Wolbachia strains are common endosymbionts in insects, with multiple strains often coexisting in the same species. The coexistence of multiple strains is poorly understood but may rely on Wolbachia organisms having diverse phenotypic effects on their hosts. As Wolbachia is increasingly being developed as a tool to control disease transmission and suppress pest populations, it is important to understand the ways in which multiple Wolbachia strains persist in natural populations and how these might then be manipulated. We have therefore investigated viral protection and the molecular basis of cytoplasmic incompatibility in two coexisting Wolbachia strains with contrasting effects on host reproduction.
KEYWORDS: Dicistroviridae, Wolbachia, antiviral protection, cytoplasmic incompatibility, male killing, virus blocking
ABSTRACT
Wolbachia infections can present different phenotypes in hosts, including different forms of reproductive manipulation and antiviral protection, which may influence infection dynamics within host populations. In populations of Drosophila pandora two distinct Wolbachia strains coexist, each manipulating host reproduction: strain wPanCI causes cytoplasmic incompatibility (CI), whereas strain wPanMK causes male killing (MK). CI occurs when a Wolbachia-infected male mates with a female not infected with a compatible type of Wolbachia, leading to nonviable offspring. wPanMK can rescue wPanCI-induced CI but is unable to induce CI. The antiviral protection phenotypes provided by the wPanCI and wPanMK infections were characterized; the strains showed differential protection phenotypes, whereby cricket paralysis virus (CrPV)-induced mortality was delayed in flies infected with wPanMK but enhanced in flies infected with wPanCI compared to their respective Wolbachia-cured counterparts. Homologs of the cifA and cifB genes involved in CI identified in wPanMK and wPanCI showed a high degree of conservation; however, the CifB protein in wPanMK is truncated and is likely nonfunctional. The presence of a likely functional CifA in wPanMK and wPanMK’s ability to rescue wPanCI-induced CI are consistent with the recent confirmation of CifA’s involvement in CI rescue, and the absence of a functional CifB protein further supports its involvement as a CI modification factor. Taken together, these findings indicate that wPanCI and wPanMK have different relationships with their hosts in terms of their protective and CI phenotypes. It is therefore likely that different factors influence the prevalence and dynamics of these coinfections in natural Drosophila pandora hosts.
IMPORTANCE Wolbachia strains are common endosymbionts in insects, with multiple strains often coexisting in the same species. The coexistence of multiple strains is poorly understood but may rely on Wolbachia organisms having diverse phenotypic effects on their hosts. As Wolbachia is increasingly being developed as a tool to control disease transmission and suppress pest populations, it is important to understand the ways in which multiple Wolbachia strains persist in natural populations and how these might then be manipulated. We have therefore investigated viral protection and the molecular basis of cytoplasmic incompatibility in two coexisting Wolbachia strains with contrasting effects on host reproduction.
INTRODUCTION
Wolbachia pipientis strains are obligate bacterial endosymbionts that naturally infect insects, including multiple Drosophila species (1–3). Wolbachia is transmitted predominantly maternally (4), with rare horizontal transmission over periods of thousands of years (5–7). The spread and establishment of a stable infection in host populations are advantageous for Wolbachia (8–10). Infection stability is dictated by three factors: transmission efficiency, penetrance of the reproductive manipulation phenotype, and the effects of infection on hosts (6, 11–15). The phenotypic manifestation of these factors can define Wolbachia organisms as both parasitic and mutualistic symbionts (15–17).
Because of its effects on host reproduction, Wolbachia can increase its prevalence within populations even at the expense of host fitness (18–20). Infection frequencies in natural populations reflect the balance of Wolbachia manipulation of host reproduction and fitness costs as well as rates of maternal transmission (18, 21). Reproductive manipulations due to Wolbachia include cytoplasmic incompatibility (CI) (22–24), male killing (MK) (25, 26), parthenogenesis (PI) (3, 27, 28), and feminizing (29, 30), all of which can act to maintain and increase the frequency of Wolbachia in populations even when there is a cost to host fitness.
