Abstract
Objectives
To perform a systematic review and network meta-analysis comparing stone-free rates following retrograde intrarenal surgery (RIRS), extracorporeal shock wave lithotripsy (SWL), and percutaneous nephrolithotomy (PCNL) treatments of renal stones.
Materials and methods
Clinical trials comparing RIRS, SWL, and PCNL for treatment of renal stones were identified from electronic databases. Stone-free rates for the procedures were compared by qualitative and quantitative syntheses (meta-analyses). Outcome variables are shown as risk ratios (ORs) with 95% credible intervals (CIs).
Results
A total of 35 studies were included in this network meta-analysis of success and stone-free rates following three different treatments of renal stones. Six studies compared PCNL versus SWL, ten studies compared PCNL versus RIRS, fourteen studies compared RIRS versus SWL, and five studies compared PCNL, SWL, and RIRS. The quality scores within subscales were relatively low-risk. Network meta-analyses indicated that stone-free rates of RIRS (OR 0.38; 95% CI 0.22–0.64) and SWL (OR 0.12; 95% CI 0.067–0.19) were lower than that of PCNL. In addition, stone-free rate of SWL was lower than that of RIRS (OR 0.31; 95% CI 0.20–0.47). Stone free rate of PCNL was also superior to RIRS in subgroup analyses including ≥ 2 cm stone (OR 4.680; 95% CI 2.873–8.106), lower pole stone (OR 1.984; 95% CI 1.043–2.849), and randomized studies (OR 2.219; 95% CI 1.348–4.009). In rank-probability test, PCNL was ranked as No. 1 and SWL was ranked as No. 3.
Conclusions
PCNL showed the highest success and stone-free rate in the surgical treatment of renal stones. In contrast, SWL had the lowest success and stone-free rate.
Introduction
Urinary tract calculi, one of the most common benign urological diseases, is seen in 12% of patients and has a recurrence rate of approximately 50% [1, 2]. Factors that may play an important role in the increase of urinary tract stone disease include increases in diagnosis of metabolic syndrome, lifestyle changes, dehydration, lack of water intake, and low urine volume [3]. Furthermore, recent studies have shown that the worldwide increase of renal colic and renal stones is affected by seasonal changes, particularly the hot season, and that global warming is capable of increasing the incidence of renal stones [4]. In particular, renal uric acid stones show a tendency to increase in hot and dry climates because of the reduction of urine excretion and urine pH [5].
The European Association of Urology (EAU) Urolithiasis Guidelines suggest that the primary treatment of renal stones <2 cm should include extracorporeal shock wave lithotripsy (SWL) and retrograde intrarenal surgery (RIRS) and that the primary treatment for renal stones >2 cm should include percutaneous nephrolithotomy (PCNL) [6]. In cases of 1–2-cm lower pole renal stones, RIRS or PCNL is recommended if there are unfavorable factors in SWL. In comparison with PCNL and RIRS, SWL plays a pivotal role in the treatment of urinary tract stones because it is the only interventional treatment with non-invasive properties [7]. In contrast with SWL, RIRS can perform stone dusting and fragmentation under endoscopic direct vision and has the advantage of being able to directly remove the fragmented stone using a stone basket [8]. PCNL is the standard treatment for large, renal stones (>2 cm) and can also be considered as a treatment option for large stones with resistance to shock waves [9]. Though prospective studies and a meta-analysis of the three treatments along with their advantages and disadvantages have been reported, a network meta-analysis that compares all three treatments at the same time has not yet been reported. Network meta-analysis is a research method that can compare multiple treatments using direct comparison and indirect comparison methods [10–12]. Therefore, we performed a systematic review and a network meta-analysis analysis that compares the success as well as the stone-free rates of SWL, RIRS, and PCNL.
Materials and methods
Inclusion criteria
Published clinical studies that were in accordance with the following criteria were included: (i) study design assessed two or three methods, including SWL, PCNL, and RIRS, to treat renal stones; (ii) baseline characteristics of patients from two or three groups were matched, including the total number of subjects and the values of each index; (iii) outcomes of SWL, PCNL, and RIRS were analyzed by stone-free or success rates according to each group; (iv) standard indications for SWL, PCNL, and RIRS to treat renal stones were accepted; (v) endpoint outcome parameters also included complication rate; (vi) the full text of the study was available in English. This report was prepared in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (accessible at http://www.prisma-statement.org/) [13]. The protocol for this study is shown in S1 Table.
Search strategy
A literature search of all publications before 31 June 2016 was performed using EMBASE and PubMed. Additionally, a cross-reference search of eligible articles was performed to identify studies that were not found during the computerized search. The proceedings of appropriate meetings were also searched. Combinations of the following MeSH terms and keywords were used: extracorporeal shock wave lithotripsy, shock wave lithotripsy, percutaneous nephrolithotomy, nephrolithotomy, percutaneous, flexible ureteroscopy, flexible ureterorenoscopy, retrograde intrarenal surgery, renal stone, urolithiasis, rate, and stone-free (S2 Table).
Data extraction
Two researcher (DYC and DHK) screened all titles and abstracts identified by the search strategy. Two other researchers (HDJ and JKK) independently evaluated the full text of each paper to determine whether it met the inclusion criteria. Disagreements were resolved by discussion until a consensus was reached or by arbitration mediated by another researcher (JYL).
Quality assessment for studies
When the final group of articles was agreed upon, two researchers independently examined the quality of each article using the Downs and Black checklist. The Downs and Black checklist was developed for the purpose of quality assessment of both randomized and nonrandomized studies of health interventions [14]. The checklist consists of five subscales: reporting, internal validity bias, internal validity confounding, external validity, and power. Because six items in the original list were related to intervention, randomization, and power calculation, and not all of the studies examined were randomized studies, the scores for these six items were counted as zero, as suggested in a previous study [15]. Therefore, the maximum quality score was 31 points. A higher score was considered to be an indicator of a good quality study.
Heterogeneity tests
Heterogeneity of included studies was examined using the Q statistic and Higgins’ I2 statistic [16]. Higgins’ I2 measures the percentage of total variation due to heterogeneity rather than chance across studies. Higgins’ I2 was calculated as follows:
in which “Q” is Cochran's heterogeneity statistic and “df” is the degrees of freedom.
An I2 with I degrees of freedom represents substantial heterogeneity [17]. For the Q statistic, heterogeneity was deemed to be significant for p<0.10 [18]. If there was evidence of heterogeneity, the data were analyzed using a random-effects model. Studies in which positive results had been confirmed were assessed with a pooled specificity using 95% CIs. In addition, L’Abbe plot and Galbraith’s radial plot were created to evaluate heterogeneity [19, 20].
Ethics statement
The study was exempt from requiring the participants’ written informed consent because this is systematic review and network meta-analysis. The approval of the Institutional Review Board was also exempted.
Statistical analysis
Outcome variables measured at specific time points were compared in terms of odds ratios (OR) or mean differences with 95% CIs using a network meta-analysis. Analyses were based on non-informative priors for effect sizes and precision. Convergence and lack of auto-correlation were confirmed after four chains and a 50,000-simulation burn-in phase. Finally, direct probability statements were derived from an additional 100,000-simulation phase. The probability that each group had the lowest rate of clinical events was assessed by Bayesian Markov Chain Monte Carlo modeling. Sensitivity analyses were performed by repeating the main computations with a fixed-effect method. Model fit was appraised by computing and comparing estimates for deviance and deviance information criterion. All statistical analyses were performed with R (R version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org) and the associated meta, netmeta, pcnetmeta, and gemtc packages for pairwise and network meta-analyses.
Results
Eligible studies
The database search retrieved 35 articles covering 237 studies for potential inclusion in meta-analysis. Eight articles were excluded according to the inclusion/exclusion criteria; three had no data on stone-free rate, three were reviews, and two reported case series. The remaining 35 articles were included in the qualitative and quantitative syntheses using pairwise and network meta-analyses (Fig 1).
Fig 1. Flow diagram of evidence acquisition.
Thirteen studies were ultimately included in the qualitative and quantitative review that used pairwise and network meta-analyses.
Data corresponding to confounding factors derived from each study are summarized in Table 1. Six studies compared PCNL and SWL [21–26]. Ten trials reported outcomes between PCNL and RIRS [27–36]. Fourteen studies compared outcomes between RIRS and SWL [37–50]. Five articles compared PCNL, SWL, and RIRS [51–55] (Fig 2). Stone-free rates of enrolled studies are summarized in Table 1.
