Abstract
Rationale:
For patients with asymptomatic high-grade carotid stenosis, clinical investigations have focused on preventing cerebral infarction, yet stenosis that reduces cerebral blood flow may independently impair cognition. Whether revascularization of a hemodynamically significant carotid stenosis can alter the course of cognitive decline has never been investigated in the context of a randomized clinical trial.
Hypothesis:
Among patients randomized in the Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis (CREST-2) trials, the magnitude of treatment differences (revascularization versus medical management alone) with regard to cognition will differ between those with flow impairment compared to those without flow impairment.
Sample size:
We will enroll approximately 500 patients from CREST-2, of which we anticipate 100 will have hemodynamic impairment. We estimate 93% power to detect a clinically meaningful treatment difference of 0.5 SD.
Methods and design:
We will use perfusion-weighted magnetic resonance imaging to stratify by hemodynamic status. Linear regression will compare treatment differences, controlling for baseline cognitive status, age, depression, prior cerebral infarcts, silent infarction, white matter hyperintensity volume, and cerebral microbleeds.
Study outcomes:
The primary outcome is change in cognition at one year. Secondary outcomes include silent infarction, change in white matter hyperintensity volume, number of cerebral microbleeds, and cortical thickness over one year.
Discussion:
If cognitive impairment can be shown to be reversible by revascularization, then we can redefine ‘‘symptomatic carotid stenosis’’ to include cognitive impairment and identify a new population of patients likely to benefit from revascularization.
Keywords: Carotid endarterectomy, carotid stenting, asymptomatic carotid stenosis, cerebral blood flow, cognitive impairment, magnetic resonance imaging, perfusion weighted imaging
Introduction
Vascular cognitive impairment is often described as a step-wise or progressive disease resulting from accumulated ischemic injury.1–4 Cerebral hemodynamic impairment in patients with high-grade carotid artery stenosis can also impair cognition when there is low cerebral blood flow (CBF) on the side of stenosis, even if no clinically evident stroke has occurred.5–7 Although there is suggestive evidence from case series that hemodynamic impairment affects cognition in patients with carotid occlusive disease, treatment of this condition to reverse cognitive decline has never been tested in the context of a randomized trial. The Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis – Hemodynamics (CREST-H) study is designed to address this knowledge gap.
Rationale
Preclinical data suggest that there is a direct effect of cerebral hypoperfusion on cognition from carotid occlusive disease and that it is reversible. Middle-aged rats underwent bilateral common carotid plus left subclavian artery ligation to produce ‘‘chronic cerebrovascular insufficiency.’’ This resulted in low CBF, selective hippocampal CA1 damage, and poorer performance on a water maze test.8 Rats that were de-occluded at 1–2 weeks by releasing the carotid ligation showed CBF levels similar to intact controls, limited CA1 damage, and improved performance on the water maze test. This model was the first to suggest that a state of ‘‘chronic ischemia’’ from large vessel occlusion might reverse with restoration of flow.
In human studies, hemodynamic impairment in high-grade stenosis is associated with lower cognitive scores. An increased probability of developing cognitive deterioration has been demonstrated in patients with unilateral asymptomatic severe carotid stenosis compared to healthy controls (OR = 14.66 (95% CI 7.51–28.59); p<0.001).9 We previously demonstrated that hemodynamic impairment among a cohort of patients with unilateral carotid artery occlusion was an independent predictor of cognitive impairment.7 We recently showed a direct correlation between reduced blood flow in the cortex on the side of high-grade carotid artery stenosis and cortical thinning.10
Clinically, reversal of cognitive impairment with revascularization has been demonstrated only in case series.11,12 A recent study reported 137 patients with >70% internal carotid artery (ICA) stenosis who had transient ischemic attack within the last six months and underwent carotid endarterectomy (CEA). At baseline, these patients demonstrated cognitive impairments specific to the side of occlusion. At six months post-op, cognitive performance was improved and correlated with improvement in cerebral hemodynamics by breath holding index (adj R2>0.70).12 Finally, a meta-analysis of 16 studies found overall improvements in the modified mini-mental state exam and tests of attention/psychomotor speed and memory after carotid stenting.13
Study organization and funding
CREST-H is a National Institute of Neurological Disorders and Stroke (NINDS)-sponsored ancillary study to the NINDS-sponsored Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Study (CREST-2, clinicaltrials.gov NCT02089217).14 CREST-2 is a pair of outcome-blinded, Phase 3 randomized clinical trials in patients with asymptomatic high-grade carotid artery stenosis, assessing stroke and death rates in patients assigned to CEA plus intensive medical management (IMM) versus IMM alone, and those assigned to carotid artery stenting plus IMM versus IMM alone. CREST-2 includes a neurocognitive battery, measuring cognitive status at baseline and yearly up to four years. Once a patient is enrolled and randomized into CREST-2, the patient is approached for CREST-H enrollment. Following CREST-H consent and cognitive assessment and prior to beginning any CREST-2 treatment, the patient undergoes an MRI perfusion scan to determine whether there is hemodynamic flow impairment. Those with flow impairment receive another perfusion scan at one-year follow-up.