MK endosymbionts affect transmission by terminating male embryos, which can increase the fitness of the remaining embryos if processes such as sibling competition are operating (reviewed in reference 25). Drosophila-specific Wolbachia strains that induce MK include those found within populations of Drosophila bifasciata, Drosophila innubila, and Drosophila pandora (12, 25, 31–34). CI increases Wolbachia infection frequencies, as progeny from crosses between uninfected females and infected males are inviable (8; reviewed in referneces 10 and 35). The CI phenotype is thought to involve a modification-rescue system where a maternal Wolbachia infection rescues the modification induced by a paternal Wolbachia infection during reproduction (3, 13, 24, 36). CI is observed in crosses between infected males and noninfected females where the paternal infection modifies the resulting zygote, characterized by early mitotic defects and disruption of paternal chromosome processing (37–41) not rescued by a maternal Wolbachia infection, resulting in nonviable zygote development (3, 10). A number of Wolbachia infections in Drosophila cause CI (42–44), particularly those associated with strain wRi infection (3, 45).
Cytoplasmic incompatibility factors A (CifA) and B (CifB) are involved in the CI phenotype (46–48; reviewed in reference 49). These proteins are encoded by syntenic loci within Wolbachia’s WO prophage region, and the genes appear to be codiverging (46, 48, 50, 51) and coevolving (46, 48). As current evidence suggests that CifA functions in both modification and rescue and that CifB functions strictly in modification (46–48), this system follows a two-by-one modification (CifA and CifB)-rescue (CifA) model.
Although Wolbachia is often considered to have fitness costs on hosts, it can also have fitness benefits, increasing the prevalence and stability of Wolbachia infection in host populations (14, 32, 44, 52, 53). Examples of Wolbachia-mediated effects that could create a selective advantage include nutritional provisioning (17, 54), increasing host fecundity (32, 55, 56), and providing protection against host pathogens (53, 57, 58; reviewed in references 59, to ,61). However, beneficial host fitness impacts may clash with other aspects of Wolbachia phenotypes. For instance, the density of Wolbachia often correlates with negative effects on host life history, for example, life span (16, 62, 63), but also correlates with the strength of antivirus protection provided by some (21, 64, 65) but not all (33) strains of Wolbachia.
Several Wolbachia strains with different effects on hosts can cooccur in the same populations. For instance, in Drosophila, MK strains often occur at a low frequency in populations, whereas CI strains may be more common in the same population. Furthermore, the dynamics of Wolbachia infections may change over time; they may initially invade a population using reproductive manipulation strategies, and once reaching an infection threshold, reproductive manipulation may evolve toward neutrality and degrade (44, 66–68).
Drosophila pandora is a newly described member of the Drosophila ananassae species complex (69). Two Wolbachia strains coexist in D. pandora populations. These infections display high maternal transmission and a combined infection frequency approaching 100% in surveyed populations, with strains wPanMK and wPanCI found at low and high incidence, respectively (31). According to multilocus sequence typing system (MLST) analysis (70) the two strains are genetically distinct, with one causing near complete CI (wPanCI) and the other causing MK (wPanMK) (31).
The reproductive manipulation strategies of Wolbachia infecting D. pandora are complex. At high temperatures, wPanMK-infected males emerge. These males do not induce CI; however, wPanMK females are able to rescue the CI induced by wPanCI, as crosses between differentially infected individuals are compatible (31). This suggests that either high temperatures reduce CI penetrance, by reducing Wolbachia density (18, 25, 34, 71; reviewed in references 72 and 73), or wPanMK is able only to rescue and not to induce CI, a phenomenon observed in a subset of Wolbachia strains (13, 66, 74). Investigation of the CI genotypes of wPanCI and wPanMK would give insight into the involvement of recently described cif genes and their potential role in the CI phenotype and indicate whether this has the potential to affect the infection dynamics in D. pandora populations. This builds on genomic data for wPanCI (45) that indicate intact cif genes similar to those from wRi infections of Drosophila.