Table 1. Enrolled studies for current meta-analysis.
| Category | Study | Year | Methods | Study Design | Inclusion Criteria | No. of Patients | Follow-up | Definition of Stone-free | Stone-free Patients (No.) | Stone-free Rate (%) | Complication (No.) | Quality Assessment | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clavien I-II |
Clavien III-IV | ||||||||||||
| PCNL vs. SWL | Netto et al. [21] | 1991 | PCNL | Retrospective | ≤ 3 cm, single or multiple stones | 23 | 3 months | Complete removal | 22 | 95.7 | 3 | 0 | 13 |
| SWL | 24 | 3 months | 19 | 79.2 | 1 | 0 | |||||||
| Havel et al. [22] | 1998 | PCNL | Retrospective | Solitary lower pole caliceal calculi | 73 | 1 day | Not stated | 53 | 72.6 | 51 | 5 | 15 | |
| SWL | 587 | 3 months | 335 | 57.1 | 88 | 5 | |||||||
| Albala et al. [23] | 2001 | PCNL | Randomized controlled | Symptomatic lower pole, ≤ 3 cm |
55 | 3 months | Not stated | 52 | 94.5 | 12.0 | 2 | 14 | |
| SWL | 52 | 3 months | 19 | 36.5 | 6.0 | 1 | |||||||
| Preminger et al. [24] | 2006 | PCNL | Randomized controlled | Solitary lower pole stone, ≤ 3cm |
47 | 3 months | Not stated | 45 | 95.7 | Not stated | 14 | ||
| SWL | 54 | 3 months | 19 | 35.2 | |||||||||
| Yuruk et al. [25] | 2010 | PCNL | Randomized controlled | Asymptomatic lower caliceal, ≤ 2 cm |
31 | 3 months | Not stated | 30 | 96.8 | 2.0 | 0 | 13 | |
| SWL | 31 | 3 months | 17 | 54.8 | 2.0 | 0 | |||||||
| Hassan et al. [26] | 2015 | PCNL | Retrospective | 2 to 3 cm, renal pelvis stone | 170 | Not stated | Not stated | 162 | 95.3 | 13.0 | 0 | 17 | |
| SWL | 167 | Not stated | 115 | 68.9 | 4.0 | 0 | |||||||
|
PCNL vs. RIRS |
Hyams et al. [27] | 2009 | PCNL | Retrospective | 2–3 cm, renal stone | 20 | 3 months | < 4 mm | 20 | 100.0 | Not stated | 13 | |
| RIRS | 19 | 3 months | 18 | 94.7 | |||||||||
| Akman et al. [28] | 2011 | PCNL | Retrospective | 2–4 cm, renal stone | 34 | 3 months | Not stated | 33 | 97.1 | 4.0 | 1 | 14 | |
| RIRS | 34 | 3 months | 32 | 94.1 | 3.0 | 1 | |||||||
| Bozkurt et al. [29] | 2011 | PCNL | Retrospective | 1.5–2 cm, renal stone | 42 | 2 procedures | Not stated | 41 | 97.6 | 7.0 | 0 | 13 | |
| RIRS | 37 | 2 procedures | 35 | 94.6 | 4.0 | 0 | |||||||
| Bryniarski et al. [30] | 2012 | PCNL | Randomized controlled | Renal pelvis stone, ≥ 2 cm | 32 | 3 weeks | Not stated | 30 | 93.8 | Not stated | 14 | ||
| RIRS | 32 | 3 weeks | 24 | 75.0 | |||||||||
| Jung et al. [31] | 2015 | PCNL | Retrospective | 1.5–3 cm, lower pole stone | 44 | 1 month | < 3 mm | 37 | 84.1 | 5.0 | 2 | 16 | |
| RIRS | 44 | 1 month | 41 | 93.2 | 1.0 | 1 | |||||||
| Karakoyunlu et al. [32] | 2015 | PCNL | Randomized controlled | Renal pelvis stone, > 2 cm | 30 | Final procedures | Complete removal | 26 | 86.7 | 15.0 | 0 | 17 | |
| RIRS | 30 | Final procedures | 20 | 66.7 | 19.0 | 0 | |||||||
| Koyuncu et al. [33] | 2015 | PCNL | Retrospective | Lower pole stones, ≥ 2 cm | 77 | Final procedures | Complete removal | 74 | 96.1 | 4.0 | 1 | 14 | |
| RIRS | 32 | Final procedures | 29 | 90.6 | 3.0 | 0 | |||||||
| Bas et al. [34] | 2015 | PCNL | Retrospective | Symptomatic stone-bearing calyceal diverticula | 29 | 3 months | Less than 3 mm | 24 | 82.8 | 3.0 | 3 | 13 | |
| RIRS | 25 | 3 months | 19 | 76.0 | 4.0 | 1 | |||||||
| Zengin et al. [35] | 2015 | PCNL | Retrospective | Kidney stones, ≥ 2–3 cm | 74 | 1 month | Less than 2 mm | 71 | 95.9 | 8.0 | 2 | 14 | |
| RIRS | 80 | 1 month | 65 | 81.3 | 7.0 | 0 | |||||||
| Ozayar et al. [36] | 2016 | PCNL | Prospective | Lower pole stone, ≤ 2 cm | 30 | Not stated | Not stated | 28 | 93.3 | Not stated | 13 | ||
| RIRS | 26 | 23 | 88.5 | ||||||||||
| SWL vs. RIRS | Pearle et al. [37] | 2008 | SWL | Randomized controlled | Isolated lower pole stone, < 1 cm |
26 | 3 months | Complete removal | 9 | 34.6 | 6.0 | 1 | 12 |
| RIRS | 32 | 3 months | 16 | 50.0 | 6.0 | 1 | |||||||
| Koo et al. [38] | 2011 | SWL | Retrospective | Lower pole renal calculi, ≤ 2 cm | 51 | Final procedures | Complete removal | 30 | 58.8 | 2.0 | 2 | 15 | |
| RIRS | 37 | Final procedures | 24 | 64.9 | 1.0 | 3 | |||||||
| El-Nahas et al. [39] | 2012 | SWL | Retrospective | Lower pole stones, 1 to 2 cm | 62 | 3 months | Complete removal | 42 | 67.7 | 2.0 | 1 | 16 | |
| RIRS | 37 | 3 months | 32 | 86.5 | 4.0 | 1 | |||||||
| Salem et al. [40] | 2013 | SWL | Randomized controlled | Renal stone, ≤ 2 cm |
30 | 3 months | < 3 mm | 17 | 59.7 | 7.0 | 0 | 14 | |
| RIRS | 30 | 3 months | 29 | 96.7 | 5.0 | 0 | |||||||
| Sener et al. [41] | 2014 | SWL | Randomized controlled | Lower pole stones, < 1 cm | 70 | 3 months | Not stated | 64 | 91.4 | 3.0 | 1 | 16 | |
| RIRS | 70 | 3 months | 70 | 100.0 | 3.0 | 0 | |||||||
| Singh et al. [42] | 2014 | SWL | Randomized controlled | Inferior calyceal stones, 1 to 2 cm | 35 | 1 month | Not stated | 17 | 48.3 | 15.0 | 2 | 14 | |
| RIRS | 35 | 1 month | 29 | 82.9 | 10.0 | 1 | |||||||
| Burr et al. [43] | 2015 | SWL | Retrospective | Lower pole stones | 93 | 6–12 weeks | Less than 3 mm |
23 | 24.7 | 3.0 | 0 | 14 | |
| RIRS | 68 | 6–12 weeks | 63 | 92.6 | 4.0 | 0 | |||||||
| Kumar et al. [44] | 2015 | SWL | Randomized controlled | Lower calyceal calculi, ≤ 2 cm | 90 | 3 months | Radiologic absence of stone | 74 | 82.2 | 6.0 | 0 | 15 | |
| RIRS | 90 | 3 months | 78 | 86.7 | 10.0 | 0 | |||||||
