standard of care for those with severe symptoms(16). However, in COPD, symptoms of anxiety and depression often overlap with fatigue or dyspnea, thus complicating screening. Furthermore, medication treatment of anxiety in those with impaired lung function and chronic hypercapnia is often impeded by provider concerns about respiratory suppression (17). GOLD guidelines are inconclusive regarding the pharmacologic treatment of anxiety and depressive symptoms in COPD(18), and the paucity of data on pharmacologic treatment in the broader population of smokers makes it difficult to examine gaps that could exist between general population guidelines and clinical practice in smokers with and without COPD.
We analyzed data from current and former smokers with and without COPD in the multisite Genetic Epidemiology of COPD (COPDGene) study which was designed to discover genetic factors that contribute to the development of COPD(19). We compared the frequency of anxiety symptoms, depressive symptoms, and use of commonly prescribed anxiolytic-hypnotics and antidepressants across GOLD severity stages, including participants with a history of smoking without COPD. We then identified demographic and clinical characteristics associated with having unmedicated symptoms. We hypothesized that anxiety symptoms, depressive symptoms, anxiolytic-hypnotic use, and antidepressant use would be highest in smokers with COPD compared to those without COPD. We also hypothesized that demographic characteristics such as African American race and clinical characteristic such as higher respiratory symptom burden would identify smokers at increased risk for having unmedicated symptoms.
METHODS
Participants
We analyzed data from the Genetic Epidemiology Study of COPD (COPDGene), which has been previously described(19). The COPDGene study recruited current and former smokers (≥10 pack-years) from 21 centers across the United States. This analysis used data from participants with available Hospital Anxiety and Depression Scale (HADS) and psychiatric medication data collected only during a Phase 2 study visit. The Institutional Review Board at all sites approved the study, and participants provided written informed consent.
Defining Symptoms and Medication Use
The HADS is a self-administered questionnaire with anxiety (HADS-A) and depression (HADS-D) subscales, each comprised of 7 items rated on a 4-point Likert scale (0 to 3)(20). HADS subscale scores range from 0 to 21, with higher scores indicating more severe symptoms. A score ≥8 on each subscale is the most commonly used threshold for clinically elevated anxiety or depressive symptoms(21). This threshold is associated with clinically important COPD outcomes including exacerbations and identifies anxiety and depression by standardized neuropsychiatric interview with acceptable sensitivity and specificity(22, 23).
Subjects reported current use of psychiatric medications by generic and/or trade names. After pharmacist (MJ) adjudication, members of the study team (FW, KH, ASI, GK) developed the coding scheme used in this analysis. We organized medications by mechanism of action into “commonly prescribed anxiolytic-hypnotics” and “commonly prescribed antidepressants” as follows: commonly prescribed anxiolytic-hypnotics (Lorazepam, Flurazepam, Triazolam, Clonazepam, Chlordiazepoxide, Temazepam, Oxazepam, Clorazepate, Diazepam, Alprazolam, Quazepam, Estazolam); commonly prescribed antidepressants (Selective Serotonin Reuptake Inhibitors [SSRIs] and Serotonin-Norepinephrine Reuptake Inhibitors [SNRIs])(24). SSRIs included Citalopram, Escitalopram, Fluvoxamine, Paroxetine, Fluoxetine, Sertraline, and Fluoxetine/Olanzapine. SNRIs included Duloxetine, Venlafaxine, Levomilnacipran, Desvenlafaxine, and Milnacipran. We performed the analysis with and without the Norepinephrine-Dopamine Reuptake Inhibitor, Bupropion, as it is routinely used for tobacco cessation.
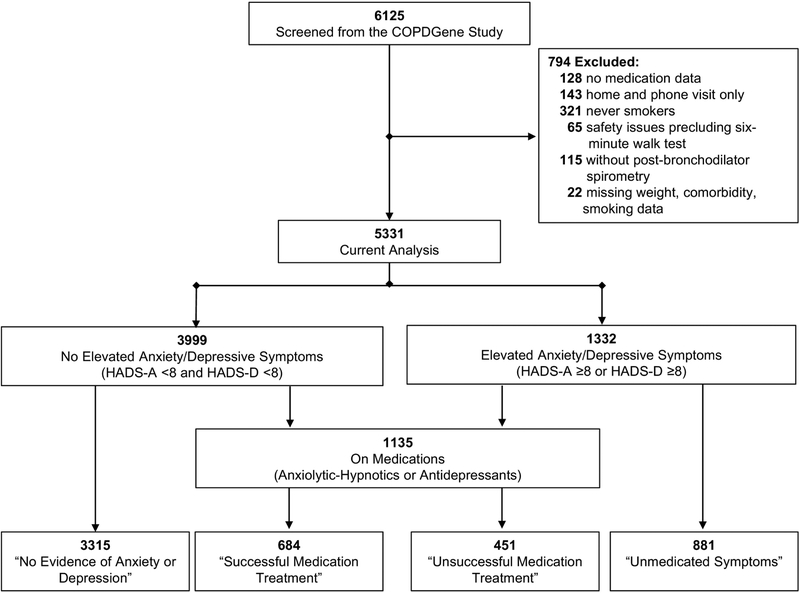
We referred to commonly prescribed anxiolytic-hypnotics or antidepressants as “Medications”. We categorized subjects without currently elevated symptoms of anxiety or depression and who were not on medications as having “No Evidence of Anxiety or Depression”. We categorized subjects “On Medications” as either having “Unsuccessful Medication Treatment” (on medications with elevated symptoms) or “Successful Medication Treatment” (on medications without elevated symptoms). We defined subjects with elevated anxiety or depressive symptoms but not taking medications as having “Unmedicated Symptoms” (Figure 1).