To help understand the persistence of wPanCI and wPanMK infections in D. pandora, this study investigated the molecular mechanism behind wPanMK’s ability to rescue but not induce CI by comparing CI genotypes in the MK and CI strains. The study also determined whether the provision of protection against host pathogens has the potential to influence observed infection dynamics in host populations by investigating wPanCI- and wPanMK-mediated antiviral protection utilizing Cricket paralysis virus (CrPV), a natural pathogen of Drosophila (75).
RESULTS
Presence of type I cif homologs in wPanCI and wPanMK.
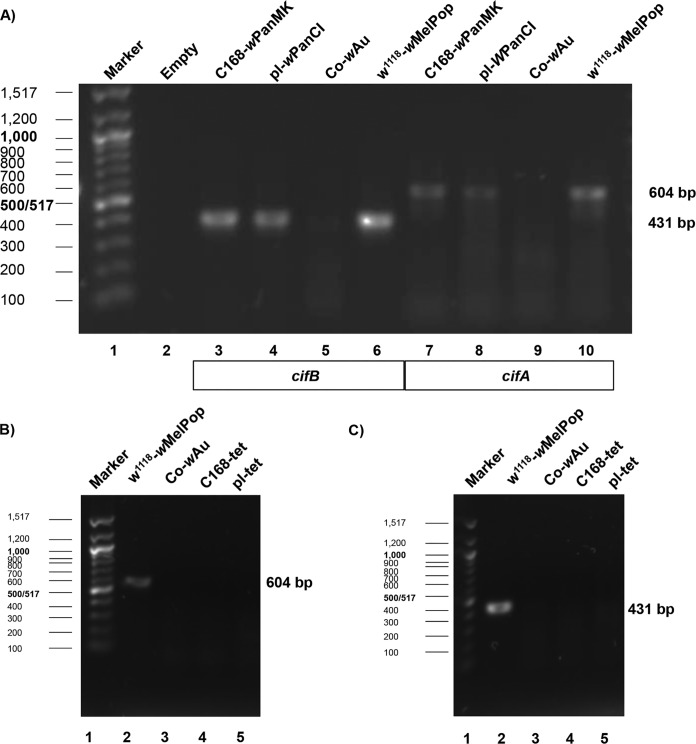
While wPanMK expresses MK, it is able to rescue CI induced by wPanCI, suggesting that it may retain genetic elements of the CI system, cifA and cifB. To determine the presence or absence of the cif genes in both wPanCI and wPanMK, DNA from Co-wAu (negative control), w1118-wMelPop (positive control), pl-wPanCI, and C168-wPanMK flies was analyzed by PCR with primers targeting internal conserved regions of cifA and cifB (Table 1, PCR primers CifA and CifB). PCR products were analyzed by electrophoresis. Bands corresponding to the expected ∼604-bp cifA and ∼431-bp cifB PCR products were identified in both pl-wPanCI and C168-wPanMK samples (Fig. 1A), indicating that at least parts of both the cifA and cifB loci are present. No corresponding bands were identified in pl-Tet or C168-Tet samples, confirming that the cifA and cifB genes were associated with the Wolbachia genomes and not the associated host D. pandora genomes (Fig. 1B and C).
TABLE 1.
