EBV causes tumors in multiple organs, particularly in the oro- and nasopharyngeal area but also in the digestive system. This virus enters the body in the oropharynx and establishes a chronic infection in this area. The observation that the virus causes tumors in the digestive system implies that the infected cells can move to this organ. We found that EBV infection induces the expression of integrin beta 7 (ITGB7), an integrin that associates with integrin alpha 4 to form the LPAM-1 dimer. LPAM-1 is key for homing of B cells to the gastrointestinal tract, suggesting that induction of this molecule is the mechanism through which EBV-infected cells enter this organ. In favor of this hypothesis, we could also detect EBV-infected cells in the lymph nodes adjacent to the colon and in the appendix.
KEYWORDS: EBV, LPAM-1, posttransplant lymphoproliferative disorder, mucosa-associated lymphoid tissue
ABSTRACT
Epstein-Barr virus (EBV) infects the oropharynx but, surprisingly, frequently induces B cell proliferation in the gut of immunosuppressed individuals. We found that EBV infection in vitro induces the expression of the LPAM-1 integrin on tonsillar B cells and increases it on peripheral blood cells. Similarly, LPAM-1 was induced in the tonsils of patients undergoing primary infectious mononucleosis. EBV-induced LPAM-1 bound to the MAdCAM-1 addressin, which allows B cell homing to the gastrointestinal mucosa-associated lymphoid tissue (GALT). Thus, we hypothesized that EBV-induced LPAM-1 could induce relocation of infected B cells from the tonsil to the GALT. In situ hybridization with an EBER-specific probe revealed the frequent presence of EBV-infected cells in the pericolic lymph nodes of healthy individuals. Relocation of infected B cells into the GALT would expand the EBV reservoir, possibly protecting it from T cells primed in the oropharynx, and explain why EBV induces lymphoid tumors in the gut.
IMPORTANCE EBV causes tumors in multiple organs, particularly in the oro- and nasopharyngeal area but also in the digestive system. This virus enters the body in the oropharynx and establishes a chronic infection in this area. The observation that the virus causes tumors in the digestive system implies that the infected cells can move to this organ. We found that EBV infection induces the expression of integrin beta 7 (ITGB7), an integrin that associates with integrin alpha 4 to form the LPAM-1 dimer. LPAM-1 is key for homing of B cells to the gastrointestinal tract, suggesting that induction of this molecule is the mechanism through which EBV-infected cells enter this organ. In favor of this hypothesis, we could also detect EBV-infected cells in the lymph nodes adjacent to the colon and in the appendix.
INTRODUCTION
Epstein-Barr virus (EBV) infects a large majority of the world population (1). The virus is spread through saliva and efficiently infects B lymphocytes that undergo transformation through the expression of nine viral latent genes that belong to the EBNA (Epstein-Barr virus nuclear antigen) and LMP (latent membrane protein) families. This cellular proliferation is usually transitory in immunocompetent individuals, although it can be quite extensive in tonsils of individuals undergoing infectious mononucleosis (IM) (1).
Even if the EBV infection causes no pathology, the virus remains silent in infected subjects (1). Patients with immune deficiencies, e.g., transplant recipients treated with immunosuppressive drugs or HIV-infected individuals, are at increased risk of B cell lymphoproliferations driven by EBV (see Van Krieken et al., Raphael et al., and Swerdlow et al. in reference 2 for reviews). These disorders commonly invade the gastrointestinal tract, in particular the colon. A recent series of patients with posttransplant lymphoproliferative disease (PTLD) showed gastrointestinal (GI) tract involvement in 56% of the cases (3). However, the origin of the infected cells remains unclear because the virus enters the body through the oropharynx and is initially found in the tonsil, where it persists and from which it recirculates in the peripheral blood (1). Previous investigations showed that EBV-infected cells are 20 times more frequent in the Waldeyer ring than in the spleen or in mesenteric lymph nodes around the liver and spleen (4). Indeed, lesions such as plasmacytic hyperplasia and infectious mononucleosis-like lymphoproliferations that are encountered in children or EBV-naive adult solid-organ recipients frequently present in tonsils and adenoids, but this does not explain the high frequency of gut PTLDs (see Swerdlow et al. in reference 2). We have readdressed this issue and performed molecular investigations that revealed that EBV infection induces the expression of integrin beta 7 (ITGB7), an integrin that associates with integrin alpha 4 (ITGA4) to form the LPAM-1 dimer. LPAM-1 binds to the addressin MAdCAM-1 that is expressed on the surface of venules of the mucosa-associated lymphoid tissue (MALT) and is key for homing of B cells to the gastrointestinal tract (5). Furthermore, we found EBV-infected cells, sometimes in large numbers, in paracolic lymph nodes, an organ that had not been precisely investigated before, and in the appendix.
RESULTS
EBV infection induces LPAM-1 expression in B cells.