| Sener et al. [45] | 2015 | SWL | Randomized controlled | Asymptomatic lower pole, < 1 cm |
50 | 3 months | Not stated | 45 | 90.0 | 4.0 | 2 | 15 | |
| RIRS | 50 | 3 months | 46 | 92.0 | 8.0 | 6 | |||||||
| Tauber et al. [46] | 2015 | SWL | Retrospective | Renal stone, ≤ 1.5 cm | 165 | 6–12 weeks | Radiologic absence of stone | 71 | 43.0 | 9.0 | 10 | 14 | |
| RIRS | 161 | 6–12 weeks | 134 | 83.2 | 6.0 | 11 | |||||||
| Vilches et al. [47] | 2015 | SWL | RCT | Lower pole stone, ≤ 1.5 cm | 31 | 2 months | Less than 3 mm |
15 | 48.4 | 19.0 | 0 | 15 | |
| RIRS | 24 | 2 months | 17 | 70.8 | 17.0 | 0 | |||||||
| Yuruk et al. [48] | 2015 | SWL | Retrospective | Renal stone in solitary kidney patients | 30 | 3 months | Radiologic absence of stone | 22 | 73.3 | 4.0 | 11 | 14 | |
| RIRS | 18 | 3 months | 12 | 66.7 | 2.0 | 5 | |||||||
| Gokce et al. [49] | 2016 | SWL | Retrospective | horsehoe kidney (16.8±4.4 mm) (lower 12, pelvis upper 32) |
44 | 6 weeks | Less than 3 mm |
21 | 47.7 | 8.0 | 0 | 14 | |
| RIRS | 23 | 6 weeks | 17 | 73.9 | 7.0 | 0 | |||||||
| Javanmard et al. [50] | 2016 | SWL | RCT | Renal stone, 0.6 cm-2 cm | 60 | 3 months | Radiologic absence of stone | 45 | 75.0 | 13.0 | 5 | 16 | |
| RIRS | 60 | 3 months | 52 | 86.7 | 5.0 | 0 | |||||||
| PCNL vs. SWL vs. RIRS | Aboutaleb et al. [51] | 2012 | PCNL | Retrospective | Lower calyceal stone, 1–2 cm | 19 | 2 days | < 3 mm considered insignificant | 17 | 89.5 | 6.0 | 0 | 15 |
| SWL | 24 | Not stated | 15 | 62.5 | 10.0 | 0 | |||||||
| RIRS | 13 | 2 days | 11 | 84.6 | 6.0 | 0 | |||||||
| Resorlu et al. [52] | 2013 | PCNL | Retrospective | Radiolucent renal calculi, 1–2 cm |
140 | 1 procedure | Not stated | 128 | 91.4 | 28.0 | 3 | 17 | |
| SWL | 251 | Final session | 167 | 66.5 | 19.0 | 0 | |||||||
| RIRS | 46 | 1 procedure | 40 | 87.0 | 5.0 | 0 | |||||||
| Ozturk et al. [53] | 2013 | PCNL | Retrospective | Renal stone, 1.5–2 cm |
144 | Not stated | Less than 3 mm |
135 | 93.8. | 14.0 | 5 | 16 | |
| SWL | 221 | 4 months | 168 | 76.0 | 5.0 | 2 | |||||||
| RIRS | 38 | Not stated | 28 | 73.7 | 1.0 | 1 | |||||||
| Bas et al. [54] | 2014 | PCNL | Retrospective | Renal pelvis stone, ≥ 2 cm | 50 | 1 month | Not stated | 49 | 98.0 | 4.0 | 2 | 17 | |
| SWL | 52 | Mean 2.6 sessions |
45 | 86.5 | 3.0 | 1 | |||||||
| RIRS | 47 | 1 month | 43 | 91.5 | 2.0 | 1 | |||||||
| Kumar et al. [55] | 2015 | PCNL | RCT | Radiolucent lower pole renal calculi, 1–2 cm | 41 | 3 months | Less than 4 mm |
39 | 95.1 | 10.0 | 0 | 18 | |
| SWL | 42 | 3 months | 31 | 73.8 | 3.0 | 0 | |||||||
| RIRS | 43 | 3 months | 37 | 86.0 | 4.0 | 0 | |||||||
PCNL, percutaneous nephrolithotomy; SWL, shock wave lithotripsy; RIRS, retrograde intrarenal surgery
PCNL (1,205 cases), SWL (2,342 cases), RIRS (1,281 cases)
Fig 2. Network plots for included studies.

Six studies compared PCNL versus SWL. Six studies reported outcomes between PCNL and RIRS. Eight studies compared outcomes between RIRS and SWL. Four studies demonstrated the comparison for PCNL, SWL, and RIRS.
Quality assessment
The results of quality assessment based on the Downs and Black checklist are shown in Table 1. The median of the total quality scores was 14.8. Overall, the quality scores within subscales were relatively low. In most studies, external validity was not satisfactory for both significant and insignificant groups.
Heterogeneity and inconsistency assessment and publication bias
Forest plots of the pairwise meta-analysis of SWL, PCNL, and RIRS are shown in Figs 3, 4 and 5, respectively. There was no heterogeneity between PCNL and RIRS; however, there was heterogeneity between PCNL and SWL and between SWL and RIRS in each study. Thus, random-effect models were applied using the Mantel–Haenszel method for PCNL and SWL analysis and SWL and RIRS comparison (Figs 4 and 5). After selection of effect models, little heterogeneity was noted in L’Abbe plots and radial plots (Figs 6 and 7).
Fig 3. Pairwise meta-analysis of success rate in PCNL and RIRS.
Pooled data assessment of stone-free rate between PCNL and RIRS showing a significantly higher stone-free rate with PCNL (OR 2.31; 95% CI 1.45–3.67; P<0.001).
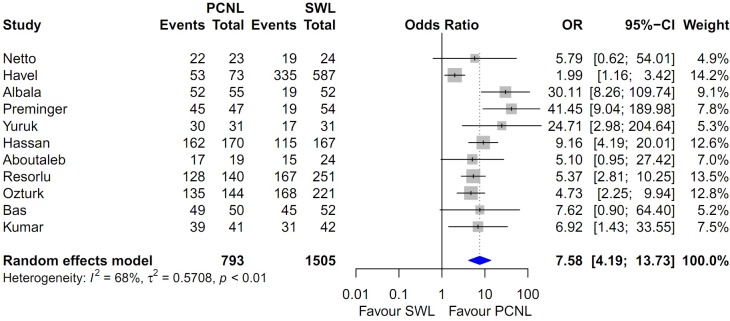
Fig 4. Pairwise meta-analysis of success rate in PCNL and SWL.
Results show that the stone-free rate of PCNL was superior to SWL (OR 7.71; 95% CI 4.08–14.57; P<0.001).
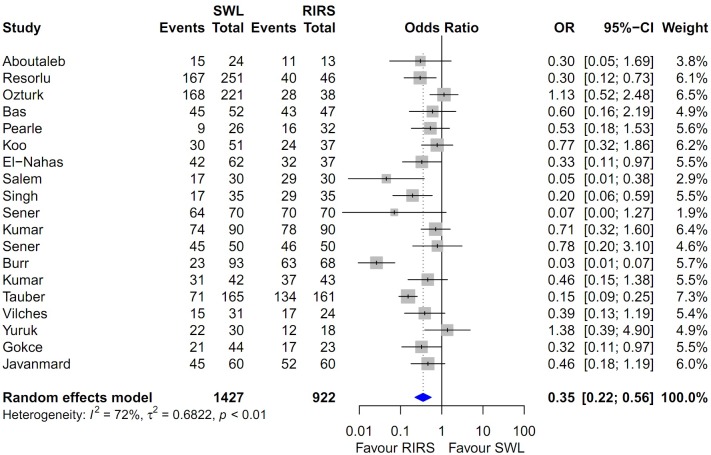
Fig 5. Pairwise meta-analysis of success rate in SWL and RIRS.
Results show that the stone-free rate of SWL was lower than RIRS (OR 60.46; 95% CI 0.30–0.71; P<0.001).
Fig 6.
L’Abbe plots of success rate between RIRS and PCNL (A), SWL and PCNL (B) and RIRS and SWL (C). Little heterogeneity was noted in L’Abbe plots.
Fig 7.
Radial plots of success rate between RIRS and PCNL (A), SWL and PCNL (B), and RIRS and SWL (C). Little heterogeneity was noted in radial plots.
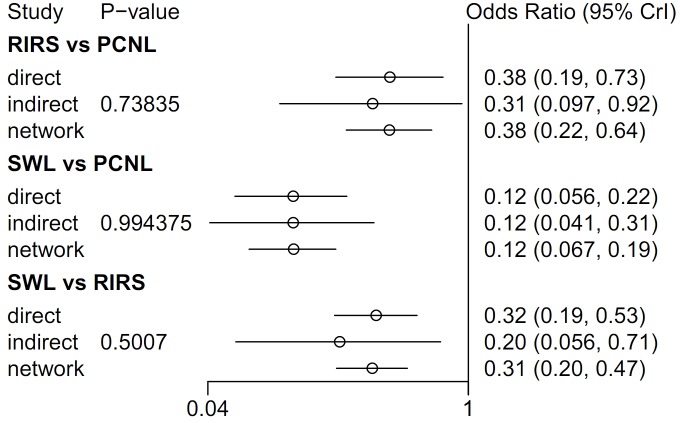
In node-splitting analysis, no inconsistency was demonstrated in direct, indirect, or network comparison (Fig 8). A net-heat plot showed that there was also little inconsistency in the whole network (Fig 9).
Fig 8. Network meta-analysis for success rate of RIRS, PCNL, SWL, and node-splitting analyses of inconsistency.
In node-splitting analysis, no inconsistency was demonstrated in direct, indirect, or network comparison.
Fig 9. Net-heat plot for inconsistency.
