Bovine herpesvirus 1 (BoHV-1) is an ubiquitous pathogen affecting cattle populations worldwide. Infection can result in complicated, polymicrobial infections due to the immunosuppressive properties of the virus. Available vaccines limit disease severity and spread but do not prevent infection. The financial and animal welfare ramifications of BoHV-1 are significant. In order to develop more effective prevention and treatment regimens, a more complete understanding of the initial steps in viral infection is necessary. We recently identified a low pH endocytosis pathway for BoHV-1. Here, we examine the role of cellular factors responsible for membrane integrity and repair in alphaherpesviral entry. This study allows comparisons of the BoHV-1 entry pathway with those of other alphaherpesviruses (pseudorabies virus [PRV] and herpes simplex virus 1 [HSV-1]). Lastly, this is the first report of sphingomyelin and lysosomal sphingomyelinase playing a role in the entry of a herpesvirus. The results may lead to the development of more effective prevention and treatment regimens.
KEYWORDS: bovine herpesvirus 1, endocytosis, herpes simplex virus, herpesviruses, membranes, pseudorabies virus, sphingomyelin, viral entry
ABSTRACT
Bovine herpesvirus 1 (BoHV-1) is an alphaherpesvirus that causes disease in cattle populations worldwide. Sphingomyelin (SM) is the most abundant sphingolipid in the mammalian cell membrane, where it preferentially associates with cholesterol to form lipid raft domains. SM is a substrate for the lysosome-resident enzyme acid sphingomyelinase, which plays a role in cell membrane repair following injury. Treatment of cells with noncytotoxic concentrations of Staphylococcus aureus-derived sphingomyelinase successfully reduced cell surface-exposed sphingomyelin but did not significantly inhibit BoHV-1 entry and infection, as measured by the beta-galactosidase reporter assay. Interestingly, entry of the porcine alphaherpesvirus pseudorabies virus (PRV) was inhibited by sphingomyelin-depletion of cells. Treatment of BoHV-1 particles with sphingomyelinase inhibited viral entry activity, suggesting that viral SM plays a role in BoHV-1 entry, while cellular SM does not. Treatment of cells with noncytotoxic concentrations of the functional inhibitors of host acid sphingomyelinase, imipramine and amitriptyline, which induce degradation of the cellular enzyme, did not significantly inhibit BoHV-1 entry. In contrast, inhibition of cellular acid sphingomyelinase inhibited PRV entry. Entry of the human alphaherpesvirus herpes simplex virus 1 (HSV-1) was independent of both host SM and acid sphingomyelinase, in a manner similar to BoHV-1. Together, the results suggest that among the alphaherpesviruses, there is variability in entry requirements for cellular sphingomyelin and acid sphingomyelinase activity.
IMPORTANCE Bovine herpesvirus 1 (BoHV-1) is an ubiquitous pathogen affecting cattle populations worldwide. Infection can result in complicated, polymicrobial infections due to the immunosuppressive properties of the virus. Available vaccines limit disease severity and spread but do not prevent infection. The financial and animal welfare ramifications of BoHV-1 are significant. In order to develop more effective prevention and treatment regimens, a more complete understanding of the initial steps in viral infection is necessary. We recently identified a low pH endocytosis pathway for BoHV-1. Here, we examine the role of cellular factors responsible for membrane integrity and repair in alphaherpesviral entry. This study allows comparisons of the BoHV-1 entry pathway with those of other alphaherpesviruses (pseudorabies virus [PRV] and herpes simplex virus 1 [HSV-1]). Lastly, this is the first report of sphingomyelin and lysosomal sphingomyelinase playing a role in the entry of a herpesvirus. The results may lead to the development of more effective prevention and treatment regimens.
INTRODUCTION
Bovine herpesvirus 1 (BoHV-1) is an alphaherpesvirus that causes disease in cattle around the globe (1–4). BoHV-1 infection induces severe ulcerative rhinotracheitis, pustular vulvovaginitis and balanoposthitis, abortion, transient immunosuppression, and, critically, lifelong latent infection (5–7). Virus-mediated suppression of host immune function is believed to play a key role in development of the polymicrobial disorder known as bovine respiratory disease complex or “shipping fever,” which results in over 500 million dollars a year in losses to the United States beef industry (8, 9). BoHV-1 entry and infection is initiated by virus interaction with cellular membranes mediated by viral glycoproteins gB, gC, and gD (6, 10, 11). While BoHV-1 gC is not required for replication, gC mediates attachment by binding to cell surface heparan sulfate proteoglycans (12, 13). BoHV-1 gB and gD are essential receptor-binding proteins involved in membrane fusion and cell penetration (6, 14–18). BoHV-1 gD interacts with cellular receptors nectin-1 and poliovirus receptor (Pvr) (19–21). Recently, host cell endocytosis and endosomal low pH were demonstrated to be critical for BoHV-1 entry (22). BoHV-1 gB or a gH/gL complex may also interact with cellular membrane proteins, including the putative alphaherpesvirus gB-receptor PILRα (23–28).
As obligatory intracellular organisms, viruses are fully dependent on the host cell. In order to replicate, a virus must transfer its genome from the cell exterior to the interior, and to propagate, the virus must transmit its genome from infected to uninfected cells (29). In order to successfully perform these functions, viruses exploit normal cellular pathways. In eukaryotic cells, endocytosis is a continuous process in which vesicles made of plasma membrane components are internalized, playing a critical role in a number of homeostatic functions (30). To gain access to the cell interior, viruses can hijack endocytosis pathways for movement across host cell membranes (31–33). Alphaherpesviruses, including bovine herpesvirus 1, pseudorabies virus, and herpes simplex virus 1 utilize endocytosis for successful entry (22, 34–36). Many additional cellular determinants of virus entry remain undefined and may offer considerable potential for the development of novel therapeutics.