Primer sequences used in PCR and qPCR/RT-qPCRa
| Assay | Primer target (reference) | Primer sequence (5′ → 3′) |
Product length (bp) | Efficiency | |
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| PCR | CifA | AAGTGGAGCGAAGGGGTAGA | CAGGAAAGCAACCTTTGGCA | 604 | NA |
| CifB | GTGCAAGTGCCTAATGCTG | TACCTTGCCTCGTCTTGC | 430 | NA | |
| CifA-CifB | TAGCGTTGTTTTCATGATATAGGGA | ACAACCAACGCGTAAATATGGA | 5,086 | NA | |
| qPCR | Act88F (92) | ATCGAGCACGGCATCATCAC | CACGCGCAGCTCGTTGTA | 74 | 1.92 |
| Wsp (65) | GCATTTGGTTATAAAATGGACGA | GGAGTGATAGGCATATCTTCAAT | NA | 1.68 | |
| CrPV | TGCCCCCTTCACAGTAACCT | GGAGTTCCTGTTCCGTCCTG | 150 | 2.00 | |
| CifA | TGGTCCTGTGTTTGATTTCTGGA | TCTACCCCTTCGCTCCACTT | 111 | 2.08 | |
| CifB | ATTGACCTTGACTTGTCTCCTGG | GCTCTAGCGCGGTTTAATGC | 140 | 1.89 | |
NA, not applicable.
FIG 1.
PCR confirmation of the presence of cifA and cifB in wPanCI and wPanMK in Drosophila pandora (A) and PCR confirmation that the cifA (B) and cifB (C) genes are associated with the Wolbachia genomes and not the associated host Drosophila pandora genomes. One percent agarose gel electrophoresis with ethidium bromide staining was used to show DNA fragments amplified by PCR that targeted internal sections of the genes cifA (604 bp) and cifB (431 bp). PCR was conducted on DNA extracted from pools of 5 flies from each fly line. (A) Lanes 3 to 6, PCR products obtained using cifB PCR primers. Lanes 7 to 10, PCR products obtained using cifA PCR primers. Lane 1, 100-bp ladder (NEB); lane 2, empty; lanes 3 and 7, C168-wPanMK; lanes 4 and 8, pl-wPanCI; lanes 5 and 9 Co-wAu (negative control); lanes 6 and 10 w1118-wMelPop (positive control). There are clear bands in lanes 3 and 4 of approximately 431 bp, showing that C168-wPanMK and pl-wPanMK are positive for the presence of the conserved internal section of cifA. There are also bands of approximately 604 bp in lanes 7 and 8, showing that C168-wPanMK and pl-wPanMK are positive for the presence of the conserved internal region of cifB. (B) PCR products obtained using cifA (604-bp) PCR primers. Lane 1, 100-bp ladder (NEB); lane 2, 10 w1118-wMelPop (positive control); lane 3, Co-wAu (negative control); lane 4, C168-Tet; lane 5, pl-Tet. The absence of bands in lanes 4 and 5 shows that cifA is not present in the genome of C168 or pl flies. (C) PCR products obtained using cifB (431-bp) PCR primers. Lane 1, 100-bp ladder (NEB); lane 2, 10 w1118-wMelPop (positive control); lane 3, Co-wAu (negative control); lane 4, C168-Tet; lane 5, pl-Tet. The absence of bands in lanes 4 and 5 shows that cifB is not present in the genome of C168 or pl flies.
Having confirmed the presence of cifA and cifB homologs in wPanCI and wPanMK, gene expression was estimated using reverse transcription-quantitative PCR (RT-qPCR) on total RNA isolated from host ovaries. Both cifA and cifB were expressed in pl-wPanCI ovaries, where cifA expression was 7-fold higher than that of cifB (P = 0.002 by the nonparametric Mann-Whitney test) (Fig. 2), consistent with previous studies on other Wolbachia strains (48). Expression of the cif genes was analyzed in C168-wPanMK across multiple biological replicates; cifA was consistently expressed in all samples analyzed, but cifB expression was detected at low levels in one sample and was below the limit of detection in three of the four biological replicates, and therefore expression levels could not be quantitatively compared (Fig. 2). Taking these results together, we interpret them to show that cifB expression is low in C168-wPanMK in comparison to the expression of cifA.
FIG 2.