We anticipate including approximately 75 sites from among the CREST-2 participating sites to achieve recruitment over the four-year enrollment period. To facilitate implementation, CREST-H utilizes the existing infrastructure of CREST-2, including the Clinical Coordinating Center (Mayo Clinic, Jacksonville, FL), the Statistical and Data Coordinating Center (University of Alabama at Birmingham, Birmingham, AL), and the Vascular Imaging Core lab (University of Maryland, Baltimore). Two new organizational units include: a Perfusion Imaging Core Lab at the University of California at Los Angeles and a Structural Imaging Core Lab at Mayo Clinic, Rochester, MN. A central institutional review board which approved this study currently manages the human subjects’ protection process for approximately 40% of the CREST-2 sites.
Methods
Patient population
From the total of 2480 patients to be enrolled in the two CREST-2 trials approximately 500 will be enrolled in CREST-H. Enrollment into either the surgical or the stenting trial for CREST-2 is allowed since the comparison groups for CREST-H will be revascularization versus IMM alone, regardless of revascularization method. Inclusion and exclusion criteria are listed in Tables 1 and 2. Although CREST-2 has no minimum education requirement and no upper age limit, patients older than 86 years at baseline or with less than eight years of education are excluded from CREST-H due to lack of adequate cognitive norms.
Table 1.
CREST-H inclusion criteria
| 1. Randomization into CREST-2 (all CREST-2 inclusion criteria apply)14 |
| 2. Age 35–86 years |
| 3. Patient agrees to complete baseline MRI scan and another at 1 year if needed |
Table 2.
CREST-H exclusion criteria
| 1. Unable to undergo MRI (e.g. non-compatible metal in body, pacemaker) |
| 2. Known allergy to gadolinium contrast dye |
| 3. Creatinine ≥2.5 mg/dl or GFR < 30 cc/min |
| 4. Contralateral ICA stenosis >70% by MRA, CTA or Doppler ultrasound |
| 5. Pre-existing diagnosis of dementia |
| 6. History of severe head trauma defined by loss of consciousness >30 min, or seizure |
| 7. Major depression |
| 8. Education less than 8 years |
ICA: internal carotid artery; MRA: magnetic resonance angiography; CTA: computerized tomographic angiograpy
Primary outcome.
The primary outcome is the change in cognitive score from baseline to one year. The primary assessment is an ‘‘interaction hypothesis’’ where the difference in cognitive outcome by treatment arm among those with hemodynamic impairment will be compared with the treatment difference in the group without hemodynamic impairment (see Figure 1). This interaction will also be assessed at years 2, 3, and 4.
Figure 1.
