The genetic responses of bacteria to depletion of proton motive force (PMF), and their effects on drug resistance, are poorly understood. PMF drives export of many antibiotics, but the energy cost may decrease fitness when antibiotics are absent. Our evolution experiment reveals genetic mechanisms of adaptation to the PMF uncoupler CCCP, including selection for increased CCCP efflux but also against the expression of PMF-driven pumps for drugs not present. The results have implications for our understanding of the gut microbiome, which experiences high levels of organic acids that decrease PMF.
KEYWORDS: CCCP, acrAB, antibiotic resistance, cecR, emrA, evolution, gadE, mprA (emrR), proton motive force, ybhR
ABSTRACT
Experimental evolution of Escherichia coli K-12 with benzoate, a partial uncoupler of the proton motive force (PMF), selects for mutations that decrease antibiotic resistance. We conducted experimental evolution in the presence of carbonyl cyanide m-chlorophenylhydrazone (CCCP), a strong uncoupler. Cultures were serially diluted daily 1:100 in LBK medium containing 20 to 150 µM CCCP buffered at pH 6.5 or at pH 8.0. After 1,000 generations, the populations tolerated up to 150 µM CCCP. Sequenced isolates had mutations in mprA (emrR), which downregulates the EmrAB-TolC pump that exports CCCP. A mprA::kanR deletion conferred growth at 60 μM CCCP, though not at the higher levels resisted by evolved strains (150 µM). Some mprA mutant strains also had point mutations affecting emrA, but deletion of emrA abolished the CCCP resistance. Thus, CCCP-evolved isolates contained additional adaptations. One isolate lacked emrA or mprA mutations but had mutations in cecR (ybiH), whose product upregulates drug pumps YbhG and YbhFSR, and in gadE, which upregulates the multidrug pump MdtEF. A cecR::kanR deletion conferred partial resistance to CCCP. Other multidrug efflux genes that had mutations included ybhR and acrAB. The acrB isolate was sensitive to the AcrAB substrates chloramphenicol and tetracycline. Other mutant genes in CCCP-evolved strains include rng (RNase G) and cyaA (adenylate cyclase). Overall, experimental evolution revealed a CCCP-dependent fitness advantage for mutations increasing CCCP efflux via EmrA and for mutations that may deactivate proton-driven pumps for drugs not present (cecR, gadE, acrAB, and ybhR). These results are consistent with our previous report of drug sensitivity associated with evolved benzoate tolerance.
IMPORTANCE The genetic responses of bacteria to depletion of proton motive force (PMF), and their effects on drug resistance, are poorly understood. PMF drives export of many antibiotics, but the energy cost may decrease fitness when antibiotics are absent. Our evolution experiment reveals genetic mechanisms of adaptation to the PMF uncoupler CCCP, including selection for increased CCCP efflux but also against the expression of PMF-driven pumps for drugs not present. The results have implications for our understanding of the gut microbiome, which experiences high levels of organic acids that decrease PMF.
INTRODUCTION
The proton motive force (PMF) is diminished or abolished by uncouplers of oxidative phosphorylation such as carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (1). Uncouplers such as CCCP are hydrophobic molecules with an acidic proton that reside in the cell’s inner membrane. The molecule shuttles protons across the membrane via protonation/deprotonation (1–3). The proton flux equilibrates both the transmembrane pH difference (ΔpH) and the transmembrane electrical potential (Δψ) and thus depletes PMF (1, 2). Respiration runs a futile cycle, as protons continue to be pumped but PMF is not maintained (4–6). The uncoupler effect is most pronounced during growth at low external pH, where the electron transport system is upregulated and a higher PMF is maintained (7).
The PMF powers many low-level multidrug resistance (MDR) efflux pumps, which provide bacteria with a first-line defense against antibiotics and other growth-inhibiting molecules (8, 9). The effect of uncouplers on MDR efflux is poorly understood. Experimental evolution in benzoic acid, a membrane-permeant aromatic acid and partial uncoupler, incurs the loss of antibiotic resistance and decreased expression of multidrug efflux pumps (10). For example, benzoate stress selects for mutations that delete or downregulate the Gad acid fitness island, including the mdtEF drug efflux system (10, 11). At a high concentration, benzoic acid partly uncouples PMF (12, 13) and thus could increase the fitness cost of efflux pumps driven by proton flux. Other evolution experiments on pH stress reveal surprising fitness tradeoffs, such as the loss of amino acid decarboxylases that are highly induced by acid (10, 14–16).
It was of interest, therefore, to test the fitness effect of long-term exposure to a strong uncoupler, CCCP, that more completely abolishes PMF. One system of interest for CCCP tolerance is EmrAB-TolC. EmrA, EmrB, and TolC form a multidrug efflux pump that exports CCCP and various ionophores and antibiotics (17–19). The emrAB operon is upregulated by MprA (EmrR) (20). MprA binds CCCP and becomes inactivated, allowing higher expression and activity of EmrA and EmrB. It was unknown whether long-term CCCP exposure would select for increased activity of this multidrug efflux pump or its regulators or for loss of this CCCP-responsive system, and perhaps other proton-driven MDR pumps, as was found in the benzoate evolution experiment (10).
Thus, we performed experimental evolution to test the long-term effects of exposure to a full uncoupler, CCCP. We included the factor of external pH in CCCP tolerance by conducting serial dilution of Escherichia coli at pH 6.5 and 8.0 with increasing concentrations of CCCP. After 1,000 bacterial generations, we sequenced the CCCP-evolved isolates and analyzed their mutations.
RESULTS
CCCP-evolved populations show increased relative fitness in the presence of CCCP.
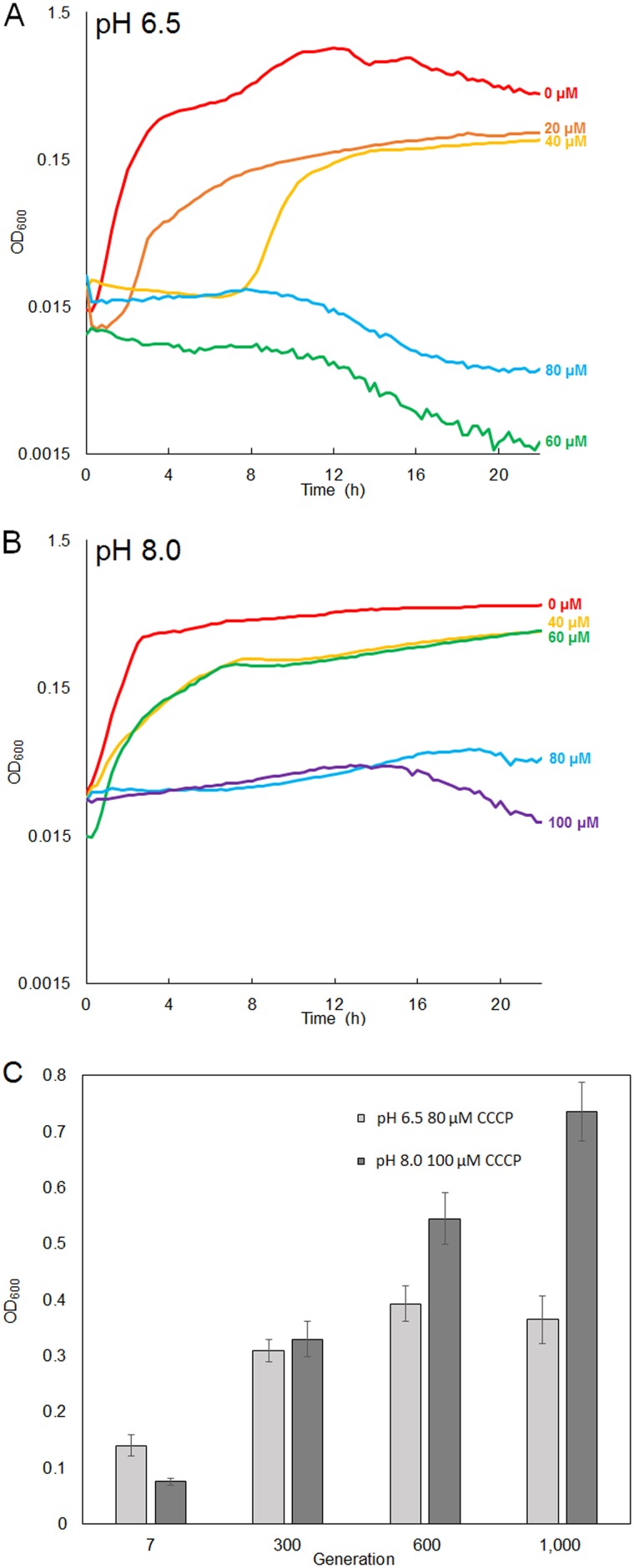
To investigate the selection effects of CCCP on E. coli, populations of strain W3110 were subcultured daily with CCCP in medium buffered at pH 6.5 or at pH 8.0 (Table 1). The initial CCCP concentrations, 20 µM for low pH and 60 µM for high pH, were determined by culturing W3110 in a range of CCCP concentrations at pH 6.5 and at pH 8.0 (Fig. 1A and B). The ancestral strain W3110 failed to grow consistently above 40 µM CCCP (pH 6.5) or above 60 µM CCCP (pH 8.0). Culture densities at 16 h showed that 20 µM CCCP at pH 6.5, and 50 µM CCCP at pH 8.0, resulted in a significant decrease of growth without full loss of viability. Although we had started the populations at pH 8.0 with 60 µM CCCP, we decreased it to 50 µM after 20 generations due to this poor growth. From this point forward, the CCCP concentration was increased in a stepwise fashion, reaching 150 µM CCCP by generation 1000 (Table 1).
TABLE 1.
Generation number at increases in CCCP concentration during evolution of E. coli
| Generation no. | New CCCP concn (µM) for: |
|
|---|---|---|
| pH 6.5 condition | pH 8.0 condition | |
| 0 | 20 | 60 |
| 20 | 20 | 50 |
| 66 | 30 | 60 |
| 299 | 40 | 70 |
| 345 | 50 | 80 |
| 523 | 60 | 90 |
| 536 | 70 | 100 |
| 556 | 80 | 110 |
| 616 | 120 | 130 |
| 782 | 150 | 150 |
FIG 1.