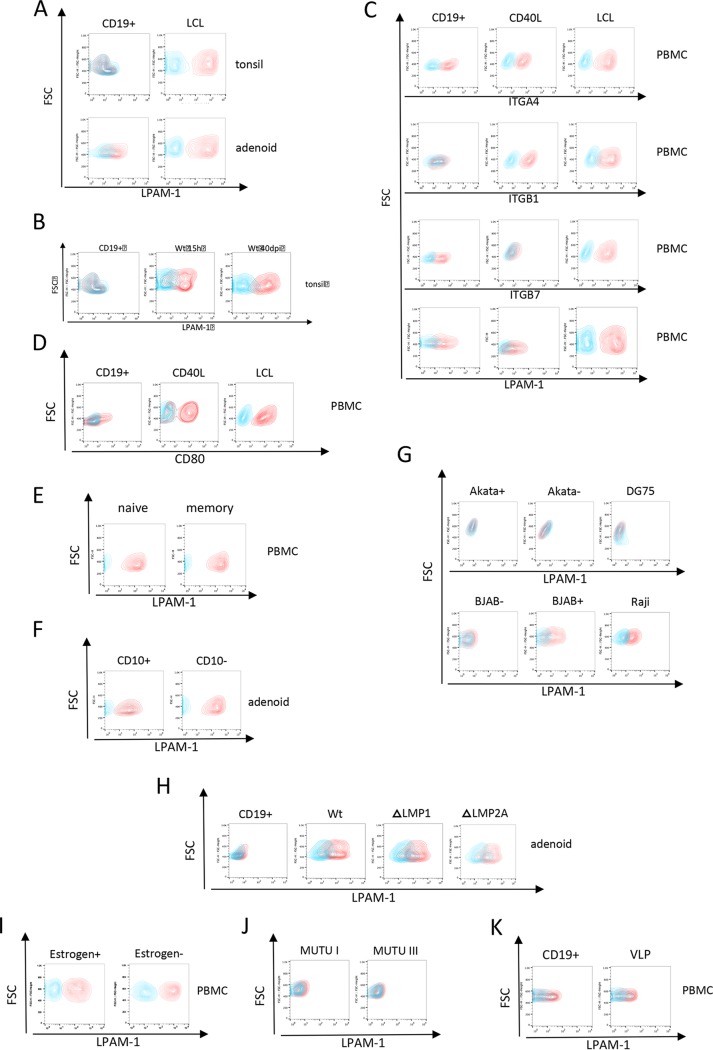
We first performed transcriptome sequencing (RNA-seq) on four B cell lines generated by EBV infection and filtered the list of transcripts for molecules involved in homing. This approach identified CCR7, CD44, CD99, F11R, SELPLG, GLG1, CXCR4, PECAM1, LPAM-1 (ITGA4 and ITGB7), L-selectin (SELL), LFA-1 (ITGB2 and ITGAL), and VLA-4 (ITGA4 and ITGB1) as potentially upregulated by EBV infection (Table 1) (5). CD44, CCR7, SELL, LFA-1, and VLA-4 were known to be targets of the virus and its LMP-1 protein, but ITGB7 and LPAM-1 have not been reported before as EBV targets (6, 7). Flow cytometry analyses were first performed with B cells from adenoids and from tonsils that were or were not exposed to EBV. Noninfected tonsillar B cells did not express LPAM-1, as previously reported, and adenoid cells expressed this molecule only weakly (Fig. 1A) (8). However, EBV infection of adenoid and tonsillar B cells induced the expression of LPAM-1 strongly, demonstrating that exposure to the virus induced the expression of this integrin pair in hitherto negative cells or in cells weakly expressing it. The induction of LPAM-1 became visible 15 h after EBV infection (Fig. 1B). We then repeated and extended this assay with resting peripheral blood B cells, the same B cells stimulated with CD40L/interleukin-4 (IL-4) or infected with EBV. The peripheral blood B cells expressed ITGA4, ITGB7, and LPAM-1 but not ITGB1, as previously described (Fig. 1C) (8, 9). Expression of ITGB7 on these cells is expected, as they include recirculating cells from the gastrointestinal mucosa-associated lymphoid tissue (GALT). CD40L/IL-4-stimulated B cells lost expression of ITGB7 and LPAM-1 but expressed ITGA4 and ITGB1. Peripheral blood B cells transformed by EBV expressed all these integrins, thereby confirming the results of the RNA-seq assay. Importantly, EBV-transformed blasts expressed ITGB7 and LPAM-1 at levels clearly higher than those of the resting peripheral blood B cells, an observation in line with the infection experiments performed with adenoid cells. We then compared the activation status of CD40L/IL-4-stimulated B cells and EBV-infected cells using CD80 expression as a readout. This assay showed that the B cell populations were activated at comparable levels, suggesting that the differences in integrin expression between these populations could not be ascribed to a general activation level (Fig. 1D). We then infected naive and memory B cells isolated from peripheral blood and transformed by EBV that were found to express LPAM-1 at high levels after transformation (Fig. 1E). Therefore, EBV infection induces ITGB7 expression in B cells independently of their initial differentiation status. Infection of CD10-positive and CD10-negative cells from an adenoid sample also enhanced ITGB7 and LPAM-1, although the effect was stronger in the CD10-negative population (Fig. 1F). We extended our investigations to the EBV-negative Burkitt’s lymphoma cell lines DG75, BJAB, and Akata (EBV-negative clone), which were found to be LPAM-1 negative (Fig. 1G). Similar results were obtained with the EBV-positive cell line Akata. However, we found a weak expression of LPAM-1 on the Burkitt’s lymphoma cell line Raji that was one order of magnitude lower than that in lymphoblastoid cell lines (LCLs). We infected BJAB cells with a recombinant B95-8 virus and selected the transfected cells with hygromycin. This generated a cell line that expressed the green fluorescent protein (GFP) gene that is cloned onto the recombinant viral genome in more than 98% of the cells. Fluorescence-activated cell sorting (FACS) staining of this cell line showed that it weakly expressed LPAM-1 at the levels seen in Raji cells. Thus, expression of ITGB7 after EBV infection is observed with primary B cells and irregularly and weakly in Burkitt’s lymphoma cell lines (compare Fig. 1C and G).
TABLE 1.
