Abstract
Gorham-Stout disease (GSD) was first described by Gorham and colleagues in 1954, but its precise mechanism and cause remain to be elucidated. In this condition, voluminous and potentially fatal chylous effusions into the thorax can occur. Herein, we describe a case of GSD in which the patient presented with massive pleural effusions and mottled osteolytic bone lesions. We performed multiple operations, including thoracic duct ligation using video-assisted thoracoscopic surgery and thoracotomic decortication, but these procedures did not succeed in preventing recurrent pleural effusion and chest wall lymphedema. After administering sirolimus (0.8 mg/m2, twice a day) and propranolol (40 mg, twice a day), the process of GSD in this patient has been controlled for more than 2 years.
Keywords: Gorham-Stout disease, Pleural effusion, Sirolimus, Chylothorax
Case report
An 11-year-old girl weighing 39.4 kg, without any specific medical or family history, was admitted with fever, cough, and dyspnea. A chest radiograph and computed tomography (CT) scan showed a massive right-sided pleural effusion with mediastinal shifting (Fig. 1). A closed thoracotomy drained a dark brownish exudative effusion that was found to be rich in fat content with no microorganism growth, proving its chylous origin. A CT scan showed multiple osteolytic lesions with resorption of cortical bone involving the right clavicle and first rib, as well as tiny splenic cysts; overall, these features were consistent with Gorham-Stout disease (GSD). A laboratory blood test showed elevated alkaline phosphatase and eosinophilia, although without clinical significance. These findings were also consistent with reported cases of GSD [1].
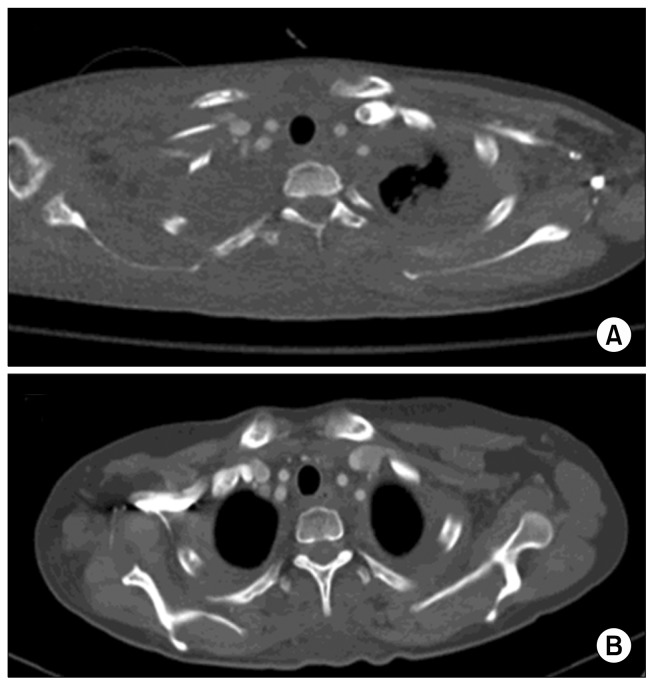
Fig. 1.