(A and B) Culture densities attained by populations of strain W3110 serially diluted and exposed to increasing CCCP concentrations. Microplate 200-µl populations were diluted in LBK medium containing 100 mM Na-PIPES, pH 6.5 (A), and 100 mM TAPS, pH 8.0 (B). (C) Mean endpoints at t = 16 h for evolving populations cultured with 80 µM CCCP at pH 6.5 (light bars) and with 100 µM CCCP at pH 8.0 (dark bars). At pH 6.5 and 80 µM CCCP, 8 samples were excluded from the generation 10 OD600 data because no growth occurred. Tukey’s test gave a P value of <0.05 for growth of generations 300, 600, and 1000 compared to growth of generation 7 at both pH 6.5 with 80 µM CCCP and pH 8.0 with 100 µM CCCP.
Figure 1C compares the 16-h endpoint culture densities attained by the evolving populations. Aliquots were obtained from frozen plates, starting from the first plate stored and followed by populations frozen at succeeding generations up to 1,000. The pH 8.0 populations showed a steeper increase in fitness than those exposed to CCCP at pH 6.5, where fitness leveled off after 600 generations.
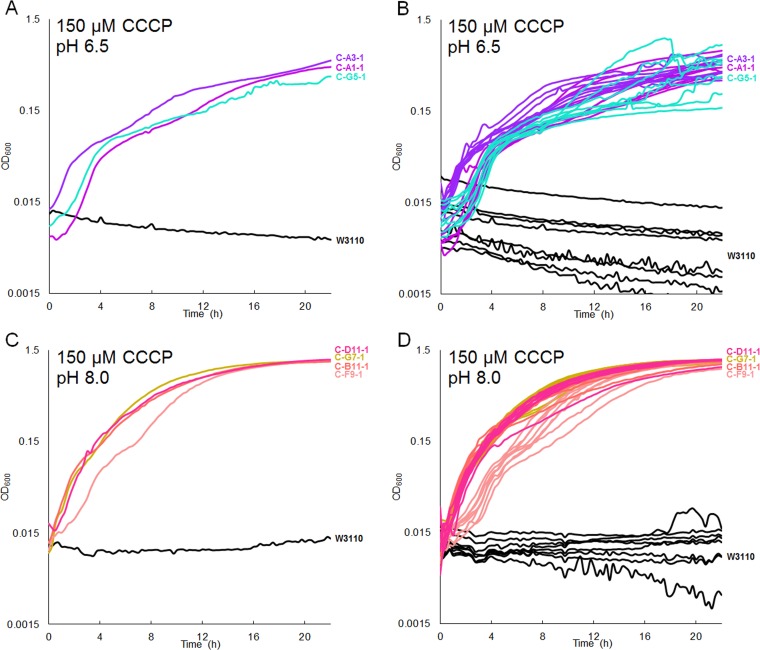
After 1,000 generations, isolates were obtained from selected microplate populations by sequential restreaks. Isolated strains are named by the position on the plate and isolate number; for example, strain C-A1-1 was the first CCCP-evolved strain from the well in row A and column 1. Strain names are listed in Table 2. Figure 2 shows growth curves obtained for isolates from populations following evolution at pH 6.5 (Fig. 2A and B) or at pH 8.0 (Fig. 2C and D). For each isolate, eight replicate curves were obtained. Figure 2A and C show the curve exhibiting median density at 16 h for each strain and condition; Fig. 2B and D show all eight replicate curves. Isolates that had evolved at pH 6.5 (C-A1-1, C-A3-1, and C-G5-1) as well as isolates that had evolved at pH 8.0 (C-B11-1, C-D11-1, C-F9-1, and C-G7-1) showed an increase in tolerance to 150 µM CCCP.
TABLE 2.
Strains generated by experimental evolution or by P1 phage transduction
| Strain | Population isolate | Description or genotype | Generation |
|---|---|---|---|
| W3110 | Escherichia coli K-12 | ||
| JLSC0001 | C-A1-1 | W3110 evolved at pH 6.5 with CCCP | 1000 |
| JLSC0005 | C-E1-1 | W3110 evolved at pH 6.5 with CCCP | 1000 |
| JLSC0009 | C-A3-1 | W3110 evolved at pH 6.5 with CCCP | 1000 |
| JLSC0010 | C-B3-1 | W3110 evolved at pH 6.5 with CCCP | 1000 |
| JLSC0013 | C-E3-1 | W3110 evolved at pH 6.5 with CCCP | 1000 |
| JLSC0016 | C-H3-1 | W3110 evolved at pH 6.5 with CCCP | 1000 |
| JLSC0017 | C-A5-1 | W3110 evolved at pH 6.5 with CCCP | 1000 |
| JLSC0023 | C-G5-1 | W3110 evolved at pH 6.5 with CCCP | 1000 |
| JLSC0024 | C-H5-1 | W3110 evolved at pH 6.5 with CCCP | 1000 |
| JLSC0028 | C-D7-1 | W3110 evolved at pH 8.0 with CCCP | 1000 |
| JLSC0031 | C-G7-1 | W3110 evolved at pH 8.0 with CCCP | 1000 |
| JLSC0033 | C-A9-1 | W3110 evolved at pH 8.0 with CCCP | 1000 |
| JLSC0038 | C-F9-1 | W3110 evolved at pH 8.0 with CCCP | 1000 |
| JLSC0042 | C-B11-1 | W3110 evolved at pH 8.0 with CCCP | 1000 |
| JLSC0044 | C-D11-1 | W3110 evolved at pH 8.0 with CCCP | 1000 |
| JLSC0058 | C-B11-3 | W3110 evolved at pH 8.0 with CCCP | 2000 |
| JLSC0059 | C-B11-4 | W3110 evolved at pH 8.0 with CCCP | 2000 |
| JLS1718 | W3110 ΔmprA::kanR | ||
| JLS1740 | W3110 ΔybiH::kanR | ||
| JLS1809 | W3110 ΔadhE::kanR | ||
| JLS1706 | W3110 ΔemrA::kanR | ||
| JLS1701 | JLSC0001 (C-A1-1) ΔemrA::kanR | ||
| JLS1702 | JLSC0009 (C-A3-1) ΔemrA::kanR | ||
| JLS1703 | JLSC0023 (C-G5-1) ΔemrA::kanR | ||
| JLS1704 | JLSC0042 (C-B11-1) ΔemrA::kanR | ||
| JLS1705 | JLSC0044 (C-D11-1) ΔemrA::kanR | ||
| JLS1733 | JLSC0042 (C-B11-1) rng+ |
FIG 2.
Isolates from CCCP-evolved populations show increased tolerance for 150 µM CCCP. (A and B) Low-pH-evolved strains in medium buffered with 100 mM Na-PIPES, pH 6.5; (C and D) high-pH-evolved strains in medium buffered with 100 mM TAPS, pH 8.0. Panels A and C show representative curves with median density at 16 h; panels B and D show all 8 replicates. For the growth of all low-pH-evolved strains compared to W3110 (A and B), Tukey’s test gave a P value of <0.05. Tukey’s test also gave a significant P value of <0.05 for growth of all high-pH-evolved strains compared to W3110 (C and D).
Genomes of CCCP-evolved strains show independent recurring mutations in common genes.
The genomes of CCCP-evolved strains were resequenced and analyzed using the computational pipeline breseq (see Materials and Methods) to characterize mutations acquired over the course of the evolution (Table 3 shows selected mutations; see Table S1 in the supplemental material for all mutations). Isolate C-B3-1 behaved as a mutator, showing an approximately 20-fold-higher mutation frequency than the other strains; this strain contained a mutation to mutS (see Table S2 in the supplemental material). Isolates C-E1-1 and C-A1-1 were genetically highly similar, indicating that the strains were nearly clones. This high degree of similarity may have occurred via inadvertent transfer during in the subculturing process. For these reasons, isolates C-B3-1 and C-E1-1 were excluded from further study.
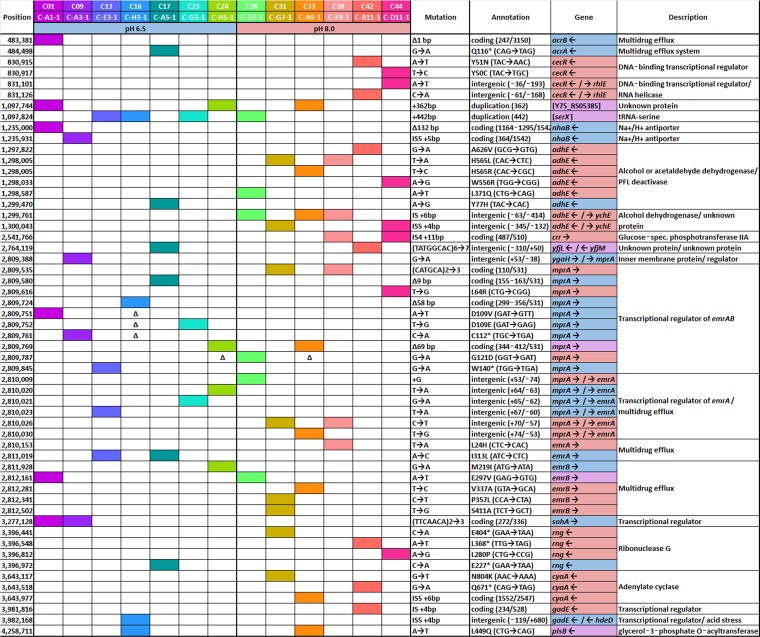
TABLE 3.
Selected mutations after evolution with CCCP at pH 6.5 or at pH 8.0a
Selected genes are listed, including those for which a mutation occurred in more than one independently evolved population. The full list of mutations found is in Table S1 in the supplemental material. The presence of a Δ indicates that these bases were covered by a deleted sequence.
For seven CCCP-evolved populations at pH 6.5 and six at pH 8.0, all mutations predicted by breseq are shown in Table S1. Selected mutations are shown in Table 3, including all genes that showed mutations in more than one population and all mutations in the emrAB operon or its repressor gene mprA. Table 3 is organized by condition, with mutations found in populations cultured at pH 6.5 shaded blue and mutations found at high pH shaded red. Mutations that occurred in both the low-pH and high-pH projects are shaded purple.