Expression of B cell transcripts involved in lymphocyte B homing in four EBV-infected cell lines
| Transcript | Value (RPKM) for samplea: |
Mean | SD | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| ITGA4 | 27.50 | 25.50 | 18.58 | 19.00 | 22.64 | 4.53 |
| ITGAL | 8.36 | 16.16 | 13.01 | 19.07 | 14.15 | 4.59 |
| ITGB1 | 11.34 | 9.05 | 8.07 | 8.91 | 9.34 | 1.40 |
| ITGB2 | 14.91 | 7.67 | 17.23 | 9.74 | 12.39 | 4.44 |
| ITGB3 | 3.02 | 2.27 | 5.12 | 4.59 | 3.75 | 1.33 |
| ITGB7 | 12.74 | 13.25 | 13.57 | 11.76 | 12.83 | 0.79 |
| SELL | 38.46 | 105.06 | 46.65 | 82.10 | 68.07 | 31.09 |
| SELPLG | 3.15 | 7.09 | 18.16 | 16.48 | 11.22 | 7.26 |
| GLG1 | 7.88 | 8.20 | 6.46 | 8.00 | 7.64 | 0.79 |
| CD44 | 12.87 | 18.50 | 18.39 | 27.25 | 19.25 | 5.95 |
| CCR7 | 8.17 | 37.54 | 30.38 | 49.69 | 31.44 | 17.45 |
| CCR9 | 0.02 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 |
| CCR10 | 1.71 | 1.02 | 0.93 | 0.67 | 1.08 | 0.44 |
| CXCR4 | 2.96 | 8.78 | 11.06 | 8.85 | 7.91 | 3.47 |
| CXCR5 | 0.09 | 0.18 | 0.01 | 0.07 | 0.09 | 0.07 |
| PECAM1 | 1.30 | 2.00 | 1.53 | 2.51 | 1.83 | 0.54 |
| CD99 | 17.89 | 17.24 | 20.91 | 20.00 | 19.01 | 1.73 |
| F11R | 3.68 | 2.54 | 6.92 | 5.59 | 4.69 | 1.95 |
| JAM3 | 0.66 | 1.11 | 0.83 | 1.05 | 0.91 | 0.21 |
RPKM, reads per kilobase per million mapped reads.
FIG 1.
Expression of integrins in various B cell populations. (A) Resting (CD19+) and EBV-infected adenoid and tonsillar B cells (LCL) were stained with antibodies against LPAM-1 (red) or the isotype control (blue) and analyzed by flow cytometry (n = 3 for each type of sample). FSC, forward scatter. (B) Resting (CD19+) adenoid B cells were exposed to EBV. Cell populations were stained with antibodies against LPAM-1 and analyzed by flow cytometry 15 h and 40 days postinfection (dpi) (n = 2). (C) The expression of ITGA4, ITGB1, ITGB7, and LPAM-1 (red) or isotype control (blue) was assessed by flow cytometry in resting blood B cells (CD19+), EBV-infected blood B cells (LCL), or blood B cells stimulated by CD40L and IL-4 (CD40L) (n = 5). PBMC, peripheral blood mononuclear cells. (D) The expression of CD80 (red) or isotype control (blue) was assessed by flow cytometry in resting blood B cells (CD19+), EBV-infected blood B cells (LCL), or blood B cells stimulated by CD40L and IL-4 (CD40L) (n = 2). (E) Three EBV-negative Burkitt’s lymphoma cell lines (Akata−, DG75, and BJAB) and two EBV-positive Burkitt’s lymphoma cell line (Akata+ and Raji) were stained for LPAM-1 (red) or the isotype control (blue) (n = 1). (F) Expression of LPAM-1 (red) or isotype control (blue) in cell lines generated by infection of memory and naive blood B cells (n = 2). (G) LPAM-1 (red) or isotype control (blue) expression in EBV-infected adenoid CD10+ and CD10− B cells (n = 3). (H) Expression of LPAM-1 in B cell populations infected with various viral mutants. Resting (CD19+) adenoid B cells were infected with either M81 wild-type virus (Wt) or an M81 mutant lacking the LMP1 or LMP2 gene (ΔLMP1 or ΔLMP2). Cell populations were stained with antibodies against LPAM-1 and analyzed by flow cytometry at day 7 postinfection. (I) EREB cells were grown in the presence (Estrogen+) or absence (Estrogen−) of estrogen. LPAM-1 (red) or isotype control (blue) expression is shown in the FACS dot plots. (J) Two EBV-positive Burkitt’s lymphoma cell lines that express the full (MUTU III; latency 3) or restricted (MUTU I; latency 1) set of viral latent proteins were stained for LPAM-1 (red) or the isotype control (blue). (K) Expression of LPAM-1 (red) or isotype control (blue) in resting (CD19+) B cells and in B cells exposed to DNA-free virus-like particles (VLP) for 24 h (n = 2).
We then attempted to identify the EBV proteins involved in the induction or amplification of ITGB7 expression. To this end, tonsillar B cells were infected with a virus mutant lacking the latent EBV protein LMP1, but we found that the infected cells expressed ITGB7 at the same levels as B cells transformed by wild-type viruses (Fig. 1H). Similar results were obtained with B cells infected with other EBV mutants lacking other viral latent proteins or noncoding RNAs, such as the EBERs or the BHRF1 and BART microRNAs (Fig. 1H and data not shown). To determine the role of EBNA2 in LPAM-1 induction, we studied EREB cells, which are peripheral blood B cells transformed with a conditional EBNA2 that is responsive to estrogen (10). Inactivation of EBNA2 after a 3-day estrogen withdrawal did not affect its expression (Fig. 1I). We expanded our analyses to a pair of Burkitt’s lymphoma cell lines that express either the full set (MUTU III, latency III) or a restricted set of latent proteins (MUTU I, latency I) but could not detect LPAM-1 in any of the samples (Fig. 1J). This suggests that the EBNA and LMP proteins, with the exception of EBNA1, are not involved in the induction of LPAM-1. Therefore, we stained an LCL generated with an EBNA1 null mutant with an antibody specific to LPAM-1 (11). This cell line expressed LPAM-1, suggesting that EBNA1 is not involved in its regulation in LCLs (data not shown). To determine whether LPAM-1 expression requires infection with a functional EBV genome, we infected adenoid B cells with EBV virus-like particles (VLPs) that are devoid of viral DNA and are therefore unable to transform infected cells. While LPAM-1 expression was found in cells infected with wild-type virus, we could not detect LPAM-1 expression after exposure to EBV VLPs (Fig. 1K). Thus, it remains unclear how the viral infection induces LPAM-1, but this process depends on the presence of the EBV genome.