Net-heat plot showing that there is little inconsistency in whole network analysis of PCNL, SWL, and RIRS.
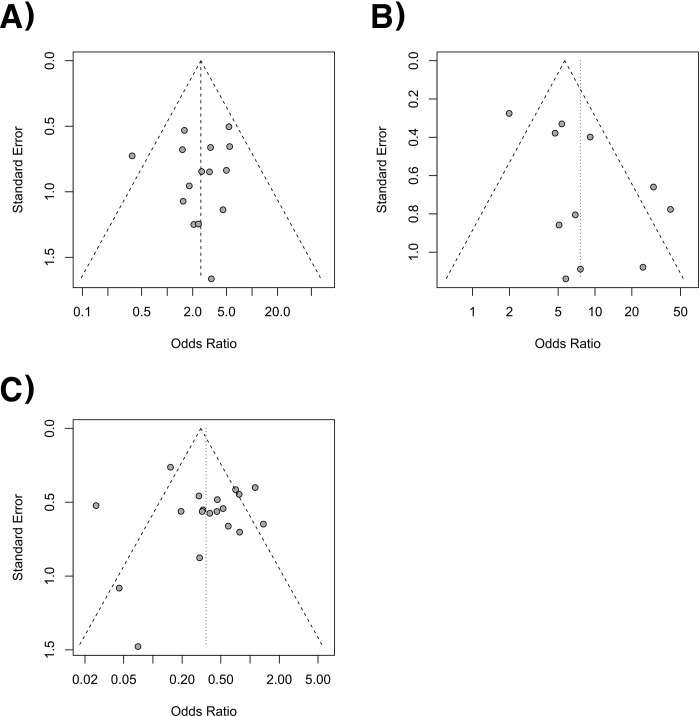
The Begg and Mazumdar rank correlation tests for each analysis showed no evidence of publication bias in the present meta-analysis between PCNL and SWL (P = 0.697). However, Egger’s regression intercept tests revealed a slight publication bias (P = 0.041). According to a rank correlation test (P = 0.520) and regression tests (P = 0.771), there was no publication bias in PCNL and RIRS. Also, no publication bias was shown for SWL versus RIRS in the rank correlation test (P = 0.421) and regression test (P = 0.855). However, there was little publication bias from funnel plots in each comparison (Fig 10).
Fig 10.
Funnel plots of success rate between RIRS and PCNL (A), SWL and PCNL (B), and RIRS and SWL (C). There were some publication bias in funnel plots.
Pairwise meta-analysis of SWL, PCNL, and RIRS for stone-free rate
Pooled data that were used to compare the stone-free rate between PCNL and RIRS showed a significantly higher stone-free rate with PCNL (OR 2.493; 95% CI 1.708–3.637; P<0.001; Fig 3). The stone-free rate of PCNL was superior to that of SWL (OR 7.583; 95% CI 4.188–13.731; P<0.001; Fig 4). The stone-free rate of SWL was lower than that RIRS (OR 0.352; 95% CI 0.223–0.557; P<0.001; Fig 5).
Network meta-analysis of SWL, PCNL, and RIRS for stone-free rate
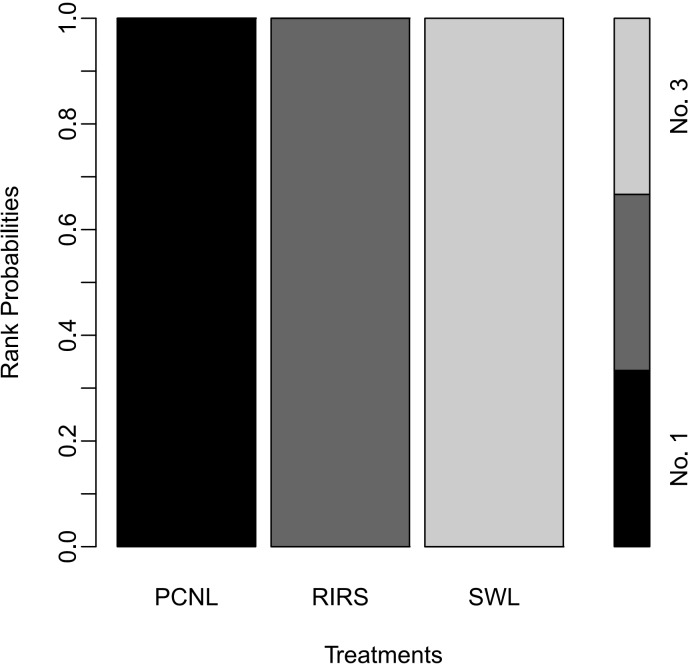
In network meta-analyses, the stone-free rate of RIRS was lower than that of PCNL (OR 0.38; 95% CI 0.22–0.64), the stone-free rate of SWL was lower than that of PCNL (0.12; 95% CI 0.067–0.19), and the stone-free rate of SWL was lower than that of RIRS (OR 0.31; 95% CI 0.20–0.47) (Fig 9). In the rank-probability test, PCNL was ranked as No. 1 and SWL was ranked as No. 3 (Fig 11). The P-score test using a frequentist method to rank treatments in the network demonstrated PCNL (P-score 1.0) was superior to RIRS (P-score 0.5) and SWL (P-score 0) in stone-free rate [56].
Fig 11. Rank-probability test of network meta-analyses.
In the rank-probability test, PCNL was ranked as No. 1 and SWL was ranked as No. 3.
Subgroup analyses using stone size, location of renal stone, and study design
In ≥ 2 cm stones, seven studies were included. There was a single study that compared PCNL to SWL, and there were six studies that demonstrated the comparison between PCNL and RIRS. In this subgroup analysis, PCNL can be superior to RIRS (OR 4.680; 95% CI 2.873–8.106) and SWL (OR 9.732; 95% CI 5.675–28.060), and RIRS can be superior to SWL (OR 2.47; 95% CI 1.076–4.614). In subgroup analysis for lower pole stones, 19 studies were enrolled. The success rate of PCNL can be higher compared to RIRS (OR 1.984; 95% CI 1.043–2.849) and SWL (OR 6.687 95% CI 4.204–10.450). In RCTs, PCNL can be superior to RIRS (OR 2.219; 95% CI 1.348–4.009) and SWL (OR 5.605; 95% CI 3.129–11.250), and RIRS can also be superior to SWL (OR 2.407; 95% CI 1868–3.773) in success rate (Table 2).
Table 2. Subgroup network meta-analysis for ≥ 2 cm stone, lower pole stones and RCTs.
PCNL, percutaneous nephrolithotomy; SWL, shock wave lithotripsy; RIRS, retrograde intrarenal surgery.
| ≥ 2 cm | PCNL | RIRS | SWL | |
|---|---|---|---|---|
| PCNL | 4.680 (2.873‒8.106) | 9.732 (5.675‒28.060) | ||
| RIRS | 0.214 (0.123‒0.348) | 2.479 (1.076‒4.614) | ||
| SWL | 0.103 (0.036‒0.176) | 0.403 (0.217‒0.930) | ||
| Lower pole | PCNL | RIRS | SWL | |
| PCNL | 1.984 (1.043‒2.849) | 6.687 (4.204‒10.450) | ||
| RIRS | 0.504 (0.351‒0.961) | 3.564 (2.398‒5.509) | ||
| SWL | 0.150 (0.096‒0.238) | 0.281 (0.182‒0.417) | ||
| RCTs | PCNL | RIRS | SWL | |
| PCNL | 2.219 (1.348‒4.009) | 5.605 (3.129‒11.250) | ||
| RIRS | 0.451 (0.249‒0.742) | 2.407 (1.868‒3.773) | ||
| SWL | 0.178 (0.089‒0.320) | 0.416 (0.265‒0.536) |
Complication Rate according to Clavien-Dindo classification
From 31 studies, rates of complication in SWL, PCNL, and RIRS were 12.5%, 20.2%, and 15.0%, respectvely. The rate of major complication in total complication cases were 15.4% in SWL, 13.8% in PCNL, and 18.3% in RIRS (Table 3).