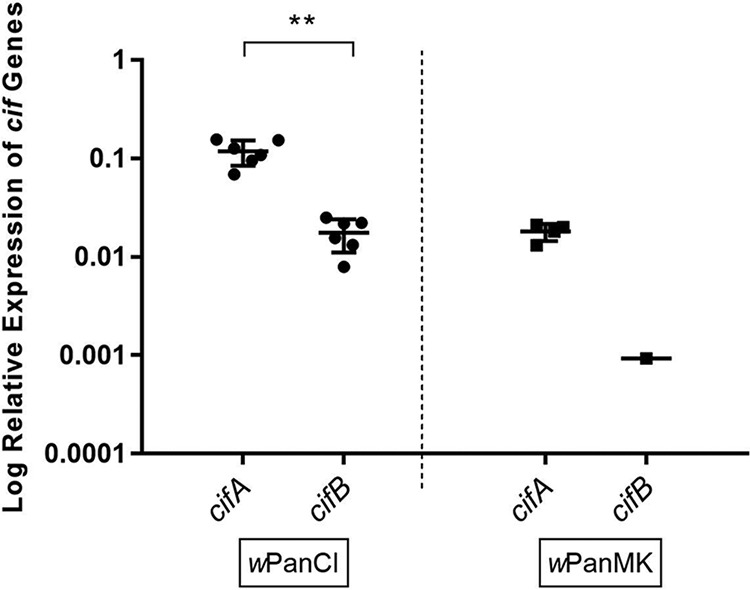
Differential expression of the cifA and cifB genes in wPanCI and wPanMK. Total RNA was extracted from 20 paired ovaries of 11- to 14-day-old female flies from three different cohorts of pl-wPanCI and C168-wPanMK Drosophila pandora lines. Relative RNA levels of the cif genes were measured via RT-qPCR and normalized against the host gene, actin88F. Graphs represent the mean and standard error of the mean (SEM) from 4 to 6 independent replicates. The difference between expression of cifA and cifB in the respective fly lines was analyzed using the nonparametric Mann-Whitney test. There is a significant difference between the expression of cifA and cifB in pl-wPanCI fly ovaries (**, P = 0.0022 by the nonparametric Mann-Whitney test). For only one of four samples was cifB expression in C168-wPanMK flies above detectable levels; expression of cifB therefore could not be statistically compared to that of cifA in C168-wPanMK flies.
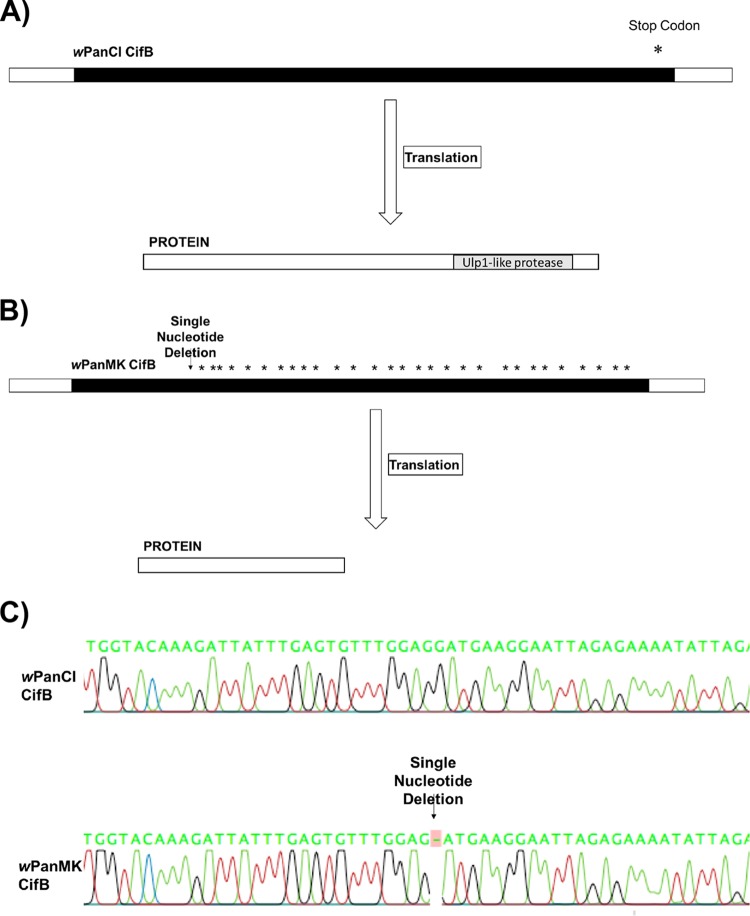
The nucleotide sequences of the cifA and cifB loci in wPanCI and wPanMK were determined, and a pairwise comparison of nucleotide and amino acid sequences was conducted. The intergenic region between cifA and cifB is highly conserved between wPanCI and wPanMK. Both the wPanCI and wPanMK intergenic regions are predicted to include the Rho-independent transcription terminator, as identified previously in wMel (76). Taken together, these results suggest that it is unlikely that the intergenic region is involved in the observed reduced expression of cifB in wPanMK. The pairwise comparison of wPanCI and wPanMK showed that their cifA nucleotide sequences had 98.7% identity and no gaps (Table 2). Similarly, the CifA amino acid sequence had 97.5% identity (Table 2). The cifB nucleotide sequences had 99.2% identity but also included one single-nucleotide deletion (Table 2; Fig. 3A). Therefore, the CifB amino acid sequences of wPanCI and wPanMK had only 44.6% identity, with many gaps (Table 2), consistent with the observed frameshift mutation at position 1213 in the nucleotide sequence (Fig. 3A). The presence of the frameshift was independently