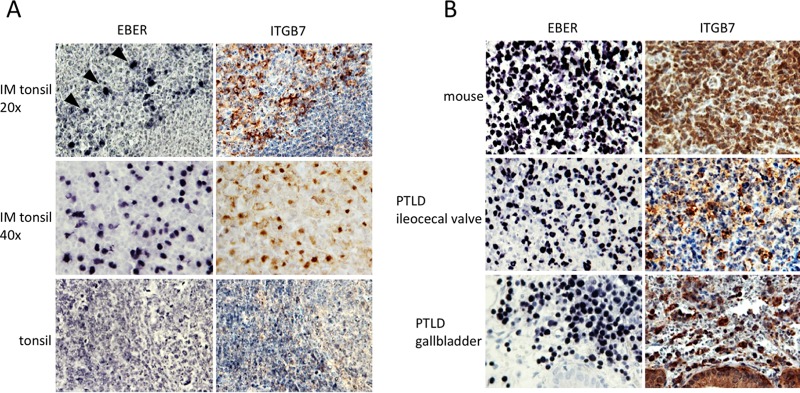
In an attempt to demonstrate that ITGB7 induction after EBV infection also takes place in vivo, we stained tissue sections from tonsils of patients with or without acute EBV infection. While EBV-negative tonsils showed no or only faint ITGB7 expression in both follicular and interfollicular B cells, confirming the data of the literature and our FACS stains, the IM tonsils strongly expressed this integrin in areas of the section where tonsillar cells were infected with EBV in the seven investigated cases (Fig. 2A), suggesting that EBV infection also induces ITGB7 in vivo. Immunostaining of tissues from tumors generated in humanized mice infected by the virus (n = 4) and of gastrointestinal PTLD cases (n = 8) revealed that these lesions all expressed ITGB7 (Fig. 2B). Thus, EBV infection induces or potentiates the expression of a molecule on activated B lymphocytes that allows entry into the mucosal immune system both in vitro and in vivo.
FIG 2.
Expression of integrins and localization of EBV-infected B cells. (A) Consecutive histological sections of a tonsil removed from a patient with IM was stained for EBER (top left) or ITGB7 (top right) (n = 7). The top and middle pictures were taken at low (×20) and high (×40) power. (Bottom) Similar investigations performed in the tonsil of a patient without EBV acute infection (n = 4). (B) The expression of ITGB7 in various EBV-infected tumors from humans or immunosuppressed mice was assessed by immunohistochemistry (n = 4 and n = 8, respectively).
EBV-infected cells can be captured by cells expressing MAdCAM-1.
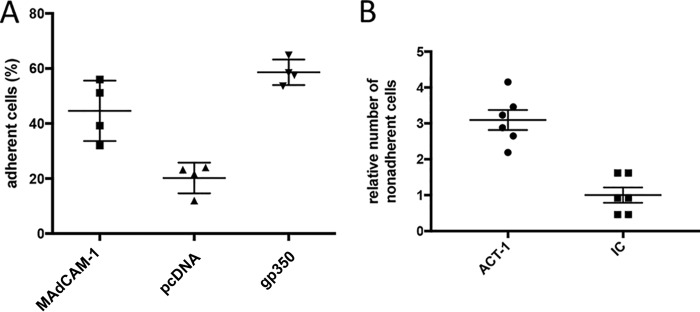
LPAM-1 interacts with MAdCAM-1, an addressin selectively expressed by the endothelial cells of high endothelial venules in the small and large intestines and the GALT, as well as in the intestinal lamina propria (12). We tested whether LPAM-1 expressed at the surface of EBV-infected B cells can interact with MAdCAM-1 by transfecting CHO cells with the human MAdCAM-1 gene and with the EBV-specific BLLF1 gene whose protein product gp350 avidly binds to CD21 expressed by B cells (1). Cells transfected with an empty pcDNA3.1 plasmid served as a negative control. We assessed the ability of these cell lines to retain EBV-infected cells. Figure 3A shows that the expression of MAdCAM-1 doubles the number of EBV-infected cells retained by CHO cells after 30 min of coculture at 4°C, with an efficacy close to the one observed after transfection of gp350. To further validate that the binding to MAdCAM-1 was attributable to LPAM-1 expression, we treated EBV-infected B cells with an antibody against LPAM-1 (Act-1) that blocks the binding to MAdCAM-1. Treatment with Act-1 led to a 3.1-fold decrease in the number of bound B cells compared to the level for cells that were treated with an isotype control antibody (Fig. 3B). We conclude that the LPAM-1 molecule expressed by EBV-infected cells interacts with MAdCAM-1, its ligand (12).
FIG 3.
EBV-transformed B cells are retained by CHO cells that express MAdCAM-1. (A) CHO cells were stably transfected with pcDNA3.1 vectors expressing MAdCAM-1 or EBV gp350 or with the pcDNA3.1 parental vector. These cell lines were incubated with 4 independent EBV-transformed B cell samples. The dot plot shows the percentage of B cells adherent to the stably transfected CHO clones. (B) Lymphocyte adhesion to CHO cells that stably express MAdCAM-1 was blocked by an antibody directed against LPAM-1 (Act-1). An antibody of the same class and type but without target specificity served as an isotype control (IC). Three replicates of two independent LCLs were analyzed, and the results are shown in the dot plot (n = 6).
