Lytic polysaccharide monooxygenases promote enzymatic depolymerization of lignocellulosic materials by microorganisms due to their ability to oxidatively cleave recalcitrant polysaccharides. The properties of these copper-dependent enzymes are currently of high scientific and industrial interest. We describe a previously uncharacterized fungal LPMO and show how reductants, which are needed to prime the LPMO by reducing Cu(II) to Cu(I) and to supply electrons during catalysis, affect enzyme efficiency and stability. The results support claims that H2O2 is a natural cosubstrate for LPMOs by demonstrating that when certain reductants are used, catalysis can be driven only by H2O2 and not by O2. Furthermore, we show how auto-inactivation resulting from endogenous generation of H2O2 in the LPMO-reductant system may be prevented. Finally, we identified a reductant that leads to enzyme activation without any endogenous H2O2 generation, allowing for improved control of LPMO reactivity and providing a valuable tool for future LPMO research.
KEYWORDS: LPMO, lignocellulose, brown rot fungi, hydrogen peroxide, lytic polysaccharide monooxygenase, wood decay
ABSTRACT
Lytic polysaccharide monooxygenases (LPMOs) are copper-dependent enzymes that perform oxidative cleavage of recalcitrant polysaccharides. We have purified and characterized a recombinant family AA9 LPMO, LPMO9B, from Gloeophyllum trabeum (GtLPMO9B) which is active on both cellulose and xyloglucan. Activity of the enzyme was tested in the presence of three different reductants: ascorbic acid, gallic acid, and 2,3-dihydroxybenzoic acid (2,3-DHBA). Under standard aerobic conditions typically used in LPMO experiments, the first two reductants could drive LPMO catalysis whereas 2,3-DHBA could not. In agreement with the recent discovery that H2O2 can drive LPMO catalysis, we show that gradual addition of H2O2 allowed LPMO activity at very low, substoichiometric (relative to products formed) reductant concentrations. Most importantly, we found that while 2,3-DHBA is not capable of driving the LPMO reaction under standard aerobic conditions, it can do so in the presence of externally added H2O2. At alkaline pH, 2,3-DHBA is able to drive the LPMO reaction without externally added H2O2, and this ability overlaps entirely the endogenous generation of H2O2 by GtLPMO9B-catalyzed oxidation of 2,3-DHBA. These findings support the notion that H2O2 is a cosubstrate of LPMOs and provide insight into how LPMO reactions depend on, and may be controlled by, the choice of pH and reductant.
IMPORTANCE Lytic polysaccharide monooxygenases promote enzymatic depolymerization of lignocellulosic materials by microorganisms due to their ability to oxidatively cleave recalcitrant polysaccharides. The properties of these copper-dependent enzymes are currently of high scientific and industrial interest. We describe a previously uncharacterized fungal LPMO and show how reductants, which are needed to prime the LPMO by reducing Cu(II) to Cu(I) and to supply electrons during catalysis, affect enzyme efficiency and stability. The results support claims that H2O2 is a natural cosubstrate for LPMOs by demonstrating that when certain reductants are used, catalysis can be driven only by H2O2 and not by O2. Furthermore, we show how auto-inactivation resulting from endogenous generation of H2O2 in the LPMO-reductant system may be prevented. Finally, we identified a reductant that leads to enzyme activation without any endogenous H2O2 generation, allowing for improved control of LPMO reactivity and providing a valuable tool for future LPMO research.
INTRODUCTION
Lignocellulosic biomass is the most abundant biogenic material on Earth. In nature, microorganisms that degrade lignocellulose utilize a plethora of different enzymes that attack the various components of the plant cell wall (lignin, cellulose, and hemicelluloses). Among these are traditional hydrolytic cellulases and hemicellulases (1), as well as oxidoreductases with a wide range of functions (2). Crucial among these oxidoreductases are enzymes called lytic polysaccharide monooxygenases (LPMOs), whose beneficial effect on biomass degradation was first described in 2005 for chitin (3) and subsequently for cellulose (4). The genomes of lignocellulose-degrading organisms often encode multiple LPMOs. These enzymes are found in all three domains of life and are classified in the carbohydrate-active enzymes (CAZy) database (5) in the auxiliary activity (AA) families 9, 10, 11, 13, 14, and 15. LPMOs are mono-copper oxidases that cause chain breaks in crystalline and amorphous polysaccharides, such as cellulose, hemicelluloses, chitin, and starch (6–9). Cellulose-active fungal LPMOs form family AA9. Depending on the organism, the number of LPMO genes in fungi can range from a few to dozens (10, 11).
Gloeophyllum trabeum is a model wood-degrading basidiomycete that causes brown rot by the selective removal of the cell wall polysaccharides without the mineralization of lignin (12). Brown rot fungi form a polyphyletic group, characterized not by their shared ancestry but by the type of decay they cause (13). While brown rot fungi are limited in their number of lignocellulose active enzymes compared to the number in other decay organisms such as white rot fungi, they are hypothesized to use a Fenton-like mechanism involving iron, oxalic acid, and phenolic secondary metabolites to generate reactive oxygen species (ROS) that depolymerize wood cell wall polysaccharides (12, 14–16). These mechanisms are well known from G. trabeum, which secretes oxalic acid to chelate transition metals and to maintain a low pH in its surroundings (12, 14, 15). While the genome of G. trabeum encodes a limited number of traditional hydrolytic cellulases and hemicellulases (nine glycoside hydrolase 3 [GH3], five GH5, and three GH10 enzymes and two GH12s, one GH74, and five GH43s, respectively) compared to the numbers in white rot fungi, it encodes a considerable number of AA family enzymes, including six LPMOs (4 AA9s and 2 AA14s), 24 glucose-methanol-choline (GMC) oxidoreductases (AA3), and 2 copper radical oxidases (AA5) (10). GMC oxidoreductases are flavoenzymes that oxidize various sugars and alcohols with the concomitant reduction of either O2 (to H2O2) or quinones/phenoxy radicals (to phenols) or other compounds (17).
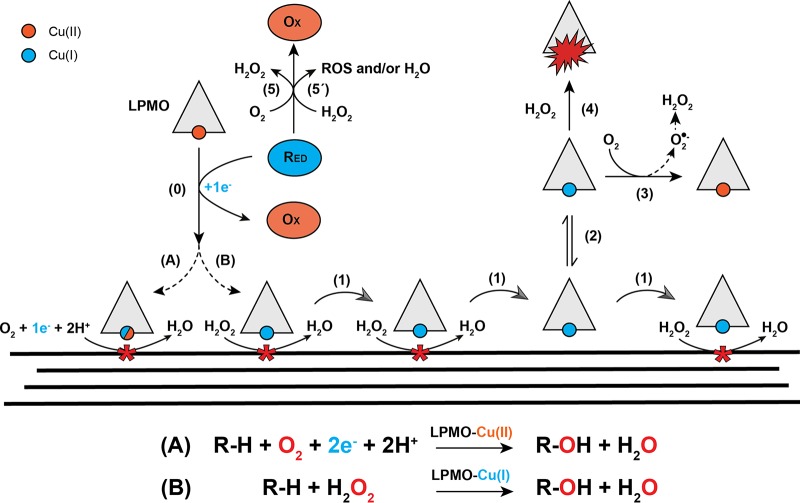
The role of H2O2 in wood-degrading fungi has traditionally been attributed to oxidative depolymerization of plant cell wall lignin by lignolytic peroxidases and polysaccharides via nonenzymatic generation of reactive oxygen species (ROS). Recent developments regarding the oxidative mechanisms of LPMOs, however, have shed new light on the potential role of H2O2 during enzymatic depolymerization of cell wall polysaccharides (2, 18). Since their identification as oxidative enzymes, LPMOs were thought to use molecular oxygen as cosubstrate (8, 19), hence the name monooxygenase (Fig. 1). The suggested mechanism for the monooxygenase reaction entails a one-electron reduction of Cu(II) to Cu(I), followed by the binding of O2 and formation of a superoxide intermediate [Cu(II)-O-O·] (20). A second electron and two protons are then required to complete the catalytic cycle, via different possible routes (6, 21), leading to incorporation of a hydroxyl group at the C-1 or C-4 in the scissile glycosidic bond, which is followed by spontaneous bond cleavage (19). This mechanism has recently been challenged by Bissaro et al. (18), who suggested that H2O2 is the natural cosubstrate of LPMOs (Fig. 1). In this scenario a one-electron “priming” reduction of the active site Cu(II) to Cu(I) is followed by a reaction with H2O2 that leads to hydrogen abstraction and subsequent hydroxylation of the substrate via different possible routes (18). Subsequent experimental (22–24) and computational (25, 26) studies have supported H2O2-driven LPMO catalysis, but the nature of the natural cosubstrate, O2 or H2O2, remains under debate.
FIG 1.