Lipid rafts are sphingolipid- and cholesterol-enriched microdomains that were first identified by their resistance to solubilization by detergents (37, 38). Lipid rafts are involved in lipid and protein sorting and endocytic trafficking in both epithelial cells and neurons and play a role in signal transduction in a variety of cell types (37, 38). Maintenance of membrane integrity is paramount for cell survival and requires the constitutive function of a multitude of pathways (39). Critical in this maintenance is the dynamic interplay between endocytic and exocytic pathways (40). Following membrane injury, cells deliver vesicles to the plasma membrane by exocytosis, followed by rapid endocytosis of membrane lesions, leading to membrane resealing (40–43). Exocytosis delivers a lysosomal enzyme, acid sphingomyelinase (ASMase), to the outer membrane leaflet, where it cleaves membrane sphingomyelin (SM) to phosphorylcholine and ceramide (44). SM is a phospholipid and is the most abundant sphingolipid component of the mammalian plasma membrane, where it preferentially associates with cholesterol to form lipid rafts (38, 45, 46). Ceramide is a potent signaling molecule that can self-associate, joining lipid rafts into larger macrodomains that promote signal transmission across membranes as well as protein concentration and receptor clustering within membranes (47, 48).
While the specific role of lipid rafts in alphaherpesvirus entry is not known, pseudorabies virus (PRV) localizes in juxtaposition with GM1, a lipid raft marker (49), and herpes simplex virus 1 (HSV-1) gB associates with lipid rafts during virus entry (50). Additionally, cholesterol is important for entry of alphaherpesviruses (49–53). BoHV-1 requires cell membrane cholesterol for entry into Madin-Darby bovine kidney (MDBK) cells and viral envelope cholesterol for infectivity (54). PRV requires cell plasma membrane cholesterol to enter swine kidney (SK) cells and viral envelope cholesterol for infectivity in SK cells (49, 55). HSV-1 requires cell membrane cholesterol for efficient infection of cells and viral envelope cholesterol for entry (50, 56, 57). Host cell cholesterol is specifically needed for the membrane fusion step of HSV-1 entry (56). Sphingomyelin and acid sphingomyelinase can influence cell entry of a number of viruses. Adenovirus both induces and hijacks the cellular processes that remove membrane wounds, and inhibition of ASMase slows endocytosis of adenovirus and reduces adenoviral infection (58). Ebola virus requires the host cell plasma membrane sphingomyelin and acid sphingomyelinase activity for successful infection (59). Exposure of ceramide by ASMase hydrolysis of sphingomyelin has been demonstrated to mediate endosomal escape by calicivirus, leading to successful infection (60). West Nile virus infection is enhanced by sphingomyelin accumulation and is decreased by depletion of sphingomyelin (61). Hepatitis C virus requires viral envelope sphingomyelin and cholesterol for successful infection (62). Finally, influenza virus requires a functional sphingomyelin synthesis pathway for the generation of infectious particles (63). The roles of sphingomyelin and acid sphingomyelinase in herpesviral entry remain undefined.
RESULTS AND DISCUSSION
BoHV-1 entry into MDBK cells is not dramatically altered by hydrolysis of cell membrane sphingomyelin.
To determine whether alteration of host cell sphingomyelin inhibits BoHV-1 infection, we employed Staphylococcus aureus-derived sphingomyelinase (SMase). When added to cells at neutral pH, SMase hydrolyzes surface-exposed sphingomyelin, leaving ceramide behind in the membrane (44). Hydrolysis of cellular SM inhibits the entry of viruses that require SM (59, 64). MDBK cells were treated with increasing concentrations of SMase for 45 min at 37°C. SMase was removed, and BoHV-1 v4a (lacZ-positive) was added to cells. Beta-galactosidase activity at 6.5 h postinfection was an indicator of viral entry. Treatment of MDBK cells with SMase did not result in robust inhibition of BoHV-1 entry (Fig. 1). The highest concentration tested (10 U/ml), inhibited BoHV-1-induced reporter expression by only 15%. In contrast, treatment of PK15 cells with SMase inhibited PRV BeBlue (lacZ-positive) entry by as much as 54% (>2-fold) at the highest concentration tested (Fig. 1). Inhibition of PRV entry by treating cells with 5 to 10 U/ml SMase was statistically significant. The modest inhibition may be due to the incomplete removal of sphingomyelin from cells. More complete depletion would likely result in cytotoxicity. These results suggest that BoHV-1 does not rely on cellular sphingomyelin for efficient entry, whereas PRV entry is partly sphingomyelin dependent.
FIG 1.
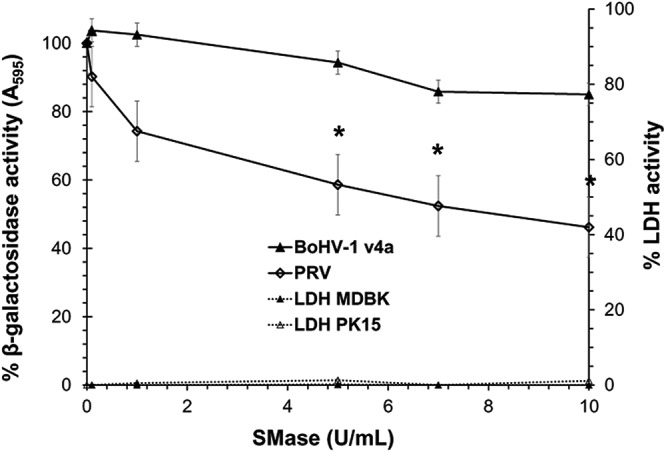
Effect of sphingomyelin depletion on BoHV-1 and PRV entry into cells. MDBK or PK15 cells were treated with SMase at the indicated concentrations and incubated at 37°C for 45 min. Cells were washed with PBS. BoHV-1 or PRV lacZ-positive strains were added, and at 45 min p.i. noninternalized virus was inactivated with medium buffered to pH 4.7. Infection proceeded for a total of 6.5 h in the presence of normal culture medium. The beta-galactosidase expression is calculated as a percentage of the activity in mock-treated cells. Values shown are the means of three independent experiments with standard errors. The P values were determined using Student’s t test. (*, P < 0.05). Cytotoxicity is shown as percent LDH activity. LDH activity was <2% for all tested concentrations of SMase on both MDBK and PK15 cells.