confirmed by conducting sequence analysis directly on PCR products from across this region of the gene. The frameshift in the wPanMK cifB homolog introduced a premature stop codon and an additional 44 stop codons within the remainder of the gene, indicating that any translated protein would be truncated and likely nonfunctional (Fig. 3B). Taken together, these results show that while there is a high degree of sequence identity between the cifB genes of wPanCI and wPanMK, this is not reflected in the amino acid sequences of the encoded proteins.
TABLE 2.
Pairwise comparison of the nucleotide and amino acid sequences of the cifA and cifB genes and protein homologs in wPanCI and wPanMKa
| Parameter | Nucleotide sequence |
Amino acid sequence |
||
|---|---|---|---|---|
| cifA (1,425 nt) | cifB (∼3,500 nt) | CifA (475 aa) | CifB (∼1,167 aa) | |
| Gaps | 0 | 1 | 0 | 177 |
| Differences | 18 | 29 | 12 | 695 |
| Identity (%) | 98.7 | 99.2 | 97.5 | 44.6 |
Analysis was conducted in CLC workbench version 7.9.1.
FIG 3.
(A and B) Schematic depiction of the cifB gene (3,501 nucleotides [nt]) and protein (1,167 amino acids [aa]) homologs in wPanCI (A) and of the homologs and deletion in wPanMK (B). The annotated Ulp1-like protease domain has been shown experimentally to be involved in modifications induced by cifB (41). (A) The nucleotide sequence contains nucleotide substitutions (not shown) but no frameshift mutations. The translated protein contains the Ulp1-like protease domain, suggesting that the protein encoded is functional. (B) The nucleotide sequence contains nucleotide substitutions (not shown) and a single nucleotide deletion at nucleotide position 1213. This deletion causes a frameshift and premature stop codon in the sequence, resulting in the truncation of the encoded protein. The truncated protein does not contain the Ulp1-like protease domain, suggesting that this protein is nonfunctional. *, stop codon. The diagram is not to scale. (C) Chromatograph showing raw sequencing data from CLC workbench depicting the single-nucleotide deletion in the wPanMK cifB homolog at nucleotide position 1213.
wPanCI and wPanMK protection phenotypes.