All populations that evolved with CCCP at pH 6.5, and five out of six that evolved at pH 8.0, showed mutations in mprA (emrR), which encodes the repressor of emrAB (Table 3) (17, 21). Of the 12 strains with mutations in mprA, there were a total of 10 unique mutations to the coding region of MprA and 6 mutations to the intergenic region between mprA and emrA. Two mutations caused early stop codons in MprA, and two other mutations caused deletions of 50 bp or greater (Table 3). These early stop codons and large deletions are likely to knock out the function of MprA. MprA represses the emrAB operon, which encodes a multidrug efflux pump that exports CCCP from the cell (18, 19, 22). Thus, mprA repressor knockouts could be associated with increased production of the EmrAB multidrug efflux pump and could mediate the increased CCCP tolerance in the CCCP-evolved strains. Nine of the thirteen strains showed additional mutations in emrA or emrB or in the intergenic region between mprA and emrA, which includes promoter control sequences (Tables 3 and S1).
No knockout insertions or deletions were seen for emrAB. All mutations in emrA or emrB consist of base substitutions. These mutations are less likely to knock out the function of the protein; rather, they are more likely to modify the function of the efflux pump. Since all these mutations are found in strains containing an mprA knockout, they might be selected for because they correct for negative effects of emrAB overexpression.
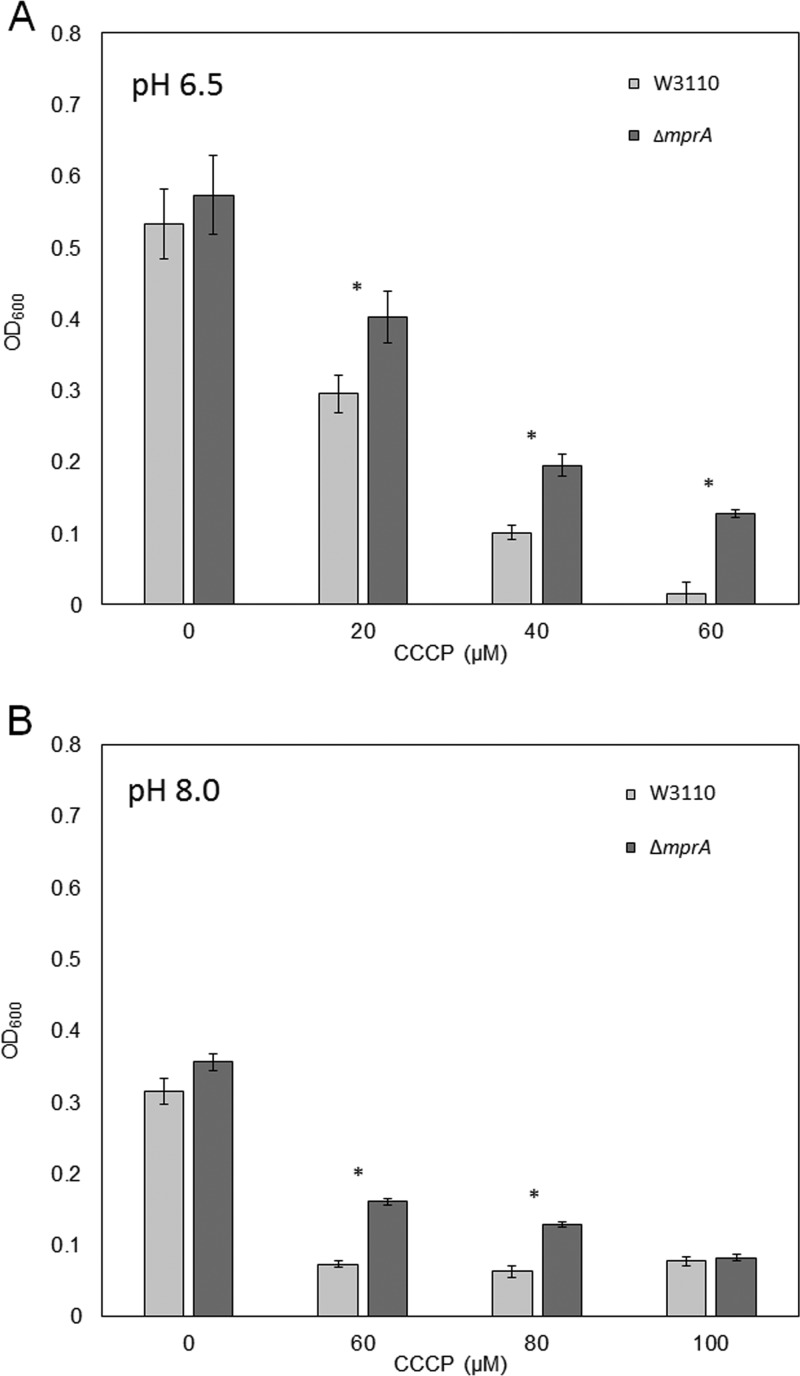
Deletion of mprA enhances growth in CCCP.
Given the fitness selection for mutations in mprA, we tested the role of MprA in CCCP tolerance by deleting mprA from the ancestral strain E. coli W3110. Both at pH 6.5 and at pH 8.0, the ΔmprA::kanR deletant grew at a higher concentration of CCCP than the ancestral strain W3110 (Fig. 3). In addition, loss of MprA caused no growth difference in the absence of CCCP. Thus, the growth improvements due to ΔmprA::kanR are associated with CCCP, probably by efflux via the EmrAB complex. The increased CCCP tolerance of the ΔmprA::kanR strain is consistent with the hypothesis that loss of MprA repressor function in CCCP-evolved isolates increases fitness in the presence of CCCP.
FIG 3.
ΔmprA increases resistance of W3110 to CCCP at both low and high pH. W3110 ΔmprA::kanR (dark gray) and W3110 (light gray) were grown with increasing concentrations of CCCP in LBK with 100 mM Na-PIPES, pH 6.5 (A), and LBK with 100 mM TAPS, pH 8.0 (B). *, significant Tukey’s test P value of <0.05.
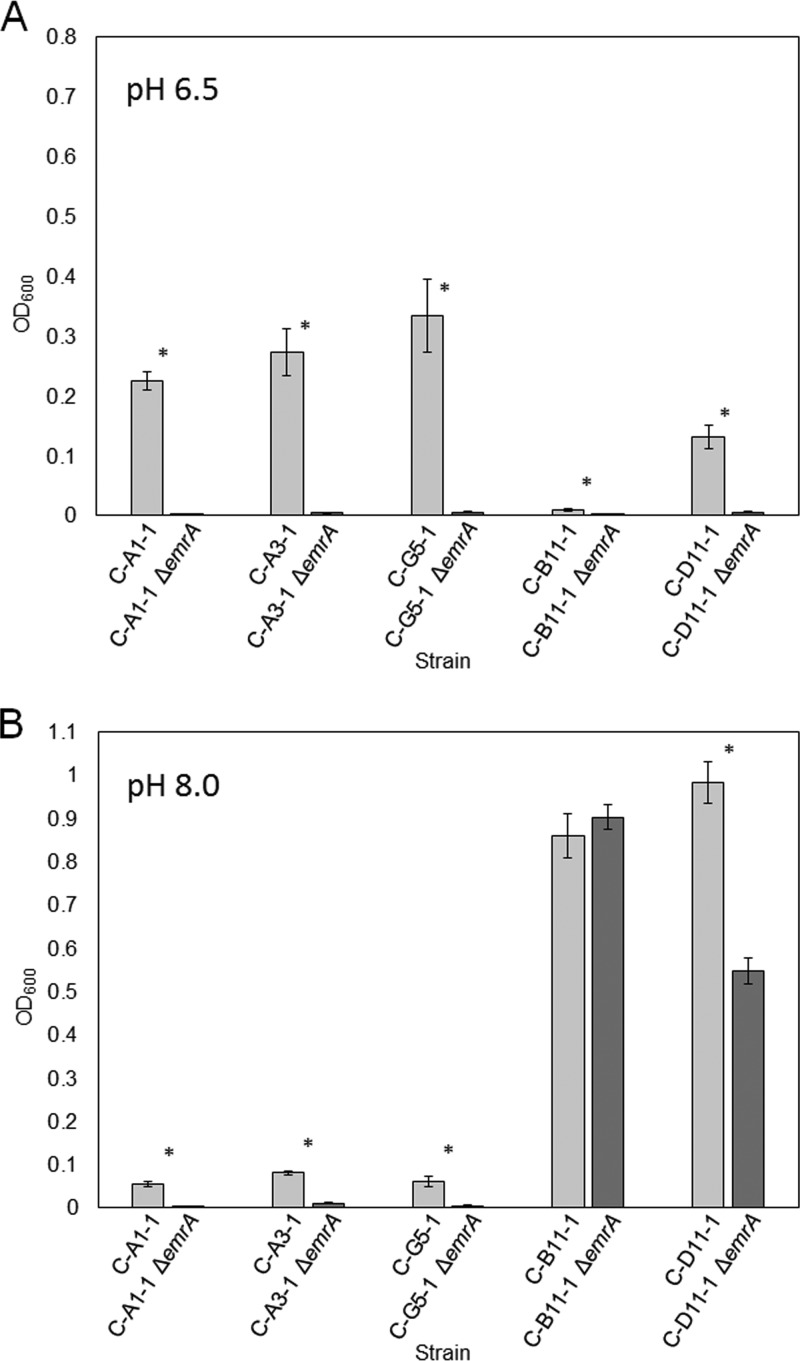
Deletion of emrA in CCCP-evolved strains decreases CCCP fitness.
Three of the CCCP-evolved strains (C-E3-1, C-A5-1, and C-F9-1) showed missense mutations in emrA. We therefore tested whether emrA activity was required for these and other CCCP-evolved strains. Deletion of emrA (by transduction of ΔemrA::kanR) in strains C-A1-1, C-A3-1, C-G5-1, and C-D11-1 substantially decreased growth compared to that of the strains with emrA intact, both at pH 6.5 and at pH 8.0 with CCCP (Fig. 4). Thus, emrA appears to be required for the CCCP fitness advantage of these evolved strains.
FIG 4.
ΔemrA decreases growth of CCCP-evolved strains in the presence of 150 µM CCCP. The growth of C-A1-1, C-A3-1, C-G5-1, C-B11-1, and C-D11-1 (light gray) with 150 µM CCCP in LBK (pH 6.5) with 100 mM Na-PIPES (A) and LBK (pH 8.0) with 100 mM TAPS (B) is compared to the growth of C-A1-1 ΔemrA::kanR, C-A3-1 ΔemrA::kanR, C-G5-1 ΔemrA::kanR, C-B11-1 ΔemrA::kanR, and C-D11-1 ΔemrA::kanR (dark gray). *, significant Tukey’s test P value of <0.05.