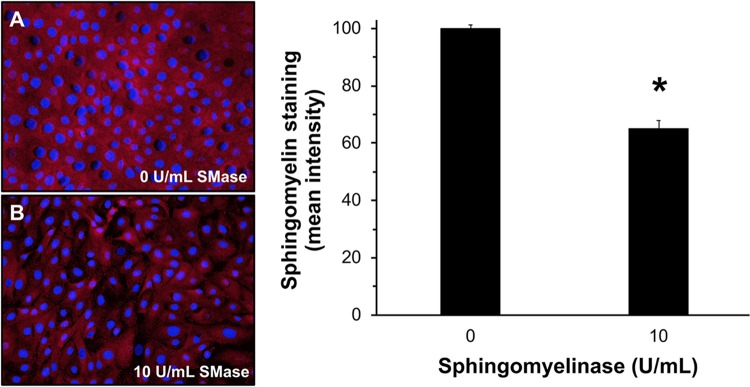
To confirm that SMase treatment reduces plasma membrane sphingomyelin, MDBK cells grown in confluent monolayers on coverslips were treated with SMase for 45 min, and then fixed with 6% paraformaldehyde. The sphingomyelin-binding protein lysenin was added to cells and detected by immunofluorescence microscopy. Lysenin binds specifically to sphingomyelin and has no cross-reactivity with other membrane phospholipids, including ceramide (65). Treatment of MDBK cells with 10 U/ml SMase reduced sphingomyelin staining intensity by 35% (Fig. 2). These results support the conclusion that BoHV-1 does not require cell membrane SM for entry.
FIG 2.
SMase treatment of MDBK cells reduces sphingomyelin levels. Confluent MDBK monolayers were treated with DMEM (A) or 10 U/ml S. aureus SMase (B) for 45 min at 37°C. Cells were fixed with 6% paraformaldehyde and incubated with 0.5 μM lysenin for 2 h. Rabbit polyclonal antibody to lysenin was added and then detected with Alexa Fluor 594-conjugated goat anti-rabbit antibody (red). Nuclei were counterstained with DAPI. Cells were visualized by fluorescence microscopy. Magnification, ×40. ImageJ software was used to measure the mean fluorescence intensity from five equal areas per sample, each containing ∼150 to 250 cells. Results are representative of two independent experiments. The P value was determined using Student’s t test. (*, P < 0.0005).
Treatment of viral particles with SMase inhibits BoHV-1 entry activity.
Depletion of BoHV-1 viral envelope cholesterol by methyl-beta-cyclodextrin significantly reduces virus infectivity (54). We next investigated the importance of SM in the BoHV-1 viral envelope for virus entry and infectivity. Increasing concentrations of S. aureus-derived SMase were added to BoHV-1 v4a for 45 min at 37°C. Reactions were diluted in culture medium to achieve a final enzyme concentration of 0.1 U/ml or less, which, when added to cells, did not inhibit BoHV-1 entry (Fig. 1). Treated BoHV-1 was added to MDBK cells. Beta-galactosidase activity at 6.5-h postinfection was an indicator of viral entry. The highest concentration of SMase tested inhibited approximately 70% of BoHV-1 entry activity (Fig. 3). The importance of viral envelope sphingomyelin to HSV-1 and PRV entry is of interest and remains to be determined. These results suggest that viral envelope SM plays a critical role in BoHV-1 infectivity. Further investigation is required to determine the precise step at which sphingomyelin acts or whether it is required for viral envelope stability.
FIG 3.
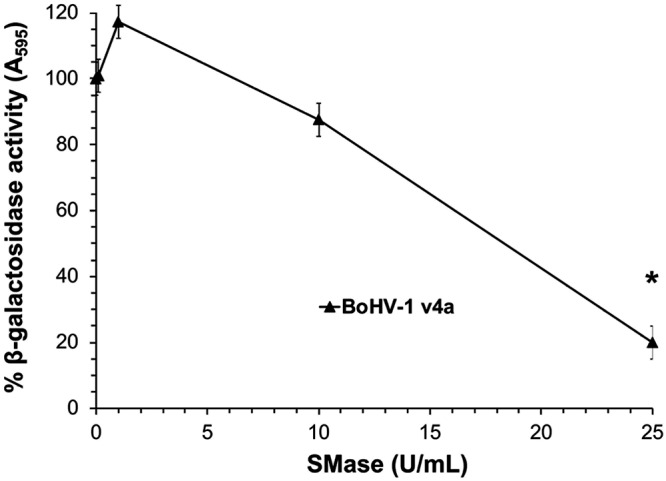
BoHV-1 entry activity is dependent on viral envelope sphingomyelin. Concentrated BoHV-1 virions were incubated at the indicated concentrations for 45 min at 37°C. Virus was diluted to a final concentration of 0.1 U/ml or less. Cells were infected with treated virus at an MOI of 2 for 6.5 h. The beta-galactosidase expression was calculated as a percentage of activity of untreated virus. Values are the means of three independent experiments with standard errors. The P value was determined using Student’s t test. (*, P < 0.0005).
Functional inhibitors of lysosomal acid sphingomyelinase do not inhibit BoHV-1 entry but do inhibit PRV entry.