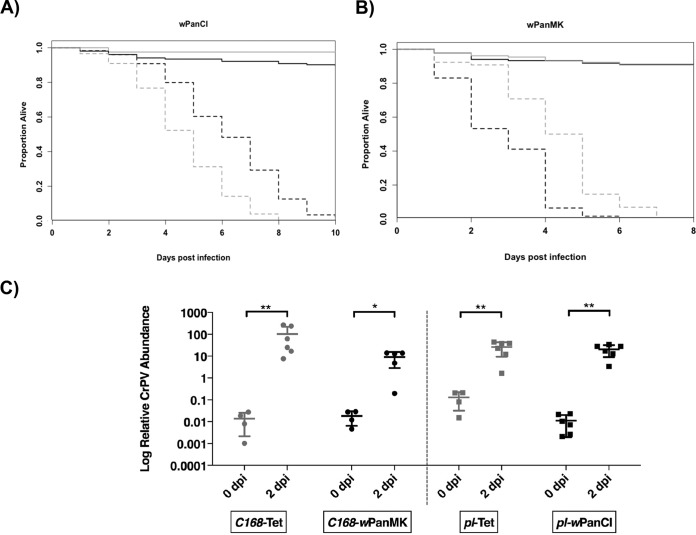
The CrPV model was used to characterize the protection phenotypes of wPanCI and wPanMK in D. pandora. The daily survival of three independent cohorts of pl-Tet, pl-wPanCI, C168-Tet, and C168-wPanMK flies was monitored after challenge with 109 IU/ml of CrPV and phosphate-buffered saline (PBS) (negative control). Comparisons between the survival of flies with and without Wolbachia were made to determine if Wolbachia affects virus-induced mortality. CrPV-induced mortality in pl-Tet was significantly different from that in pl-wPanCI (P = 0.000032 by the Cox mixed-effects model) (Fig. 4A), with pl-wPanCI flies succumbing to CrPV-induced death on average 2 days earlier than their pl-Tet counterparts. This demonstrates that wPanCI enhanced CrPV-induced mortality in this Wolbachia-fly pairing. Conversely, a significant difference was observed between the survival of C168-Tet and C168-wPanMK flies infected with CrPV (P = 0.008 by the Cox mixed-effects model), where mortality was delayed in C168-wPanMK flies on average 2 days (P = 0.008 by the Cox mixed-effects model) (Fig. 4B). Taken together, these results indicate that wPanMK protects C168 flies from CrPV-induced mortality, while wPanCI enhances CrPV-induced mortality in the pl flies.
FIG 4.
Cricket paralysis virus (CrPV)-induced mortality and viral accumulation in pl-wPanCI, pl-Tet, C168-wPanMK, and C168-Tet. (A and B) Survival curves derived from the survfit function in R studio (v. 1.0.153). Survival of three cohorts of 4- to 7-day-old females from the pl-Tet (black lines) and pl-wPanCI (gray lines) (A) and the C168-Tet (black lines) and C168-wPanMK (gray lines) (B) lines following challenge with CrPV (109 IU/ml) (dashed lines) and a PBS control (solid lines) was measured. Each cohort contained three vials of 15 flies for each Wolbachia-fly line pairing and treatment group (CrPV and PBS). The coxme function in R studio was used to determine the effect of Wolbachia infection on daily survival and also its effect on CrPV-induced mortality. (A) wPanCI infection had a significant effect on CrPV-induced mortality (P = 0.000032 by the Cox mixed-effects model), decreasing fly survival. (B) wPanMK infection had a significant effect on virus-induced mortality (P = 0.008 by the Cox mixed-effects model), increasing fly survival. (C) RT-qPCR was used to measure the abundance of CrPV RNA normalized to host gene actin88F in pools of at least 3 4- to 7-day-old females from at least 3 separate cohorts at 0 dpi and 2 dpi after challenge with CrPV (109 IU/ml). Graphs show the mean and standard deviation from at least three separate cohorts of flies. A Mann-Whitney unpaired test was used to determine if significant differences exist between the means of the groups. The relative accumulations of CrPV were significantly different between 0 and 2 dpi for each fly line (P < 0.05 by the Mann-Whitney test) but not between Wolbachia and Tet-cured lines at 2 dpi.