The one CCCP-evolved strain that lacked any emrA or mprA mutation (strain C-B11-1) showed a fitness advantage that was independent of emrA, during culture at pH 8.0 (Fig. 4B). Another strain, C-D11-1, also showed partial CCCP tolerance at pH 8.0 with emrA deleted. However, at pH 6.5, where the uncoupler effect of CCCP is greatest, all strains, including C-B11-1 required emrA for growth.
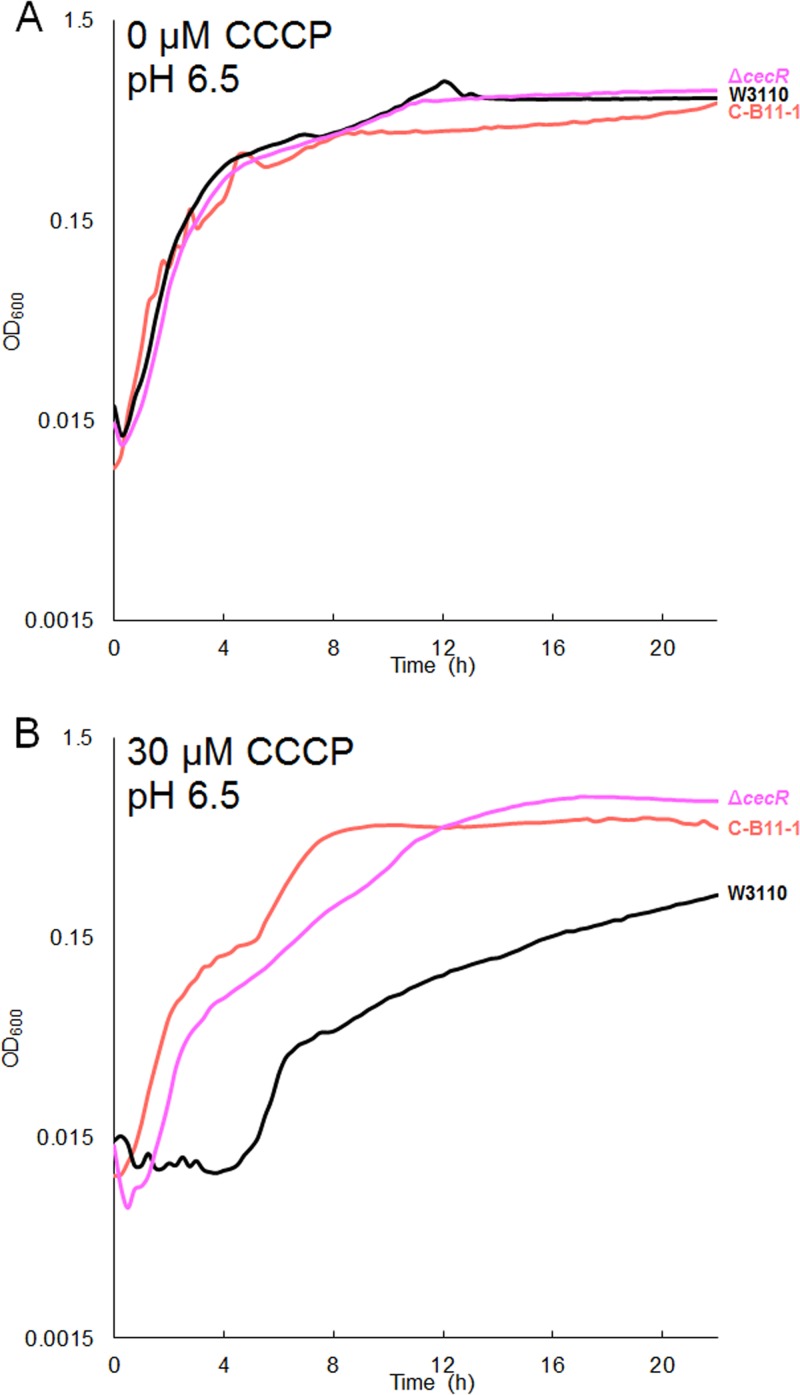
Mutations affecting cecR in strain C-B11-1 increase CCCP tolerance.
The CCCP-evolved isolate C-B11-1 contains no mutations in mprA or emrAB, so we sought to identify other major components of CCCP tolerance in this strain. C-B11-1 notably sustained an insH-mediated deletion of nearly 20 kb which includes phoE, proA and proB, perR, pepD, gpt, frsA, and crl (Table S1). In addition to this large deletion, C-B11-1 also showed a mutation to cecR (ybiH) and a mutation in the intergenic region between cecR and rhlE. Mutations to this region were also present in another high-pH- and CCCP-evolved strain, C-D11-1. CecR regulates genes that affect sensitivity to cefoperazone and chloramphenicol (23). We found that W3110 ΔcecR::kanR showed increased CCCP tolerance at pH 6.5 (Fig. 5). In the presence of 30 µM CCCP, the cecR deletion strain reached a cell density comparable to that of isolate C-B11-1, whereas the ancestral strain W3110 grew significantly less. Thus, a cecR defect could contribute a major part of the CCCP fitness advantage for C-B11-1 and for C-D11-1 (Fig. 4B).
FIG 5.
W3110 ΔcecR and C-B11-1 show enhanced growth in CCCP. Growth of C-B11-1, W3110 ΔcecR::kanR, and W3110 in 100 mM Na-PIPES, pH 6.5, with 0 µM CCCP (A) and with 30 µM CCCP (B) is shown. At 30 µM CCCP, both W3110 ΔcecR::kanR and C-B11-1 grew significantly more than W3110. ANOVA with Tukey’s test, F = 0.135 and P = 0.875 (A) and F = 93.63 and P = 3.45e−11 (B) (n = 8 for each strain).
Multidrug components gadE, acrA, and acrB had mutations in CCCP-evolved strains.
Strain C-B11-1 showed an additional mutation of interest for drug efflux, in gadE (24, 25). Also, an intergenic mutation near gadE was found in strain C-H3-1 (Table 3). GadE activates expression of the Gad acid fitness island, which includes drug efflux genes mdtFE (26). The MdtFE multidrug efflux pump is not known to transport CCCP. Negative mutation of gadE would decrease expression of mdtFE, an effect seen in evolution under benzoate stress (10).
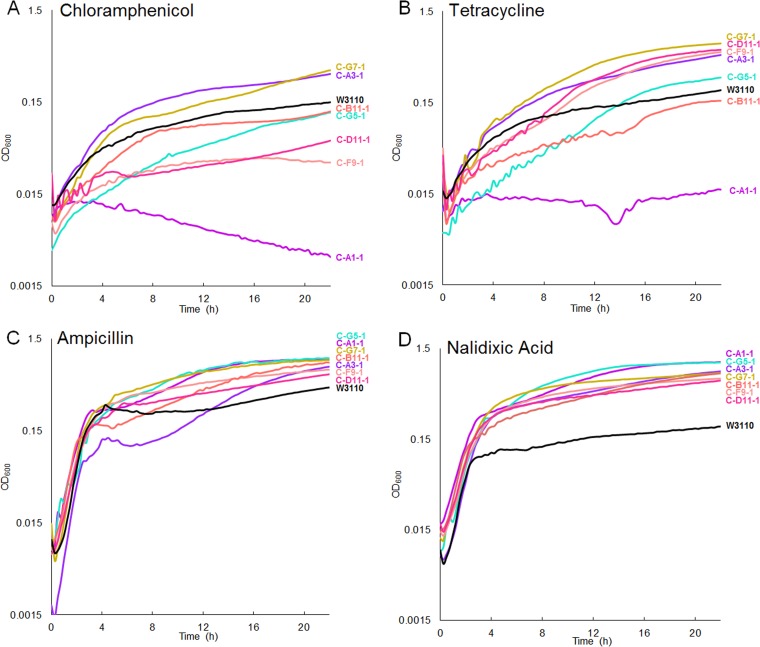
Strains C-A1-1 and C-A5-1 had mutations in acrB and acrA, respectively (27, 28); these are components of the major multidrug efflux pump AcrAB-TolC that confers resistance to chloramphenicol and tetracycline. C-A1-1 showed resistance to chloramphenicol (Fig. 6A) and tetracycline (Fig. 6B), indicating knockout of acrB function. We tested whether ΔacrB::kanR confers resistance to CCCP in the W3110 background, but we did not see an effect.
FIG 6.
Growth of CCCP-evolved strains compared to the ancestor in LBK with 100 mM MOPS (pH 7.0) and 4 µg/ml chloramphenicol (A), 1 µg/ml tetracycline (B), 1 µg/ml ampicillin (C), or 6 µg/ml nalidixic acid (D). Curves shown are representative of the 8 replicates for each strain. Endpoint OD600 data at 16 h as a measure of culture growth are shown. For chloramphenicol, growth of strains C-A1-1, C-D11-1, and C-F9-1 compared to W3110 gave a significant Tukey’s test P value of <0.05. For tetracycline, all strains except C-B11-1 and C-G5-1 had growth differing significantly from that of W3110 (P < 0.05). For ampicillin and nalidixic acid, growth of all evolved isolates compared to that of W3110 gave a Tukey’s test P value of <0.05.
Several additional genes that showed mutations in CCCP-evolved strains were tested for effects on CCCP tolerance. Four strains that evolved with CCCP at pH 8.0, including strain C-B11-1, showed unique mutations in rng, encoding RNase G (29). A C-B11-1 rng+ construct made by recombineering showed no difference in CCCP tolerance compared to the parental strain C-B11-1, cultured in 150 µM CCCP at pH 8.0. CCCP-evolved strains also showed multiple mutations in genes cyaA (adenylate cyclase) and nhaB (sodium-proton antiporter). For these genes, deletions were tested in the ancestral strain background: W3110 ΔcyaA (50 µM CCCP, pH 6.5) and W3110 ΔnhaB (0 to 50 µM CCCP, pH 7.0). None of these constructs showed significant difference in growth compared to strain W3110, but small fitness increments would require competition assays to be revealed.
Mutations in 2,000-generation isolates from population C-B11.