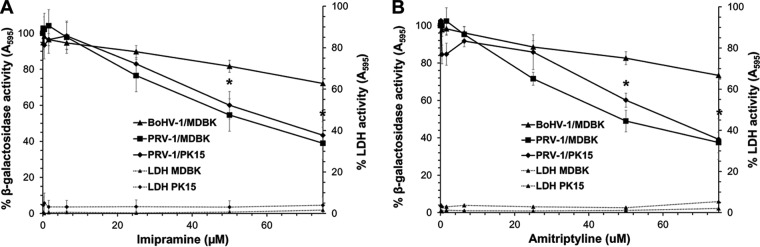
Imipramine and amitriptyline belong to a large group of organic, amphiphilic bases known as functional inhibitors of acid sphingomyelinase (FIASMAs) (66). These drugs accumulate within lysosomes and are believed to displace cellular acid sphingomyelinase from the inner lysosomal membrane leaflet. Following detachment, inactivation within the lysosome may result from proteolytic degradation (67). Inactivation of host cell acid sphingomyelinase reduces infection by a number of viruses, including adenovirus, Ebola virus, certain rhinoviruses, and measles virus. FIASMAs also block endosomal escape by feline calicivirus, porcine enteric calicivirus, and murine norovirus (58–60, 68). To determine whether cellular acid sphingomyelinase is required for alphaherpesviral entry and infection, cells were treated with increasing concentrations of either imipramine or amitriptyline for 1 h, followed by infection with lacZ-positive BoHV-1 or PRV in the continued presence of drug. A total of 75 μM imipramine or amitriptyline failed to inhibit >50% of BoHV-1-induced beta-galactosidase activity on MDBK cells (Fig. 4A and B). In contrast, the FIASMAs inhibited 56% to 62% of PRV entry into MDBK and PK15 cells. The inhibitory concentrations of the drugs were not cytotoxic to MDBK or PK15 cells under the tested conditions. These results suggest that BoHV-1 entry does not require cellular acid sphingomyelinase, while PRV is dependent on the lysosomal enzyme for efficient entry. These findings suggest that host cell membrane sphingomyelin and acid sphingomyelinase may be part of a pathway that mediates PRV entry and infection. The requirement for both cellular factors suggests that the enzymatic action of SMase on SM and the underlying cellular membrane repair pathway may be crucial to PRV entry. During rhinovirus infection of human epithelial cells, virions colocalize with ceramide-enriched domains generated by ASMase cleavage of SM (68). It remains to be determined whether PRV associates with ceramide domains. Alternately, host sphingomyelin may play a direct role in PRV membrane fusion, while fusion during entry of BoHV-1 may be sphingomyelin independent.
FIG 4.
Effect of cellular acid sphingomyelinase inactivation by FIASMAs on BoHV-1 infection. Cells were treated with imipramine (A) or amitriptyline (B) at the indicated concentrations for 1 h at 37°C. MDBK or PK15 cells were infected with BoHV-1 or PRV, respectively, in the continued presence of drug. At 6.5 h p.i., the beta-galactosidase expression was calculated as a percentage of activity in untreated, infected cells. Cytotoxicity is shown as percent LDH activity. Values are the means of two or three independent experiments with standard errors. The P value represents PRV on MDBK cells and was determined using Student’s t test. (*, P < 0.009).
Role of host cell sphingomyelin and acid sphingomyelinase in HSV-1 entry and infection of Vero cells.
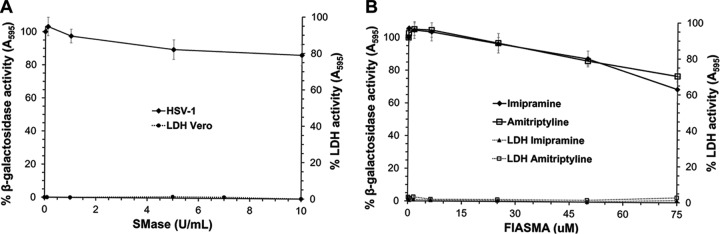
Given the disparate results for BoHV-1 and PRV, we extended the study to evaluate roles for sphingomyelin and lysosomal ASMase in the entry of the human alphaherpesvirus HSV-1. Vero cells were treated with SMase, washed, and then infected with HSV-1 tk12 (lacZ-positive) for 45 min at 37°C. The SMase-treated cells supported HSV-1 entry (Fig. 5A). Treatment with the highest concentration of SMase tested inhibited only ∼13% of HSV-1-induced beta-galactosidase activity. This suggests that HSV-1, similar to BoHV-1, does not require cellular sphingomyelin for entry and infection. BoHV-1 and HSV-1 entry both require cholesterol but not sphingomyelin, which may suggest that intact lipid rafts are not necessary for viral entry.
FIG 5.
Effects of cellular sphingomyelin depletion and FIASMAs on HSV-1 entry. (A) SMase was added to Vero cells at 37°C for 45 min. Treated and mock-treated cells were washed with PBS. HSV-1 KOS tk12 (lacZ-positive) was added, and at 45 min p.i., noninternalized virus was inactivated with medium buffered to pH 4.7. The infection proceeded for total of 6.5 h in the presence of normal culture medium. (B) Vero cells were treated with imipramine or amitriptyline for 1 h at 37°C. Cells were infected with HSV-1 in the continued presence of drugs. At 6.5 h p.i., the beta-galactosidase expression was calculated as a percentage of activity in untreated, infected cells. Values are the means of three independent experiments with standard errors. Cytotoxicity is shown as percent LDH activity.
Vero cells were treated with increasing concentrations of either imipramine or amitriptyline for 1 h followed by infection with HSV-1 in the continued presence of drug. HSV-1 entry and infection was resistant to inhibition by FIASMAs (Fig. 5B). A total of 75 μM imipramine or amitriptyline only inhibited HSV-1-induced beta-galactosidase activity ∼30% or 23%, respectively. This result suggests that HSV-1, similar to BoHV-1, does not absolutely require cellular acid sphingomyelinase activity for entry and infection. BoHV-1 and PRV belong to the Varicellovirus genus, while HSV-1 is a simplex virus. Thus, a reasonable prediction would be that BoHV-1 and PRV would respond similarly to these treatments or that HSV-1 might respond uniquely. Altogether, the results suggest that alphaherpesviruses, regardless of phylogenetic relationship, may have differential requirements for host cell membrane sphingomyelin and lysosomal sphingomyelinase for entry.