EBV-infected cells are present in paracolic mesenteric lymph nodes and in the appendix.
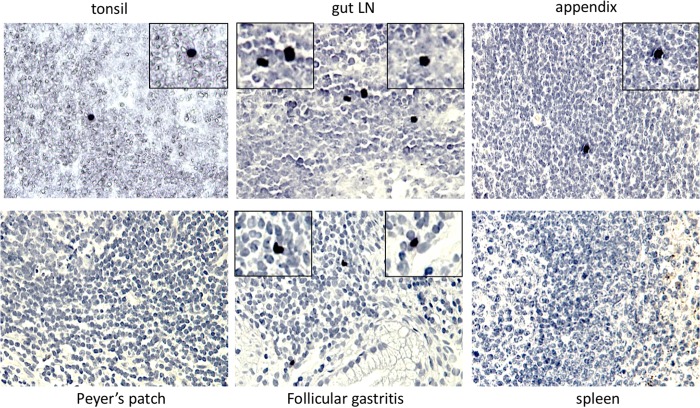
The detection of LPAM-1 at the surface of infected B cells suggested that these cells should be able to enter the GALT. Therefore, we screened a panel of lymphoid tissues from immunocompetent individuals without cancers or infections with a probe specific for the EBV-specific viral noncoding RNA EBER. This analysis detected, as expected, rare EBV-positive cells in 70% of investigated tonsils (Fig. 4 and Table 2). The frequency of infection in pericolic lymph nodes and appendices reached 40% and 44% of the investigated samples, respectively. We could not find evidence for EBER-positive cells in the investigated splenic or ileal samples (Fig. 4 and Table 2).
FIG 4.
EBV-infected cells can be detected in various lymphoid organs. Tissues from tonsils, paracolic lymph nodes, appendices, Peyer’s patches, follicular gastritis, and spleen collected from immunocompetent individuals were subjected to in situ hybridization with an EBER-specific probe (Table 2 provides the number of investigated samples).
TABLE 2.
Frequency of EBV infection in various human tissues
| Tissue | No. positive (EBER)/total no. of samples | No. of EBER-positive cells per mm2 (mean) | No. positive/total no. of samples (qPCR) | qPCR titer (mean copies/cell) |
|---|---|---|---|---|
| Gut lymph nodes | 4/10, 16/38a | 0.139 | 5/10 | 5.77E−04 |
| Tonsil | 7/10 | 0.049 | 7/10 | 3.49E−03 |
| Appendix | 4/9 | 0.066 | 4/9 | 4.06E−04 |
| Peyer’s patches | 0/5 | 0.000 | 0/5 | 0 |
| Spleen | 0/5 | 0.000 | 0/5 | 0 |
| Gastritis | 3/10 | 0.143 | 8/10 | 4.92E−03 |
For gut lymph nodes, the first set of numbers indicates number of patients, while the second set indicates number of samples.
We then determined the density of EBER-positive cells and found that it was more than 2 times higher in the pericolic lymph nodes than in tonsils or appendices (Table 2). The investigated tissues were subjected to EBV-specific quantitative PCR (qPCR) that confirmed the absence of viral DNA in the spleen and its presence in the tissues that contain EBER-positive cells. Only one sample of pericolic lymph nodes showed discrepancies between both methods, being positive by qPCR and not for EBER (Table 2). As we could investigate only a limited proportion of lymph nodes from individual healthy virus carriers, and considering the abundance of the GALT system, it is likely that at least some individuals host far more resting EBV-infected cells in the GALT than in the tonsils.
EBV can colonize acquired MALT structures in the stomach with follicular gastritis.
The identification of EBV-positive gastric carcinomas raises the question of how the virus enters the stomach. However, the normal stomach does not contain any organized lymphoid structures (13). This changes after infection with Helicobacter pylori, which can lead to the development of an acquired mucosa-associated lymphoid tissue (MALT) tissue with development of B cell follicles, generating follicular gastritis (13). We EBER stained three cases of these lesions and could detect the presence of the virus in all three cases that were also PCR positive (Fig. 4 and Table 2). We also tested seven additional samples from gastritis without B cell follicles but could not detect the virus in these tissues.
DISCUSSION
In an attempt to explain the frequent localization of EBV-associated tumors to the GI tract, we screened multiple RNA-seq libraries of EBV-infected B cells for molecules involved in B cell homing and found that this virus induced the expression of LPAM-1. Furthermore, LPAM-1 expression in B cells allowed functional interaction with cells that express MAdCAM-1, its ligand, which can be blocked by an antibody against LPAM-1 (12). This strongly suggests that EBV-infected B cells can access the GALT. Indeed, a strong expression of LPAM-1 on lymphocytes and, in particular, on B immunoblasts is sufficient for their homing to the GALT through interaction with the MAdCAM-1 addressin (8). This molecular interaction is essential, as knockout mice that lack MAdCAM-1 or ITGB7 possess a severely atrophic GALT system (14, 15).