Overview of LPMO reactions. Cu(II) in the LPMO is reduced to Cu(I) by the reductant (e.g., AscA or 2,3-DHBA) via a single-electron reduction (step 0). Reduced LPMO then performs oxidative cleavage of the cellulose substrate through one of two suggested mechanisms: the reduced LPMO uses O2 directly and requires two protons and a second electron to complete the hydroxylation of a carbon in the scissile glycosidic bond (reaction A), or the reduced LPMO uses H2O2 (reaction B). Several scenarios have been proposed for reaction A, including scenarios where the copper stays reduced in between reactions (this uncertainty is indicated by the mixed blue/red symbols for the copper); in any case, each catalytic cycle requires two externally delivered electrons as shown in the reaction scheme below the drawing. For reaction B the copper stays in the Cu(I) state and the enzyme will go through multiple catalytic cycles without a need for additional delivery of electrons. Non-substrate-bound, reduced LPMO will generate H2O2 through reduction of O2, via superoxide formed by the reaction of the reduced copper with O2 (steps 2 and 3). H2O2 production may occur through the release of superoxide from the LPMO, followed by reduction or dismutation in solution, or via delivery of a second electron and two protons to the LPMO-superoxide complex, with subsequent release of H2O2. Reaction of a reduced LPMO with H2O2 in solution, i.e., in the absence of substrate, will lead to formation of reactive oxygen species that can damage and inactivate the LPMO (step 4). Reductants can reduce O2 to H2O2 (step 5) and may also be oxidized by H2O2 (step 5′), generating reactive oxygen species (ROS) and/or water. The processes denoted by steps 5 and 5′ may be affected by the presence of transition metals in the solution. See the main text for references and further details.
No matter the true mechanism, it is clear that LPMOs need to be reduced to become active. The source of the necessary reducing power in vivo is not known, but it is well established that LPMOs are promiscuous when it comes to interacting with reductants in vitro (27, 28). Several AA3 enzymes (27, 29), including well-known CDHs (30, 31) and an AA12 pyranose dehydrogenase (32), are able to drive LPMO action. In addition to enzymatic electron donors, phenolics (28, 33) and lignin-derived compounds (34) can also drive LPMO reactions. While cellobiose dehydrogenase (CDH) sometimes is considered a natural reductant for LPMOs, several brown rot fungi, including G. trabeum, lack genes that encode CDH but have several genes encoding other AA3 family enzymes (e.g., aryl alcohol oxidases) that may reduce LPMOs (27, 29) and that are expressed during growth on lignocellulosic biomass (35). Of note, several AA3 enzymes produce H2O2 and may fuel H2O2-driven LPMO catalysis (2).
The observed promiscuity of LPMOs when it comes to reductants can likely be attributed in part to the open structure of the LPMO active site, where the copper is directly exposed on the flat catalytic surface of the enzyme. Previous studies have reported the redox potential of LPMO-Cu(II)/LPMO-Cu(I) to be 155 to 326 mV (most are >240 mV), while that of soluble copper is approximately 160 mV [Cu(II)/Cu(I)] (29).
Reactions in the presence of reductant and absence of substrate have shown that LPMOs can act as oxidases, reducing O2 to produce H2O2 (36). There are two potential pathways for the observed generation of H2O2. In one scenario, single-electron reduction of molecular oxygen in the LPMO active site is followed by the release of superoxide that will then undergo spontaneous disproportionation or react with a reductant. The other possible mechanism involves H2O2 production in the active site of the enzyme, meaning that the first single-electron reduction of molecular oxygen is followed by delivery of an additional electron and two protons to reduce copper-bound superoxide to H2O2. There is some disagreement in the literature as to why H2O2 production is not observed in reaction mixtures containing an LPMO substrate. Some investigators argue that the oxidase reaction is suppressed by the formation of a productive enzyme-substrate complex and that substrate cleavage is a monooxygenase reaction (24), whereas others argue that, even in the presence of substrate, non-substrate-bound LPMOs generate H2O2, which is not observed because substrate-bound LPMOs use the H2O2 in carrying out a peroxygenase reaction (2, 18). Of note, the role of the interaction of the LPMO with the reductant varies quite considerably between these two scenarios.
We have studied the properties of a not previously characterized LPMO, LPMO9B, from the brown rot fungus G. trabeum (GtLPMO9B), focusing on the effects of reductant, pH, and H2O2 on catalytic activity. We demonstrate how the activity of GtLPMO9B is modulated by different reductants, reductant concentrations, and supply of H2O2. Importantly, these studies revealed that a reductant not previously used in LPMO activity studies, 2,3-dihydroxybenzoic acid (2,3-DHBA), is capable of activating the LPMO for H2O2-driven catalysis but, in contrast to, e.g., ascorbic acid (AscA), cannot drive the LPMO reaction on its own. This interesting observation provides a useful tool for further LPMO studies and lends support to the proposal that H2O2 is a natural cosubstrate of LPMOs.
RESULTS AND DISCUSSION
Enzyme production.
Recombinant GtLPMO9B was successfully produced in Pichia pastoris, and the purified enzyme revealed an approximate mass of 50 kDa when analyzed by SDS-PAGE. Sequence analysis of secreted GtLPMO9B shows a predicted mass of 24.5 kDa, indicating significant posttranslational modification (glycosylation) of the recombinant protein. GtLPMO9B carries two putative N-glycosylation sites (Asn138 and Asn217) and five putative O-glycosylation sites (Thr26, Ser99, Ser100, Ser139, and Ser192), as determined by the NetNGlyc, version 1.0 (37), and NetOGlyc, version 4.0 (38), servers of the Technical University of Denmark. GtLPMO9B is a single-domain AA9 LPMO, and the closest characterized relative is GtLPMO9A-2 from G. trabeum (76.6% sequence identity), and the second closest relative with a known structure (PDB accession number 4EIS) is Neurospora crassa LPMO9M (NcLPMO9M) (54.8% identity).
A structural model of GtLPMO9B built with PHYRE2 (39) showed the presence of a typical catalytic copper site comprising two conserved histidines (His1 and His86) and a tyrosine (Tyr175) in the proximal axial coordination position (see Fig. S1 in the supplemental material). The potential glycosylation sites are not located on the catalytic surface in the enzyme model; the closest residues, Asn138 and Ser139, are more than 10 Å away from the His brace. Still, since glycosylation was considerable, we cannot exclude the possibility that the attached glycans interact with the catalytic surface.
Substrate and reductant specificity.
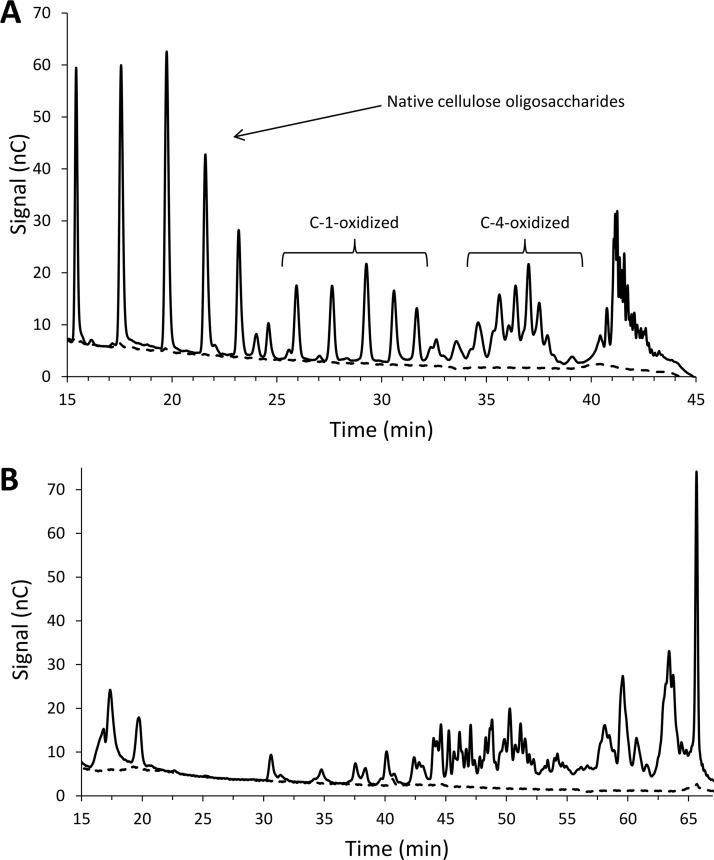
The activity of GtLPMO9B was tested on a wide range of substrates, including phosphoric acid-swollen cellulose (PASC), soluble cello-oligosaccharides (Glc5 and Glc6), konjac glucomannan, lichenan from Icelandic moss, birchwood xylan, galactomannan, wheat arabinoxylan, barley beta-glucan, ivory nut mannan, and xyloglucan from tamarind seed (tamarin xyloglucan [TXG]) using ascorbic acid (AscA) as a reducing agent. Product analysis by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) revealed reductant-dependent product formation for cellulose and xyloglucan only (Fig. 2). Figure 2A shows that GtLPMO9B produces both C-1- or C-4-oxidized cello-oligosaccharides from PASC, which implies that also native and double-oxidized oligomers are prominent (40). Of the G. trabeum LPMO9s, GtLPMO9B shares 76.6% identity with the AA9 domain of GtLPMO9A-2 (characterized by Kojima et al. [41]). Both enzymes are C-1/C-4-oxidizing LPMOs and have activity on cellulose and xyloglucan (Fig. 2) (41). GtLPMO9A-2 has broad specificity and is able to cleave the xyloglucan backbone regardless of the substitution pattern of glucosyl residues. Of note, in a previous study, GtLPMO9B was shown to increase the activity of the G. trabeum endoglucanase GtCel5B and xylanase GtXyl10G on pretreated oak and kenaf (a hemp type) (42), but in this study the activity of the LPMO alone was not analyzed.
FIG 2.