MATERIALS AND METHODS
Cells and viruses.
MDBK cells and Vero cells (American Type Culture Collection, Manassas, VA) were propagated in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Grand Island, NY) supplemented with 5% and 10% fetal bovine serum (Atlanta Biologicals, Atlanta, GA), respectively. PK15 cells (provided by Matthew Taylor, Montana State University) were propagated in DMEM (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Atlanta Biologicals). BoHV-1 strain Colorado-1 (American Type Culture Collection) was propagated on MDBK cells. A thymidine kinase-negative, beta-galactosidase-positive recombinant of BoHV-1 Colorado-1 containing the Escherichia coli lacZ gene in place of the viral thymidine kinase gene (obtained from C. Whitbeck, G. Cohen, and R. Eisenberg, University of Pennsylvania) was propagated on MDBK cells (69). PRV BeBlue (provided by Lynn Enquist, Princeton University), a PRV Becker strain derivative with the E. coli lacZ gene inserted into the gG locus (46), was propagated on PK15 cells. HSV-1 strain KOS tk12 (obtained from Patricia Spear, Northwestern University), which contains the lacZ gene under the control of an HSV-inducible promoter, was propagated on Vero cells (70).
Reagent preparation.
Stock solutions of Staphylococcus aureus-derived sphingomyelinase in a 50% buffered aqueous glycerol solution (Sigma-Aldrich, St. Louis, MO) were diluted in DMEM containing 5% (MDBK cells) or 10% (PK15 and Vero cells) fetal bovine serum (FBS) immediately prior to use. One-hundred-millimolar stock solutions of imipramine and amitriptyline (Sigma-Aldrich) were prepared in water, stored at −80°C, and diluted in DMEM immediately prior to use.
Cell viability.
Cell viability was assessed by direct measurement of lactate dehydrogenase (LDH) leakage (71). Confluent cell monolayers grown in 96-well plates were treated with experimental concentrations of drugs, 50% buffered aqueous glycerol solution, or cell culture-grade water for 6.5 h. Cells in triplicate wells were lysed, and plates were incubated for an additional 30 min. LDH leakage was determined using a Pierce LDH cytotoxicity assay kit (Thermo Scientific, Rockford, IL) following the manufacturer's instructions.
Beta-galactosidase reporter assay of alphaherpesvirus entry.
Quadruplicate wells of confluent cell monolayers grown in 96-well plates were infected with BoHV-1 v4a (MOI of ∼2), PRV BeBlue (multiplicity of infection [MOI] of ∼1) or HSV-1 KOS tk12 (MOI of ∼12) for 6.5 h at 37°C. A higher MOI for HSV-1 tk12 was required to achieve beta-galactosidase expression levels comparable to the other viruses. Cell lysates were prepared with 0.5% Igepal (Sigma-Aldrich, St. Louis, MO). Chlorophenol red-β-d-galactopyranoside (Roche Diagnostics, Indianapolis, IN) was added, and beta-galactosidase activity was read at 595 nm with a microtiter plate reader (BioTek Instruments, Winooski, VT).
Effect of SMase treatment of cells on alphaherpesvirus entry.
Confluent cell monolayers grown in 96-well plates were incubated with sphingomyelinase (Sigma-Aldrich, St. Louis, MO) at various concentrations at 37°C for 45 min. Cells were washed twice with warm PBS. Virus was added to cells. At 45 min postinoculation (p.i.), the inoculum was removed, and noninternalized virus was inactivated by the treatment of cells with culture medium buffered to pH 4.7. At 6.5 h p.i. the beta-galactosidase reporter assay for entry was performed.
Effect of SMase on BoHV-1 virions.
Extracellular, supernatant preparations of BoHV-1 v4a was incubated with SMase at various concentrations at 37°C for 45 min. Viral preparations were diluted 100- to 250-fold in culture medium to obtain a final enzyme concentration of 0.1 U/ml or less and a final virus titer of ∼3 × 107 PFU/ml. Treated virus and samples of similarly diluted, untreated virus were added to confluent MDBK cell monolayers in 96-well plates (MOI of ∼1). At 6.5 h p.i. the beta-galactosidase reporter assay for entry was performed.
Detection of cellular sphingomyelin.
Lysenin (Sigma-Aldrich, St. Louis, MO) was reconstituted in PBS and stored at −20°C. Lysenin antisera (Peptides International, Louisville, KY) was stored at −20°C and diluted 1:1,000 in blocking solution immediately prior to use. Goat anti-rabbit Alexa Fluor 594 (Thermo Fisher Scientific, Grand Island, NY) was stored at 4°C and diluted at 1:1,000 in 1% ovalbumin (Sigma-Aldrich) in PBS immediately prior to use. Confluent cell monolayers grown on coverslips were incubated with sphingomyelinase or mock-treated at 37°C for 45 min. Cells were washed twice with room temperature PBS and fixed with freshly prepared 6% paraformaldehyde (Sigma-Aldrich) in PBS at room temperature for 10 min. Cells were washed twice with PBS, and paraformaldehyde was quenched by treatment with 50 mM ammonium chloride at room temperature for 10 min. Cells were washed twice with PBS and incubated with 1% ovalbumin in PBS at room temperature. Cells were then incubated with 0.5 μg/ml lysenin prepared in 1% ovalbumin at room temperature for 2 h. Cells were washed twice with PBS and incubated with lysenin rabbit antisera at 4°C overnight. Cells were washed twice with PBS and incubated with Alexa Fluor 594-labeled goat anti-rabbit antibody for 1 h at room temperature. Cells were washed twice with PBS, and nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Roche Diagnostics, Indianapolis, IN) for 10 min at room temperature. Cells were washed twice with PBS and once with room temperature cell culture-grade water. Coverslips were mounted to slides with Fluoromount G (Thermo Fisher Scientific). Samples were visualized with a fluorescence microscope (Leica Microsystems, Inc., Buffalo Grove, IL) at ×40 magnification.