LPAM-1 is expressed by a subset of B cells located in the Peyer’s patches and in the lamina propria of the small intestine (16). This molecule is also present on peripheral blood B cells that comprise cells recirculating from the GALT (8, 9). However, LPAM-1 is expressed neither by resting nor by proliferating B cells located in the tonsil or in the spleen (8, 9, 17). We could confirm that tonsillar B cells do not express ITGB7 and that low levels of ITGB7 and LPAM-1 expression can be detected on peripheral blood B cells and cells from adenoids. However, EBV infection clearly upregulated ITGB7 and LPAM-1 expression in all of these cell types, the amplification being maximum in tonsillar B cells. LPAM-1 induction upon EBV infection took place after infection of different B cell subsets, including CD10+ and CD10− cells, as well as naive and memory B cells. INTB7 upregulation also took place in tonsils of patients with IM. Unfortunately, the ITGB7-specific antibody is not suitable for double stains with EBERs that would have been ideal to directly demonstrate that the EBV-infected cells express ITGB7. However, this protein was clearly expressed only in areas where the infected cells could be found. In contrast, B cells stimulated by CD40L and IL-4 and Burkitt’s lymphoma cells, some of which were EBV positive, did not express ITGB7/LPAM-1, although B cells stimulated by CD40L, IL-4, and LCLs expressed CD80 at similar levels. On the other hand, Raji and BJAB cells infected with a recombinant B95-8 virus expressed LPAM-1 but at much lower levels than LCLs. Thus, strong and constant LPAM-1 upregulation after EBV infection takes place only in primary B lymphocytes and is probably the result of a complex process that does not involve the key latent proteins EBNA2 and LMP-1. Furthermore, LPAM-1 induction following EBV infection bypasses the B cell priming for gut homing conferred by intestinal dendritic cells (17). This is, to our knowledge, the first example of a virus infection that can induce a change in the homing of B cells. HIV selectively infects LPAM-1-positive T-lymphocytes to access the gut in which the virus can replicate (18). However, HIV does not induce expression of the LPAM-1 integrin as EBV does.
LPAM-1 is considered to be the most important protein for lymphoid cells to access the GALT; thus, its induction could enable EBV-infected B cells to access the GALT (5). We tested this hypothesis by staining tissues from the GALT and could identify EBV-infected B cells in the appendix and in pericolic lymph nodes. Thus, we are confident that EBV-infected B cells can be found outside the blood and the oropharyngeal area. The analysis of a larger number of samples from the GALT will give more precise information on the size of the EBV reservoir in these organs.
However, we could not detect any EBV-infected cells in the Peyer’s patches of adults. It is possible that EBV-infected cells are more frequent in the larger Peyer’s patches of younger individuals (19). CCR9 and CCR10, two chemokine receptors that increase B cell retention in the small intestine or colon, are not expressed in EBV-infected B cells (Table 1) (5, 20). However, EBV-infected cells expressed L-selectin, which binds to the peripheral node addressin and CCR7, a chemokine receptor that interacts with the CCL19 and CCL21 chemokines (7). This unusual combination of integrins allowing entry in different lymphoid structures at the surface of EBV-infected cells might lead to preferential persistence in the paracolic mesenteric lymph nodes within the GALT (20).
Primary EBV infection takes place in the oropharynx, and B cells become infected in this area (1). Indeed, individuals with infectious mononucleosis have enlarged tonsils and adenoids containing a large number of EBV-infected cells (1). The detection in the present study of EBV-infected B cells in mesenteric lymph nodes implies that these infected cells originate from these oropharyngeal lymphoid organs. The homing of B cells in general from the Waldeyer ring to the GALT in humans has not been investigated in details, although one study suggested that its efficacy is poor (21). This cell migration could be greatly facilitated by an EBV infection.
The identification of EBV-infected B cells in the GI tract has implications for our understanding of the interactions between the virus and its host. Previous models have considered that the Waldeyer ring and not the mesenteric lymph nodes are the main reservoir of virus, although it was recognized that a few EBV-infected cells can be found in the latter organs (4). Importantly, this study focused on mesenteric lymph nodes adjacent to the liver and the spleen and not on the paracolic lymph nodes (4). This model was mainly based on the observation that viral replication takes place in the oropharynx, and virus-infected B cells outside the Waldeyer ring were considered dead ends in terms of virus spread (4). EBV-infected cells express not only LPAM-1 but also CD44, L-selectin, LFA-1, and VLA-4, a set of molecules that allow entry in the Waldeyer ring and inflammatory sites (12). However, EBV-infected cells are found at various levels in the peripheral blood and could include recirculating infected B cells from the oropharynx and B cells from the GALT that could return to the oropharynx.
Our findings have consequences for the pathogenesis of EBV-associated diseases. Many PTLD develop in the GI tract of immunodeficient individuals who present in 20% of the cases with enlarged mesenteric lymph nodes (22). The frequent detection of latently infected B cells in the paracolic lymph nodes or in the intestinal wall suggests that their uncontrolled proliferation gives rise to these adenopathies and are the source of the transformed cells that invade the GI tract. Interestingly, an early form of polyclonal PTLD frequently develops in the oropharyngeal areas in children (2). This fits with the concept that the virus initially enters the body through the oropharynx but establishes long-term persistence in larger areas of the body, including the GI tract. Inflammation enhances MAdCAM-1 expression (23, 24). Thus, LPAM-1 expression in inflammatory bowel disease colonic tissues could explain the reports of an increased presence of EBV-infected cells in these samples (25–27). In the same vein, EBV has been detected in patients with gastritis cases, with or without adjacent carcinoma (27). Inflammation could also explain the development of EBV-associated diffuse large B cell lymphomas in body cavities of patients with tuberculosis treated by artificial pneumothorax and in patients with osteomyelitis, metallic implant, or chronic skin ulcer (see Chan et al. in reference 2).
What would be the advantages of viral persistence in the GALT? On one hand, it would expand the reservoir of virus in infected individuals, thereby increasing the chances of the virus persisting in the infected host. On the other hand, it would also reduce the likelihood of being detected by EBV-specific T lymphocytes. Indeed, upon encounter with their cognate antigen in a lymph node, T lymphocytes become imprinted to the territory drained by this lymph node (28). Thus, if the primary encounter with the virus takes place in the tonsil, the likelihood for an EBV-infected cell to be recognized by T cells in the GALT is reduced. In favor of this hypothesis, Andrew Hislop and colleagues found that EBV-specific CD8-positive T cells home to oropharyngeal sites during IM (29). The reduced ability of the T cells to target EBV-infected cells in the gut would lead to a preferential proliferation of infected cells located in this organ in the case of immunosuppression.