Figure 1. Subjects Screened and Enrolled from the COPDGene Study.
Other Measures
We included only participants with available post-bronchodilator spirometry and categorized participants by GOLD stage as COPD [GOLD I-II (mild-moderate COPD) and GOLD III-IV (severe-very severe COPD)] or no COPD [GOLD 0 (FEV1/FVC ≥0.70 and FEV1 %-predicted ≥0.80) and GOLD PRISm (Preserved Ratio Impaired Spirometry or FEV1/FVC ≥0.70 with FEV1 %-predicted <0.80)(25)]. We defined low functional status as <350 meters on the six-minute walk test, which has been associated with increased risk for mortality(26). We defined low income as a household income <$15,000 per year(27) and health insurance as categorical. Access to ambulatory preventative care was self-reported as having access to preventative care provided in the outpatient setting rather than in the hospital. We reviewed comorbidities and generated a comorbidity count (28), including coronary artery disease, angina, heart attack, coronary artery bypass surgery, angioplasty, diabetes mellitus, congestive heart failure, stroke, transient ischemic attack, osteoarthritis, osteoporosis, compression fractures in the back, hypertension, hyperlipidemia, gastroesophageal reflux disease, stomach ulcers, obesity (body mass index ≥30), obstructive sleep apnea, hay fever, and peripheral vascular disease. We categorized participants into groups according to the 2017 GOLD “ABCD” assessment tool, which includes the modified Medical Research Council (mMRC) dyspnea scale (≥2) or the COPD Assessment Test (≥10) and a history of exacerbations in the past year (18). Group D participants have the highest symptom burden and frequency of exacerbations, especially those requiring hospitalizations (Table 2).
Table 2.
Symptoms of Anxiety and Depression and Use of Anxiolytic-Hypnotics and Antidepressants by GOLD ABCD Groups (n=5331)
| Exacerbation History | GOLD ABCD Groups | |
|---|---|---|
| ≥2 or ≥1 Leading to Hospital Admission |
C (n=1007) Anxiety Symptoms: 24.6% Depressive Symptoms: 14.1% Anxiolytic-Hypnotics: 8.8% Antidepressants: 15.3% Unmedicated Symptoms: 19.9% |
D (n=905) Anxiety Symptoms: 26.6% Depressive Symptoms: 19.7% Anxiolytic-Hypnotics: 12.4% Antidepressants: 20.0% Unmedicated Symptoms: 23.0% |
| 0 or 1 Not Leading to Hospital Admission |
A (n=2180) Anxiety Symptoms 9:2% Depressive Symptoms: 3.8% Anxiolytic-Hypnotics: 6.0% Antidepressants: 14.3% Unmedicated Symptoms: 7.5% |
B (n=1239) Anxiety Symptoms 29.2% Depressive Symptoms: 23.6% Anxiolytic-Hypnotics: 9.3% Antidepressants: 17.2% Unmedicated Symptoms: 25.0% |
| mMRC 0–1 or CAT<10 |
mMRC ≥2 or CAT ≥10 |
|
| Symptoms | ||
Abbreviations: mMRC = modified Medical Research Council Dyspnea scale; CAT = COPD Assessment Test
Statistical Analysis
We documented adherence to STROBE statement (STrengthening the Reporting of OBservational studies in Epidemiology)(29) in the online supplement (Appendix Table A.1). We reported frequency for categorical variables and mean ± standard deviation (SD) for continuous variables. We compared the frequency of elevated anxiety symptoms, depressive symptoms, use of commonly prescribed anxiolytic-hypnotics or antidepressants, and unmedicated symptoms across the entire cohort using an Omnibus Chi-square test at p<0.05 followed by post-hoc group-by-group comparisons between each GOLD stage and by GOLD ABCD groups. We compared differences in characteristics between subjects with unmedicated symptoms to those on medications using Chi-squared test for categorical variables and independent t-test for normally distributed continuous variables or Mann-Whitney U test for non-normally distributed continuous variables.
To identify characteristics independently associated with having unmedicated symptoms, we developed a mixed effects logistic regression model using the GenLinMixed function of Statistical Package for the Social Sciences (SPSS 23.0, SPSS Inc., Chicago, IL, USA) and varied the intercept of the model randomly by study center to account for site-specific differences in recruitment(30). Informed by the biopsychosocial model and prior literature, we included clinically important fixed effects that represented spheres of biological, clinical, and socioeconomic characteristics in the full model(31–34). All tests were two-sided, missing data were treated as missing, and we defined an alpha<0.05 as significant.
Role of the Funding Source
The COPDGene Study is supported by the National Institutes of Health and the COPD Foundation, and neither contributed to the design or conduct of this study.
RESULTS
Cohort Description
Of 6125 participants from the COPDGene study, 5997 (97.9%) had medication data available for coding. We excluded 143 (2.4%) subjects with home or phone visits only, 321 (5.4%) never smokers, 65 (1.1%) participants without six-minute walk test data due to safety reasons, 115 (2.0%) who did not have post-bronchodilator spirometry, and 22 (0.04%) with missing weight or smoking data (Figure 1). The current analysis included 5331 participants. The overall cohort had a mean ±SD age of 65.5±8.6 years, with 2683 (50.3%) males, nearly one third non-Hispanic African Americans, and one third current smokers (Table 1). Overall, 54.8% of participants did not have COPD [GOLD 0 and PRISm], while the remaining 45.1% had COPD [GOLD I-II and GOLD III-IV] (Table 1).
Table 1.