Products generated by GtLPMO9B. (A) HPAEC-PAD chromatogram showing soluble native and oxidized cello-oligosaccharides released from PASC. The peaks were annotated based on Isaksen et al. (46); double-oxidized oligomers elute at 40 to 43 min. (B) HPAEC-PAD chromatogram showing soluble native and oxidized xyloglucan oligomers released from tamarind xyloglucan (TXG). Reaction mixtures contained 0.2% (wt/vol) PASC or TXG, 1 μM GtLPMO9B, and 1 mM AscA (solid line) or no AscA (dashed line) and were incubated in 50 mM bis-Tris-HCl buffer (pH 6.5) for 24 h at 45°C.
Analysis of product formation after incubating GtLPMO9B with PASC for 24 h in the presence of 1 mM concentrations of various reducing agents at pH 6.5 showed C-1- or C-4-oxidized product levels similar to those obtained with AscA for gallic acid (GA), pyrogallol, caffeic acid, catechol, and hydroquinone, all of which are di-hydroxy or tri-hydroxy aromatic compounds (results not shown; note that these were endpoint measurements; kinetic data for selected reductants are discussed below). Monohydroxy coniferyl alcohol, a natural lignin precursor, gave low product yields, with peak intensities >15 times lower than those with AscA, whereas no product formation was observed in reactions with 2,3-dihydroxybenzoic acid, 3,5-dihydroxybenzoic acid, vanillic acid, guaiacol, veratryl alcohol, 2,4-hexadiene-1-ol, and 4-hydroxybenzoic acid under these standard conditions.
Activity with AscA.
The activity of GtLPMO9B with ascorbic acid (AscA) as the reducing agent was tested under aerobic conditions using either millimolar concentrations of the reductant only or multiple additions of reductant and H2O2 at micromolar concentrations. The multiple-addition strategy was used to keep the maximum concentrations of H2O2 low since high concentrations may lead to autocatalytic inactivation of the LPMO (18). Solubilized oligomeric products were treated with Trichoderma reesei Cel7A (TrCel7A) to generate a mixture containing only C-1-oxidized cellobiose (cellobionic acid or GlcGlcA) and C-4-oxidized cellobiose (Glc4gemGlc) as oxidized products. The amounts of these two oxidized disaccharides were quantified by HPAEC-PAD using in-house-prepared standards and summed to determine total product formation by the LPMO. The ratio of C-1- to C-4-oxidized products was constant in all experiments carried out at standard pH.
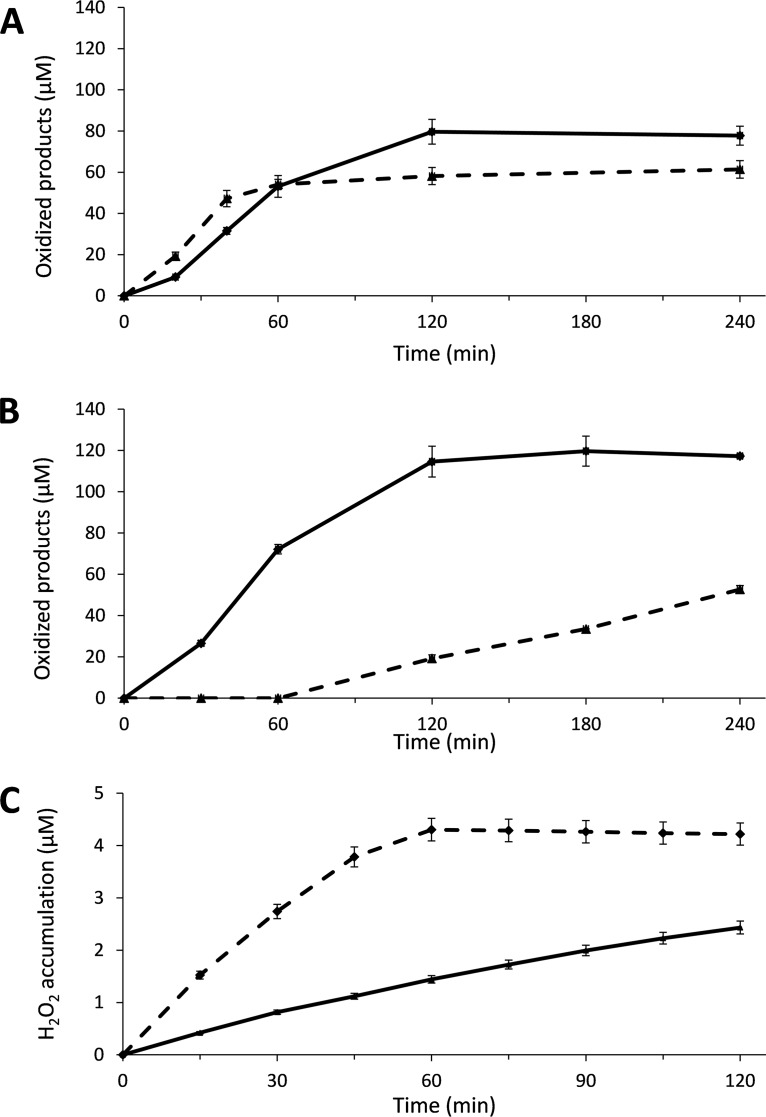
Figure 3A shows that in the initial linear phase of reactions with 1 mM AscA at pH 6.5, i.e., conditions that are generally used in LPMO research, 1 μM GtLPMO9B produced approximately 0.88 μM oxidized products per minute, and the reaction stopped after about 120 min. Increasing the concentration of AscA to 5 mM gave an increased initial rate (1.18 μM·min−1), but product formation leveled off already after 60 min, meaning that the product final yield was reduced.
FIG 3.
Cellulose degradation and H2O2 generation by GtLPMO9B with ascorbic acid (AscA) as the reductant. Reaction mixtures contained 0.2% (A) or 0.5% (B) (wt/vol) PASC and 1 μM GtLPMO9B, in 50 mM bis-Tris-HCl buffer at pH 6.5 and were incubated at 45°C and 1,000 rpm. LPMOs were activated by adding 1 mM (solid line) or 5 mM (dashed line) AscA only at time zero (A) or 15 μM AscA and 50 μM H2O2 (solid line) or 15 μM AscA only (dashed line) (and replacing the volume of H2O2 with water) every 15 min starting at time zero (B). Control reactions with only H2O2 added (no AscA) did not produce detectable amounts of oxidized products. Solubilized oxidized products were quantified as C-1-oxidized (cellobionic acid) and C-4-oxidized (Glc4gemGlc) dimers using HPAEC-PAD, and the sum of both products was calculated. (C) H2O2 accumulation in a reaction mixture containing 1 μM GtLPMO9B and 30 μM AscA without PASC (dashed line) and in a control reaction mixture containing 1 μM CuSO4 instead of the LPMO (solid line); reaction mixtures without AscA did not show H2O2 accumulation. Error bars show standard deviations (n = 3 independent experiments).
Notably, the final yield of oxidized products in reactions with millimolar concentrations of AscA (1 mM and 5 mM) remained far below the theoretical yield, which would be at least 250 μM product if molecular oxygen was limiting or 1 mM and 5 mM, respectively, if AscA was limiting. This is commonly observed for in vitro LPMO reactions. Some oxidations will be overlooked because the oxidized sites remain on the insoluble substrate (43). While this fraction may be as high as 50% in some cases (43, 44), it cannot explain why the total yield in the reaction with 5 mM AscA is only 60 μM. A more likely explanation is that the LPMOs become inactivated during the reaction due to oxidative damage in their active sites (18, 24, 45).
In the presence of O2 and AscA and in the absence of substrate, LPMOs produce H2O2 (36, 46), and it has been claimed that H2O2 production by non-substrate-bound reduced enzyme contributes to driving LPMO reactions under commonly used conditions (18). It has further been shown that accumulation of H2O2 and/or poor substrate binding correlates with LPMO inactivation (18, 23, 45). Figure 3C shows that GtLPMO9B incubated under aerobic conditions with AscA indeed produces H2O2 in the absence of substrate.
Figure 3B shows that GtLPMO9B activity may be driven by stepwise addition of H2O2 and small amounts of AscA. At AscA concentrations (15 μM per addition) that, alone, gave low LPMO activity, the reaction with added H2O2 showed a rate similar to that obtained in the reaction with 5 mM AscA. Similar to standard reactions with millimolar amounts of AscA, the reactions with added H2O2 showed enzyme inactivation. As shown in Müller et al. (47) and also below, in the section on 2,3-DHBA as a reductant, such inactivation can be overcome by lowering the H2O2 concentrations.
Activity with GA.
Of the other tested reductants, gallic acid (GA) was considered the most interesting one because it is known to react more slowly with O2 (27) and could thus perhaps lead to more controlled reactions, with less inactivation. Indeed, in contrast to results in reactions with AscA, in reactions with GA, product formation continued after 4 h (Fig. 4A). In accordance with the presumed slower generation of reactive species by GA and in contrast to what was observed with AscA (Fig. 3A), increasing the concentration of GA increased both the reaction rate and the final yield after 24 h (Fig. 4A). Figure 4C shows that H2O2 accumulation in the absence of substrate was lower for GA than for AscA (both at 30 μM concentration; the H2O2 production rates were 0.29 μM·h−1 and 4.8 μM·h−1, respectively).
FIG 4.