MATERIALS AND METHODS
Human tissues.
The samples were provided by the tissue bank of the National Center for Tumor Diseases (DZIF, Heidelberg, Germany) in accordance with the regulations of the tissue bank and the approval of the ethics committee of Heidelberg University. We collected extranodal PTLD samples that developed in the digestive system and tonsils from normal and IM patients. We also investigated paracolic lymph nodes (between 1 and 6 per patient; 3 on average), spleens, Peyer’s patches, gastric biopsy specimens, and appendices from patients without cancer or acute infections.
Lymphocyte adhesion and blocking of adhesion.
We generated CHO cell lines that stably express pcDNA3.1 expression plasmids that encode MAdCAM-1 (B1621) and carry the EBV gene BLLF1, whose protein product is gp350 (B702), after selection with G418 (1 mg/ml). Clones that express gp350 or MAdCAM-1 and controls (CHO/gp350, CHO/MAdCAM-1, and CHO/pcDNA3.1) were selected for adhesion assays in which they are cocultivated with EBV-transformed LCLs for 30 min at +4°C under constant agitation (30). Transfected CHO cells (2 × 104) were mixed with 6 × 104 EBV-infected B cells in 96-well plates. After three gentle washings with phosphate-buffered saline (PBS), the percentage of nonadherent B cells was determined by counting. For blocking experiments, 5 × 104 EBV-infected B cells were first mixed with either the LPAM-1 blocking antibody Act-1 (1:500 dilution; provided by the NIH) or with an isotype control and cocultivated with CHO/MAdCAM-1 cells for 30 min at 37°C. After three gentle washings with PBS, the percentage of nonadherent B cells was determined by counting.
Virus production.
HEK293 cells (ATCC CRL-1573) stably transfected with recombinant B95-8 or M81 EBV-bacterial artificial chromosomes were transfected with expression plasmids encoding BZLF1 (p509) and BALF4 (pRA) to induce lytic replication (31). Three days after transfection, virus supernatants were collected and filtered through a 0.45-μm filter.
B cell infections and in vitro transformation experiments.
Total CD19-positive B cells purified from the peripheral blood or tonsils from different donors were exposed to viruses for 4 h at a multiplicity of infection (MOI) of 20 virus genomes, as defined by qPCR, per target cell as described previously (31). These assays were approved by the ethics committee of Heidelberg University (392/2005). Infected cells were washed once with PBS and plated in cluster plates in RPMI supplemented with 20% fetal bovine serum. In some experiments, we performed EBV infections of CD10-positive and CD10-negative cells isolated from tonsillar CD19-positive cells using MACS beads (Miltenyi Biotec, Germany). We also used the same technology to purify CD27+/IgD− memory and CD27−/IgD+ naive B cells from the peripheral blood and infected them in parallel with the virus.
B cell stimulation with CD40-ligand and IL-4.
Freshly isolated CD19+ primary B cells were cultured on 90-Gy γ-irradiated CD40 ligand (CD40L) feeder cell layers in the presence of 25 ng/ml recombinant human IL-4 (PeproTech, Germany) for 1 week. For long-term expansion, the CD40-ligand feeder cells were replaced at least once per week, and the cells were replenished with fresh medium containing 10 ng/ml recombinant human IL-4.
Antibodies.
We used primary mouse monoclonal antibodies against ITGA4 (1:25 dilution; BioLegend), ITGB1 (1:50 dilution; BioLegend), ITGB7 (1:50 dilution; BioLegend), and LPAM-1 (clone Act-1; 1:500 dilution) (32). The secondary antibodies applied for immunofluorescence staining were goat anti-mouse coupled to Alexa 488 (1:300 dilution; BioLegend) or to phycoerythrin (1:300 dilution; BioLegend). We also used streptavidin coupled to Alexa 488 (1:200 dilution; Life Technologies) to visualize the biotinylated primary antibodies.
ACKNOWLEDGMENTS
The mouse monoclonal antibody Act-1 (11718) was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH. We thank the microarray unit of the Genomics and Proteomics Core Facility, German Cancer Research Center (DKFZ), for providing excellent services. We thank B. Kempkes for the EREB cells and A. Schepers for LCLs transformed by the EBNA1-null mutant.
S.D. was supported by the German Center for Infection Research (DZIF). We have no conflicts of interest to declare.