Cohort Demographic and Clinical Characteristics (n=5331)
| Variable | n (%) or Mean ±SD |
|---|---|
| Age (Years) | 65.5±8.6 |
| Gender (% Male) | 2683 (50.3) |
| Race (% African American) | 1573 (29.5) |
| Income <$15,000 (%) | 1432 (26.9) |
| Health Insurance (%) | 5136 (96.3) |
| Ambulatory Preventative Care (%) | 5047 (94.7) |
| GOLD Stage | |
| GOLD 0 (%) | 2260 (42.4) |
| GOLD PRISm (%) | 662 (12.4) |
| GOLD I-II (%) | 1602 (30.1) |
| GOLD III-IV (%) | 807 (15.1) |
| Comorbidity Count | 3.4±2.4 |
| Current Smoking (%) | 2022 (37.9) |
| Oxygen Therapy (%) | 648 (12.2) |
| No Exercise in Last 3 Weeks (%) | 2473 (46.4) |
| Unintentional Weight Loss (%) | 594 (11.1) |
| Chronic Bronchitis (%) | 819 (15.4) |
| Six-minute Walk Distance (m) | 392.4±131.9 |
| Cardiopulmonary Rehabilitation in the Last 5 Years (%) | 337 (6.7) |
| Inhaled bronchodilators (%) | 5197 (97.5) |
| Inhaled corticosteroids (%) | 1328 (24.9) |
| GOLD ABCD Groups* | |
| Group A | 2180 (40.9) |
| Group B | 1239 (23.2) |
| Group C | 1007 (18.9) |
| Group D | 905 (17.0) |
| Anxiety or Depressive Symptoms | 1332 (25.0) |
| Anxiety Symptoms (HADS-Anxiety ≥8) | 1052 (19.7) |
| Depressive Symptoms (HADS-Depression ≥8) | 696 (13.1) |
| Both Anxiety and Depressive Symptoms | 416 (7.8) |
| Commonly Prescribed Anxiolytic-Hypnotics or Antidepressants | 1135 (21.3) |
| Anxiolytic-Hypnotics | 446 (8.4) |
| Antidepressants | 859 (16.1) |
| Selective Serotonin Reuptake Inhibitors | 650 (12.2) |
| Serotonin-Norepinephrine Reuptake Inhibitors | 219 (4.1) |
| Anxiolytic-Hypnotic and Antidepressant | 170 (3.2) |
| Norepinephrine-Dopamine Reuptake Inhibitor (Bupropion) | 224 (4.2) |
See Table 2 for classification. GOLD Group D has highest symptom burden and history of frequent exacerbations.
HADS-Anxiety ≥8 or HADS-Depression ≥8 and not an anxiolytic-hypnotic or antidepressant
Abbreviations: GOLD = Global Initiative for Obstructive Lung Disease; PRISm = Preserved Ratio with Impaired Spirometry; HADS = Hospital Anxiety and Depression Scale
Symptoms and Use of Commonly Prescribed Medications
Overall, 1332 (25.0%) subjects reported elevated anxiety/depressive symptoms [n=1052 (19.7%) with HADS-Anxiety ≥8; n=696 (13.1%) with HADS-Depression ≥8; and n=416 (7.8%) with comorbid anxiety and depressive symptoms]. Frequency of medication use is shown in Table 1. Of the 1332 participants with anxiety or depressive symptoms, 881 (66.1%) had unmedicated symptoms, which represented 16.5% of the overall cohort (Figure 1 and Table 1).
Symptoms and Medications Across GOLD Stages and ABCD Groups
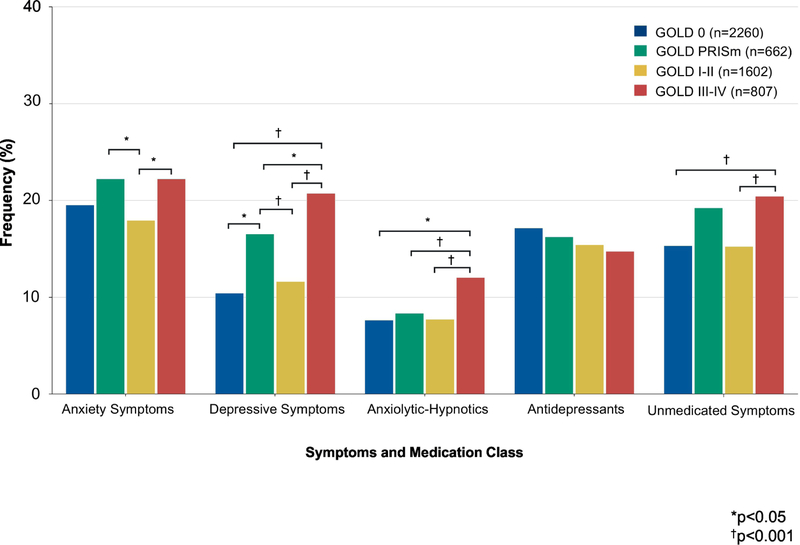
Anxiety symptoms were frequent in all GOLD stages with little variation (GOLD 0: 19.5%; GOLD PRISm: 22.2%; GOLD I-II: 17.9%; and GOLD III-IV: 22.2%, p=0.03). In contrast, the frequency of depressive symptoms differed by GOLD stage (Figure 2). The highest frequency of depressive symptoms occurred in participants with severe-very severe COPD (GOLD 0: 10.4%; GOLD PRISm: 16.5%; GOLD I-II: 11.6%; and GOLD III-IV: 20.7%, p<0.001).
Figure 2. Symptoms of Anxiety and Depression and Use of Anxiolytic-Hypnotics and Antidepressants by GOLD Stage.