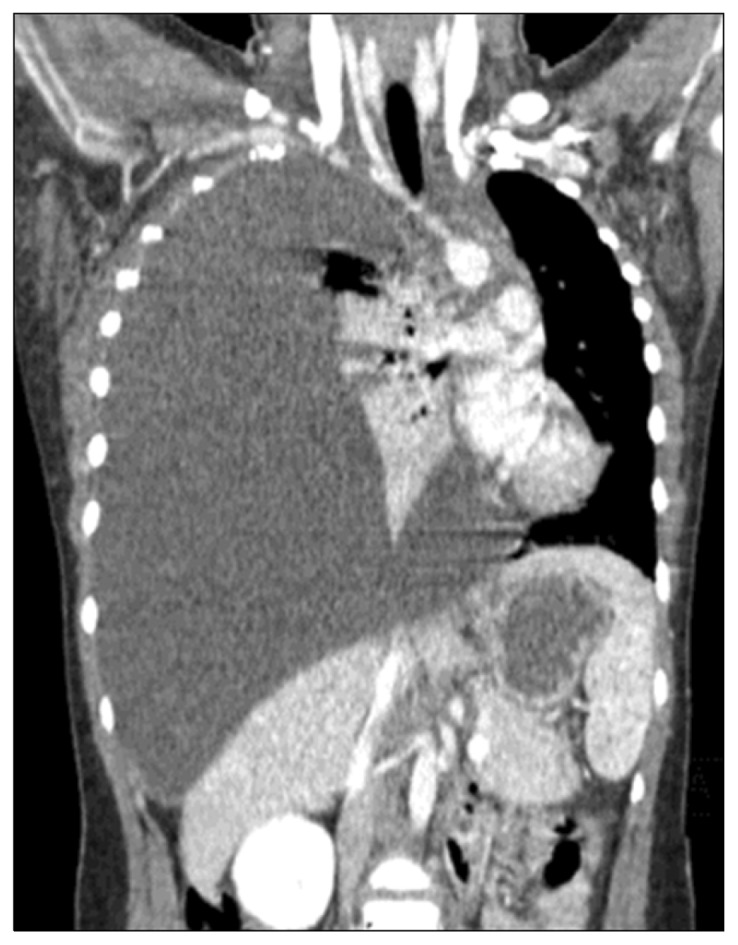
A chest computed tomography scan showed a massive right-sided pleural effusion with mediastinal shifting.
She was put on parenteral hyperalimentation and somatostatin administration, but 2–3 L of daily chest tube drainage persisted, and lymphoscintigraphy showed abnormal radioactivity at the T11–T12 levels of the spine, suggesting chyle leakage. She was then transferred to our medical facility for video-assisted thoracoscopic surgery (VATS) thoracic duct ligation. The operative findings via VATS revealed that the mediastinum was filled with chyle with active leakage, as well as atrophic changes in the surrounding connective and fat tissue. The thoracic duct was identified at the level of T11 and ligated, yielding an immediate intraoperative decrease in chyle leakage. Adhesive materials were used in the surrounding tissue to prevent leakage recurrence.
The amount of drainage through the chest tube occasionally approached 1 L/day, but the average amount decreased to about 200–300 mL/day after surgery. She was put on a regular diet from time to time, but doing so resulted in an immediate increase in the left chest tube drainage (up to 1.5 L/day). Thirty days after surgery, the left chest tube showed a daily drainage of about 50–100 mL per day, and the drain was successfully removed 41 days after the initial procedure. She was eventually discharged with mild, loculated pleural effusions in the right pleural cavity and her left side clear of effusion. However, 5 months after discharge, a chest radiograph revealed increased effusions on both sides that required drainage (Fig. 2). Radiotherapy was considered because several successful cases have been reported in the literature, but due to the progression of osteolytic lesions in the patient’s right scapula, right clavicle, T1–2 spinous process, and right first and second ribs, the decision was made to conduct conservative management via tube drainage. However, the chest tube drainage did not decrease, and the patient underwent decortication on both sides via thoracotomy for the loculated effusions. The operative findings included multiple septate effusions with a bloody color from the apex to diaphragm, as well as severe pleural thickening and massive adhesions. The initial drainage in the operating room was 3.5 L on the left side and 1 L on the right side. Her vascular endothelial growth factor level, measured via an enzyme-linked immunosorbent assay kit, was 74 pg/mL, and she was started on propranolol, followed by sirolimus a month later after a thorough review of the literature and identification of a relevant case report. Propranolol was administered, at 40-mg doses twice a day. Sirolimus was administered at 0.8 mg/m2 twice a day and titrated based on a trough level goal of 9 to 12 μg/L. The major adverse effects of sirolimus are dysmenorrhea and galactose intolerance. And the major adverse effects of propranolol are bradycardia and orthopedic hypotension. The plan was made to determine the duration of the medication based on the patient’s clinical course, since cases of recurrence after discontinuation of medication have been reported [2]. A week after the initial administration of sirolimus, we performed lymphangiography with embolization of the right thoracic lymphatic vessels. Thirty days after the initial administration of sirolimus, both chest tubes were removed, and as of 2 years of follow-up, she has not undergone any additional thoracotomies for drainage and her osteolytic lesions have not worsened. The patient is still currently taking propranolol and sirolimus, and her symptoms of GSD are well-controlled.