Activity of GtLPMO9B with gallic acid (GA) as a reductant. The reaction mixtures used in the experiments shown in panels A and B were incubated at 45°C and 1,000 rpm, and mixtures contained 1 μM enzyme, 50 mM bis-Tris-HCl buffer at pH 6.5, 0.2% (A) or 0.5% (B) PASC (wt/vol), and 1 mM (solid line) GA or 5 mM (dashed line) GA added only at time zero (A) or with repetitive addition of 15 μM GA and 50 μM H2O2 every 15 min, starting at time zero (B). Solubilized oxidized products were quantified as C-1-oxidized (cellobionic acid) and C-4-oxidized (Glc4gemGlc) dimers using HPAEC-PAD, and the sum of the two was calculated. Note the difference in time scale between the graphs shown in panels A (24 h) and B (240 min). (C) H2O2 accumulation in a reaction mixture containing 1 μM GtLPMO9B and 30 μM GA without PASC (dashed line) and in a control reaction mixture containing 1 μM CuSO4 instead of the LPMO (solid line); reaction mixtures without GA did not show H2O2 accumulation. Error bars show standard deviations (n = 3 independent experiments).
To investigate whether this difference could be attributed to H2O2 scavenging by GA, we incubated 30 μM reductant (AscA, GA, or 2,3-DHBA) with 30 μM H2O2 for 1 h (pH 6.5 at room temperature) (Fig. S2). This revealed that while AscA and 2,3-DHBA removed 54% and 31% of H2O2, respectively, GA removed 94%. We interpret these observations to indicate that GA is protecting the LPMO from excessive H2O2 generated in the reaction with PASC (Fig. 4A), explaining the prolonged longevity of the enzyme in the reaction. Notably, this finding highlights the fact that the Amplex Red assay should be used with caution to determine absolute H2O2 production by LPMOs (36) in the presence of reductants. Reductants may react with H2O2, which will mask H2O2 production by LPMOs, and, thus, the values obtained in the Amplex Red assay reflect the total accumulation and not production. These observations underpin the impact of the choice of reductant on the activity and longevity of LPMOs.
Interestingly, replacing 15 μM AscA with 15 μM GA in reactions with multiple additions of reductant and H2O2 (Fig. 4B) revealed much less difference between the reductants. Compared to results with reaction mixtures containing millimolar amounts of GA, this H2O2-based setup gave fast kinetics and progress curves with GA that were quite similar to those obtained with AscA under the same conditions. The observation that GA and AscA gave similar results when applied at low concentrations in reactions with added H2O2 indicated that a difference in reductant performance in standard O2-driven reactions is not due to differences in the ability to carry out the first priming reduction of the LPMO but, rather, to a difference in the ability to promote generation and accumulation of H2O2 in the reaction mixture.
Activity with 2,3-DHBA and hydrogen peroxide.
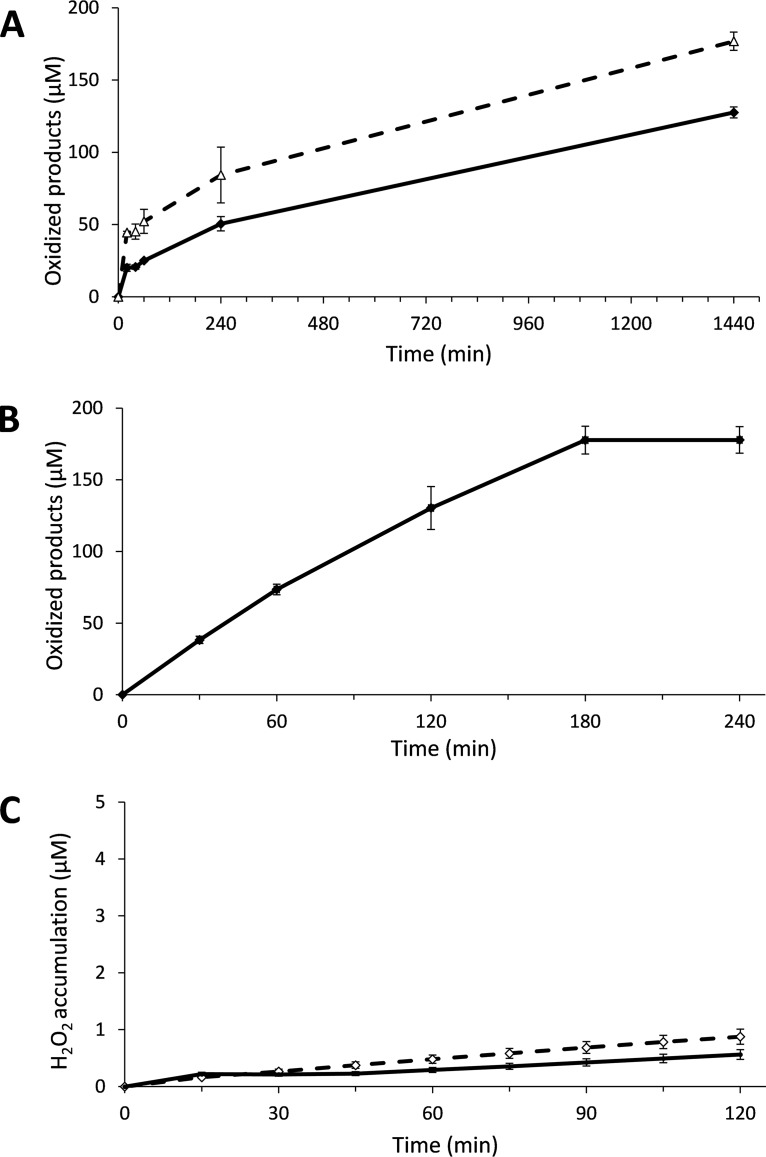
Considering the observation that GA was equally as effective as AscA in reactions with added H2O2, we then tested whether something similar might apply to the reductants that were not able to drive LPMO reactions under standard (aerobic, no added H2O2) conditions. Of three reductants tested, 2,3-dihydroxybenzoic acid (2,3-DHBA), 3,5-DHBA, and vanillic acid, one, 2,3-DHBA, indeed led to the formation of oxidized products in reaction mixtures with added H2O2 at pH 6.5 (Fig. 5). This experiment shows that 2,3-DHBA was able to prime GtLPMO9B but was unable to drive LPMO reaction without the added H2O2. Accordingly, under the conditions used, H2O2 production in a reaction without substrate at pH 6.5 was not detected (see Fig. 6, discussed in detail below). This finding supports the notion that LPMO activity requires H2O2, which either can be generated in the reaction, as in standard reactions with AscA, or needs to be added, as in reactions with 2,3-DHBA.
FIG 5.
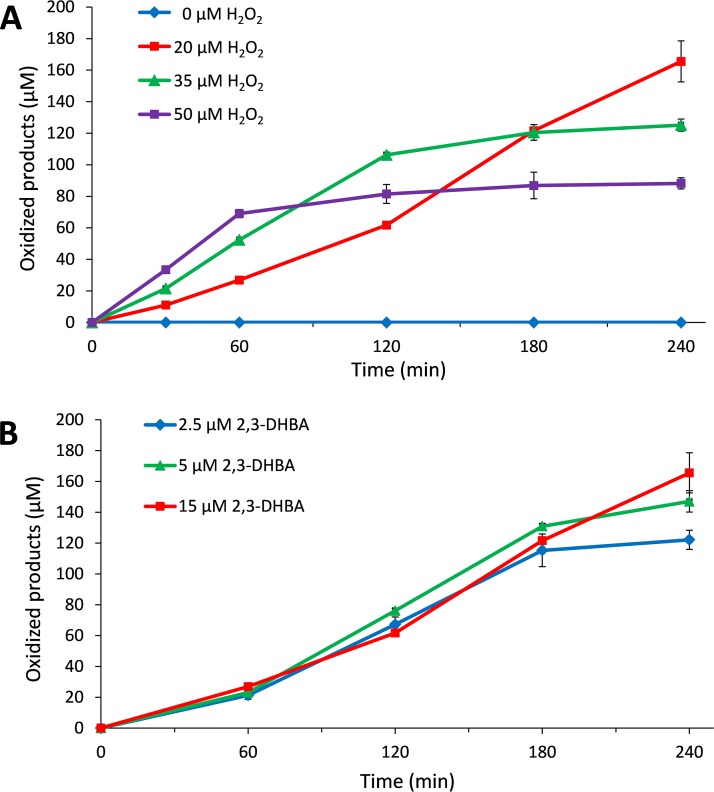
Activity of GtLPMO9B with gradual addition of 2,3-dihydroxybenzoic acid (2,3-DHBA) and H2O2. The reaction mixtures were incubated at 45°C and 1,000 rpm and contained 1 μM enzyme, 50 mM bis-Tris-HCl buffer at pH 6.5, and 0.5% (wt/vol) PASC and were subjected to repetitive additions, every 15 min, starting at time zero, of 15 μM 2,3-DHBA and either 0, 20, 35 or 50 μM H2O2 (indicated in the figure) (A) or 20 μM H2O2 and either 2.5, 5 or 15 μM 2,3-DHBA (B). Solubilized oxidized products were quantified as C-1-oxidized (cellobionic acid) and C-4-oxidized (Glc4gemGlc) dimers using HPAEC-PAD, and the sum of the two was calculated. Error bars show standard deviations (n = 3 independent experiments).
FIG 6.