Effect of FIASMAs on alphaherpesviral entry.
Confluent cell monolayers grown in 96-well plates were incubated with imipramine or amitriptyline (Sigma-Aldrich, St. Louis, MO) at various concentrations for 1 h. Virus was added, and cells were incubated in the continued presence of agent for 6.5 h.
ACKNOWLEDGMENTS
We thank Amanda Foreman for early contributions to this work and Massaro Ueti for use of the fluorescence microscope. We also thank Gary Cohen, Roselyn Eisenberg, Lynn Enquist, Patricia Spear, Subramaniam Srikumaran, Naomi Taus, Matthew Taylor, and J. Charles Whitbeck for generous gifts of reagents.
This work was supported by Public Health Service grants AI119159 (A.V.N.) and AI007025 (G.P.) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Leon FC, Diez FG, Ferri FR, Vizcaino LL, Gijon FC, Gimeno EJ, Sein CZ, Rodriguez JMSV, Madrigal JJC, Gomez PC, Schudel A. 2005. The translation into Spanish of the OIE Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees): problems, solutions and conclusions. Rev Sci Tech 24:1095–1104. [PubMed] [Google Scholar]
- 2.Constable P, Hinchcliff KW, Done S, Gruenberg W. 2017. Veterinary medicine: a textbook of the diseases of cattle, sheep, pigs, goats and horses, 11th ed Saunders Ltd; Philadelphia, PA. [Google Scholar]
- 3.Rock DL, Reed DE. 1982. Persistent infection with bovine herpesvirus type 1: rabbit model. Infect Immun 35:371–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winkler MT, Doster A, Jones C. 2000. Persistence and reactivation of bovine herpesvirus 1 in the tonsils of latently infected calves. J Virol 74:5337–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones C, Chowdhury S. 2007. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Anim Health Res Rev 8:187–205. doi: 10.1017/S146625230700134X. [DOI] [PubMed] [Google Scholar]
- 6.Babiuk LA, van Drunen Littel-van den Hurk S, Tikoo SK. 1996. Immunology of bovine herpesvirus 1 infection. Vet Microbiol 53:31–42. [DOI] [PubMed] [Google Scholar]
- 7.Srikumaran S, Kelling CL, Ambagala A. 2007. Immune evasion by pathogens of bovine respiratory disease complex. Anim Health Res Rev 8:215–229. doi: 10.1017/S1466252307001326. [DOI] [PubMed] [Google Scholar]
- 8.Jones C, Chowdhury S. 2010. Bovine herpesvirus type 1 (BHV-1) is an important cofactor in the bovine respiratory disease complex. Vet Clin North Am Food Anim Pract 26:303–321. doi: 10.1016/j.cvfa.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Gershwin LJ, Van Eenennaam AL, Anderson ML, McEligot HA, Shao MX, Toaff-Rosenstein R, Taylor JF, Neibergs HL, Womack J, Bovine Respiratory Disease Complex Coordinated Agricultural Project Research T . 2015. Single pathogen challenge with agents of the bovine respiratory disease complex. PLoS One 10:e0142479. doi: 10.1371/journal.pone.0142479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang XP, Babiuk LA, Littel-van den Hurk SV, Fitzpatrick DR, Zamb TJ. 1991. Bovine herpesvirus-1 attachment to permissive cells is mediated by its major glycoprotein-gi, glycoprotein-giii, and glycoprotein-giv. J Virol 65:1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, van Drunen Littel-van den Hurk S, Babiuk LA, Liang X. 1995. Characterization of cell-binding properties of bovine herpesvirus 1 glycoproteins B, C, and D: identification of a dual cell-binding function of gB. J Virol 69:4758–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang X, Babiuk LA, Zamb TJ. 1992. An in vivo study of a glycoprotein gIII-negative bovine herpesvirus 1 (BHV-1) mutant expressing beta-galactosidase: evaluation of the role of gIII in virus infectivity and its use as a vector for mucosal immunization. Virology 189:629–639. doi: 10.1016/0042-6822(92)90586-E. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki K, Matsuzaki T, Sugahara Y, Okada J, Hasebe M, Iwamura Y, Ohnishi M, Kanno T, Shimizu M, Honda E. 1991. BHV-1 adsorption is mediated by the interaction of glycoprotein gIII with heparinlike moiety on the cell surface. Virology 181:666–670. [DOI] [PubMed] [Google Scholar]
- 14.Keil GM, Hohle C, Giesow K, Konig P. 2005. Engineering glycoprotein B of bovine herpesvirus 1 to function as transporter for secreted proteins: a new protein expression approach. J Virol 79:791–799. doi: 10.1128/JVI.79.2.791-799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ll YH, Van Drunen little-Van Den Hurk S, Liang XP, Babiuk LA. 1997. Functional analysis of the transmembrane anchor region of bovine herpesvirus 1 glycoprotein gB. Virology 228:39–54. doi: 10.1006/viro.1996.8372. [DOI] [PubMed] [Google Scholar]
- 16.Alves Dummer L, Pereira Leivas Leite F, van Drunen Littel-van den Hurk S. 2014. Bovine herpesvirus glycoprotein D: a review of its structural characteristics and applications in vaccinology. Vet Res 45:111. doi: 10.1186/s13567-014-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chase CC, Carter-Allen K, Lohff C, Letchworth GJ III, 1990. Bovine cells expressing bovine herpesvirus 1 (BHV-1) glycoprotein IV resist infection by BHV-1, herpes simplex virus, and pseudorabies virus. J Virol 64:4866–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miethke A, Keil GM, Weiland P, Mettenleiter TC. 1995. Unidirectional complementation between glycoprotein-B homologs of pseudorabies virus and bovine herpesvirus-1 is determined by the carboxyterminal part of the molecule. J Gen Virol 76:1623–1635. doi: 10.1099/0022-1317-76-7-1623. [DOI] [PubMed] [Google Scholar]