Table 3. Complication rates from studies according to Clavien-Dindo classification.
| Methods | Complication | ||||||
|---|---|---|---|---|---|---|---|
| Total | Clavien Grades I-II (Minor) | Clavien Grades III-IV (Major) | |||||
| No. of patients | N | % | N | % | N | % | |
| SWL | 2,288 | 287 | 12.5 | 243 | 84.7 | 44 | 15.3 |
| PCNL | 1,076 | 217 | 20.2 | 187 | 86.2 | 30 | 13.8 |
| RIRS | 1,204 | 180 | 15.0 | 147 | 81.7 | 33 | 18.3 |
Discussion
The use of minimally invasive techniques like SWL, PCNL, and RIRS, has developed dramatically despite the continued high incidence and recurrence of urinary tract stone disease. [57]. The minimally invasive techniques for treatment of renal stones, have continuously improved over the last 30 years, and new procedures are being introduced as a result of the combination of instruments and technology that is now taking place. Since Fernstrom and Johansson introduced PCNL as the surgical treatment for patients with large and complex renal calculi for the first time in 1976 [58], PCNL has been considered as the standard surgery for the treatment of renal stones >2 cm [9]. The procedure was developed in the sequential order of tubeless PCNL, supine PCNL, and mini-PCNL [59–61]. Further changes in the PCNL procedure led to the recent development of endoscopic combined intrarenal surgery (ECIRS) [62]. The first experience of SWL was reported in 1984, when Chaussy and his colleagues performed SWL on 852 patients [63]. Until recently, the advancement of patient selection, shock wave delivery, and the new lithotripter design were the reasons why SWL is was still the primary treatment for non-lower pole renal stones <2 cm [7]. RIRS has achieved rapid development since the 1990’s when the holmium:yttrium aluminum garnet (YAG) laser system was introduced [64]. The development of the recently introduced small-aperture digital video scope (Flex-Xc; Karl Storz Endoskope, Tuttlingen, Germany, URF-V2; Olympus Corp, Tokyo, Japan) and the single-use video scope (LithoVue; Boston Scientific, Marlborough, MA, USA) has led to the popularization of RIRS by improving both the image quality as well as durability [65, 66].
In most cases of non-symptomatic kidney stones, observation is sufficient. However, treatment is recommended in cases in which stones are continuously increasing in size, there is a high risk of additional stone formation, there is obstruction due to the stones, infection, pain, or hematuria, or stones are >1.5 cm. Treatment is also recommended if it is desired with regard to the patient’s social situation [67]. As mentioned earlier, the EAU guideline suggests SWL and RIRS for the primary treatment of renal stones <2 cm, and PCNL for the primary treatment for stones >2 cm. In general, PCNL is more invasive than RIRS and SWL and has relatively large complications related to hemorrhaging. Though the procedure of SWL is relatively safe, there is a possibility of repeated treatment. RIRS is also expanding in use due to the gradual development of related systems, but there can be technical difficulties and surgical complications may occur. Hence, there are advantages and disadvantages for each interventional treatment, and it is extremely important to find and perform the best treatment for the individual patient with the renal stones.
Perhaps stone-free rate is one of the first things to consider when choosing among treatments that have their own advantages and disadvantages. This report is the first of a network meta-analysis on the success or stone-free rates of SWL, PCNL, and RIRS. A pairwise meta-analysis comparing each method has already been reported several times. In the pairwise meta-analysis of PCNL and RIRS reported in 2015, the complication rate (OR 1.61; 95% CI 1.11–2.35), hemoglobin drop (MD 0.87; 95% CI 0.51–1.22), and the hospital stay (MD 1.28; 95% CI 0.79–1.77) of RIRS showed better results than PCNL [68]. However, the stone-free rate of PCNL was higher than that of RIRS (OR 2.19; 95% CI 1.53–3.13, P<0.001). In our study, the pairwise meta-analysis of PCNL and RIRS showed better results of PCNL in terms of the stone-free rate (OR 2.31; 95% CI 1.45–3.67). Either in the network meta-analysis, RIRS showed a lower stone-free rate than PCNL (OR 0.36; 95% CI 0.19–0.68). In another study, Zhang and colleagues performed pairwise meta-analyses of SWL, PCNL, and RIRS for the lower pole renal stone, and found that PCNL shows a higher stone-free rate than SWL and RIRS, and there is no difference in the stone-free rates of SWL and RIRS (OR 1.97; 95% CI 0.98–3.95) [69]. Our results also show PCNL had the best stone-free rate, but the results for SWL and RIRS differ between our study and that of Zhang et al. These authors argue that residual fragments should be considered more seriously for the lower pole stone than for other locations because gravity plays a crucial role in the clearance of the residual stone fragments. In particular, they predict that the increase in laser dusting without stone extraction in the mini-PCNL and RIRS treatments will play a role in lowering the stone-free rate to values similar to that for the fragments clearance using SWL, and that this prediction explains why the stone-free rate does not differ between SWL and RIRS treatments in their study. Donaldson et al reported meta-analysis on clinical effectiveness of SWL, RIRS and PCNL for lower pole stone [70]. They concluded that PCNL and RIRS were superior to SWL in clearing the stones within 3 months. In their study, they used pair-wise meta-analysis for the outcomes in patients with only lower pole stone. We also performed subgroup analyses with lower pole stone data using Bayesian network meta-analysis and the results of our study also demonstrated similarities to those by Donaldson et al., but we reaffirmed the superiority of PCNL and RIRS using network meta-analysis. In EAU guidelines, in lower pole stone, PCNL and RIRS should be recommended as the first-line treatment [6]. In our analysis, the reason why RIRS showed a higher stone-free rate than SWL was because our research included all renal stones regardless of their location, whereas the analysis performed by Zhang and colleagues included only lower pole renal stones. Furthermore, our results may differ from those of their research because a higher number of studies were included in our meta-analysis. The technical development of RIRS can be another reason for the differing results. A recent survey of 414 surgeons indicates that the dusting technique using high-power holmium laser is popular and that this technique is judged to be a help in improving the stone-free rate of RIRS [71]. The lower pole stone has been reported to be used in 55.8% of cases of translocation using the stone basket. In the case of RIRS and even focusing on the lower pole stones, stones <2 cm may increase the stone-free rate through translocation [72].
There was no difference in the stone-free rate (RR 0.95; 95% CI 0.88–1.02, P = 0.15) shown in the pairwise meta-analysis of RIRS and PCNL for renal stones >2 cm reported by Zheng et al [73]. This is quite different from our meta-analysis results because Zheng and colleagues did not provide a clear quality assessment, there was a factor of publication bias, and it is presumed that the suitability of the effect model was not evaluated using the Labble plot. These conflicting results indicate that additional research is still needed.
Finally, without factoring the size and location of renal stones, the results presented in our study show that PCNL treatment resulted in the highest stone-free rate and SWL exhibited the lowest stone-free rate. Our study is unique in that three treatments were analyzed simultaneously using a network meta-analysis model. Furthermore, our study is judged to have great value because it is the first study to derive the superiority of a treatment using the rank test and because only studies with low bias and high quality were included in the analysis using quality assessment. Especially, in large stone (> 2 cm) and lower pole stone, PCNL can be superior to RIRS and SWL. EAU guidelines also recommended PCNL as the first-line treatment in large stone and lower pole stones. So far, the success rates of RIRS and SWL seem to not exceed that of PCNL. Based on our results, further research for treatments with higher stone-free rates will be necessary in the future.
The recently presented ECIRS is a treatment comprising a combination of PCNL and RIRS and is predicted to be capable of achieving a higher stone-free rate [74]. PCNL and RIRS should be the mainstay of interventional therapy for patients with renal stones. However, for some patients with bilateral disease, ECIRS may also be an effective treatment rather than bilateral PCNL or RIRS [75, 76]. Although PCNL is the most effect interventional therapy with the highest stone-free rate, careful patient selection is required because of the high invasiveness of this treatment. Indeed, recent reports highlight the advantage of reduced invasiveness in mini-PCNL and ultramini-PCNL treatments [77] and successful results in treatments with ECIRS performed with mini-PCNL [78]. In summary, PCNL is the most effective treatment, and RIRS is able to compensate for a lower stone-free rate than PCNL. For patients with a low stone-free rate in the recently presented nephrolithometry score [79], increasing the stone-free rate by using ECIRS should be the goal of interventional therapy in the future [76].
A limitation of our study is that no subgroup analysis was performed on the size and location of the renal stones. In the event that a subgroup analysis is performed, there is a possibility it may lead to different outcomes because the recommended treatments vary depending on the size and location of the renal stones. Some degree of publication bias was also a limitation of this study. However, Sutton et al. reviewed 48 articles from the Cochrane Database of Systematic Reviews and showed publication or related biases were common within the sample of meta-analyses assessed [80].
Another limitation is that the results reflected only the efficacy aspect of the stone-free rate and did not take into account the safety aspect of the treatments. Discriminating between merits and drawbacks of the treatment for a patient is clearly an important decision. Further studies that address these limitations are needed in the future.
Conclusions
PCNL for renal stones resulted in the highest success and stone-free rate and ranked the highest of the treatments analyzed. In contrast, SWL ranked the lowest of the treatments because of its lowest success and stone-free rates. The complexity of individual patients considered in this meta-analysis may have played a role in the results. Future analyses should include patient selection criteria such as renal stone location.
Supporting information
(DOCX)
(DOCX)
Data Availability
All relevant data are within paper and its Supporting Information files.