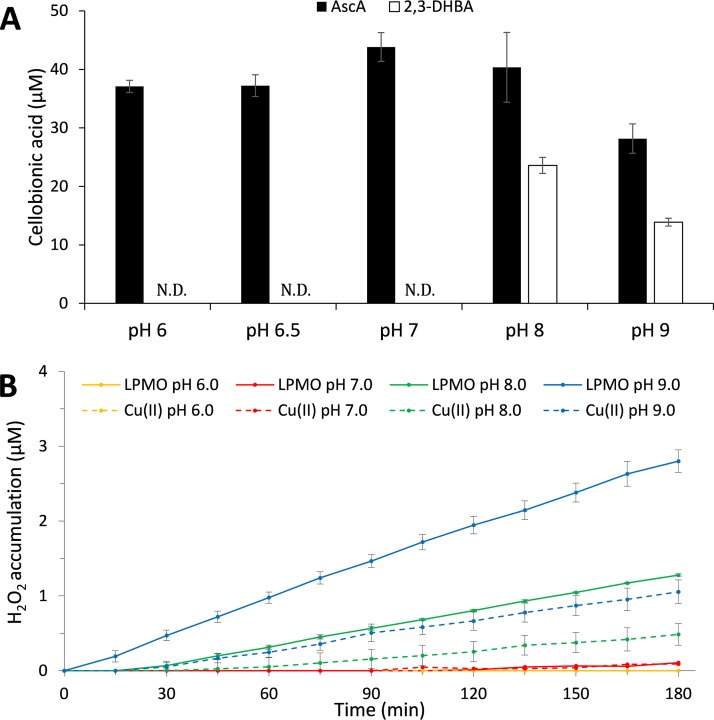
pH dependency of LPMO activity. (A) Product formation in reaction mixtures containing 1 μM GtLPMO9B on 0.5% (wt/vol) PASC and 1 mM AscA or 2,3-DHBA as reductants at pH 6.0 to 9.0 after incubation for 24 h at 45°C. Only cellobionic acid (C-1-oxidized dimers) was analyzed due to instability of C-4-oxidized dimers (Glc4gemGlc) at alkaline pH. ND, not detected. (B) H2O2 accumulation in reaction mixtures containing 1 μM GtLPMO9B and 30 μM 2,3-DHBA at pH 6.0 to 9.0, and in control reaction mixtures containing 1 μM Cu(II) (CuSO4) instead of the LPMO. At each tested pH, H2O2 accumulation in reaction mixtures containing the reductant only (not shown) was similar to, but slightly lower than, H2O2 accumulation in the control reactions with CuSO4. Note that this experiment, which was primarily done to show the effect of pH on the ability of 2,3-DHBA to drive LPMO reactions, shows only end points, and thus differences in initial rates and enzyme inactivation are not visible in the data shown in panel A. Error bars show standard deviations (n = 3 independent experiments).
Experiments with various amounts of added H2O2 showed that larger amounts led to faster initial enzyme rates and faster inactivation (Fig. 5A). Estimated initial reaction rates were 0.52 μM·min−1, 0.88 μM·min−1, and 1.30 μM·min−1, in reaction mixtures supplied with 20, 35, and 50 μM H2O2 every 15 min, respectively. Enzyme inactivation, which is likely due to accumulation of H2O2 in solution, became visible after 60 min in the reaction with 50 μM H2O2, after 120 min in the reaction with 35 μM H2O2, and not at all (within the 240 min sampling period) in the reaction with 20 μM H2O2. In agreement with recent studies describing how LPMO activity may be controlled and maintained during cellulose saccharification by controlled addition of H2O2 (47), the present results show that keeping the H2O2 concentration at an appropriate, low concentration is essential for LPMO performance.
Figure 5B shows that, within the tested range of 2.5 to 15 μM, in reactions with 20 μM H2O2, the reductant (2,3-DHBA) concentration had a relatively modest effect on LPMO performance, including the initial rate of the reaction. When 5 or 2.5 μM 2,3-DHBA combined with 20 μM H2O2 feeds was used, 147 and 122 μM soluble oxidized products were detected after 240 min, i.e., 1.8- and 3.1-fold more than the total amount of added reductant (80 and 40 μM, resulting from 16 additions of 5 μM or 2.5 μM each, respectively). This confirms the notion that only nonstoichiometric amounts of reductant (with respect to amounts of oxidized products) are required when the LPMO is fueled with H2O2. After 240 min, the cumulative H2O2 concentration would be 320 μM, which would imply that in the most stable reaction depicted in Fig. 5A (15 μM 2,3-DHBA, 20 μM H2O2 curve), giving a product yield after 240 min of 165 μM, 51.5% of the H2O2 has been converted to soluble oxidized cello-oligomers. Of note, only soluble oxidized sites were measured, whereas existing data (43, 48) indicate that in the early phases of LPMO reactions, a considerable fraction of oxidized sites may remain in the insoluble fraction. Thus, the actual level of incorporation of H2O2 into oxidized sites will be higher than 51.5%, and the present data are compatible with an expected 1:1 stoichiometry between the amount of added H2O2 and the amount of generated oxidized sites.
The effect of pH on the LPMO–AscA/2,3-DHBA systems.
As the redox potential of 2,3-DHBA is strongly affected by pH (49), we tested the activity of GtLPMO9B (1 μM) with either 1 mM AscA or 2,3-DHBA at pH 6.0, 6.5 (the pH used in the experiments described above), 7.0, 8.0, and 9.0 on 0.5% PASC, in the presence of O2 only for 24 h. The reactions were evaluated based on C-1-oxidized products only as C-4-oxidized products are unstable at alkaline pH (40). AscA was able to drive GtLPMO9B action in the full pH range of 6.0 to 9.0. On the other hand, while 2,3-DHBA was not able to drive the LPMO reaction at pH 6.5 in the absence of H2O2 (Fig. 5A and 6A), it was able to do so at pH 8.0 and 9.0 (Fig. 6A). Importantly, Fig. 6B shows that the pH dependency of LPMO activity (Fig. 6A) correlates with the pH dependency of the ability of the GtLPMO9B/2,3-DHBA system to generate H2O2: production of H2O2 was detected at pH 8.0 and 9.0 but not at pH 6.0 and 7.0. Of note, the same type of pH dependency applies to control reactions with CuSO4 (Fig. 6B), albeit with lower H2O2 production rates than with reaction mixtures containing the LPMO.
Since the redox potential of 2,3-DHBA becomes less positive with increasing pH (49), possibly as a result of deprotonation of a phenolic hydroxyl group (49), it is conceivable that increased pH leads to an increased ability to generate H2O2 by LPMO-dependent or LPMO-independent reduction of O2 (Fig. 1). Figure 6B shows that accumulation of H2O2 is much higher in reaction mixtures containing the LPMO and 2,3-DHBA than in control reaction mixtures lacking the enzyme. Thus, by far most of the H2O2 generated in the LPMO-containing reaction mixtures is generated by the LPMO and not by enzyme-independent processes, i.e., direct oxidation of 2,3-DHBA, possibly catalyzed by free copper in solution. Apparently, at pH 6 to 7, neither LPMO-dependent nor LPMO-independent H2O2 generation is sufficient to drive the reaction. However, at this pH, 2,3-DHBA does reduce the LPMO, allowing H2O2-driven catalysis.
Concluding remarks.
In this study, we analyzed the properties of the second of a total of four predicted family AA9 LPMOs encoded by the genome of Gloeophyllum trabeum, GtLPMO9B. The substrate specificity of this single-domain LPMO is similar to that of the previously characterized GtLPMO9A-2 (41).
The interplay of LPMOs with reducing compounds, reactive oxygen species, and other redox enzymes is of great current interest (2, 27, 28). We therefore used GtLPMO9B to study some of these issues, which led to the discovery of a reductant, 2,3-DHBA, which had remarkable effects on LPMO catalytic activity.
The present data indicate that, at pH 6.5, 2,3-DHBA reduces Cu(II) to Cu(I) via a single-electron reduction and that this reduced copper center, in what is now a primed LPMO, reacts with externally supplied H2O2 to perform catalytic cleavage of the cellulose substrate. The absence of LPMO activity in the absence of externally supplied H2O2 correlates with the lack of H2O2 generation by the 2,3-DHBA–LPMO redox system. It is remarkable that H2O2 production was not detected at pH 6 or 7 since a reduced LPMO [Cu(I)] will react with molecular oxygen to generate superoxide (20) and since redox potentials suggest that a reductant that is capable of reducing the LPMO (E0 of +155 to 326 mV) would also be capable of reducing superoxide to H2O2 (E0 of +890 mV) (60). One explanation could be that the superoxide remains bound to the now-oxidized copper ion [Cu(II)], which would strongly affect its redox potential.
We would argue that the present results support the notion that H2O2 is the natural substrate for LPMOs (18), but it must be noted that the option that LPMOs can use molecular oxygen directly cannot be dismissed (24, 50). Interestingly, regardless of the true nature of the oxygen cosubstrate, the present data show that GtLPMO9B at pH 6 to 7, with 2,3-DHBA acting as electron donor, is not able to reduce this cosubstrate to a species powerful enough to carry out hydrogen abstraction from the polysaccharide substrate. Use of 2,3-DHBA enables good control of LPMO activity, and we expect that this reductant will be useful in future LPMO studies. Preliminary studies with 2,3-DHBA and another LPMO, C-4-oxidizing NcLPMO9C from Neurospora crassa, gave results similar to those described above (Fig. S3). It is worth noting that, in contrast to use of AscA, e.g., use of 2,3-DHBA may allow reducing the LPMO without creating conditions that lead to enzyme inactivation, and this can be done under aerobic conditions.
MATERIALS AND METHODS
Cloning and expression of GtLPMO9B.