Anxiety symptoms, depressive symptoms, anxiolytic-hypnotic use, antidepressant use, and unmedicated symptoms across GOLD severity stages using ANOVA followed by post-hoc group-by-group comparisons. *p<0.05; †p<0.001
Anxiolytic-hypnotic use differed significantly across GOLD stages (GOLD 0: 7.6%; GOLD PRISm: 8.3%; GOLD I-II: 7.7%; and GOLD III-IV: 12.0%, p<0.001) but there was no significant variation in the frequency of antidepressant use (GOLD 0: 17.1%; GOLD PRISm: 16.2%; GOLD I-II: 15.4%; and GOLD III-IV: 14.7%, p=0.35). Finally, the frequency of unmedicated symptoms differed across GOLD stages, with the highest frequency in GOLD PRISm and GOLD III-IV stage COPD (GOLD 0: 15.3%; GOLD PRISm: 19.2%; GOLD I-II: 15.2%; and GOLD III-IV: 20.4%, p<0.001, Figure 2).
As shown in Table 2, anxiety symptoms were significantly more frequent in GOLD groups B, C, and D than group A (A: 9.2%; B: 29.2%; C: 24.6%; and D: 26.6%, p<0.001), while depressive symptoms were significantly more frequent in GOLD groups B and D than in groups A or C (A: 3.8%; B: 23.6%; C: 14.1%; and D: 19.7%, p<0.001). Anxiolytic-hypnotic use was most frequent in GOLD group D (A: 6.0%; B: 9.3%; C: 8.8%; and D: 12.4%, p<0.001), as was antidepressant use (A: 14.3%; B: 17.2%; C: 15.3%; and D: 20.0%, p=0.001).
Characteristics Associated with Unmedicated Symptoms
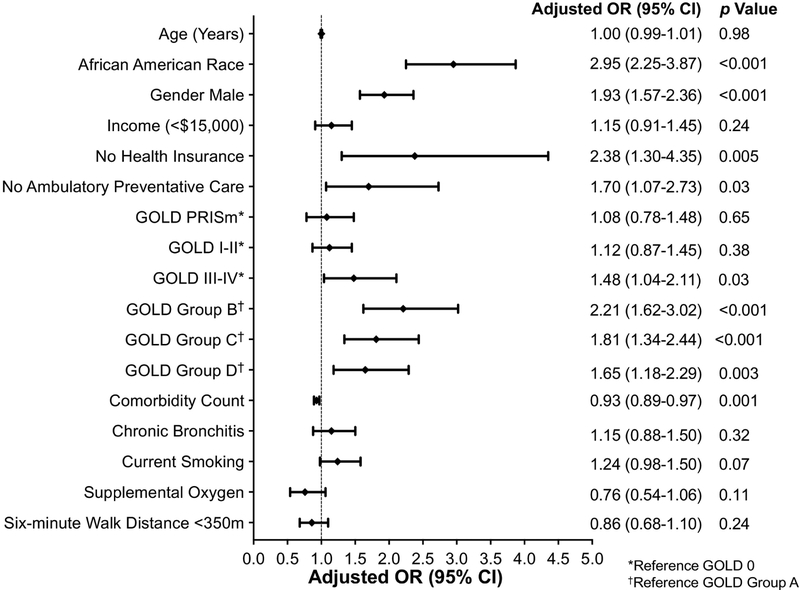
Along with the random effect of study center, we included the following clinically important variables based on the biopsychosocial model and the literature as fixed effects in the full model: age, gender, race, income, health insurance, preventative care, GOLD severity stages (reference GOLD 0), GOLD ABCD groups (reference group A), comorbidity count, chronic bronchitis, current smoking, supplemental oxygen, and six-minute walk distance <350 meters. Demographic characteristics that were associated with an increased risk of having unmedicated symptoms versus being on medications included: African American race (Adjusted OR 2.95, 95% CI 2.25–3.87, p<0.001), male gender (Adjusted OR 1.93, 95% CI 1.57–2.36, p<0.001), lack of health insurance (Adjusted OR 2.38, 95% CI 1.30–4.35, p=0.005), and no ambulatory preventative care (Adjusted OR 1.70, 95% CI 1.07–2.73, p=0.03) (Figure 3 and Appendix Table A.2). Clinical characteristics associated with an increased risk for having unmedicated symptoms included having GOLD III-IV stages of COPD (GOLD 0=reference; adjusted OR 1.48, 95% CI 1.04–2.11, p=0.03) and higher GOLD ABCD groups, with the highest risk for unmedicated symptoms in group B (reference group A; adjusted OR 2.21, 95% CI 1.62–3.02, p<0.001). We re-ran the analyses including Bupropion, a medication comonly prescribed for smoking cessation, and only GOLD III-IV stages lost statistical significance (p=0.32).
Figure 3. Characteristics Associated with Unmedicated Anxiety or Depressive Symptoms.
Mixed effects logistic regression model with unmedicated symptoms as the dependent variable compared to being on medications, study center as the random effect, and clinically important variables included in the full model. See Appendix 2 for full model. *Reference GOLD 0. †Reference GOLD Group A.
DISCUSSION
We found that significant unmet mental health care needs exist in smokers with and without COPD. In particular, significant variation exists in the frequency of depressive symptoms and the use of commonly-prescribed anxiolytic-hypnotics across GOLD severity stages. This is the first demonstration that specific subgroups of smokers are at an increased risk for having unmedicated symptoms of anxiety and depression, and the data could aid stratification of those at highest risk for having unmedicated symptoms.