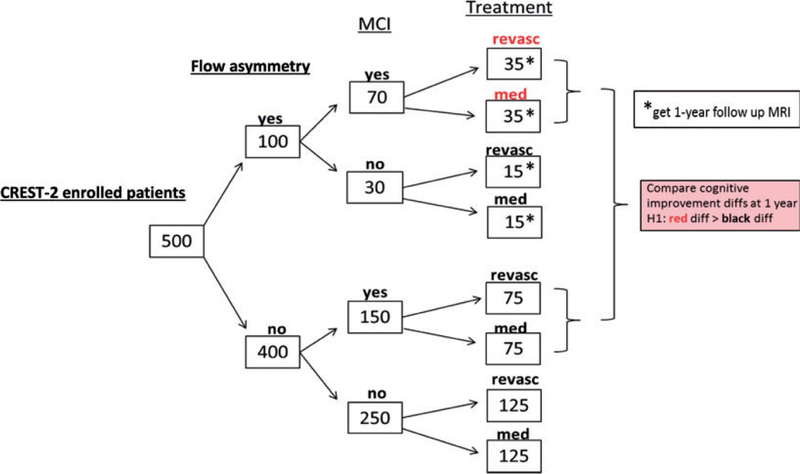
CREST-H study design.
Secondary outcomes
Correlation between cognitive change and perfusion change. We hypothesize that improvement in cognition following revascularization among those with baseline hemodynamic impairment will correlate with improvement in cerebral perfusion.
Silent infarcts and increased white matter hyperintensity volume (WMHV) at one year. We hypothesize that the number of new silent infarcts and the WMHV at one year will be greater in the IMM-only group versus the revascularization group and may help explain changes in cognition.
Exploratory aims will assess cognitive change using alternative imaging measures – time-to-peak (TTP) delay >4 s, mean transit time, maximum perfusion change (Tmax), CBF, and cerebral blood volume.
We will also measure cortical thickness in the target hemisphere versus the hemisphere supplied by the contralateral carotid artery.10,15
Data collection
Cognitive assessments.
The CREST-2 telephone-based cognitive test battery uses well-validated measures in aural format, with certified test administrators (intertester reliability ~98%). To make the CREST-2 battery more sensitive and specific to large vessel cerebral vascular disease, we added Oral Trail Making A & B, which enriches the assessment of executive function. Cognitive assessments take place after CREST-2 randomization but must take place prior to revascularization or up to and including the 44-day CREST-2 follow-up visit if the patient is randomized to IMM alone. At baseline and at each yearly test interval, a composite (mean) z-score is derived from published normative samples for each test element.16 The change in the mean composite z-score from baseline will be calculated, adjusting for age, education, and depression. The test battery is administered identically for all CREST-2 and CREST-H enrolled patients, but the elements used to generate the composite z-score will differ in CREST-H, based on known sensitivity to hemodynamic compromise. Table 3 shows the CREST-2 and CREST-H batteries, with the domain and behavior outcome measures for each, indicating which measures will be used to determine the primary cognitive outcomes, respectively.
Table 3.
CREST-2/H cognitive assessment.
| Domain | Test | Behavior outcome | CREST-2 composite | CREST-H composite |
|---|---|---|---|---|
| Learning | CERAD Word List Learning | Sum of 3 trials | × | |
| Attention | Digit Span | Number of sequences correctly repeated (forward + backward) | × | |
| Memory | CERAD Delayed Recall | Number correct | × | × |
| Animal Fluency | Number correct in 1 min | × | × | |
| Executive Function | Letter Fluency (Controlled Oral Word Association) | Number correct in 1 min for each of F, A, and S | × | × |
| Oral Trail Making A & B | Time to complete Part B | × | × |
x: measure used in the composite z-score to determine outcome.
CERAD: Center to Establish a Registry for Alzheimer’s Disease.
Image acquisition.
MR images will be obtained at each performance site according to parameters established by CREST-H investigators. Field strength of 1.5 T or 3.0 T is allowable, although 3.0 T is preferred. A dynamic contrast susceptibility MR perfusion weighted image (PWI) will be used to stratify patients by hemodynamic status at baseline.
For secondary aims we will also evaluate: (1) WMHV by fluid attenuation inversion recovery (FLAIR), (2) cerebral microbleeds, defined as ≥2 mm hypointense lesions on gradient echo (GRE), (3) silent infarction, defined as hyperintense lesions with a dark center on FLAIR images ≥3 mm on one-year MRI not present on baseline FLAIR, (4) acute silent infarct defined by >1 mm hyperintense lesion on diffusion weighted imaging, and (5) cortical thickness measured by T1 high-resolution imaging. Table 4 lists the general acquisition parameters for each sequence. Specific parameters by MR scanner manufacturer and field strength are provided to sites to standardize across scanner types. Total scan time is under 30 min.