Because C-B11-1 uniquely contained no mprA or emrAB mutations and contained a mutation to gadE, we decided to sequence two later isolates from the C-B11 population (C-B11-3 and C-B11-4) after a total of 2,000 generations (see Table S3 in the supplemental material). Cultures of the later isolates showed growth curves comparable to those for C-B11-1. These later isolates retained most of the mutations found in C-B11-1, including the nearly 20-kb deletion of 28 genes that was unique to population C-B11. At between 1,000 and 2,000 generations, the C-B11 strains acquired additional mutations affecting genes ybhR, lptD, tsx, gltA, cfa, mgrB, pykA, cpsB, ydhN, and yjjU. Gene ybhR encodes a subunit of the cefoperazone efflux complex regulated by CecR (23). This result is thus consistent with the enhanced fitness shown by the cecR mutation found in the C-B11 lineage. The mgrB gene is acid inducible, via the PhoPQ response (30), and shows mutations selected after high-pH evolution (16). The gene lptD forms part of the lipopolysaccharide (LPS) transport slide, a hydrophobic intermembrane hole for the transport of LPS to the surface of the bacterium, (31), which could be relevant for CCCP-membrane interactions.
CCCP-evolved strains show altered resistance to antibiotics.
Exposure to the partial uncoupler benzoate selects for strains sensitive to chloramphenicol and tetracycline (10). We therefore tested the growth of the CCCP-evolved strains in the presence of various antibiotics. Strain C-A1-1 (which contains an acrB mutation) was very sensitive to chloramphenicol and tetracycline (Fig. 6A and B), similar to the result seen for benzoate-evolved strains (10). In addition to the acrB mutation, strain C-A1-1 also had mutations in sohA and in rpoB that resemble the pattern of mutations selected in the benzoate-evolved strains (10). The strain did not, however, show loss of resistance to ampicillin (Fig. 6C), another substrate of AcrAB-TolC (32). Other CCCP-evolved isolates showed marginal degrees of gain or loss of resistance to chloramphenicol, tetracycline, and ampicillin (Fig. 6).
Nalidixic acid is a target of the EmrAB-TolC multidrug efflux pump (18), so mutations increasing expression of the function of this pump might increase resistance to the substrate antibiotics. In the presence of nalidixic acid, all CCCP-evolved strains grew to higher levels than the ancestor (Fig. 6D). Strain C-B11-1 does not contain mutations known to affect EmrAB, but it contains a mutation affecting CecR, which may increase resistance to nalidixic acid (23).
Evolved strains show small pH growth effects independent of CCCP.
Serial culture at low or high pH is known to shift the growth range (14, 16). Thus, we tested the effect of pH alone during CCCP evolution at low or high pH. The CCCP-evolved strains showed marginal changes in pH dependence, as represented by culture density at 16 h. Strains evolved at pH 6.5 showed growth indistinguishable from that of the ancestral W3110 at pH 6.5 (Fig. 7A). At pH 8.0, we saw a small significant increase in density during stationary phase (Fig. 7B). Strains evolved at pH 8.0 consistently showed a small increase in stationary-phase growth at either pH 6.5 (Fig. 7C) or pH 8.0 (Fig. 7D).
FIG 7.
OD600 values for CCCP-evolved strains cultured over 22 h at pH 6.5 or at pH 8.0. Low-pH-evolved strains were cultured with 100 mM K-PIPES, pH 6.5 (A), and with 100 mM TAPS, pH 8.0 (B). High-pH-CCCP-evolved strains were cultured with 100 mM K-PIPES pH 6.5 (C), and with 100 mM TAPS, pH 8.0 (D). Each curve represents the median 16-h culture density of eight replicates. At pH 6.5, growth of C-A1-1 and C-H3-1 is significantly different from growth of W3110 (Tukey’s test, P < 0.05) (A). At pH 8.0, the W3110 16-h culture density differs significantly from those of all strains but C-H5-1 (P < 0.05; ANOVA with Tukey post hoc test, F = 34.698, P < 0.001; n = 8 for each strain) (B). At both pH 6.5 (C) and pH 8.0 (D), growth of all high-pH-evolved strains is significantly different from growth of the W3110 ancestor (Tukey’s test, P < 0.05).
In order to test whether these pH-dependent improvements in growth reflect some other factor associated with the stress of growing in a microplate, we conducted a growth curve with aeration in baffled flasks. We selected two evolved strains from the low-pH and high-pH conditions and measured their growth compared to that of the ancestor at pH 6.5 [100 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES)] and pH 8.0 {100 mM [tris(hydroxymethyl)methylamino]propanesulfonic acid (TAPS)}, respectively, over the course of 8 h using a growth assay conducted under highly aerobic conditions. We saw no significant growth differences between ancestral and evolved populations (data not shown).
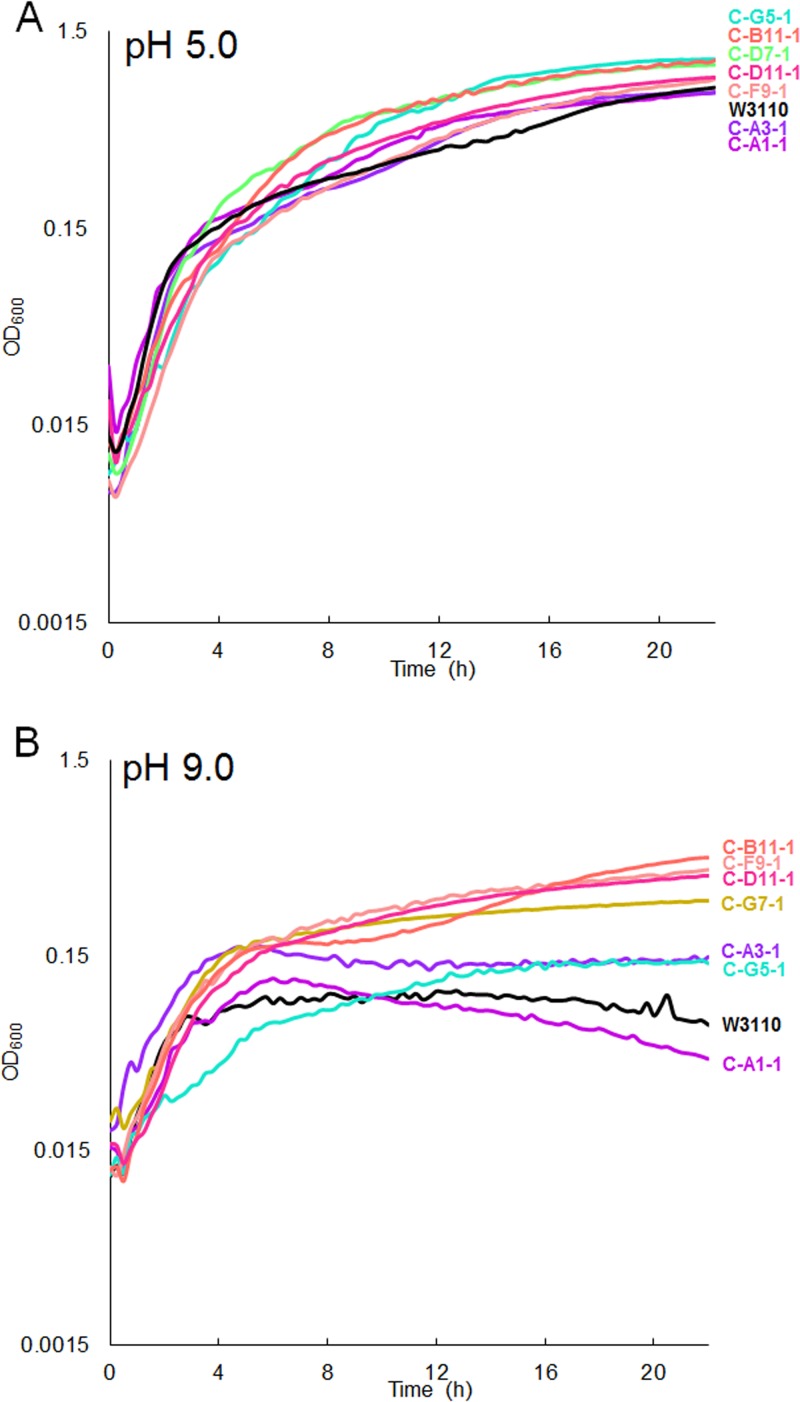
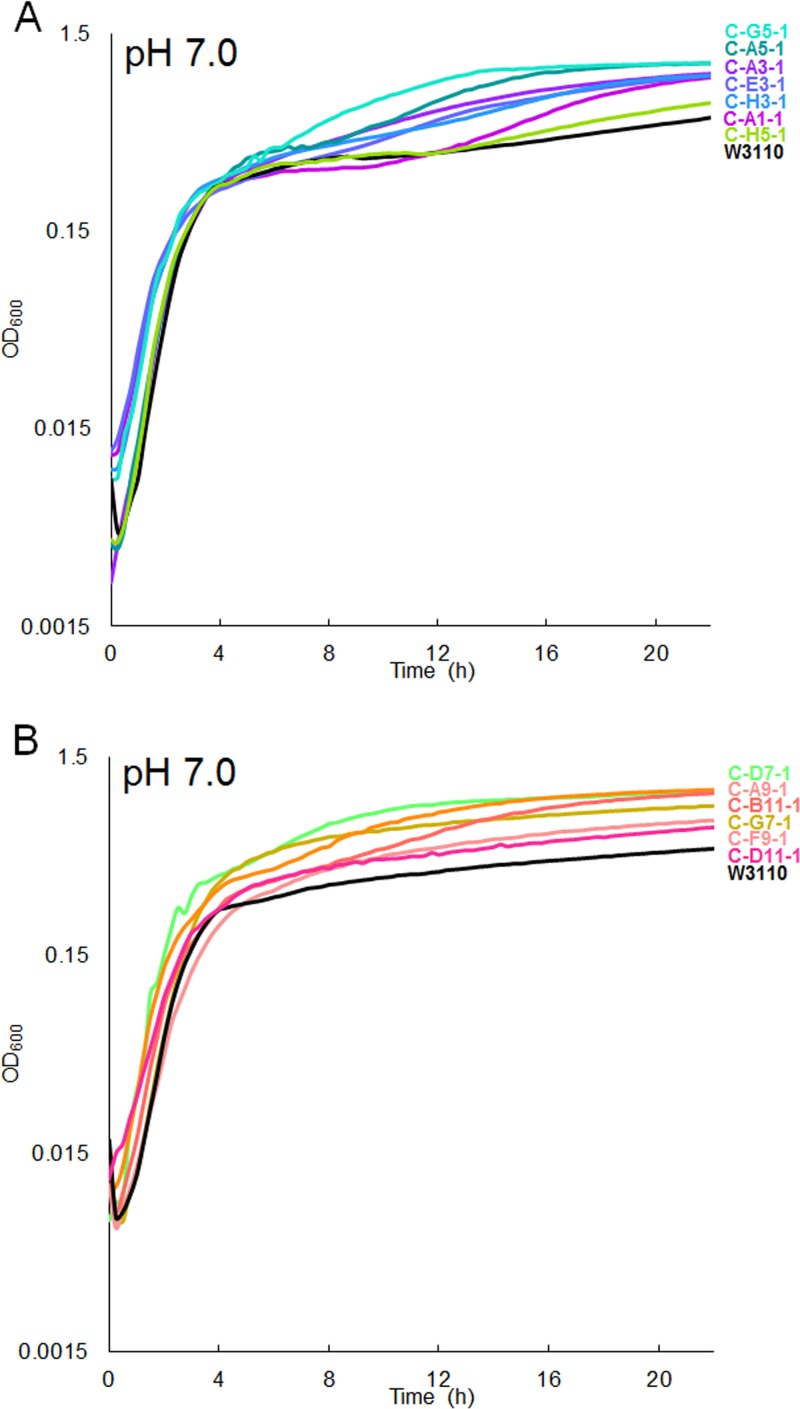
To determine whether fitness differences occur under more extreme acidic and basic conditions, growth of CCCP-evolved strains was tested at pH 5.0 (100 mM morpholineethanesulfonic acid [MES]) (Fig. 8A) and pH 9.0 [100 mM N-(1,1-dimethyl-2-hydroxyethyl)-3-amino-2-hydroxypropanesulfonic acid (AMPSO)] (Fig. 8B). Tests at pH 5.0 showed small significant differences. At pH 9.0, the pH 8.0-evolved strains (C-B11-1, C-D11-1, C-F9-1, and C-G7-1) grew to a higher density than the ancestor; these results show some shifting of growth range in favor of the pH during serial culture. The pH 6.5-evolved strains (C-A1-1, C-A3-1, and C-G5-1) showed smaller, mixed effects (Fig. 8B). At pH 7.0, most of the CCCP-evolved strains showed marginal or no differences from the ancestor (Fig. 9).