The gene encoding GtLPMO9B (UniProt accession number S7RK00) including its native signal sequence was codon optimized for Pichia pastoris (GenScript, NJ, USA). The synthetic gene was inserted into the pPink-GAP vector, which was then transformed into P. pastoris PichiaPink strain 4 cells (Invitrogen, CA, USA), as described earlier (51). Transformants were screened for protein production in BMGY medium (containing 1% [vol/vol] glycerol).
The best-producing transformant was grown in 25 ml of buffered minimal glycerol complex (BMGY) medium (containing 1% [vol/vol] glycerol) overnight in a 100-ml shake flask at 29°C and 200 rpm. The culture was then used to inoculate 500 ml of BMGY medium (containing 1% [vol/vol] glycerol) in a 2-liter baffled shake flask. The culture was incubated at 29°C and 200 rpm for 48 h. After 24 h of incubation the medium was resupplemented with 1% (vol/vol) glycerol. After 48 h the culture was centrifuged at 4°C and 10,000 × g for 10 min to remove the cells. The supernatant was filtered through a 0.2-μm-pore-size polyethersulfone membrane (Millipore, MA, USA), dialyzed against 50 mM bis-Tris-HCl buffer (pH 6.5), and concentrated to 100 ml with a VivaFlow 50 tangential crossflow concentrator (molecular weight cutoff [MWCO] of 10 kDa; Sartorius Stedim Biotech, Germany).
Purification of recombinant protein.
The recombinant GtLPMO9B protein was purified in a two-step protocol using hydrophobic interaction chromatography (HIC) followed by size exclusion chromatography (SEC), as described previously (52). Purified GtLPMO9B was copper saturated by incubating the enzyme with an excess of Cu(II)SO4 (at ∼3:1 molar ratio of copper/enzyme) for 30 min at room temperature as described previously (53). The Cu(II)-loaded sample of GtLPMO9B was buffer exchanged to 50 mM bis-Tris-HCl buffer (pH 6.5), using Amicon Ultra centrifugal filters (MWCO of 3 kDa; Merck Millipore, Burlington, MA, USA). The resulting protein solution was filtered through a sterile 0.22-μm-pore-size Millex-GV filter (Merck Millipore, Burlington, MA, USA) and stored at 4°C. Protein concentrations were determined by measuring absorbance at 280 nm in a spectrophotometer, calculating the concentration based on the extinction coefficient (ε = 55,475 M−1⋅cm−1; calculated using ProtParam [54]).
Screening of substrates and reductants.
Activity of GtLPMO9B was tested on the following substrates: phosphoric acid-swollen cellulose (PASC) prepared from Avicel as described previously (55), konjac glucomannan, galactomannan, ivory nut mannan, xyloglucan from tamarind seed, lichenan from Icelandic moss, wheat arabinoxylan, and barley beta-glucan, all purchased from Megazyme (Bray, Ireland), and birchwood xylan, obtained from Sigma-Aldrich (St. Louis, MO, USA). Substrate specificity was tested by setting up reaction mixtures (100 μl) containing 0.2% (wt/vol) substrate, 1 μM GtLPMO9B, and 1 mM ascorbic acid (AscA) in 50 mM bis-Tris-HCl buffer, pH 6.5. The samples were incubated in an Eppendorf ThermoMixer C (Eppendorf, Hamburg, Germany) at 45°C and 1,000 rpm for 24 h. Control reactions were performed in the absence of ascorbic acid. Reactions were stopped by separating the soluble fractions from the insoluble substrates by filtration using a 96-well filter plate (Millipore) operated with a vacuum manifold.
Activity assays with PASC, under the conditions described above, were also done with the following alternative reducing agents: gallic acid, pyrogallol, caffeic acid, catechol, hydroquinone, coniferyl alcohol, 2,3-dihydroxybenzoic acid, 3,5-dihydroxybenzoic acid, vanillic acid, guaiacol, veratryl alcohol, 2,4-hexadiene-1-ol, and 4-hydroxybenzoic acid. All reducing agents were purchased from Sigma-Aldrich.
GtLPMO9B activity on PASC: time series and quantification of oxidized products.
Reaction mixtures (600 μl) contained 1 μM GtLPMO9B, 0.2% (wt/vol) PASC, and 1 mM reductant in 50 mM bis-Tris-HCl buffer, pH 6.5. The reaction mixtures were incubated in an Eppendorf ThermoMixer C (Eppendorf, Hamburg, Germany) at 45°C and 1,000 rpm. At various time points (20, 40, 60, 120, and 240 min), 100-μl samples were taken and boiled for 5 min. For gallic acid, a final sample was taken after 24 h. Separation of soluble and insoluble material was achieved by centrifugation at 11,000 × g for 10 min. The soluble fractions (25 μl) were mixed with 24 μl of 150 mM sodium-acetate buffer (pH 4.5) and 1 μl of TrCel7A from Trichoderma reesei (∼1 μM) and incubated for 24 h at 37°C. The treatment with TrCel7A converts soluble oxidized cello-oligomers to cellobionic acid (C-1 oxidized) and Glc4gemGlc (C-4 oxidized). Soluble fractions treated in this way were subsequently analyzed using high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD). The amount of released oxidized products was quantified using the following standards: 4-hydroxy-β-d-xylo-Hexp-(1→4)-β-d-Glcp (Glc4gemGlc), prepared as described previously (56), and cellobionic acid, prepared by treating cellobiose with cellobiose dehydrogenase from Myriococcum thermophilum (MtCDH) (57). All experiments were performed in triplicate.
The effect of pH on GtLPMO9B activity.
GtLPMO9B (1 μM) was incubated with 0.5% (wt/vol) PASC in 50 mM buffer solution with 1 mM reductant in a final volume of 100 μl. For the reaction mixtures at pH 6.0, 6.5, or 7.0, 50 mM bis-Tris-HCl was used as the buffering agent, while 50 mM Tris-HCl was used for the reaction mixtures at pH 8.0 or 9.0. The samples were incubated in an Eppendorf ThermoMixer at 45°C and 1,000 rpm for 24 h. The reactions were stopped by boiling for 5 min. Samples were hydrolyzed with TrCel7A as previously described, and oxidized product was quantified as cellobionic acid. All reactions were performed in triplicate.
H2O2 as cosubstrate.
In reaction mixtures with added H2O2, 1 μM GtLPMO9B was mixed with 0.5% (wt/vol) PASC in 50 mM bis-Tris-HCl buffer (pH 6.5), and various initial concentrations of reductant and H2O2 (2.5 and 20, 5 and 35, or 15 and 50 μM, respectively) were added. GtLPMO9B was premixed with PASC in the buffer (588 μl), after which we added first 6 μl of freshly prepared reductant solution with a concentration of 0.25, 0.5, or 1.5 mM reductant and then 6 μl of a freshly prepared 2, 3.5, or 5 mM H2O2 stock solution. Reductant and H2O2, in that order, were subsequently added to the reaction mixtures every 15 min for a total period of 240 min. Added volumes were adapted to the changes in total reaction volume that were due to previous additions and sampling so that the added amounts corresponded to final concentrations identical to the concentrations at time (t) 0. Reactions were stopped by boiling and freezing (−20°C) prior to further analysis. The amount of released oxidized product was quantified as described above. In control experiments, either H2O2 or reductant was replaced with water. All reactions were performed aerobically and in triplicate.
H2O2 production in the absence of substrate at different pHs.
The production of H2O2 by GtLPMO9B at different pHs in the absence of substrate was monitored using an assay with Amplex Red and horseradish peroxidase (HRP), both purchased from Sigma-Aldrich (St. Louis, USA), according to the method of Kittl et al. (36). In brief, 100-μl reaction mixtures were prepared in 96-well microtiter plates containing 1 μM GtLPMO9B, 5 U of horseradish peroxidase, 50 μM Amplex Red, and 30 μM reductant in the appropriate buffer. The reactions were initiated by addition of the reductant, followed by real-time measurement of absorbance at 540 nm for 120 min in a Thermo Scientific Multiscan FC microplate reader at room temperature. For pH 6.0, 6.5, and 7.0, 50 mM bis-Tris-HCl buffer was used, while at pH 8.0 and 9.0, 50 mM Tris-HCl buffer was used. In control reaction mixtures, 1 μM GtLPMO9B was replaced by either 1 μM CuSO4 or water. Standard curves for quantification of H2O2 were made in the concentration range of 0.5 to 20 μM, at the pHs used in the experiment. All reactions were performed in triplicate.
H2O2 scavenging by reductants.
Reductants (AscA, GA, and 2,3-DHBA) were incubated with H2O2 at a 1:1 molar ratio (30 μM) in 50 mM bis-Tris-HCl buffer, pH 6.5, for 60 min at room temperature. In the control reaction, reductant was replaced with water. H2O2 concentrations were determined by a single time point measurement of (maximal) conversion of Amplex Red (50 μM starting concentration) to resorufin by 5 U of HRP using a Multiscan FC microplate reader (540 nm). H2O2 quantification was achieved using a standard curve covering the range of 1 to 30 μM.
Detection of oxidized products.
Oxidized products were analyzed using high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD). HPAEC was performed on a Dionex ICS5000 system, equipped with a CarboPac PA1 analytical column (2 by 250 mm) and a CarboPac PA1 guard column (2 by 50 mm), using a 50-min gradient for cellulosic (58) and a 75-min gradient (59) for hemicellulosic substrates. Chromatograms were recorded and analyzed using Chromeleon, version 7.0, software (Thermo Fisher Scientific, Waltham, MA, USA).