Funding Statement
This study was supported by a faculty research grant from the Yonsei University College of Medicine (6-2016-0119) to Dr. Joo Yong Lee.The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Teichman JM. Clinical practice. Acute renal colic from ureteral calculus. N Engl J Med. 2004;350(7):684–93. Epub 2004/02/13. 10.1056/NEJMcp030813 . [DOI] [PubMed] [Google Scholar]
- 2.Kang DH, Cho KS, Ham WS, Chung DY, Kwon JK, Choi YD, et al. Ureteral stenting can be a negative predictor for successful outcome following shock wave lithotripsy in patients with ureteral stones. Investigative and clinical urology. 2016;57(6):408–16. Epub 2016/11/17. 10.4111/icu.2016.57.6.408 ; PubMed Central PMCID: PMCPmc5109799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong Y, Cook P, Roderick P, Somani BK. Metabolic Syndrome and Kidney Stone Disease: A Systematic Review of Literature. J Endourol. 2016;30(3):246–53. Epub 2015/11/19. 10.1089/end.2015.0567 . [DOI] [PubMed] [Google Scholar]
- 4.Geraghty RM, Proietti S, Traxer O, Archer M, Somani BK. Worldwide Impact of Warmer Seasons on the Incidence of Renal Colic and Kidney Stone Disease: Evidence from a Systematic Review of Literature. J Endourol. 2017. Epub 2017/03/25. 10.1089/end.2017.0123 . [DOI] [PubMed] [Google Scholar]
- 5.Trinchieri A, Montanari E. Prevalence of renal uric acid stones in the adult. Urolithiasis. 2017. Epub 2017/03/05. 10.1007/s00240-017-0962-5 . [DOI] [PubMed] [Google Scholar]
- 6.Turk C, Petrik A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur Urol. 2016;69(3):475–82. Epub 2015/09/08. 10.1016/j.eururo.2015.07.041 . [DOI] [PubMed] [Google Scholar]
- 7.Lawler AC, Ghiraldi EM, Tong C, Friedlander JI. Extracorporeal Shock Wave Therapy: Current Perspectives and Future Directions. Curr Urol Rep. 2017;18(4):25 Epub 2017/03/02. 10.1007/s11934-017-0672-0 . [DOI] [PubMed] [Google Scholar]
- 8.Lee YJ, Bak DJ, Chung JW, Lee JN, Kim HT, Yoo ES, et al. Is it necessary to actively remove stone fragments during retrograde intrarenal surgery? Investigative and clinical urology. 2016;57(4):274–9. Epub 2016/07/21. 10.4111/icu.2016.57.4.274 ; PubMed Central PMCID: PMCPmc4949691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JY, Jeh SU, Kim MD, Kang DH, Kwon JK, Ham WS, et al. Intraoperative and postoperative feasibility and safety of total tubeless, tubeless, small-bore tube, and standard percutaneous nephrolithotomy: a systematic review and network meta-analysis of 16 randomized controlled trials. BMC urology. 2017;17(1):48 Epub 2017/06/29. 10.1186/s12894-017-0239-x ; PubMed Central PMCID: PMCPmc5488341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897–900. Epub 2005/10/15. 10.1136/bmj.331.7521.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914 Epub 2013/05/16. 10.1136/bmj.f2914 . [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Cho KS, Ham WS, Lee H, Kwon JK, Choi YD, et al. Comparison of High, Intermediate, and Low Frequency Shock Wave Lithotripsy for Urinary Tract Stone Disease: Systematic Review and Network Meta-Analysis. PLoS One. 2016;11(7):e0158661 10.1371/journal.pone.0158661 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097 Epub 2009/07/22. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomura K, Nakao M, Morimoto T. Effect of smoking on hearing loss: quality assessment and meta-analysis. Preventive medicine. 2005;40(2):138–44. Epub 2004/11/10. 10.1016/j.ypmed.2004.05.011 . [DOI] [PubMed] [Google Scholar]
- 15.Macfarlane TV, Glenny AM, Worthington HV. Systematic review of population-based epidemiological studies of oro-facial pain. Journal of dentistry. 2001;29(7):451–67. Epub 2002/01/26. . [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. Epub 2003/09/06. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JY, Kang DH, Chung DY, Kwon JK, Lee H, Cho NH, et al. Meta-Analysis of the Relationship between CXCR4 Expression and Metastasis in Prostate Cancer. World J Mens Health. 2014;32(3):167–75. Epub 2015/01/22. 10.5534/wjmh.2014.32.3.167 ; PubMed Central PMCID: PMCPmc4298820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleiss JL. Analysis of data from multiclinic trials. Control Clin Trials. 1986;7(4):267–75. Epub 1986/12/01. . [DOI] [PubMed] [Google Scholar]
- 19.L'Abbe KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987;107(2):224–33. Epub 1987/08/01. . [DOI] [PubMed] [Google Scholar]
- 20.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7(8):889–94. Epub 1988/08/01. . [DOI] [PubMed] [Google Scholar]
- 21.Netto NR Jr., Claro JF, Lemos GC, Cortado PL. Renal calculi in lower pole calices: what is the best method of treatment? J Urol. 1991;146(3):721–3. . [DOI] [PubMed] [Google Scholar]
- 22.Havel D, Saussine C, Fath C, Lang H, Faure F, Jacqmin D. Single stones of the lower pole of the kidney. Comparative results of extracorporeal shock wave lithotripsy and percutaneous nephrolithotomy. Eur Urol. 1998;33(4):396–400. . [DOI] [PubMed] [Google Scholar]
- 23.Albala DM, Assimos DG, Clayman RV, Denstedt JD, Grasso M, Gutierrez-Aceves J, et al. Lower pole I: a prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis-initial results. J Urol. 2001;166(6):2072–80. . [DOI] [PubMed] [Google Scholar]
- 24.Preminger GM. Management of lower pole renal calculi: shock wave lithotripsy versus percutaneous nephrolithotomy versus flexible ureteroscopy. Urol Res. 2006;34(2):108–11. 10.1007/s00240-005-0020-6 . [DOI] [PubMed] [Google Scholar]
- 25.Yuruk E, Binbay M, Sari E, Akman T, Altinyay E, Baykal M, et al. A prospective, randomized trial of management for asymptomatic lower pole calculi. J Urol. 2010;183(4):1424–8. 10.1016/j.juro.2009.12.022 . [DOI] [PubMed] [Google Scholar]
- 26.Hassan M, El-Nahas AR, Sheir KZ, El-Tabey NA, El-Assmy AM, Elshal AM, et al. Percutaneous nephrolithotomy vs. extracorporeal shockwave lithotripsy for treating a 20–30 mm single renal pelvic stone. Arab J Urol. 2015;13(3):212–6. 10.1016/j.aju.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyams ES, Shah O. Percutaneous nephrostolithotomy versus flexible ureteroscopy/holmium laser lithotripsy: cost and outcome analysis. J Urol. 2009;182(3):1012–7. Epub 2009/07/21. 10.1016/j.juro.2009.05.021 . [DOI] [PubMed] [Google Scholar]
- 28.Akman T, Binbay M, Ozgor F, Ugurlu M, Tekinarslan E, Kezer C, et al. Comparison of percutaneous nephrolithotomy and retrograde flexible nephrolithotripsy for the management of 2–4 cm stones: a matched-pair analysis. BJU Int. 2012;109(9):1384–9. Epub 2011/11/19. 10.1111/j.1464-410X.2011.10691.x . [DOI] [PubMed] [Google Scholar]
- 29.Bozkurt OF, Resorlu B, Yildiz Y, Can CE, Unsal A. Retrograde intrarenal surgery versus percutaneous nephrolithotomy in the management of lower-pole renal stones with a diameter of 15 to 20 mm. J Endourol. 2011;25(7):1131–5. Epub 2011/06/11. 10.1089/end.2010.0737 . [DOI] [PubMed] [Google Scholar]
- 30.Bryniarski P, Paradysz A, Zyczkowski M, Kupilas A, Nowakowski K, Bogacki R. A randomized controlled study to analyze the safety and efficacy of percutaneous nephrolithotripsy and retrograde intrarenal surgery in the management of renal stones more than 2 cm in diameter. J Endourol. 2012;26(1):52–7. Epub 2011/10/19. 10.1089/end.2011.0235 . [DOI] [PubMed] [Google Scholar]