FIG 8.
OD600 values for CCCP-evolved strains cultured over 22 h at with 100 mM MES, pH 5.0 (A), and with 100 mM AMPSO, pH 9.0 (B). With 100 mM MES (pH 5.0), W3110 differs significantly from all evolved strains except A3-1 (P < 0.05; ANOVA with Tukey post hoc test, F = 23.481, P < 0.001; n = 8 for each strain) (A). With 100 mM AMPSO (pH 9.0), all low-pH-CCCP-evolved strains differ from all high-pH-evolved strains (P < 0.05; ANOVA with Tukey post hoc test, F = 34.9, P = 2e−16; n = 8 for each strain) (B).
FIG 9.
OD600 values for CCCP-evolved strains cultured with 100 mM MOPS, pH 7.0, over 22 h. (A) Strains evolved at low pH; W3110 differs significantly from all strains but H5-1 (P < 0.05; ANOVA with Tukey post hoc test, F = 15.925, P < 0.001; n = 8 for each strain). (B) Strains evolved at high pH; W3110 differs significantly from A9-1 and B11-1 (P < 0.05; ANOVA with Tukey post hoc test, F = 10.721, P < 0.001; n = 8 for each strain).
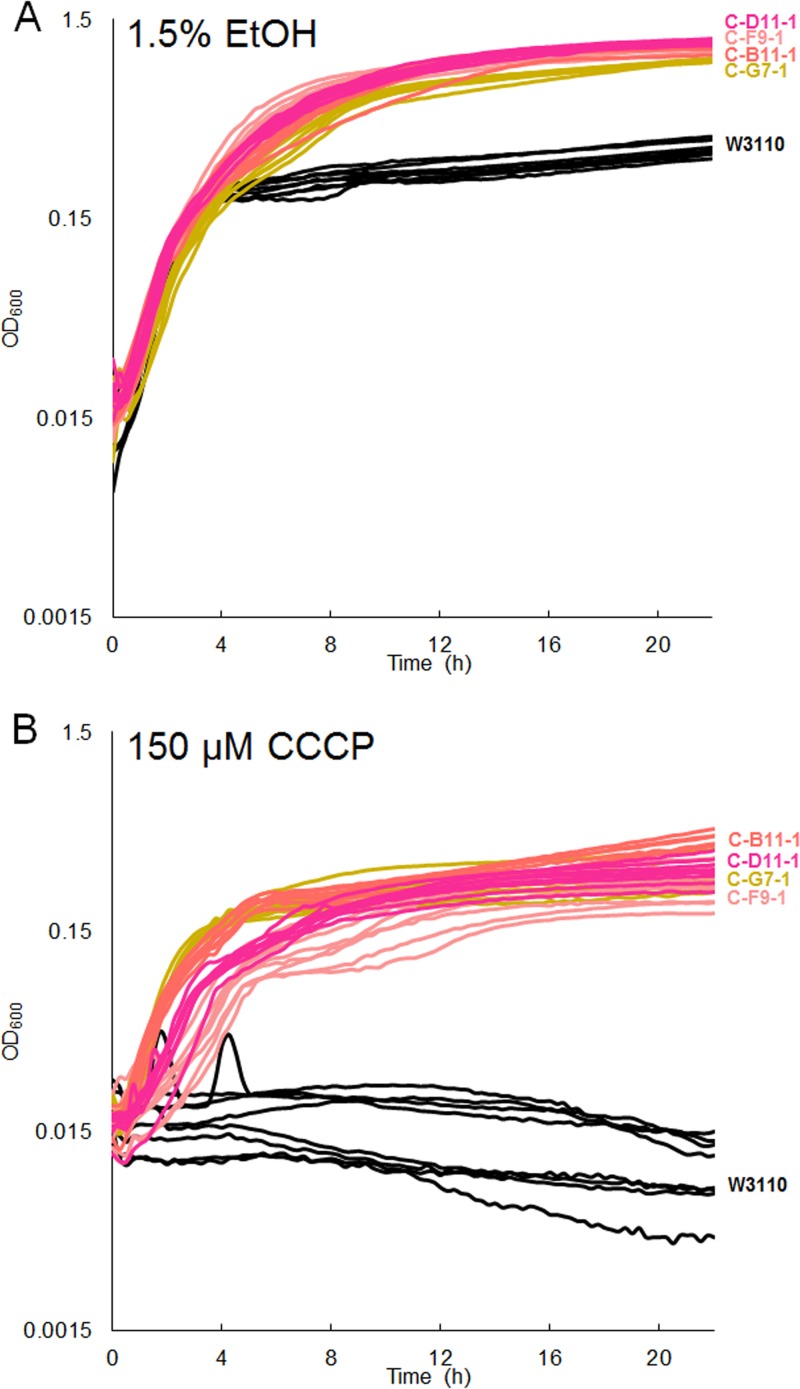
Evolved strains show mutations to adhE.
Mutations occurred in adhE (acetaldehyde coenzyme A [acetaldehyde-CoA] dehydrogenase) in the strains that evolved in CCCP at pH 8.0. The CCCP stock had been dissolved in ethanol, leading to 1.5% ethanol at the highest concentration used in growth media (150 µM CCCP for both pH 8.0 and pH 6.5). We tested the ability of the high-pH-evolved strains to grow in 1.5% ethanol and found that evolved strains grew to a significantly higher optical density (OD) than the ancestor (Fig. 10A). We also tested the CCCP tolerance of our strains using a stock dissolved in dimethyl sulfoxide (DMSO) instead of ethanol. The CCCP fitness advantage remained in the absence of ethanol (Fig. 10B).
FIG 10.
Growth of high-pH-CCCP-evolved strains in LBK with 100 mM TAPS, pH 8.0, and 1.5% ethanol (EtOH) (A) or 150 μM CCCP dissolved in DMSO (B). Growth of all evolved strains in both 1.5% ethanol and 150 µM CCCP differed significantly from growth of W3110 (Tukey’s test, P < 0.05).
DISCUSSION
In our experimental evolution of E. coli with CCCP, all strains but one (C-B11-1) showed mutations to mprA, the repressor of emrAB. The emrAB genes encode a multidrug efflux transport system that has been previously linked to CCCP resistance (17). Deleting the repressor may increase expression or activity of CCCP efflux via EmrAB-TolC (17, 19). The upregulation of EmrAB in our strains is consistent with their increased resistance to nalidixic acid (Fig. 6D), another substrate of the efflux pump (17, 33). The emrAB genes also showed point mutations in many of the strains. These emrA and emrB point mutations probably arose secondary to the mprA mutations that derepress emrAB expression. The appearance of secondary mutations contingent on fitness changes from an earlier mutation is a common feature of experimental evolution (34).
Besides the apparent mprA knockouts, CCCP selected for mutations affecting several drug efflux pumps that are not known to transport CCCP. Strain C-B11-1 (lacking mutations in mprA) attained a comparable fitness increase via mutations in the regulator gadE (activates mtdEF) or cecR (represses ybhG [resistance to chloramphenicol and cefoperazone]). Other strains showed mutations in MDR components acrAB and ybhR. The decreased expression or activity of non-CCCP drug pumps is comparable to our previous results for evolution in the presence of benzoate. Table 4 compiles all the MDR-related mutations we have found to be selected during evolution with CCCP or with benzoate (10). In contrast, microplate experimental evolution under other conditions does not show loss of multiple drug pumps (15, 16). The one exception might be mutations decreasing mdtEF expression in the Gad acid fitness island during evolution in near-extreme acid, but acid response components of Gad could also affect fitness in this case.
TABLE 4.