We demonstrated that 25% of smokers in the COPDGene cohort have anxiety or depressive symptoms. Considering symptoms and medication use together, 38% of current and former smokers had some evidence of anxiety and depression, which is similar to other smoker populations(2, 35). Our data reveal a discordance between the frequency of symptoms and the use of medications to treat those symptoms across GOLD stages, including smokers without COPD. Anxiety symptoms are prevalent in all GOLD stages; however, depressive symptoms disproportionately affect smokers in GOLD PRISm and GOLD III-IV stages. Not assessing anxiety and depressive symptoms in smokers without COPD misses those from GOLD 0 and GOLD PRISm stages who also have emotional symptoms. On the other hand, GOLD ABCD grouping, which accounts for dyspnea, quality of life, and exacerbation history, may provide a more effective way of discerning smokers with symptoms of anxiety and depression. We found that anxiety symptoms are over three times more frequent in GOLD groups B, C, and D compared to group A, and depressive symptoms are six times more frequent in GOLD group D compared to group A. The magnitude of differences in psychological symptoms and medication use between GOLD groups were greater than we initially hypothesized, and future studies should validate this finding.
Overall, 8% of the cohort was on a commonly-prescribed anxiolytic-hypnotic, while 16% was on a commonly-prescribed antidepressant. Existing literature raises concerns that benzodiazepine use may cause respiratory suppression or adverse outcomes in COPD, particularly in older patients and those with chronic hypercapnia, which may limit use of benzodiazepines(36–38). GOLD guidelines mention these concerns but do not provide conclusive recommendations regarding pharmacologic treatment of anxiety and depressive symptoms in smokers(18). The data confirm our initial hypothesis that gaps between mental healthcare guidelines and clinical practice exist.
Anxiolytic-hypnotics provide quick relief of anxiety symptoms, and the distress associated with worsening dyspnea may explain, in part, why anxiolytic-hypnotics are more frequent in severe-very severe COPD and in group D (39). We found that anxiolytic-hypnotics are prescribed at nearly twice the frequency in these groups. Guidelines instead recommend SSRIs and SNRIs for sustained control of both anxiety and depressive symptoms in general populations(16, 24), and recent data suggest that SSRIs and SNRIs improve adherence to COPD inhalers(24, 40). We discovered a higher frequency of depressive symptoms without an increased frequency of antidepressant use in those with severe-very severe COPD. Reasons for this are likely multifactorial including lack of awareness of treatment recommendations, provider inexperience initiating and titrating antidepressants in smokers, or bias that symptoms of depression are expected in the context of advanced illness(15, 41). Depressive symptoms in severe-very severe COPD may be due to patients’ reaction to loss of function; however, in depression following a major life loss, antidepressant medications successfully improve symptoms(42). Our data support this in that 60.2% of smokers on medications did not report elevated anxiety or depressive symptoms (Figure 1). Future work is needed to examine why antidepressant use does not match the higher frequency of symptoms in severe to very severe COPD and GOLD PRISm.
Unmedicated symptoms affected nearly one in five smokers, or one in three who had elevated anxiety or depressive symptoms in our cohort. Lack of health insurance was associated with a high risk for not receiving medication treatment in the context of elevated anxiety or depressive symptoms. Prior data have shown that the uninsured are at an increased risk for having anxiety and depression(43) and that mental health-related outcomes improve for these patients after expansion of health insurance coverage, likely related in part to reduced constraints and expanded range of available mental health services when insured(44–46). We also discovered that significant racial and gender disparities in unmet mental healthcare needs exist in smokers. Though we were limited by the lack of data on Hispanics in the COPDGene study, our results are consistent with other studies showing that African Americans have a significantly lower utilization of mental health care services(12, 47). The association between male gender and unmedicated symptoms may indicate a degree of stigma, stoicism regarding emotional symptoms, lack of access to mental health services for men in particular (12, 47).
We must consider several limitations in this analysis. First, we did not have information about anxiety or depression diagnoses. Instead, we used a self-reported measure of anxiety and depressive symptoms that is commonly used in patients with COPD and has been examined in multiple settings(22, 48). Second, we did not have access to data on psychotherapy or palliative care, and the rate of referral to cardiopulmonary rehabilitation was low (6.7%). Furthermore, we did not know the specific indication for a psychiatric medication, and it is possible that subjects may have previously tried psychiatric medications, did not report medication use, or were noncompliant. Self-report may have thus introduced recall bias. Still, medications are a cornerstone of treatment for elevated anxiety and depressive symptoms, and no large cohort studies to date have reported the frequency of psychiatric medication use among current and former smokers with and without COPD nor done so in the context of elevated anxiety and depressive symptoms. Our clear approach to classifying medications into commonly-prescribed anxiolytic-hypnotics and antidepressants(10) is a key contribution of the manuscript that is clinically relevant, maximizes likelihood that participants took these medications for psychiatric conditions, is generalizable, and influences future trial designs. Our results fill gaps in the literature and identify a subpopulation of smokers at highest risk for not receiving medication treatment and may benefit from further assessment.
CONCLUSIONS
Significant unmet mental healthcare needs exist in smokers especially those with severe-very severe COPD and with higher symptom burden or exacerbation history. Smokers from all GOLD severity stages struggle with anxiety symptoms, but a discordance exists in that those with severe-very severe COPD have the highest frequency of depressive symptoms yet receive the highest frequency of anxiolytichypnotics. One in five current and former smokers have unmedicated symptoms, and demographic and clinical characteristics identify those at greatest need. Future studies investigating the impact of unmedicated symptoms on disease outcomes are warranted.
Highlights.
Significant unmet mental healthcare needs exist in current and former smokers.
Symptoms and treatment of anxiety/depression vary across COPD severity stages.
One in five smokers have unmedicated anxiety/depressive symptoms.
Subgroups of smokers are at risk for unmedicated anxiety/depressive symptoms.
Characteristics identify smokers at highest risk for having unmedicated symptoms.
Acknowledgments
Primary Funding Source: National Institutes of Health and The COPD Foundation.