- 31.Jung GH, Jung JH, Ahn TS, Lee JS, Cho SY, Jeong CW, et al. Comparison of retrograde intrarenal surgery versus a single-session percutaneous nephrolithotomy for lower-pole stones with a diameter of 15 to 30 mm: A propensity score-matching study. Korean J Urol. 2015;56(7):525–32. Epub 2015/07/16. 10.4111/kju.2015.56.7.525 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karakoyunlu N, Goktug G, Sener NC, Zengin K, Nalbant I, Ozturk U, et al. A comparison of standard PCNL and staged retrograde FURS in pelvis stones over 2 cm in diameter: a prospective randomized study. Urolithiasis. 2015;43(3):283–7. Epub 2015/04/04. 10.1007/s00240-015-0768-2 . [DOI] [PubMed] [Google Scholar]
- 33.Koyuncu H, Yencilek F, Kalkan M, Bastug Y, Yencilek E, Ozdemir AT. Intrarenal Surgery vs Percutaneous Nephrolithotomy in the Management of Lower Pole Stones Greater than 2 cm. Int Braz J Urol. 2015;41(2):245–51. 10.1590/S1677-5538.IBJU.2015.02.09 WOS:000354634500009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bas O, Ozyuvali E, Aydogmus Y, Sener NC, Dede O, Ozgun S, et al. Management of calyceal diverticular calculi: a comparison of percutaneous nephrolithotomy and flexible ureterorenoscopy. Urolithiasis. 2015;43(2):155–61. 10.1007/s00240-014-0725-5 . [DOI] [PubMed] [Google Scholar]
- 35.Zengin K, Tanik S, Karakoyunlu N, Sener NC, Albayrak S, Tuygun C, et al. Retrograde intrarenal surgery versus percutaneous lithotripsy to treat renal stones 2–3 cm in diameter. Biomed Res Int. 2015;2015:914231 10.1155/2015/914231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozayar E, Gulec H, Bayraktaroglu M, Tutal ZB, Kurtay A, Babayigit M, et al. Comparison of Retrograde Intrarenal Surgery and Percutaneous Nephrolithotomy: From the View of an Anesthesiologist. J Endourol. 2016;30(2):184–8. 10.1089/end.2015.0517 . [DOI] [PubMed] [Google Scholar]
- 37.Pearle MS, Lingeman JE, Leveillee R, Kuo R, Preminger GM, Nadler RB, et al. Prospective randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol. 2008;179(5 Suppl):S69–73. 10.1016/j.juro.2008.03.140 . [DOI] [PubMed] [Google Scholar]
- 38.Koo V, Young M, Thompson T, Duggan B. Cost-effectiveness and efficiency of shockwave lithotripsy vs flexible ureteroscopic holmium:yttrium-aluminium-garnet laser lithotripsy in the treatment of lower pole renal calculi. BJU Int. 2011;108(11):1913–6. 10.1111/j.1464-410X.2011.10172.x . [DOI] [PubMed] [Google Scholar]
- 39.El-Nahas AR, Ibrahim HM, Youssef RF, Sheir KZ. Flexible ureterorenoscopy versus extracorporeal shock wave lithotripsy for treatment of lower pole stones of 10–20 mm. BJU Int. 2012;110(6):898–902. 10.1111/j.1464-410X.2012.10961.x . [DOI] [PubMed] [Google Scholar]
- 40.Salem A, Saad I, Emran A, Abdelhakiem M, Abdelrazzak O, Abdelkader M. Laser Lithotripsy Versus Eswl for Lower Calyceal Renal Stones. J Urology. 2013;189(4):E751–E. 10.1016/j.juro.2013.02.2192 WOS:000320281602397. [DOI] [Google Scholar]
- 41.Sener NC, Imamoglu MA, Bas O, Ozturk U, Goktug HN, Tuygun C, et al. Prospective randomized trial comparing shock wave lithotripsy and flexible ureterorenoscopy for lower pole stones smaller than 1 cm. Urolithiasis. 2014;42(2):127–31. 10.1007/s00240-013-0618-z . [DOI] [PubMed] [Google Scholar]
- 42.Singh BP, Prakash J, Sankhwar SN, Dhakad U, Sankhwar PL, Goel A, et al. Retrograde intrarenal surgery vs extracorporeal shock wave lithotripsy for intermediate size inferior pole calculi: a prospective assessment of objective and subjective outcomes. Urology. 2014;83(5):1016–22. 10.1016/j.urology.2013.12.026 . [DOI] [PubMed] [Google Scholar]
- 43.Burr J, Ishii H, Simmonds N, Somani BK. Is flexible ureterorenoscopy and laser lithotripsy the new gold standard for lower pole renal stones when compared to shock wave lithotripsy: Comparative outcomes from a University hospital over similar time period. Cent Eur J Urol. 2015;68(2):183–6. 10.5173/ceju.2015.509 WOS:000363946600014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Vasudeva P, Nanda B, Kumar N, Das MK, Jha SK. A Prospective Randomized Comparison Between Shock Wave Lithotripsy and Flexible Ureterorenoscopy for Lower Caliceal Stones < = 2 cm: A Single-Center Experience. Journal of Endourology. 2015;29(5):575–9. 10.1089/end.2013.0473 WOS:000354037000016. [DOI] [PubMed] [Google Scholar]
- 45.Sener NC, Bas O, Sener E, Zengin K, Ozturk U, Altunkol A, et al. Asymptomatic lower pole small renal stones: shock wave lithotripsy, flexible ureteroscopy, or observation? A prospective randomized trial. Urology. 2015;85(1):33–7. 10.1016/j.urology.2014.08.023 . [DOI] [PubMed] [Google Scholar]
- 46.Tauber V, Wohlmuth M, Hochmuth A, Schimetta W, Krause FS. Efficacy Management of Urolithiasis: Flexible Ureteroscopy versus Extracorporeal Shockwave Lithotripsy. Urol Int. 2015;95(3):324–8. 10.1159/000439356 . [DOI] [PubMed] [Google Scholar]
- 47.Vilches RM, Aliaga A, Reyes D, Sepulveda F, Mercado A, Moya F, et al. Comparison between retrograde intrarenal surgery and extracorporeal shock wave lithotripsy in the treatment of lower pole kidney stones up to 15 mm. Prospective, randomized study. Actas Urol Esp. 2015;39(4):236–42. 10.1016/j.acuro.2014.08.003 WOS:000355239400006. [DOI] [PubMed] [Google Scholar]
- 48.Yuruk E, Binbay M, Ozgor F, Sekerel L, Berberoglu Y, Muslumanoglu AY. Comparison of shockwave lithotripsy and flexible ureteroscopy for the treatment of kidney stones in patients with a solitary kidney. J Endourol. 2015;29(4):463–7. 10.1089/end.2014.0613 . [DOI] [PubMed] [Google Scholar]
- 49.Gokce MI, Tokatli Z, Suer E, Hajiyev P, Akinci A, Esen B. Comparison of shock wave lithotripsy (SWL) and retrograde intrarenal surgery (RIRS) for treatment of stone disease in horseshoe kidney patients. Int Braz J Urol. 2016;42(1):96–100. 10.1590/S1677-5538.IBJU.2015.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Javanmard B, Kashi AH, Mazloomfard MM, Jafari AA, Arefanian S. Retrograde Intrarenal Surgery Versus Shock Wave Lithotripsy for Renal Stones Smaller Than 2 cm: A Randomized Clinical Trial. Urol J. 2016;13(5):2823–8. WOS:000388158300002. [PubMed] [Google Scholar]
- 51.Aboutaleb H, El-Shazly M, Badr Eldin M. Lower pole midsize (1–2 cm) calyceal stones: outcome analysis of 56 cases. Urol Int. 2012;89(3):348–54. Epub 2012/08/28. 10.1159/000341557 . [DOI] [PubMed] [Google Scholar]
- 52.Resorlu B, Unsal A, Ziypak T, Diri A, Atis G, Guven S, et al. Comparison of retrograde intrarenal surgery, shockwave lithotripsy, and percutaneous nephrolithotomy for treatment of medium-sized radiolucent renal stones. World J Urol. 2013;31(6):1581–6. Epub 2012/11/28. 10.1007/s00345-012-0991-1 . [DOI] [PubMed] [Google Scholar]
- 53.Ozturk U, Sener NC, Goktug HNG, Nalbant I, Gucuk A, Imamoglu MA. Comparison of Percutaneous Nephrolithotomy, Shock Wave Lithotripsy, and Retrograde Intrarenal Surgery for Lower Pole Renal Calculi 10–20 mm. Urologia Internationalis. 2013;91(3):345–9. 10.1159/000351136 WOS:000325832600018. [DOI] [PubMed] [Google Scholar]
- 54.Bas O, Bakirtas H, Sener NC, Ozturk U, Tuygun C, Goktug HN, et al. Comparison of shock wave lithotripsy, flexible ureterorenoscopy and percutaneous nephrolithotripsy on moderate size renal pelvis stones. Urolithiasis. 2014;42(2):115–20. Epub 2013/10/29. 10.1007/s00240-013-0615-2 . [DOI] [PubMed] [Google Scholar]