Multidrug pumps and regulators showing mutations selected by long-term exposure to benzoate or to CCCPa
| Evolution conditions | Mutation | Coding | Gene(s)b | Function related to MDR |
|---|---|---|---|---|
| CCCP, pH 6.5 | Δ1 bp | Coding (nt 247/3150) | acrB ← | MDR efflux (Tet, Chl, Amp, Nal, Rif) |
| G→A | Q116 (CAG→TAG) | acrA ← | MDR efflux (Tet, Chl, Amp, Nal, Rif) | |
| CCCP, pH 8.0 | T→C | I321V (ATT→GTT) | ybhR ← | MDR efflux (Cef) |
| A→T | Y51N (TAC→AAC) | cecR ← | MDR regulator of YbhFSR, YbhG (Cef, Chl) | |
| T→C | Y50C (TAC→TGC) | cecR ← | MDR regulator of YbhFSR, YbhG (Cef, Chl) | |
| A→T | Intergenic (−36/−193) | cecR ← / → rhlE | MDR regulator of YbhFSR, YbhG (Cef, Chl) | |
| C→A | Intergenic (−61/−168) | cecR ← / → rhlE | MDR regulator of YbhFSR, YbhG (Cef, Chl) | |
| Benzoate, pH 6.5 | IS5 (+) | Coding (nt 79–82/267) | ariR (ymgB) → | Acid stress; upregulates Gad regulon (mdtEF) |
| CCCP, pH 6.5 | (C)5→6 | Coding (nt 936/993) | sapD ← | Putrescine efflux |
| Benzoate, pH 6.5 | Δ6,115 bp | Coding inclusive deletion | [ydeA]–[ydeH] | MarRAB multidrug efflux regulon |
| C→A | L191M (CTG→ATG) | mdtA → | MDR efflux (fluoroquinolones, macrolides) | |
| IS5 (+) | Coding (nt 430–433/1539) | emrY ← | MDR efflux (Tet) | |
| IS2 (–) | Coding (635–639/1173) | emrA → | MDR efflux (fluoroquinolones) | |
| CCCP, pH 8.0 | T→C | Intergenic (−91/−59) | cpxP ← / → cpxR | MDR regulator (with CpxA) |
| Benzoate, pH 6.5 | G→A | G92S (GGC→AGC) | cpxA → | Sensory kinase with CpxR |
| G→T | R106L (CGT→CTT) | cpxA → | Sensory kinase with CpxR | |
| A→G | N107S (AAC→AGC) | cpxA → | Sensory kinase with CpxR | |
| Δ14,146 bp | InsH IS5 mediated | gadX–[yhiS] | gadE, gadXW, mdtFE (fluoroquinolones, penams, macrolides) | |
| Δ7,921 bp | InsH IS5 mediated | gadX–gadE | gadE, gadXW, mdtFE (fluoroquinolones, penams, macrolides) | |
| Δ78 bp | Coding (nt 42–119/825) | gadX → | Upregulates mdtFE | |
| G→T | L199F (TTG→TTT) | gadX → | Upregulates mdtFE | |
| IS5 (+) | Coding (nt 626–629/825) | gadX → | Upregulates mdtFE | |
| Δ10,738 bp | InsH mediated | [gadW]–slp | gadX, mdtFE, gadE | |
| CCCP, pH 8.0 | IS | Coding (nt 234/528) | gadE ← | Upregulates mdtEF |
| CCCP, pH 6.5 | IS5 | Intergenic (−119/+680) | gadE ← / ← hdeD | Upregulates mdtEF |
| Benzoate, pH 6.5 | A→G | S34P (TCC→CCC) | rob ← | Upregulates marA regulon |
| CCCP, pH 6.5 | C→T | E94K (GAA→AAA) | arcA ← | Anaerobic; upregulates mdtEF |
| CCCP, pH 8.0 | T→G | T3P (ACC→CCC) | arcA ← | Anaerobic; upregulates mdtEF |
Includes CCCP data from the present paper, as well as benzoate data from reference 10. Abbreviations: nt, nucleotides; Amp, ampicillin; Cef, cefoperazone; Chl, chloramphenicol; Nal, nalidixic acid; Rif, rifampin; Tet, tetracycline.
Arrows next to genes indicate the direction of transcription within the genome; brackets represent only partial deletion of the gene coding sequence.
Overall, our results add to a pattern of selection against drug efflux pumps and their regulators under evolution with an uncoupler or a partial uncoupler (such as benzoate). However, unlike CCCP, benzoate acts at relatively high aqueous concentrations that equilibrate across the membrane. These concentrations cannot be effluxed by multidrug efflux pumps; therefore, adaptation to the presence of benzoate and other permeant acids may be more dependent upon mutations that decrease transmembrane proton flux through unneeded pumps. Organic permeant acids are found in high concentrations in the gut. These organic acids decrease PMF for enteric bacteria in the human microbiome. As such, our findings have implications for the potential application of microbiome evolution in the human intestinal tract. We are following up this work with a more-extended comparison of the relative fitness costs of drug pump genes, using flow cytometry competition assays (35).
MATERIALS AND METHODS
Experimental evolution with CCCP at pH 6.5 and 8.0.
For experimental evolution, E. coli K-12 W3110 populations were cultured at 37°C in a 96-well microplate in modified LBK medium (10 g/liter tryptone, 5 g/liter yeast extract, and 7.45 g/liter KCl) (14). The medium was modified with buffers to compare the effects of CCCP at low pH versus high pH. The pH 6.5 medium contained 100 mM Na-PIPES (0.55 M PIPES buffer and 1.4 M NaOH), and the pH 8.0 medium contained 100 mM TAPS. The initial and subsequent microplates contained 32 evolving populations of 200 µl each. These included 16 populations with 20 µM CCCP buffered at pH 6.5 and 16 populations with 50 µM CCCP buffered at pH 8.0. The well cultures grew to stationary phase and after a total of 22 h were diluted 1:100 daily, undergoing approximately 6.6 generations per day. A multichannel pipettor with filter tips was used for daily transfer of 2 µl culture into 200 µl fresh medium. Over the course of their serial dilutions, the concentration of CCCP was increased, with the pH 6.5 condition increasing from 20 μM to 150 μM and the pH 8.0 condition increasing from 50 μM to 150 μM CCCP (Table 1).
After the populations were cultured for approximately 1,000 generations, clonal isolates were obtained from microplate well populations. Of the populations evolved at pH 6.5, seven isolates from different wells were selected for whole-genome sequencing, and of those at pH 8.0, six isolates were selected for whole-genome sequencing. Clonal isolates were generated by streaking samples three times on LBK plates. Each isolate was given a population designation: “C” for CCCP evolution, the alphanumeric position in the well plate, and a digit for the isolate number from the given well population.
Whole-genome sequencing and sequence analysis.
The genomes of fourteen isolates from generation 1000 and the ancestor were sequenced (see Table S1 in the supplemental material). DNA extraction was performed using the Epicentre Masterpure purification kit. The genomes were validated and quantitated using the Illumina TruSeq Nano DNA library preparation kit by the Research and Technology Support Facility at Michigan State University. Sequencing was then performed utilizing the Illumina MiSeq platform in 2- by 300-bp format, resulting in 20 to 25 million read pairs. Read alignment and mutation predictions were performed using the computational pipeline breseq (v.0.28.1, v.0.30.0, and v.0.30.2) with default parameters (36, 37). The reads were mapped to the E. coli K-12 W3110 reference (accession no. NC_007779.1). Mutations found in both the ancestor and evolved strains were excluded from analysis.
Knockouts in genes of interest.
To determine the fitness impact of specific genes on growth in CCCP, certain genes were deleted from both the ancestor and evolved strains. Donor E. coli strains containing kanR insertion knockouts were obtained from the Keio collection (38). Deletion strains with the W3110 background were constructed by P1 phage transduction (10). Knockout constructs were confirmed by PCR amplification of the kanR insert and were checked against the recipient and donor strains using X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) for lacZ+ (W3110 background). Colony PCR was performed using Lucigen CloneID colony PCR master mix to determine whether the kanR cassette had been inserted into the correct gene and that the recipient strain was as expected. Primers included gene of interest forward direction, gene of interest reverse direction, internal kanamycin (KAN) gene forward, internal KAN gene reverse, and primer KT for within the KAN marker.
Recombineering.
In order to generate strain C-B11-1 rng+, a section of gene rng containing the nonsense mutation in CCCP-evolved isolate C-B11-1 was replaced with the wild-type sequence using recombineering (39, 40). A counterselectable cat-sacB cassette was amplified from colonies of E. coli strain XLT241 using primers with 5′ homology to regions surrounding the rng mutation site in C-B11-1 (5′-CAGCGCCGAAAAACCATTAACGCTGGTTTTCACCCGGTCTTGTGACGGAAGATCACTTCGCAGAATA-3′ and 5′-ATAATGAAGATCACCGCCGCCGAGTGCTGCACTCGCTGGAGCAATCAAAGGGAAAACTGTCCATAT-3′). Isolate C-B11-1 transformed with the heat-inducible pSIM6::ampR plasmid was cultured to mid-log phase at 32°C and then transferred to 42°C to induce recombineering proteins. Cells were then made electrocompetent and electroporated with the cat-sacB PCR product containing edges homologous to regions flanking the rng mutation site in B11-1. The cells were grown out at 32°C for 3 to 5 h and plated on LB with 10 µg/ml chloramphenicol. Colonies were then screened for chloramphenicol resistance and sucrose sensitivity. To replace the cat-sacB cassette with the wild-type rng sequence, successful colonies were made electrocompetent and electroporated with a DNA oligonucleotide containing the wild-type sequence of the region replaced by cat-sacB as well as homology to the flanking regions (5′-CCATTAACGCTGGTTTTCACCCGGTCTTTGCTCAACGCCTGCTCCAGCGAGTGCAGCACTCGGCGGCGGTG-3′). The cells were then plated on LB lacking NaCl and containing 6% sucrose. Colonies were further screened for resistance to sucrose and chloramphenicol sensitivity. The rng sequence of the new construct was then confirmed by PCR sequencing (5′-GCATGGTGGACACGAACAAT-3′ and 5′-CGCTGGAACGCAAAGTAGAA-3′).
Growth assays.
Batch culture growth was assayed under semiaerobic conditions based on previous procedures (10, 15, 16). A 96-well microplate was filled with the desired media and inoculated at a concentration of 1:200 from an overnight culture in each test. Eight replicates from two independent overnight cultures (4 each) were performed for each strain within a single plate. Microplates were placed in the SpectraMax 384 spectrophotometer and cultured for 22 h at 37°C, with the OD at 600 nm (OD600) measured every 15 min. Each entire set of growth curves (including 8 replicates for each strain under comparison) was run in triplicate.
Growth curve determinations for antibiotic tests were conducted by inoculation from overnight cultures in LBK–100 mM MOPS (morpholinepropanesulfonic acid), pH 7.0. These antibiotic growth curve determinations were conducted in LBK–100 mM MOPS (pH 7.0) supplemented with 1 µg/ml ampicillin, 1 µg/ml tetracycline, 4 µg/ml chloramphenicol, or 6 µg/ml nalidixic acid.
For growth curves under aeration, 125-ml baffled flasks containing 20 ml of LBK (pH 8.0 [100 mM TAPS] or pH 6.5 [100 mM Na-PIPES]) were inoculated 1:200 from overnight cultures. The cultures (three per strain) were incubated in a water bath set to 37°C with rotation at 200 rpm. Aliquots (200 µl) were removed from the baffled flasks and read in a 96-well plate (wavelength, 600 nm) every thirty minutes for 8 h.