Table 4.
MRI general scanning parameters.
| Sequence | Orientation | Time | Slice (mm) | Gap | Slices | TR | TE | T1 | FOV (cm) | Frequency | Phase | Mode |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High Res T1 | Sagittal | 7:23 | 1.2 | 0 | 160 | NA | Full min | 900 | 24 | 192 | 192 | 3D |
| T2 FLAIR | Axial | 4:36 | 4 | 0 | 36 | 11,000 | 147 | 2460 | 22 | 256 | 192 | 2D |
| DWI/ADC | Axial | 0:50 | 4 | 0 | 36 | 10,000 | Min | NA | 22 | 128 | 256 | 2D |
| 2D PC scout | Coronal | 1:33 | 90 | 0 | 1 | 40 | Min | NA | 22 | 256 | 224 | 2D |
| MRA Intra | Axial | 4:18 | 1.4 (0.7) | 0 | 3 × 32 | Min | Min | NA | 18 | 384 | 224 | 3D |
| Perfusion | Axial | 3:00 | 5 | 0 | Max for TR | 2225 | 60 | NA | 24 | 128 | 96 | 2D |
| GRE | Axial | 0:09 | 4 | 0 | 36 | 1700 | Full min | NA | 22 | 128 | 128 | 2D |
FLAIR: fluid attenuation inversion recovery; DWI: diffusion weighted imaging; MRA: magnetic resonance angiography; GRE: gradient echo; TR: repetition time; TE: echo time; ADC: apparent diffusion coefficient; FOV: field of view.
Image de-identification and blinding.
To ensure that the PWI information from CREST-H does not produce bias in the parent trial, the site PIs for CREST-2 agree to remain blinded to any perfusion scan information and for PWI files not to be processed or read locally.
Image processing.
PWI source images will be processed with a semi-automated system that computes quantitative perfusion maps using deconvolution of tissue and arterial signals in an expedited manner, yielding standardized data regardless of the acquisition system at each site. Hemodynamic impairment is defined as TTP > 2 s in the middle and anterior cerebral artery territories of the ipsilateral hemisphere to the carotid lesion compared with the same territory in the opposite hemisphere. We will quantify the change in the TTP volume at one year, comparing the revascularization versus the medical arm, using continuous values. Voxel-based changes in these serial perfusion values may provide greater detail about the severity, topography, and heterogeneity of the potentially ischemic field. Co-registered, voxel-based changes in serial perfusion values will also be explored with multiparametric values.
Sample size
It is anticipated that at least 20% (100/500) of patients enrolled in CREST-H will have hemodynamic impairment. Based on preliminary studies, 70 of these will have baseline cognitive impairment to ≤–1.0 SD below age-matched norms. Such levels of cognition are consistent with a measurable decline in cognition not yet reaching criteria for frank dementia. In the flow-impairment group, we estimate a 0.6 SD treatment difference between those assigned to revascularization versus IMM alone. In contrast, we estimate that there will be no treatment difference among those with no flow impairment. There is 93% power to detect a differential treatment effect (i.e. interaction) of 0.5 SD, a clinically meaningful effect.17–19
Statistical analysis
Linear regression analysis will be used to estimate the magnitude of the treatment differences with regard to cognition among those with baseline flow impairment versus those without baseline flow impairment, with covariates age, baseline cognitive performance, depression, prior cerebral infarcts, WMHV, and microbleeds. The (two-tailed) significance level will be p = 0.05.
The primary hypothesis statistical model will be
where C1 is the cognitive z-score at year 1, C0 is the cognitive z-score at baseline, T is the treatment indicator variable, F is the flow impairment variable, and βi is the regression parameters to be estimated. The parameter of interest for the primary hypothesis is then β3 that would assess if the magnitude of treatment difference in the change in cognitive score between baseline and one year is similar for those with versus those without flow impairment. Change in z-score will be correlated with the continuous measure of the hemodynamic impairment change.
For analysis of incident silent infarction we will use linear regression if the number of new infarcts is large, or Poisson Regression if the number is smaller (considered less likely). The analysis of the change in WMH will use linear regression.