- 55.Kumar A, Kumar N, Vasudeva P, Jha SK, Kumar R, Singh H. A Prospective, Randomized Comparison of Shock Wave Lithotripsy, Retrograde Intrarenal Surgery and Miniperc for Treatment of 1 to 2 cm Radiolucent Lower Calyceal Renal Calculi: A Single Center Experience. J Urology. 2015;193(1):160–4. 10.1016/j.juro.2014.07.088 WOS:000346171500041. [DOI] [PubMed] [Google Scholar]
- 56.Rucker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58 Epub 2015/08/01. 10.1186/s12874-015-0060-8 ; PubMed Central PMCID: PMCPmc4521472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim BS. Recent advancement or less invasive treatment of percutaneous nephrolithotomy. Korean J Urol. 2015;56(9):614–23. Epub 2015/09/15. 10.4111/kju.2015.56.9.614 ; PubMed Central PMCID: PMCPmc4565895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernstrom I, Johansson B. Percutaneous pyelolithotomy. A new extraction technique. Scand J Urol Nephrol. 1976;10(3):257–9. Epub 1976/01/01. . [DOI] [PubMed] [Google Scholar]
- 59.Wickham JE, Miller RA, Kellett MJ, Payne SR. Percutaneous nephrolithotomy: one stage or two? Br J Urol. 1984;56(6):582–5. Epub 1984/12/01. . [DOI] [PubMed] [Google Scholar]
- 60.Uria JV, Santamaria EL, Rodriguez SV, Llop JT, Baquero GA, Lassa JA. Percutaneous nephrolithotomy: simplified technique (preliminary report). Arch Esp Urol. 1987;40:177–80. [PubMed] [Google Scholar]
- 61.Helal M, Black T, Lockhart J, Figueroa TE. The Hickman peel-away sheath: alternative for pediatric percutaneous nephrolithotomy. J Endourol. 1997;11(3):171–2. Epub 1997/06/01. 10.1089/end.1997.11.171 . [DOI] [PubMed] [Google Scholar]
- 62.Scoffone CM, Cracco CM, Cossu M, Grande S, Poggio M, Scarpa RM. Endoscopic combined intrarenal surgery in Galdakao-modified supine Valdivia position: a new standard for percutaneous nephrolithotomy? Eur Urol. 2008;54(6):1393–403. Epub 2008/08/22. 10.1016/j.eururo.2008.07.073 . [DOI] [PubMed] [Google Scholar]
- 63.Chaussy C, Schuller J, Schmiedt E, Brandl H, Jocham D, Liedl B. Extracorporeal shock-wave lithotripsy (ESWL) for treatment of urolithiasis. Urology. 1984;23(5 Spec No):59–66. Epub 1984/05/01. . [DOI] [PubMed] [Google Scholar]
- 64.Cho SY. Current status of flexible ureteroscopy in urology. Korean J Urol. 2015;56(10):680–8. Epub 2015/10/27. 10.4111/kju.2015.56.10.680 ; PubMed Central PMCID: PMCPmc4610894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeong JY, Kim JC, Kang DH, Lee JY. Digital Videoscopic Retrograde Intrarenal Surgeries for Renal Stones: Time-to-Maximal Stone Length Ratio Analysis. Yonsei Med J. 2018;59(2):303–9. Epub 2018/02/13. 10.3349/ymj.2018.59.2.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho SY, Lee JY, Shin DG, Seo IY, Yoo S, Park HK. Evaluation of Performance Parameters of the Disposable Flexible Ureterorenoscope (LITHOVUE) in Patients with Renal Stones: A Prospective, Observational, Single-arm, Multicenter Study. Scientific reports. 2018;8(1):9795 Epub 2018/06/30. 10.1038/s41598-018-28247-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brandt B, Ostri P, Lange P, Kvist Kristensen J. Painful caliceal calculi. The treatment of small nonobstructing caliceal calculi in patients with symptoms. Scand J Urol Nephrol. 1993;27(1):75–6. Epub 1993/01/01. . [DOI] [PubMed] [Google Scholar]
- 68.De S, Autorino R, Kim FJ, Zargar H, Laydner H, Balsamo R, et al. Percutaneous nephrolithotomy versus retrograde intrarenal surgery: a systematic review and meta-analysis. Eur Urol. 2015;67(1):125–37. Epub 2014/07/30. 10.1016/j.eururo.2014.07.003 . [DOI] [PubMed] [Google Scholar]
- 69.Zhang W, Zhou T, Wu T, Gao X, Peng Y, Xu C, et al. Retrograde Intrarenal Surgery Versus Percutaneous Nephrolithotomy Versus Extracorporeal Shockwave Lithotripsy for Treatment of Lower Pole Renal Stones: A Meta-Analysis and Systematic Review. J Endourol. 2015;29(7):745–59. Epub 2014/12/23. 10.1089/end.2014.0799 . [DOI] [PubMed] [Google Scholar]
- 70.Donaldson JF, Lardas M, Scrimgeour D, Stewart F, MacLennan S, Lam TB, et al. Systematic review and meta-analysis of the clinical effectiveness of shock wave lithotripsy, retrograde intrarenal surgery, and percutaneous nephrolithotomy for lower-pole renal stones. Eur Urol. 2015;67(4):612–6. Epub 2014/12/03. 10.1016/j.eururo.2014.09.054 . [DOI] [PubMed] [Google Scholar]
- 71.Dauw CA, Simeon L, Alruwaily AF, Sanguedolce F, Hollingsworth JM, Roberts WW, et al. Contemporary Practice Patterns of Flexible Ureteroscopy for Treating Renal Stones: Results of a Worldwide Survey. J Endourol. 2015;29(11):1221–30. Epub 2015/07/15. 10.1089/end.2015.0260 . [DOI] [PubMed] [Google Scholar]
- 72.Schuster TG, Hollenbeck BK, Faerber GJ, Wolf JS Jr. Ureteroscopic treatment of lower pole calculi: comparison of lithotripsy in situ and after displacement. J Urol. 2002;168(1):43–5. Epub 2002/06/07. . [PubMed] [Google Scholar]
- 73.Zheng C, Xiong B, Wang H, Luo J, Zhang C, Wei W, et al. Retrograde intrarenal surgery versus percutaneous nephrolithotomy for treatment of renal stones >2 cm: a meta-analysis. Urol Int. 2014;93(4):417–24. Epub 2014/08/30. 10.1159/000363509 . [DOI] [PubMed] [Google Scholar]
- 74.Cracco CM, Scoffone CM. ECIRS (Endoscopic Combined Intrarenal Surgery) in the Galdakao-modified supine Valdivia position: a new life for percutaneous surgery? World J Urol. 2011;29(6):821–7. Epub 2011/11/08. 10.1007/s00345-011-0790-0 . [DOI] [PubMed] [Google Scholar]
- 75.Kwon O, Park J, Cho MC, Son H, Jeong H, Cho SY. Feasibility of single-session endoscopic combined intrarenal surgery for ipsilateral large renal stones and retrograde intrarenal surgery for contralateral renal stones: Initial experience. Int J Urol. 2017;24(5):377–82. Epub 2017/03/11. 10.1111/iju.13313 . [DOI] [PubMed] [Google Scholar]
- 76.Jung HD, Kim JC, Ahn HK, Kwon JH, Han K, Han WK, et al. Real-time simultaneous endoscopic combined intrarenal surgery with intermediate-supine position: Washout mechanism and transport technique. Investigative and clinical urology. 2018;59(5):348–54. Epub 2018/09/06. 10.4111/icu.2018.59.5.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghani KR, Andonian S, Bultitude M, Desai M, Giusti G, Okhunov Z, et al. Percutaneous Nephrolithotomy: Update, Trends, and Future Directions. Eur Urol. 2016;70(2):382–96. Epub 2016/02/16. 10.1016/j.eururo.2016.01.047 . [DOI] [PubMed] [Google Scholar]
- 78.Taguchi K, Hamamoto S, Okada A, Mizuno K, Tozawa K, Hayashi Y, et al. First case report of staghorn calculi successfully removed by mini-endoscopic combined intrarenal surgery in a 2-year-old boy. Int J Urol. 2015;22(10):978–80. Epub 2015/07/04. 10.1111/iju.12860 . [DOI] [PubMed] [Google Scholar]
- 79.Okhunov Z, Friedlander JI, George AK, Duty BD, Moreira DM, Srinivasan AK, et al. S.T.O.N.E. nephrolithometry: novel surgical classification system for kidney calculi. Urology. 2013;81(6):1154–9. Epub 2013/04/02. 10.1016/j.urology.2012.10.083 . [DOI] [PubMed] [Google Scholar]
- 80.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. Bmj. 2000;320(7249):1574–7. Epub 2000/06/14. ; PubMed Central PMCID: PMCPmc27401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within paper and its Supporting Information files.