- 19.Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev Med Virol 10:305–319. [DOI] [PubMed] [Google Scholar]
- 20.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618–1620. [DOI] [PubMed] [Google Scholar]
- 21.Spear PG, Eisenberg RJ, Cohen GH. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 22.Pastenkos G, Lee B, Pritchard SM, Nicola AV. 2018. Bovine herpesvirus 1 entry by a low pH endosomal pathway. J Virol 92:e00839-18. doi: 10.1128/JVI.00839-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arii J, Uema M, Morimoto T, Sagara H, Akashi H, Ono E, Arase H, Kawaguchi Y. 2009. Entry of herpes simplex virus 1 and other alphaherpesviruses via the paired immunoglobulin-like type 2 receptor alpha. J Virol 83:4520–4527. doi: 10.1128/JVI.02601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Drunen Littel-van den Hurk S, Khattar S, Tikoo SK, Babiuk LA, Baranowski E, Plainchamp D, Thiry E. 1996. Glycoprotein H (gII/gp108) and glycoprotein L form a functional complex which plays a role in penetration, but not in attachment, of bovine herpesvirus 1. J Gen Virol 77:1515–1520. doi: 10.1099/0022-1317-77-7-1515. [DOI] [PubMed] [Google Scholar]
- 25.Fuller AO, Santos RE, Spear PG. 1989. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol 63:3435–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer G, Hanon E, Georlette D, Pastoret PP, Thiry E. 1998. Bovine herpesvirus type 1 glycoprotein H is essential for penetration and propagation in cell culture. J Gen Virol 79:1983–1987. doi: 10.1099/0022-1317-79-8-1983. [DOI] [PubMed] [Google Scholar]
- 27.Khattar SK, van Drunen Littel-van den Harke S, Attah-Poku SK, Babiuk LA, Tikoo SK. 1996. Identification and characterization of a bovine herpesvirus-1 (BHV-1) glycoprotein gL which is required for proper antigenicity, processing, and transport of BHV-1 glycoprotein gH. Virology 219:66–76. [DOI] [PubMed] [Google Scholar]
- 28.Schroder C, Keil GM. 1999. Bovine herpesvirus 1 requires glycoprotein H for infectivity and direct spreading and glycoproteins gH(W450) and gB for glycoprotein D-independent cell-to-cell spread. J Gen Virol 80:57–61. doi: 10.1099/0022-1317-80-1-57. [DOI] [PubMed] [Google Scholar]
- 29.Marsh M, Helenius A. 2006. Virus entry: open sesame. Cell 124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mellman I. 1996. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol 12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 31.Cossart P, Helenius A. 2014. Endocytosis of viruses and bacteria. Cold Spring Harb Perspect Biol 6:a016972. doi: 10.1101/cshperspect.a016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrow E, Nicola AV, Liu J. 2013. Multiscale perspectives of virus entry via endocytosis. Virol J 10:177. doi: 10.1186/1743-422X-10-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicola AV, Aguilar HC, Mercer J, Ryckman B, Wiethoff CM. 2013. Virus entry by endocytosis. Adv Virol 2013:469538. doi: 10.1155/2013/469538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicola AV, McEvoy AM, Straus SE. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol 77:5324–5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicola AV. 2016. Herpesvirus entry into host cells mediated by endosomal low pH. Traffic 17:965–975. doi: 10.1111/tra.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller JL, Weed DJ, Lee BH, Pritchard SM, Nicola AV. 2018. Low pH endocytic entry of the porcine alphaherpesvirus pseudorabies virus. J Virol doi: 10.1128/JVI.01849-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rietveld A, Simons K. 1998. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim Biophys Acta 1376:467–479. [DOI] [PubMed] [Google Scholar]
- 38.Brown DA, London E. 1998. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 39.McNeil PL, Steinhardt RA. 1997. Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol 137:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Idone V, Tam C, Andrews NW. 2008. Two-way traffic on the road to plasma membrane repair. Trends Cell Biol 18:552–559. doi: 10.1016/j.tcb.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bi GQ, Alderton JM, Steinhardt RA. 1995. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol 131:1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyake K, McNeil PL. 1995. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol 131:1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, Schuchman E, Tabas I, Andrews NW. 2010. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol 189:1027–1038. doi: 10.1083/jcb.201003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckmann N, Sharma D, Gulbins E, Becker KA, Edelmann B. 2014. Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons. Front Physiol 5:331. doi: 10.3389/fphys.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koivusalo M, Jansen M, Somerharju P, Ikonen E. 2007. Endocytic trafficking of sphingomyelin depends on its acyl chain length. Mol Biol Cell 18:5113–5123. doi: 10.1091/mbc.e07-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIntosh TJ, Simon SA, Needham D, Huang CH. 1992. Structure and cohesive properties of sphingomyelin/cholesterol bilayers. Biochemistry 31:2012–2020. [DOI] [PubMed] [Google Scholar]