Fig. 2.
(A, B) A chest radiograph revealed increased effusions on both sides.
Written informed consents were obtained from the patient.
Discussion
The development of chylothorax in GSD is a rare and possibly fatal complication. The histology and pathophysiology of this disease still remain an area for further research. GSD can occur at any age without a sex predilection or familial inheritance pattern, which must be considered when evaluating the possibility of other familial osteolytic disorders in the differential diagnosis [3]. It is characterized by aggressively progressive, yet non-neoplastic, vascular and lymphatic channel proliferation and possible resorption of one or more skeletal bones. These changes can remain undetected until they manifest through clinical symptoms, such as pathologic bone fracture, subcutaneous swelling, and even pericardial effusion or chylothorax, which can be life-threatening if uncontrolled [4]. Chylothorax, a rare complication of GSD, usually presents with shoulder girdle or thoracic vertebral bony osteolysis [1]. Chylothorax in GSD patients is thought to stem from either the direct invasion of lymphatic dysplasia to the thoracic duct or the invasion of pre-existing bone lesions into the pleural cavity [1]. Several methods of treating GSD have been reported, including radiotherapy, surgical interventions, bisphosphonates, and interferon therapy [5]. In our case, surgical thoracic duct ligation seemed effective initially, but recurrence occurred soon after. Further tube drainage and pleurodesis provided no benefit and only resulted in pleural adhesions, which required even further surgical interventions. Sirolimus, a mammalian target of rapamycin inhibitor, integrates signals from p23k/AKT pathway to coordinate proper cell growth and cell proliferation. Sirolimus exerts its effects by inhibiting motor signaling. It express the vascular endothelial growth factor which is a key regulator of angiogenesis and lymphangiogenesis [6]. A case report described its use in a patient with GSD with massive chylous pleural effusions [7]. Propranolol, a non-selective β-adrenergic receptor antagonist, has shown inconclusive findings in the literature, but it is starting to play a role in the treatment of lymphatic anomalies [2].
Our surgical intervention, thoracic duct ligation, initially seemed helpful, but anterolateral chylothorax and chest wall lymphedema developed. However, it may have been preferable to consider pleurectomy to relieve the effusions caused by pleural lymphatic dysplasia when performing the first operation. The role of surgery in the chylous manifestations of GSD appears limited in cases with this type of lymphatic malformation and osteolytic disease. Systemic treatment, including sirolimus medication, should be considered to control disease progression.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Tie ML, Poland GA, Rosenow EC., 3rd Chylothorax in Gorham’s syndrome: a common complication of a rare disease. Chest. 1994;105:208–13. doi: 10.1378/chest.105.1.208. [DOI] [PubMed] [Google Scholar]
- 2.Wu JK, Hooper ED, Laifer-Narin SL, et al. Initial experience with propranolol treatment of lymphatic anomalies: a case series. Pediatrics. 2016;138:e20154545. doi: 10.1542/peds.2015-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gondivkar SM, Gadbail AR. Gorham-Stout syndrome: a rare clinical entity and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e41–8. doi: 10.1016/j.tripleo.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 4.Kiran DN, Anupama A. Vanishing bone disease: a review. J Oral Maxillofac Surg. 2011;69:199–203. doi: 10.1016/j.joms.2010.05.088. [DOI] [PubMed] [Google Scholar]
- 5.Jayaprakash B, Prajeesh B, Nair DS. Gorham’s disease. J Assoc Physicians India. 2013;61:941–3. [PubMed] [Google Scholar]
- 6.Wang S, Lu J, You Q, Huang H, Chen Y, Liu K. The mTOR/AP-1/VEGF signaling pathway regulates vascular endothelial cell growth. Oncotarget. 2016;7:53269–76. doi: 10.18632/oncotarget.10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer SL, Wei S, Merrow AC, Pressey JG. Gorham-Stout disease successfully treated with sirolimus and zoledronic acid therapy. J Pediatr Hematol Oncol. 2016;38:e129–32. doi: 10.1097/MPH.0000000000000514. [DOI] [PubMed] [Google Scholar]