REFERENCES
- 1.Rickinson AB, Kieff E. 2001. Epstein-Barr virus, p 2575–2627. In Knipe DMHP, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Anonymous. 2008. WHO classification of tumours of haematopoietic and lymphoid tissues. IARC, Lyon, France. [PubMed] [Google Scholar]
- 3.Evens AM, David KA, Helenowski I, Nelson B, Kaufman D, Kircher SM, Gimelfarb A, Hattersley E, Mauro LA, Jovanovic B, Chadburn A, Stiff P, Winter JN, Mehta J, Van Besien K, Gregory S, Gordon LI, Shammo JM, Smith SE, Smith SM. 2010. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol 28:1038–1046. doi: 10.1200/JCO.2009.25.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laichalk LL, Hochberg D, Babcock GJ, Freeman RB, Thorley-Lawson DA. 2002. The dispersal of mucosal memory B cells: evidence from persistent EBV infection. Immunity 16:745–754. doi: 10.1016/S1074-7613(02)00318-7. [DOI] [PubMed] [Google Scholar]
- 5.Salmi M, Jalkanen S. 2005. Lymphocyte homing to the gut: attraction, adhesion, and commitment. Immunol Rev 206:100–113. doi: 10.1111/j.0105-2896.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 6.Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E. 1993. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol 67:2209–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahir-McFarland ED, Carter K, Rosenwald A, Giltnane JM, Henrickson SE, Staudt LM, Kieff E. 2004. Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J Virol 78:4108–4119. doi: 10.1128/JVI.78.8.4108-4119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postigo AA, Sanchez-Mateos P, Lazarovits AI, Sanchez-Madrid F, de Landazuri MO. 1993. Alpha 4 beta 7 integrin mediates B cell binding to fibronectin and vascular cell adhesion molecule-1. Expression and function of alpha 4 integrins on human B lymphocytes. J Immunol 151:2471–2483. [PubMed] [Google Scholar]
- 9.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. 1994. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol 153:517–528. [PubMed] [Google Scholar]
- 10.Kempkes B, Spitkovsky D, Jansen-Durr P, Ellwart JW, Kremmer E, Delecluse HJ, Rottenberger C, Bornkamm GW, Hammerschmidt W. 1995. B-cell proliferation and induction of early G1-regulating proteins by Epstein-Barr virus mutants conditional for EBNA2. EMBO J 14:88–96. doi: 10.1002/j.1460-2075.1995.tb06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humme S, Reisbach G, Feederle R, Delecluse HJ, Bousset K, Hammerschmidt W, Schepers A. 2003. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc Natl Acad Sci U S A 100:10989–10994. doi: 10.1073/pnas.1832776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butcher EC, Picker LJ. 1996. Lymphocyte homing and homeostasis. Science 272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 13.Isaacson PG, Norton AJ. 1994. Extranodal lymphomas, vol 1 Churchill Livingstone, London, United Kingdom. [Google Scholar]
- 14.Wagner N, Lohler J, Kunkel EJ, Ley K, Leung E, Krissansen G, Rajewsky K, Muller W. 1996. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature 382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 15.Schippers A, Leuker C, Pabst O, Kochut A, Prochnow B, Gruber AD, Leung E, Krissansen GW, Wagner N, Muller W. 2009. Mucosal addressin cell-adhesion molecule-1 controls plasma-cell migration and function in the small intestine of mice. Gastroenterology 137:924–933. doi: 10.1053/j.gastro.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 16.Kilshaw PJ, Murant SJ. 1991. Expression and regulation of beta 7(beta p) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur J Immunol 21:2591–2597. doi: 10.1002/eji.1830211041. [DOI] [PubMed] [Google Scholar]
- 17.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. 2006. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 18.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS. 2008. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol 9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 19.Cornes JS. 1965. Peyer's patches in the human gut. Proc R Soc Med 58:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorfu G, Rivera-Nieves J, Ley K. 2009. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med 9:836–850. doi: 10.2174/156652409789105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansen FE, Baekkevold ES, Carlsen HS, Farstad IN, Soler D, Brandtzaeg P. 2005. Regional induction of adhesion molecules and chemokine receptors explains disparate homing of human B cells to systemic and mucosal effector sites: dispersion from tonsils. Blood 106:593–600. doi: 10.1182/blood-2004-12-4630. [DOI] [PubMed] [Google Scholar]
- 22.Pickhardt PJ, Siegel MJ. 1999. Posttransplantation lymphoproliferative disorder of the abdomen: CT evaluation in 51 patients. Radiology 213:73–78. doi: 10.1148/radiology.213.1.r99oc2173. [DOI] [PubMed] [Google Scholar]
- 23.Salmi M, Andrew DP, Butcher EC, Jalkanen S. 1995. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J Exp Med 181:137–149. doi: 10.1084/jem.181.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR, Newman W, Ringler DJ. 1997. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 25.Yanai H, Shimizu N, Nagasaki S, Mitani N, Okita K. 1999. Epstein-Barr virus infection of the colon with inflammatory bowel disease. Am J Gastroenterol 94:1582–1586. doi: 10.1111/j.1572-0241.1999.01148.x. [DOI] [PubMed] [Google Scholar]
- 26.Spieker T, Herbst H. 2000. Distribution and phenotype of Epstein-Barr virus-infected cells in inflammatory bowel disease. Am J Pathol 157:51–57. doi: 10.1016/S0002-9440(10)64516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan JL, Shen YJ, Morgan DR, Thorne LB, Kenney SC, Dominguez RL, Gulley ML. 2012. Epstein-Barr virus infection is common in inflamed gastrointestinal mucosa. Dig Dis Sci 57:1887–1898. doi: 10.1007/s10620-012-2116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigmundsdottir H, Butcher EC. 2008. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol 9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hislop AD, Kuo M, Drake-Lee AB, Akbar AN, Bergler W, Hammerschmitt N, Khan N, Palendira U, Leese AM, Timms JM, Bell AI, Buckley CD, Rickinson AB. 2005. Tonsillar homing of Epstein-Barr virus-specific CD8+ T cells and the virus-host balance. J Clin Investig 115:2546–2555. doi: 10.1172/JCI24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zepeda-Moreno A, Taubert I, Hellwig I, Hoang V, Pietsch L, Lakshmanan VK, Wagner W, Ho AD. 2011. Innovative method for quantification of cell-cell adhesion in 96-well plates. Cell Adh Migr 5:215–219. doi: 10.4161/cam.5.3.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai MH, Raykova A, Klinke O, Bernhardt K, Gartner K, Leung CS, Geletneky K, Sertel S, Munz C, Feederle R, Delecluse HJ. 2013. Spontaneous lytic replication and epitheliotropism define an Epstein-Barr virus strain found in carcinomas. Cell Rep 5:458–470. doi: 10.1016/j.celrep.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Lazarovits AI, Moscicki RA, Kurnick JT, Camerini D, Bhan AK, Baird LG, Erikson M, Colvin RB. 1984. Lymphocyte activation antigens. I. A monoclonal antibody, anti-Act I, defines a new late lymphocyte activation antigen. J Immunol 133:1857–1862. [PubMed] [Google Scholar]