SOURCES OF SUPPORT
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf. The COPDGene Study is supported by NHLBIU01 HL089897 and U01 HL089856. The COPDGene study (NCT00608764) is also supported by the COPD Foundation through contributions made to an Industry Advisory Committee comprised of AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, and Sunovion. ASI acknowledges support in part by a T32 training grant (T32HS013852) and an institutional UAB K12 in Patient Centered Outcomes Research (K12HS023009) from the Agency for Healthcare Research and Quality. KFH acknowledges grant support from the NHLBI (K23HL095658). VK has received grant support from the NHLBI, has been on advisory boards for CSA Medical, Concert Pharmaceuticals, AstraZeneca, and Gala Therapeutics, has been a peer reviewer for MedScape, and has received monetary stipends from the American Board of Internal Medicine for serving on the Critical Care Exam Board. MTD acknowledges grants from NIH and the Department of Defense, consulting fees from AstraZeneca, Boerhinger Ingelheim, Genentech, GlaxoSmithKline, and PneumRx/BTG and contracted clinical trial funding from AstraZeneca, Boerhinger Ingelheim, GlaxoSmithKline, Yungjin, PneumRx/BTG, Pulmonx, Novartis, and Boston Scientific. SPB acknowledges grant support from the NIH (K23HL133438), and his institution receives research funding from ProterixBio Inc. CHM would like to acknowledge NIH grant support from the NHLBI (K23HL128936–01 and 3R01HL122438–02S1). NAH has received honoraria for serving as an advisor or consultant for Astra Zeneca, GSK, Boehringer Ingelheim, Sanofi/Regeneron and Novartis. His institution has received research grant support on his behalf from GSK, Boehringer Ingelheim, Astra Zeneca and Genentech. BJM reports funding from the NHLBI for the COPDGene study; grants and personal fees from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, and Sunovian; personal fees for DSMB from Spiration and Baxalta; CME personal fees from Consensus Medical Education, Integrity Medical Education, WebMD, National Jewish Health, American College of Chest Physicians, Projects in Knowledge, Hybrid Communications, SPIRE Learning, Peer Review Institute, and Medscape; personal fees and other from Mt. Sinai Medical Center; royalties from Up-To-Date; personal fees from Novartis; personal fees from CSL Bering; other from Cleveland Clinic; grants from Pearl; and personal fees from Verona, outside the submitted work. NAH has received honoraria for serving as an advisor or consultant for Astra Zeneca, GSK, Boehringer Ingelheim, Sanofi/Regeneron and Novartis. His institution has received research grant support on his behalf from GSK, Boehringer Ingelheim, Astra Zeneca and Genentech. KEH, GLK, FSW, MRJ, EAR, HFA, KEL, MGF, GS, and RAW have no conflicts to report.
Appendix Table A.1. STROBE Statement - Checklist of Items That Should be Included in Reports of Cohort Studies
| Item No | Recommendation | ||||
|---|---|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract (p. 1) | |||
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found (pp. 4–5) | |||||
| Introduction | |||||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported (p. 6) | |||
| Objectives | 3 | State specific objectives, including any prespecified hypotheses (P-7) | |||
| Methods | |||||
| Study design | 4 | Present key elements of study design early in the paper (pp. 7–8) | |||
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection (p. 7–8) | |||
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up, (pp. 7–8) | |||
| (b) For matched studies, give matching criteria and number of exposed and unexposed (n/a) | |||||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable (pp. 8–10) | |||
| Data sources/measurement | 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group, (pp. 8–11) | |||
| Bias | 9 | Describe any efforts to address potential sources of bias (pp. 10–11) | |||
| Study size | 10 | Explain how the study size was arrived at (pp. 8–10) | |||
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why. (p.10–11) | |||
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding (pp. 10–11) | |||
| (b) Describe any methods used to examine subgroups and interactions (pp. 10–11) | |||||
| (c) Explain how missing data were addressed (p.11) | |||||
| (d) If applicable, explain how loss to follow-up was addressed (n/a) | |||||
| (e) Describe any sensitivity analyses (n/a) | |||||
| Results | |||||
| Participants | 13 | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed (p. 11) | |||
| (b) Give reasons for non-participation at each stage (n/a) | |||||
| (c) Consider use of a flow diagram (Figure 1) | |||||
| Descriptive data | 14 | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders (p. 11) | |||
| (b) Indicate number of participants with missing data for each variable of interest (p. 11) | |||||
| (c) Summarize follow-up time (eg, average and total amount) (n/a) | |||||
| Outcome data | 15 | Report numbers of outcome events or summary measures over time (pp. 11–13) | |||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included (Appendix Table 2) | |||
| (b) Report category boundaries when continuous variables were categorized (pp. Table 1) | |||||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period (n/a) | |||||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses (pp. 11–13) | |||
| Discussion | |||||
| Key results | 18 | Summarize key results with reference to study objectives (pp. 14–15) | |||
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias (pp. 17–18) | |||
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence (p. 17–18) | |||
| Generalizability | 21 | Discuss the generalizability (external validity) of the study results (p. 17–18) | |||
| Other information | |||||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based (pp. 11,19) | |||
Appendix Table A.2. Characteristics Associated with Unmedicated Anxiety or Depressive Symptoms as Compared to Being on Medications – A Mixed Effects Logistic Regression Model (n=2016)
|
p Value |
p Value |
|
|---|---|---|
| Age (Years) | <0.001 | 0.98 |
| African American Race | <0.001 | <0.001 |
| Male Gender | <0.001 | <0.001 |
| Income <$15,000 | <0.001 | 0.24 |
| No Health Insurance | <0.001 | 0.005 |
| No Ambulatory Preventative Care | <0.001 | 0.03 |
| GOLD PRISm* | 0.09 | 0.65 |
| GOLD I-II* | 0.59 | 0.38 |
| GOLD III-IV* | 0.03 | 0.03 |
| GOLD B† | <0.001 | <0.001 |
| GOLD C† | <0.001 | <0.001 |
| GOLD D† | <0.001 | 0.003 |
| Comorbidity Count | <0.001 | 0.001 |
| Chronic Bronchitis | 0.009 | 0.32 |
| Current Smoking | <0.001 | 0.07 |
| Supplemental Oxygen | 0.03 | 0.11 |
| Six-minute Walk Distance <350m | 0.08 | 0.24 |
Reference GOLD 0.