- 47.Gulbins E, Kolesnick R. 2003. Raft ceramide in molecular medicine. Oncogene 22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 48.van Blitterswijk WJ, van der Luit AH, Veldman RJ, Verheij M, Borst J. 2003. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem J 369:199–211. doi: 10.1042/BJ20021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desplanques AS, Nauwynck HJ, Vercauteren D, Geens T, Favoreel HW. 2008. Plasma membrane cholesterol is required for efficient pseudorabies virus entry. Virology 376:339–345. doi: 10.1016/j.virol.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bender FC, Whitbeck JC, Ponce de Leon M, Lou H, Eisenberg RJ, Cohen GH. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J Virol 77:9542–9552. doi: 10.1128/JVI.77.17.9542-9552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hambleton S, Steinberg SP, Gershon MD, Gershon AA. 2007. Cholesterol dependence of varicella-zoster virion entry into target cells. J Virol 81:7548–7558. doi: 10.1128/JVI.00486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Favoreel HW, Van den Broeke C, Desplanques A, Deruelle M, Van Minnebruggen G, Nauwynck H, Glorieux S, Van Opdenbosch N, De Regge N. 2010. Alphaherpesvirus use and misuse of cellular actin and cholesterol. Vet Microbiol 143:2–7. doi: 10.1016/j.vetmic.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Azab W, Lehmann MJ, Osterrieder N. 2013. Glycoprotein H and alpha4beta1 integrins determine the entry pathway of alphaherpesviruses. J Virol 87:5937–5948. doi: 10.1128/JVI.03522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu L, Ding X, Tao J, Wang J, Zhao X, Zhu G. 2010. Critical role of cholesterol in bovine herpesvirus type 1 infection of MDBK cells. Vet Microbiol 144:51–57. doi: 10.1016/j.vetmic.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desplanques AS, Pontes M, De Corte N, Verheyen N, Nauwynck HJ, Vercauteren D, Favoreel HW. 2010. Cholesterol depletion affects infectivity and stability of pseudorabies virus. Virus Res 152:180–183. doi: 10.1016/j.virusres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Wudiri GA, Schneider SM, Nicola AV. 2017. Herpes simplex virus 1 envelope cholesterol facilitates membrane fusion. Front Microbiol 8:2383. doi: 10.3389/fmicb.2017.02383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wudiri GA, Pritchard SM, Li H, Liu J, Aguilar HC, Gilk SD, Nicola AV. 2014. Molecular requirement for sterols in herpes simplex virus entry and infectivity. J Virol 88:13918–13922. doi: 10.1128/JVI.01615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luisoni S, Suomalainen M, Boucke K, Tanner LB, Wenk MR, Guan XL, Grzybek M, Coskun U, Greber UF. 2015. Co-option of membrane wounding enables virus penetration into cells. Cell Host Microbe 18:75–85. doi: 10.1016/j.chom.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Miller ME, Adhikary S, Kolokoltsov AA, Davey RA. 2012. Ebolavirus requires acid sphingomyelinase activity and plasma membrane sphingomyelin for infection. J Virol 86:7473–7483. doi: 10.1128/JVI.00136-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shivanna V, Kim Y, Chang KO. 2015. Ceramide formation mediated by acid sphingomyelinase facilitates endosomal escape of caliciviruses. Virology 483:218–228. doi: 10.1016/j.virol.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martín-Acebes MA, Gabandé-Rodríguez E, García-Cabrero AM, Sánchez MP, Ledesma MD, Sobrino F, Saiz J-C. 2016. Host sphingomyelin increases West Nile virus infection in vivo. J Lipid Res 57:422–432. doi: 10.1194/jlr.M064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aizaki H, Morikawa K, Fukasawa M, Hara H, Inoue Y, Tani H, Saito K, Nishijima M, Hanada K, Matsuura Y, Lai MM, Miyamura T, Wakita T, Suzuki T. 2008. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J Virol 82:5715–5724. doi: 10.1128/JVI.02530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tafesse FG, Sanyal S, Ashour J, Guimaraes CP, Hermansson M, Somerharju P, Ploegh HL. 2013. Intact sphingomyelin biosynthetic pathway is essential for intracellular transport of influenza virus glycoproteins. Proc Natl Acad Sci U S A 110:6406–6411. doi: 10.1073/pnas.1219909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zha X, Pierini LM, Leopold PL, Skiba PJ, Tabas I, Maxfield FR. 1998. Sphingomyelinase treatment induces ATP-independent endocytosis. J Cell Biol 140:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamaji A, Sekizawa Y, Emoto K, Sakuraba H, Inoue K, Kobayashi H, Umeda M. 1998. Lysenin, a novel sphingomyelin-specific binding protein. J Biol Chem 273:5300–5306. [DOI] [PubMed] [Google Scholar]
- 66.Kornhuber J, Tripal P, Reichel M, Muhle C, Rhein C, Muehlbacher M, Groemer TW, Gulbins E. 2010. Functional inhibitors of acid sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. Cell Physiol Biochem 26:9–20. doi: 10.1159/000315101. [DOI] [PubMed] [Google Scholar]
- 67.Kolzer M, Werth N, Sandhoff K. 2004. Interactions of acid sphingomyelinase and lipid bilayers in the presence of the tricyclic antidepressant desipramine. FEBS Lett 559:96–98. doi: 10.1016/S0014-5793(04)00033-X. [DOI] [PubMed] [Google Scholar]
- 68.Grassme H, Riehle A, Wilker B, Gulbins E. 2005. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem 280:26256–26262. doi: 10.1074/jbc.M500835200. [DOI] [PubMed] [Google Scholar]
- 69.Miller JM, Whetstone CA, Bello LJ, Lawrence WC, Whitbeck JC. 1995. Abortion in heifers inoculated with a thymidine kinase-negative recombinant of bovine herpesvirus 1. Am J Vet Res 56:870–874. [PubMed] [Google Scholar]
- 70.Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 71.Korzeniewski C, Callewaert DM. 1983. An enzyme-release assay for natural cytotoxicity. J Immunol Methods 64:313–320. [DOI] [PubMed] [Google Scholar]