Statistics.
Means are reported with standard error of the mean (SEM). Statistics were computed using R packages. Samples were compared using analysis of variance (ANOVA) tests with Tukey post hoc tests.
Accession number(s).
Sequence data have been uploaded in the NCBI Sequence Read Archive (SRA) under accession number SRP157768.
ACKNOWLEDGMENTS
This work was supported by award MCB-1613278 from the National Science Foundation and by summer science funds from Kenyon College.
We thank Ellen Broeren for excellent technical support.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02792-18.
REFERENCES
- 1.Mitchell P. 2011. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim Biophys Acta Bioenerg 1807:1507–1538. doi: 10.1016/j.bbabio.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Kasianowicz J, Benz R, McLaughlin S. 1984. The kinetic mechanism by which CCCP (carbonyl cyanide m-chlorophenylhydrazone) transports protons across membranes. J Membr Biol 82:179–190. doi: 10.1007/BF01868942. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin SG, Dilger JP. 1980. Transport of protons across membranes by weak acids. Physiol Rev 60:825–863. doi: 10.1152/physrev.1980.60.3.825. [DOI] [PubMed] [Google Scholar]
- 4.Gould JM. 1979. Respiration-linked proton transport, changes in external pH, and membrane energization in cells of Escherichia coli. J Bacteriol 138:176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lobritz MA, Belenky P, Porter CBM, Gutierrez A, Yang JH, Schwarz EG, Dwyer DJ, Khalil AS, Collins JJ. 2015. Antibiotic efficacy is linked to bacterial cellular respiration. Proc Natl Acad Sci U S A 112:8173–8180. doi: 10.1073/pnas.1509743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diez-Gonzalez F, Russell JB. 1997. Effects of carbonylcyanide-m-chlorophenylhydrazone (CCCP) and acetate on Escherichia coli O157:H7 and K-12: uncoupling versus anion accumulation. FEMS Microbiol Lett 151:71–76. doi: 10.1111/j.1574-6968.1997.tb10396.x. [DOI] [PubMed] [Google Scholar]
- 7.MacLeod RA, Wisse GA, Stejskal FL. 1988. Sensitivity of some marine bacteria, a moderate halophile, and Escherichia coli to uncouplers at alkaline pH. J Bacteriol 170:4330–4337. doi: 10.1128/jb.170.9.4330-4337.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du D, Wang-Kan X, Neuberger A, van Veen HW, Pos KM, Piddock LJV, Luisi BF. 2018. Multidrug efflux pumps: structure, function and regulation. Nat Rev Microbiol 16:523–539. doi: 10.1038/s41579-018-0048-6. [DOI] [PubMed] [Google Scholar]
- 9.Schuldiner S. 2018. The Escherichia coli effluxome. Res Microbiol 169:357–362. doi: 10.1016/j.resmic.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Creamer KE, Ditmars FS, Basting PJ, Kunka KS, Hamdallah IN, Bush SP, Scott Z, He A, Penix SR, Gonzales AS, Eder EK, Camperchioli DW, Berndt A, Clark MW, Rouhier KA, Slonczewski JL. 2017. Benzoate- and salicylate-tolerant strains of Escherichia coli K-12 lose antibiotic resistance during laboratory evolution. Appl Environ Microbiol 83:e02736-16. doi: 10.1128/AEM.02736-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Z, Shan Y, Pan Q, Gao X, Yan A. 2013. Anaerobic expression of the gadE-mdtEF multidrug efflux operon is primarily regulated by the two-component system ArcBA through antagonizing the H-NS mediated repression. Front Microbiol 4:194. doi: 10.3389/fmicb.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norman C, Howell KA, Millar AH, Whelan JM, Day DA. 2004. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiol 134:492–501. doi: 10.1104/pp.103.031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slonczewski JL, Macnab RM, Alger JR, Castle AM. 1982. Effects of pH and repellent tactic stimuli on protein methylation levels in Escherichia coli. J Bacteriol 152:384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harden MM, He A, Creamer K, Clark MW, Hamdallah I, Martinez KA, Kresslein RL, Bush SP, Slonczewski JL. 2015. Acid-adapted strains of Escherichia coli K-12 obtained by experimental evolution. Appl Environ Microbiol 81:1932–1941. doi: 10.1128/AEM.03494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He A, Penix SR, Basting PJ, Griffith JM, Creamer KE, Camperchioli D, Clark MW, Gonzales AS, Sebastian Chávez EJ, George NS, Bhagwat AA, Slonczewski JL. 2017. Acid evolution of Escherichia coli K-12 eliminates amino acid decarboxylases and reregulates catabolism. Appl Environ Microbiol 83:e00442-17. doi: 10.1128/AEM.00442-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamdallah I, Torok N, Bischof KM, Majdalani N, Chadalavada S, Mdluli N, Creamer KE, Clark M, Holdener C, Basting PJ, Gottesman S, Slonczewski JL. 2018. Experimental evolution of Escherichia coli K-12 at high pH and RpoS induction. Appl Environ Microbiol 84:e00520-18. doi: 10.1128/AEM.00520-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lomovskaya O, Lewis K, Matin A. 1995. EmrR is a negative regulator of the Escherichia coli multidrug resistance pump EmrAB. J Bacteriol 177:2328–2334. doi: 10.1128/jb.177.9.2328-2334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lomovskaya O, Lewis K. 1992. emr, an Escherichia coli locus for multidrug resistance. Proc Natl Acad Sci U S A 89:8938–8942. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikaido H. 1996. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol 178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong A, Gottman A, Park C, Baetens M, Pandza S, Matin A. 2000. The EmrR protein represses the Escherichia coli emrRAB multidrug resistance operon by directly binding to its promoter region. Antimicrob Agents Chemother 44:2905–2907. doi: 10.1128/AAC.44.10.2905-2907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooun A, Tomashek JJ, Lewis K. 1999. Purification and ligand binding of EmrR, a regulator of a multidrug transporter. J Bacteriol 181:5131–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gage DJ, Neidhardt FC. 1993. Adaptation of Escherichia coli to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol. J Bacteriol 175:7105–7108. doi: 10.1128/jb.175.21.7105-7108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamanaka Y, Shimada T, Yamamoto K, Ishihama A. 2016. Transcription factor CecR (YbiH) regulates a set of genes affecting the sensitivity of Escherichia coli against cefoperazone and chloramphenicol. Microbiology 162:1253–1264. doi: 10.1099/mic.0.000292. [DOI] [PubMed] [Google Scholar]
- 24.Hommais F, Krin E, Coppée JY, Lacroix C, Yeramian E, Danchin A, Bertin P. 2004. GadE (YhiE): a novel activator involved in the response to acid environment in Escherichia coli. Microbiology 150:61–72. doi: 10.1099/mic.0.26659-0. [DOI] [PubMed] [Google Scholar]
- 25.Seo SW, Kim D, O’Brien EJ, Szubin R, Palsson BO. 2015. Decoding genome-wide GadEWX-transcriptional regulatory networks reveals multifaceted cellular responses to acid stress in Escherichia coli. Nat Commun 6:7970. doi: 10.1038/ncomms8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Xiao M, Horiyama T, Zhang Y, Li X, Nishino K, Yan A. 2011. The multidrug efflux pump MdtEF protects against nitrosative damage during the anaerobic respiration in Escherichia coli. J Biol Chem 286:26576–26584. doi: 10.1074/jbc.M111.243261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okusu H, Ma D, Nikaido H. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol 178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baucheron S, Tyler S, Boyd D, Mulvey MR, Chaslus-Dancla E, Cloeckaert A. 2004. AcrAB-TolC directs efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium DT104. Antimicrob Agents Chemother 48:3729–3735. doi: 10.1128/AAC.48.10.3729-3735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deana A, Belasco JG. 2004. The function of RNase G in Escherichia coli is constrained by its amino and carboxyl termini. Mol Microbiol 51:1205–1217. doi: 10.1046/j.1365-2958.2003.03905.x. [DOI] [PubMed] [Google Scholar]
- 30.Roggiani M, Yadavalli SS, Goulian M. 2017. Natural variation of a sensor kinase controlling a conserved stress response pathway in Escherichia coli. PLoS Genet 13:1007101. doi: 10.1371/journal.pgen.1007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Gu Y, Dong H, Wang W, Dong C. 2015. Trapped lipopolysaccharide and LptD intermediates reveal lipopolysaccharide translocation steps across the Escherichia coli outer membrane. Sci Rep 5:11883. doi: 10.1038/srep11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim SP, Nikaido H. 2010. Kinetic parameters of efflux of penicillins by the multidrug efflux transporter AcrAB-TolC of Escherichia coli. Antimicrob Agents Chemother 54:1800–1806. doi: 10.1128/AAC.01714-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulavik MC, Houseweart C, Cramer C, Jiwani N, Murgolo N, Greene J, DiDomenico B, Shaw KJ, Miller GH, Hare R, Shimer G. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob Agents Chemother 45:1126–1136. doi: 10.1128/AAC.45.4.1126-1136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blount ZD, Lenski RE, Losos JB. 2018. Contingency and determinism in evolution: replaying life’s tape. Science 362:eaam5979. doi: 10.1126/science.aam5979. [DOI] [PubMed] [Google Scholar]
- 35.Gullberg E, Cao S, Berg OG, Ilbäck C, Sandegren L, Hughes D, Andersson DI. 2011. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog 7:e1002158. doi: 10.1371/journal.ppat.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deatherage DE, Barrick JE. 2014. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol Biol 1151:165–188. doi: 10.1007/978-1-4939-0554-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deatherage DE, Traverse CC, Wolf LN, Barrick JE. 2015. Detecting rare structural variation in evolving microbial populations from new sequence junctions using breseq. Front Genet 5:468. doi: 10.3389/fgene.2014.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharan SK, Thomason LC, Kuznetsov SG, Court DL. 2009. Recombineering: a homologous recombination-based method of genetic engineering. Nat Protoc 4:206–223. doi: 10.1038/nprot.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomason LC, Sawitzke JA, Li X, Costantino N, Court DL. 2014. Recombineering: genetic engineering in bacteria using homologous recombination. Curr Protoc Mol Biol 106:1.16.1–1.16.39. doi: 10.1002/0471142727.mb0116s106. [DOI] [PubMed] [Google Scholar]