Reference GOLD Group A.
Notes: Unmedicated Anxiety and Depressive Symptoms = Anxiety/depressive symptoms and not on a commonly prescribed anxiolytic-hypnotic or antidepressant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kamal R, Cox C, Rousseau D, Kaiser Family F. Costs and Outcomes of Mental Health and Substance Use Disorders in the US. JAMA. 2017;318(5):415. [DOI] [PubMed] [Google Scholar]
- 2.Yohannes AM, Mullerova H, Hanania NA, Lavoie K, Tal-Singer R, Vestbo J, et al. Long-term Course of Depression Trajectories in Patients With COPD: A 3-Year Follow-up Analysis of the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints Cohort. Chest. 2016;149(4):916–26. [DOI] [PubMed] [Google Scholar]
- 3.Panagioti M, Scott C, Blakemore A, Coventry PA. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. International journal of chronic obstructive pulmonary disease. 2014;9:1289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jasek JP, Williams JM, Mandel-Ricci J, Johns M. Trends in smoking among adults with serious psychological distress during comprehensive tobacco control in New York City, 2003–2012. Tob Control. 2015;24(6):622–3. [DOI] [PubMed] [Google Scholar]
- 5.Sung HY, Prochaska JJ, Ong MK, Shi Y, Max W. Cigarette smoking and serious psychological distress: a population-based study of California adults. Nicotine Tob Res. 2011;13(12):1183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emre N, Topal K, Bozkurt N, Topaktas E. Mental health screening and increased risk for anxiety and depression among treatment-seeking smokers. Tob Induc Dis. 2014;12(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin RD, Wall MM, Garey L, Zvolensky MJ, Dierker L, Galea S, et al. Depression among current, former, and never smokers from 2005 to 2013: The hidden role of disparities in depression in the ongoing tobacco epidemic. Drug Alcohol Depend. 2017;173:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker ER, Cummings JR, Hockenberry JM, Druss BG. Insurance status, use of mental health services, and unmet need for mental health care in the United States. Psychiatr Serv. 2015;66(6):578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olfson M, Blanco C, Marcus SC. Treatment of Adult Depression in the United States. JAMA Intern Med. 2016;176(10):1482–91. [DOI] [PubMed] [Google Scholar]
- 10.Qaseem A, Barry MJ, Kansagara D, Clinical Guidelines Committee of the American College of P. Nonpharmacologic Versus Pharmacologic Treatment of Adult Patients With Major Depressive Disorder: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2016;164(5):350–9. [DOI] [PubMed] [Google Scholar]
- 11.Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Borkovec TD, Rickels K, et al. Consensus statement on generalized anxiety disorder from the International Consensus Group on Depression and Anxiety. J Clin Psychiatry. 2001;62 Suppl 11:53–8. [PubMed] [Google Scholar]
- 12.De Luca SM, Blosnich JR, Hentschel EA, King E, Amen S. Mental Health Care Utilization: How Race, Ethnicity and Veteran Status are Associated with Seeking Help. Community Ment Health J. 2016;52(2):174–9. [DOI] [PubMed] [Google Scholar]
- 13.Wagena EJ, Kant I, Huibers MJ, van Amelsvoort LG, Swaen GM, Wouters EF, et al. Psychological distress and depressed mood in employees with asthma, chronic bronchitis or emphysema: a population-based observational study on prevalence and the relationship with smoking cigarettes. Eur J Epidemiol. 2004;19(2):147–53. [DOI] [PubMed] [Google Scholar]
- 14.Weinberger AH, Bandiera FC, Leventhal AM, Dierker LC, Gbedemah M, Tidey JW, et al. Socioeconomic Disparities in Smoking Among U.S. Adults With Depression, 2005–2014. Am J Prev Med. 2018;54(6):765–75. [DOI] [PubMed] [Google Scholar]
- 15.Herzog DP, Wagner S, Ruckes C, Tadic A, Roll SC, Harter M, et al. Guideline adherence of antidepressant treatment in outpatients with major depressive disorder: a naturalistic study. Eur Arch Psychiatry Clin Neurosci. 2017;267(8):711–21. [DOI] [PubMed] [Google Scholar]
- 16.Sartorius N, Baghai TC, Baldwin DS, Barrett B, Brand U, Fleischhacker W, et al. Antidepressant medications and other treatments of depressive disorders: a CINP Task Force report based on a review of evidence. Int J Neuropsychopharmacol. 2007;10 Suppl 1:S1–207. [DOI] [PubMed] [Google Scholar]
- 17.Norwood R, Balkissoon R. Current perspectives on management of co-morbid depression in COPD. COPD. 2005;2(1):185–93. [DOI] [PubMed] [Google Scholar]
- 18.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–82. [DOI] [PubMed] [Google Scholar]
- 19.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 21.Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. 2003;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu W, Collet JP, Shapiro S, Lin Y, Yang T, Platt RW, et al. Independent effect of depression and anxiety on chronic obstructive pulmonary disease exacerbations and hospitalizations. Am J Respir Crit Care Med. 2008;178(9):913–20. [DOI] [PubMed] [Google Scholar]
- 23.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. [DOI] [PubMed] [Google Scholar]
- 24.Gartlehner G, Gaynes BN, Amick HR, Asher GN, Morgan LC, Coker-Schwimmer E, et al. Comparative Benefits and Harms of Antidepressant, Psychological, Complementary, and Exercise Treatments for Major Depression: An Evidence Report for a Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2016;164(5):331–41. [DOI] [PubMed] [Google Scholar]
- 25.Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cote CG, Casanova C, Marin JM, Lopez MV, Pinto-Plata V, de Oca MM, et al. Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J. 2008;31(3):571–8. [DOI] [PubMed] [Google Scholar]
- 27.Semega J, Fontenot K, Kollar M 2017;Pages Accessed at United States Census Bureau at https://www.census.gov/data/tables/2017/demo/income-poverty/p60-259.html on May 2, 2018.