Supplementary Material
ACKNOWLEDGMENTS
This work was financed by the Research Council of Norway 243663/E50 BioMim and the Norwegian Institute for Bioeconomy Research.
We are grateful to Piotr Chylenski for insightful comments and suggestions.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02612-18.
REFERENCES
- 1.Cragg SM, Beckham GT, Bruce NC, Bugg TDH, Distel DL, Dupree P, Etxabe AG, Goodell BS, Jellison J, McGeehan JE, McQueen-Mason SJ, Schnorr K, Walton PH, Watts JEM, Zimmer M. 2015. Lignocellulose degradation mechanisms across the tree of life. Curr Opin Chem Biol 29:108–119. doi: 10.1016/j.cbpa.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissaro B, Várnai A, Røhr ÅK, Eijsink VGH. 2018. Oxidoreductases and reactive oxygen species in conversion of lignocellulosic biomass. Microbiol Mol Biol Rev 82:e00029-18. doi: 10.1128/MMBR.00029-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaaje-Kolstad G, Horn SJ, van Aalten DM, Synstad B, Eijsink VGH. 2005. The non-catalytic chitin-binding protein CBP21 from Serratia marcescens is essential for chitin degradation. J Biol Chem 280:28492–28497. doi: 10.1074/jbc.M504468200. [DOI] [PubMed] [Google Scholar]
- 4.Forsberg Z, Vaaje‐Kolstad G, Westereng B, Bunæs AC, Stenstrøm Y, Mackenzie A, Sørlie M, Horn SJ, Eijsink VGH. 2011. Cleavage of cellulose by a CBM33 protein. Protein Sci 20:1479–1483. doi: 10.1002/pro.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. 2014. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beeson WT, Vu VV, Span EA, Phillips CM, Marletta MA. 2015. Cellulose degradation by polysaccharide monooxygenases. Annu Rev Biochem 84:923–946. doi: 10.1146/annurev-biochem-060614-034439. [DOI] [PubMed] [Google Scholar]
- 7.Couturier M, Ladevèze S, Sulzenbacher G, Ciano L, Fanuel M, Moreau C, Villares A, Cathala B, Chaspoul F, Frandsen KE, Labourel A, Herpoël-Gimbert I, Grisel S, Haon M, Lenfant N, Rogniaux H, Ropartz D, Davies GJ, Rosso M-N, Walton PH, Henrissat B, Berrin J-G. 2018. Lytic xylan oxidases from wood-decay fungi unlock biomass degradation. Nat Chem Biol 14:306–310. doi: 10.1038/nchembio.2558. [DOI] [PubMed] [Google Scholar]
- 8.Vaaje-Kolstad G, Westereng B, Horn SJ, Liu Z, Zhai H, Sørlie M, Eijsink VGH. 2010. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 330:219–222. doi: 10.1126/science.1192231. [DOI] [PubMed] [Google Scholar]
- 9.Leggio LL, Simmons TJ, Poulsen J-CN, Frandsen KE, Hemsworth GR, Stringer MA, Von Freiesleben P, Tovborg M, Johansen KS, De Maria L. 2015. Structure and boosting activity of a starch-degrading lytic polysaccharide monooxygenase. Nat Commun 6:5961. doi: 10.1038/ncomms6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Górecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TKA, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, et al. 2012. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719. doi: 10.1126/science.1221748. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Z, Liu H, Wang C, Xu J-R. 2013. Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genomics 14:274. doi: 10.1186/1471-2164-14-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerem Z, Jensen KA, Hammel KE. 1999. Biodegradative mechanism of the brown rot basidiomycete Gloeophyllum trabeum: evidence for an extracellular hydroquinone‐driven Fenton reaction. FEBS Lett 446:49–54. doi: 10.1016/S0014-5793(99)00180-5. [DOI] [PubMed] [Google Scholar]
- 13.Riley R, Salamov AA, Brown DW, Nagy LG, Floudas D, Held BW, Levasseur A, Lombard V, Morin E, Otillar R, Lindquist EA, Sun H, LaButti KM, Schmutz J, Jabbour D, Luo H, Baker SE, Pisabarro AG, Walton JD, Blanchette RA, Henrissat B, Martin F, Cullen D, Hibbett DS, Grigoriev IV. 2014. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc Natl Acad Sci U S A 111:9923–9928. doi: 10.1073/pnas.1400592111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen KA, Houtman CJ, Ryan ZC, Hammel KE. 2001. Pathways for extracellular Fenton chemistry in the brown rot basidiomycete Gloeophyllum trabeum. Appl Environ Microbiol 67:2705–2711. doi: 10.1128/AEM.67.6.2705-2711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jellison J, Chandhoke V, Goodell B, Fekete FA. 1991. The isolation and immunolocalization of iron-binding compounds produced by Gloeophyllum trabeum. Appl Microbiol Biotechnol 35:805–809. doi: 10.1007/BF00169899. [DOI] [Google Scholar]
- 16.Xu G, Goodell B. 2001. Mechanisms of wood degradation by brown-rot fungi: chelator-mediated cellulose degradation and binding of iron by cellulose. J Biotechnol 87:43–57. doi: 10.1016/S0168-1656(00)00430-2. [DOI] [PubMed] [Google Scholar]
- 17.Cavener DR. 1992. GMC oxidoreductases: a newly defined family of homologous proteins with diverse catalytic activities. J Mol Biol 223:811–814. doi: 10.1016/0022-2836(92)90992-S. [DOI] [PubMed] [Google Scholar]
- 18.Bissaro B, Røhr ÅK, Müller G, Chylenski P, Skaugen M, Forsberg Z, Horn SJ, Vaaje-Kolstad G, Eijsink VGH. 2017. Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat Chem Biol 13:1123. doi: 10.1038/nchembio.2470. [DOI] [PubMed] [Google Scholar]
- 19.Beeson WT, Phillips CM, Cate JH, Marletta MA. 2012. Oxidative cleavage of cellulose by fungal copper-dependent polysaccharide monooxygenases. J Am Chem Soc 134:890–892. doi: 10.1021/ja210657t. [DOI] [PubMed] [Google Scholar]
- 20.Kjaergaard CH, Qayyum MF, Wong SD, Xu F, Hemsworth GR, Walton DJ, Young NA, Davies GJ, Walton PH, Johansen KS, Hodgson KO, Hedman B, Solomon EI. 2014. Spectroscopic and computational insight into the activation of O2 by the mononuclear Cu center in polysaccharide monooxygenases. Proc Natl Acad Sci U S A 111:8797–8802. doi: 10.1073/pnas.1408115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walton PH, Davies GJ. 2016. On the catalytic mechanisms of lytic polysaccharide monooxygenases. Curr Opin Chem Biol 31:195–207. doi: 10.1016/j.cbpa.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Petrovic DM, Bissaro B, Chylenski P, Skaugen M, Sørlie M, Jensen MS, Aachmann FL, Courtade G, Várnai A, Eijsink VGH. 2018. Methylation of the N‐terminal histidine protects a lytic polysaccharide monooxygenase from auto‐oxidative inactivation. Protein Sci 27:1636–1650. doi: 10.1002/pro.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuusk S, Bissaro B, Kuusk P, Forsberg Z, Eijsink VGH, Sørlie M, Väljamäe P. 2018. Kinetics of H2O2-driven degradation of chitin by a bacterial lytic polysaccharide monooxygenase. J Biol Chem 293:523–531. doi: 10.1074/jbc.M117.817593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hangasky JA, Iavarone AT, Marletta MA. 2018. Reactivity of O2 versus H2O2 with polysaccharide monooxygenases. Proc Natl Acad Sci U S A 115:4915–4920. doi: 10.1073/pnas.1801153115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B, Johnston EM, Li P, Shaik S, Davies GJ, Walton PH, Rovira C. 2018. QM/MM studies into the H2O2-dependent activity of lytic polysaccharide monooxygenases: evidence for the formation of a caged hydroxyl radical intermediate. ACS Catal 8:1346–1351. doi: 10.1021/acscatal.7b03888. [DOI] [Google Scholar]
- 26.Hedegård ED, Ryde U. 2018. Molecular mechanism of lytic polysaccharide monooxygenases. Chem Sci 9:3866–3880. doi: 10.1039/c8sc00426a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kracher D, Scheiblbrandner S, Felice AK, Breslmayr E, Preims M, Ludwicka K, Haltrich D, Eijsink VGH, Ludwig R. 2016. Extracellular electron transfer systems fuel cellulose oxidative degradation. Science 352:1098–1101. doi: 10.1126/science.aaf3165. [DOI] [PubMed] [Google Scholar]
- 28.Frommhagen M, Westphal AH, Van Berkel WJ, Kabel MA. 2018. Distinct substrate specificities and electron-donating systems of fungal lytic polysaccharide monooxygenases. Front Microbiol 9:1080. doi: 10.3389/fmicb.2018.01080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garajova S, Mathieu Y, Beccia MR, Bennati-Granier C, Biaso F, Fanuel M, Ropartz D, Guigliarelli B, Record E, Rogniaux H, Henrissat B, Berrin J-G. 2016. Single-domain flavoenzymes trigger lytic polysaccharide monooxygenases for oxidative degradation of cellulose. Sci Rep 6:28276. doi: 10.1038/srep28276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips CM, Beeson WT, Cate JH, Marletta MA. 2011. Cellobiose dehydrogenase and a copper-dependent polysaccharide monooxygenase potentiate cellulose degradation by Neurospora crassa. ACS Chem Biol 6:1399–1406. doi: 10.1021/cb200351y. [DOI] [PubMed] [Google Scholar]