Summary and conclusions
This ancillary study provides an unparalleled opportunity to address an unanswered question regarding the relationship between hemodynamic impairment and cognition within the rigor of a randomized clinical trial. If we can demonstrate the ability to reverse cognitive decline with a mechanical intervention in a hemodynamically impaired subset of patients, this would re-define ‘‘symptomatic’’ carotid disease to include cognition, and further our understanding of the pathophysiology of vascular cognitive impairment.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the CREST-2 and CREST-H trials for their valuable contributions. A full list of participating CREST-H investigators can be found at http://www.crest2trial.org
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NINDS R01 NS097876, U01 NS080168, and U01 NS080165. Additional support for the CREST-H study comes from NIH StrokeNet U01 NS06872.
Trial Registration: US National Institutes of Health (NIH) clinicaltrials.gov NCT03121209
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Pendlebury ST and Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 2.Hachinski V World stroke day 2008: ‘‘Little strokes, big trouble’’. Stroke 2008; 39: 2407–2420. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 2011; 42: 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatemichi TK, Desmond DW, Prohovnik I and Eidelberg D. Dementia associated with bilateral carotid occlusions: neuropsychological and haemodynamic course after extracranial to intracranial bypass surgery. J Neurol Neurosurg Psychiatry 1995; 58: 633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silvestrini M, Paolino I, Vernieri F, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology 2009; 72: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 6.Buratti L, Balucani C, Viticchi G, et al. Cognitive deterioration in bilateral asymptomatic severe carotid stenosis. Stroke 2014; 45: 2072–2077. [DOI] [PubMed] [Google Scholar]
- 7.Marshall RS, Festa JR, Cheung YK, et al. Cerebral hemodynamics and cognitive impairment: baseline data from the recon trial. Neurology 2012; 78: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Torre JC, Fortin T, Park GA, Pappas BA and Richard MT. Brain blood flow restoration ‘rescues’ chronically damaged rat ca1 neurons. Brain Res 1993; 623: 6–15. [DOI] [PubMed] [Google Scholar]
- 9.Balestrini S, Perozzi C, Altamura C, et al. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology 2013; 80: 2145–2150. [DOI] [PubMed] [Google Scholar]
- 10.Marshall RS, Asllani I, Pavol MA, Cheung YK and Lazar RM. Altered cerebral hemodyamics and cortical thinning in asymptomatic carotid artery stenosis. PLoS One 2017; 12: e0189727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lal BK, Younes M, Cruz G, Kapadia I, Jamil Z and Pappas PJ. Cognitive changes after surgery vs stenting for carotid artery stenosis. J Vasc Surg 2011; 54: 691–698. [DOI] [PubMed] [Google Scholar]
- 12.Lattanzi S, Carbonari L, Pagliariccio G, et al. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology 2018; 90: e307–e315. [DOI] [PubMed] [Google Scholar]
- 13.Antonopoulos CN, Kakisis JD, Sfyroeras GS, et al. The impact of carotid artery stenting on cognitive function in patients with extracranial carotid artery stenosis. Ann Vasc Surg 2015; 29: 457–469. [DOI] [PubMed] [Google Scholar]
- 14.Howard VJ, Meschia JF, Lal BK, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis: protocol of the crest-2 clinical trials. Int J Stroke 2017; 12: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asllani I, Slattery P, Fafard A, Pavol M, Lazar RM and Marshall RS. Measurement of cortical thickness asymmetry in carotid occlusive disease. Neuroimage Clin 2016; 12: 640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005; 25: 135–143. [DOI] [PubMed] [Google Scholar]
- 17.Unger JM, van Belle G and Heyman A. Cross-sectional versus longitudinal estimates of cognitive change in nondemented older people: a cerad study. Consortium to establish a registry for Alzheimer’s disease. J Am Geriatr Soc 1999; 47: 559–563. [DOI] [PubMed] [Google Scholar]
- 18.Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Stoddard A and Tennstedt SL. The active cognitive training trial and health-related quality of life: protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci 2006; 61: 1324–1329. [DOI] [PubMed] [Google Scholar]
- 19.Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA 2015; 314: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