- 28.Putcha N, Puhan MA, Drummond MB, Han MK, Regan EA, Hanania NA, et al. A simplified score to quantify comorbidity in COPD. PLoS One. 2014;9(12):e114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7. [DOI] [PubMed] [Google Scholar]
- 30.Oberg AL, Mahoney DW. Linear mixed effects models. Methods Mol Biol. 2007;404:213–34. [DOI] [PubMed] [Google Scholar]
- 31.Kusnanto H, Agustian D, Hilmanto D. Biopsychosocial model of illnesses in primary care: A hermeneutic literature review. J Family Med Prim Care. 2018;7(3):497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai TY, Livneh H, Lu MC, Tsai PY, Chen PC, Sung FC. Increased risk and related factors of depression among patients with COPD: a population-based cohort study. BMC Public Health. 2013;13:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JH, Park MA, Park MJ, Jo YS. Clinical characteristics and related risk factors of depression in patients with early COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:1583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez Rivera C, Costan Galicia J, Alcazar Navarrete B, Garcia-Polo C, Ruiz Iturriaga LA, Herrejon A, et al. Factors Associated with Depression in COPD: A Multicenter Study. Lung. 2016;194(3):335–43. [DOI] [PubMed] [Google Scholar]
- 35.Xu W, Collet JP, Shapiro S, Lin Y, Yang T, Platt RW, et al. Independent effect of depression and anxiety on chronic obstructive pulmonary disease exacerbations and hospitalizations. American journal of respiratory and critical care medicine. 2008;178(9):913–20. [DOI] [PubMed] [Google Scholar]
- 36.Halvorsen T, Martinussen PE. Benzodiazepine use in COPD: empirical evidence from Norway. Int J Chron Obstruct Pulmon Dis. 2015;10:1695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroll DS, Nieva HR, Barsky AJ, Linder JA. Benzodiazepines are Prescribed More Frequently to Patients Already at Risk for Benzodiazepine-Related Adverse Events in Primary Care. J Gen Intern Med. 2016;31(9):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vozoris NT, Fischer HD, Wang X, Stephenson AL, Gershon AS, Gruneir A, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332–40. [DOI] [PubMed] [Google Scholar]
- 39.Di Marco F, Verga M, Reggente M, Maria Casanova F, Santus P, Blasi F, et al. Anxiety and depression in COPD patients: The roles of gender and disease severity. Respir Med. 2006;100(10):1767–74. [DOI] [PubMed] [Google Scholar]
- 40.Wei YJ, Simoni-Wastila L, Albrecht JS, Huang TY, Moyo P, Khokhar B, et al. The association of antidepressant treatment with COPD maintenance medication use and adherence in a comorbid Medicare population: A longitudinal cohort study. Int J Geriatr Psychiatry. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiwari A, Rajan M, Miller D, Pogach L, Olfson M, Sambamoorthi U. Guideline-consistent antidepressant treatment patterns among veterans with diabetes and major depressive disorder. Psychiatr Serv. 2008;59(10):1139–47. [DOI] [PubMed] [Google Scholar]
- 42.Baghai TC, Eser D, Moller HJ. Effects of different antidepressant treatments on the core of depression. Dialogues Clin Neurosci. 2008;10(3):309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward BW, Martinez ME. Health Insurance Status and Psychological Distress among US Adults Aged 18–64 Years. Stress Health. 2015;31(4):324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phalen PL. Psychological Distress and Rates of Health Insurance Coverage and Use and Affordability of Mental Health Services, 2013–2014. Psychiatr Serv. 2016: [DOI] [PubMed] [Google Scholar]
- 45.West JC, Clarke DE, Duffy FF, Barber KD, Mojtabai R, Moscicki EK, et al. Availability of Mental Health Services Prior to Health Care Reform Insurance Expansions. Psychiatr Serv. 2016;67(9):983–9. [DOI] [PubMed] [Google Scholar]
- 46.Rowan K, McAlpine DD, Blewett LA. Access and cost barriers to mental health care, by insurance status, 1999–2010. Health Aff (Millwood). 2013;32(10):1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hahm HC, Cook BL, Ault-Brutus A, Alegria M. Intersection of race-ethnicity and gender in depression care: screening, access, and minimally adequate treatment. Psychiatr Serv. 2015;66(3):258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smid DE, Franssen FM, Houben-Wilke S, Vanfleteren LE, Janssen DJ, Wouters EF, et al. Responsiveness and MCID Estimates for CAT, CCQ, and HADS in Patients With COPD Undergoing Pulmonary Rehabilitation: A Prospective Analysis. J Am Med Dir Assoc. 2016. [DOI] [PubMed] [Google Scholar]