- 31.Langston JA, Shaghasi T, Abbate E, Xu F, Vlasenko E, Sweeney MD. 2011. Oxidoreductive cellulose depolymerization by the enzymes cellobiose dehydrogenase and glycoside hydrolase 61. Appl Environ Microbiol 77:7007–7015. doi: 10.1128/AEM.05815-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Várnai A, Umezawa K, Yoshida M, Eijsink VGH. 2018. The pyrroloquinoline-quinone dependent pyranose dehydrogenase from Coprinopsis cinerea (CcPDH) drives lytic polysaccharide monooxygenase (LPMO) action. Appl Environ Microbiol 84:e00156-18. doi: 10.1128/AEM.00156-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frommhagen M, Koetsier MJ, Westphal AH, Visser J, Hinz SW, Vincken J-P, Berkel WJ, Kabel MA, Gruppen H. 2016. Lytic polysaccharide monooxygenases from Myceliophthora thermophila C1 differ in substrate preference and reducing agent specificity. Biotechnol Biofuels 9:186. doi: 10.1186/s13068-016-0594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westereng B, Cannella D, Agger JW, Jørgensen H, Andersen ML, Eijsink VGH, Felby C. 2015. Enzymatic cellulose oxidation is linked to lignin by long-range electron transfer. Sci Rep 5:18561. doi: 10.1038/srep18561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Presley GN, Hammel KE, Ryu JS, Menke JR, Figueroa M, Hu D, Orr G, Schilling JS. 2016. Localizing gene regulation reveals a staggered wood decay mechanism for the brown rot fungus Postia placenta. Proc Natl Acad Sci U S A 113:10968–10973. doi: 10.1073/pnas.1608454113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kittl R, Kracher D, Burgstaller D, Haltrich D, Ludwig R. 2012. Production of four Neurospora crassa lytic polysaccharide monooxygenases in Pichia pastoris monitored by a fluorimetric assay. Biotechnol Biofuels 5:79. doi: 10.1186/1754-6834-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta R, Brunak S. 2002. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac Symp Biocomput 2002:310–322. doi: 10.1142/9789812799623_0029. [DOI] [PubMed] [Google Scholar]
- 38.Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT-BG, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, Gupta R, Paul Bennett E, Mandel U, Brunak S, Wandall HH, Levery SB, Clausen H. 2013. Precision mapping of the human O‐GalNAc glycoproteome through SimpleCell technology. EMBO J 32:1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westereng B, Arntzen MØ, Aachmann FL, Várnai A, Eijsink VGH, Agger JW. 2016. Simultaneous analysis of C1 and C4 oxidized oligosaccharides, the products of lytic polysaccharide monooxygenases acting on cellulose. J Chromatogr A 1445:46–54. doi: 10.1016/j.chroma.2016.03.064. [DOI] [PubMed] [Google Scholar]
- 41.Kojima Y, Várnai A, Ishida T, Sunagawa N, Petrovic DM, Igarashi K, Jellison J, Goodell B, Alfredsen G, Westereng B, Eijsink VGH, Yoshida M. 2016. Characterization of an LPMO from the brown-rot fungus Gloeophyllum trabeum with broad xyloglucan specificity, and its action on cellulose-xyloglucan complexes. Appl Environ Microbiol 82:6557–6572. doi: 10.1128/AEM.01768-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung S, Song Y, Kim HM, Bae H-J. 2015. Enhanced lignocellulosic biomass hydrolysis by oxidative lytic polysaccharide monooxygenases (LPMOs) GH61 from Gloeophyllum trabeum. Enzyme Microb Technol 77:38–45. doi: 10.1016/j.enzmictec.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Loose JS, Forsberg Z, Kracher D, Scheiblbrandner S, Ludwig R, Eijsink VGH, Vaaje‐Kolstad G. 2016. Activation of bacterial lytic polysaccharide monooxygenases with cellobiose dehydrogenase. Prot Sci 25:2175–2186. doi: 10.1002/pro.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Courtade G, Forsberg Z, Heggset EB, Eijsink VGH, Aachmann FL. 2018. The carbohydrate-binding module and linker of a modular lytic polysaccharide monooxygenase promote localized cellulose oxidation. J Biol Chem 293:13006–13015. doi: 10.1074/jbc.RA118.004269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loose JS, Arntzen MØ, Bissaro B, Ludwig R, Eijsink VGH, Vaaje-Kolstad G. 2018. Multipoint precision binding of substrate protects lytic polysaccharide monooxygenases from self-destructive off-pathway processes. Biochemistry 57:4114–4124. doi: 10.1021/acs.biochem.8b00484. [DOI] [PubMed] [Google Scholar]
- 46.Isaksen T, Westereng B, Aachmann FL, Agger JW, Kracher D, Kittl R, Ludwig R, Haltrich D, Eijsink VGH, Horn SJ. 2014. A C4-oxidizing lytic polysaccharide monooxygenase cleaving both cellulose and cello-oligosaccharides. J Biol Chem 289:2632–2642. doi: 10.1074/jbc.M113.530196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Müller G, Chylenski P, Bissaro B, Eijsink VGH, Horn SJ. 2018. The impact of hydrogen peroxide supply on LPMO activity and overall saccharification efficiency of a commercial cellulase cocktail. Biotechnol Biofuels 11:209. doi: 10.1186/s13068-018-1199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forsberg Z, Bissaro B, Gullesen J, Dalhus B, Vaaje-Kolstad G, Eijsink VGH. 2018. Structural determinants of bacterial lytic polysaccharide monooxygenase functionality. J Biol Chem 293:1397–1412. doi: 10.1074/jbc.M117.817130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu R, Goodell B, Jellison J, Amirbahman A. 2005. Electrochemical study of 2,3-dihydroxybenzoic acid and its interaction with Cu(II) and H2O2 in aqueous solutions: implications for wood decay. Environ Sci Technol 39:175–180. doi: 10.1021/es049714q. [DOI] [PubMed] [Google Scholar]
- 50.Simmons TJ, Frandsen KEH, Ciano L, Tryfona T, Lenfant N, Poulsen JC, Wilson LFL, Tandrup T, Tovborg M, Schnorr K, Johansen KS, Henrissat B, Walton PH, Lo Leggio L, Dupree P. 2017. Structural and electronic determinants of lytic polysaccharide monooxygenase reactivity on polysaccharide substrates. Nat Commun 8:1064. doi: 10.1038/s41467-017-01247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Várnai A, Tang C, Bengtsson O, Atterton A, Mathiesen G, Eijsink VGH. 2014. Expression of endoglucanases in Pichia pastoris under control of the GAP promoter. Microb Cell Fact 13:57. doi: 10.1186/1475-2859-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nekiunaite L, Petrović DM, Westereng B, Vaaje-Kolstad G, Hachem MA, Várnai A, Eijsink VGH. 2016. FgLPMO9A from Fusarium graminearum cleaves xyloglucan independently of the backbone substitution pattern. FEBS Lett 590:3346–3356. doi: 10.1002/1873-3468.12385. [DOI] [PubMed] [Google Scholar]
- 53.Loose JS, Forsberg Z, Fraaije MW, Eijsink VGH, Vaaje-Kolstad G. 2014. A rapid quantitative activity assay shows that the Vibrio cholerae colonization factor GbpA is an active lytic polysaccharide monooxygenase. FEBS Lett 588:3435–3440. doi: 10.1016/j.febslet.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 54.Gasteiger E, Hoogland C, Gattiker A, Wilkins MR, Appel RD, Bairoch A. 2005. Protein identification and analysis tools on the ExPASy server, p 571–607. In Walker JM. (ed), The proteomics protocols handbook. Humana Press, New York, NY. doi: 10.1385/1-59259-890-0:571. [DOI] [Google Scholar]
- 55.Wood TM. 1988. Preparation of crystalline, amorphous, and dyed cellulase substrates. Methods Enzymol 160:19–25. doi: 10.1016/0076-6879(88)60103-0. [DOI] [Google Scholar]
- 56.Müller G, Várnai A, Johansen KS, Eijsink VGH, Horn SJ. 2015. Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions. Biotech Biofuels 8:187. doi: 10.1186/s13068-015-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zámocký M, Schümann C, Sygmund C, O'Callaghan J, Dobson ADW, Ludwig R, Haltrich D, Peterbauer CK. 2008. Cloning, sequence analysis and heterologous expression in Pichia pastoris of a gene encoding a thermostable cellobiose dehydrogenase from Myriococcum thermophilum. Protein Expr Purif 59:258–265. doi: 10.1016/j.pep.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Westereng B, Agger JW, Horn SJ, Vaaje-Kolstad G, Aachmann FL, Stenstrøm YH, Eijsink VGH. 2013. Efficient separation of oxidized cello-oligosaccharides generated by cellulose degrading lytic polysaccharide monooxygenases. J Chromatogr A 1271:144–152. doi: 10.1016/j.chroma.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 59.Agger JW, Isaksen T, Várnai A, Vidal-Melgosa S, Willats WG, Ludwig R, Horn SJ, Eijsink VGH, Westereng B. 2014. Discovery of LPMO activity on hemicelluloses shows the importance of oxidative processes in plant cell wall degradation. Proc Natl Acad Sci U S A 111:6287–6292. doi: 10.1073/pnas.1323629111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood PM. 1988. The potential diagram for oxygen at pH 7. Biochem J 253:287–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.