Wolbachia-mediated protection has been associated with either increased host resistance against or increased host tolerance to viral accumulation (65). To investigate which of these models is consistent with wPanMK-mediated protection, the accumulations of CrPV genomic RNA in flies with and without Wolbachia were compared. CrPV accumulated between 0 and 2 days postinfection (dpi) for both fly lines with and without Wolbachia (C168-Tet, P = 0.0095; C168-wPanMK, P = 0.159; pl-Tet, P = 0.0095; and pl-wPanCI, P = 0.0022 [Mann-Whitney test]). There was no significant difference in accumulation between pl-Tet and pl-wPanCI at 2 dpi (P > 0.05 by the Mann-Whitney test) or between C168-Tet and C168-wPanMK (P > 0.05 by the Mann-Whitney test), indicating that infection with Wolbachia does not affect CrPV accumulation in either fly line. Overall, this suggests that the observed effects of wPanMK and wPanCI on fly survival are unrelated to viral accumulation and may therefore be associated with their effect on tolerance.
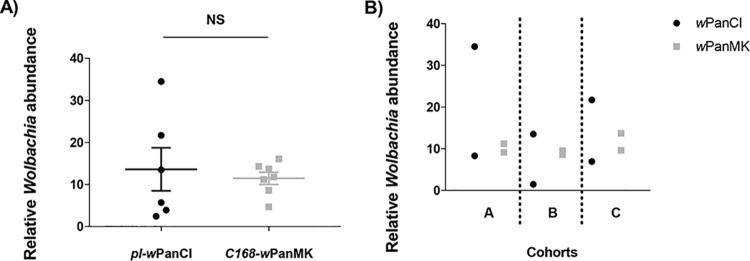
There is a large amount of evidence supporting a density-dependent model of Wolbachia-mediated protection, with higher Wolbachia densities associated with greater levels of protection (56, 57, 62, 77–79). To analyze whether differences in Wolbachia density may contribute to the observed differential protection, the relative Wolbachia abundances in pl-wPanCI and C168-wPanMK were compared. To moderate for high levels of variation in Wolbachia density, multiple separate cohorts of flies were analyzed. Using a standard qPCR assay, it was shown that there were equivalent Wolbachia densities in wPanMK and wPanCI (P = 0.83 by the Mann-Whitney test) (Fig. 5A). Wolbachia density between cohorts varied considerably among pl-wPanCI flies, while Wolbachia density in wPanMK showed less variation (Fig. 5A). This variation may be attributed to variation between individual flies within the sampled cohorts (Fig. 5B). While these results are not necessarily inconsistent with the model of density-dependent protection, they indicate that whole-fly density does not explain the disparity between the protection phenotypes of wPanCI and wPanMK.
FIG 5.
Equivalent abundance of Wolbachia in Drosophila pandora infected with wPanCI and wPanMK and high degree of variation in Wolbachia density between individual flies. qPCR was used to measure the relative densities of wPanCI and wPanMK in pools of 5 flies from the pl-wPanCI and C168-wPanMK strains. Pools were collected from three separate cohorts (cohorts A, B, and C), and amplification of the wsp gene was used as the proxy for Wolbachia density and normalized to that of host gene actin88F in qGene. (A) Mean and standard error of the mean (SEM) of Wolbachia abundance. Analysis of the data using a nonparametric Mann-Whitney test indicated no significant difference between the abundances of Wolbachia in pl-wPanCI and C168-wPanMK flies (P = 0.83). The variation in Wolbachia density appears to be greater in pl-wPanCI flies than in C168-wPanMK flies as indicated by a higher range and SEM. (B) The points on the graph represent results from two technical replicates with five different flies from the same cohort. Variation represents variation in the abundance of Wolbachia present in the five different flies sampled from the cohort. This can be seen as a reflection of variation in Wolbachia abundance between individual flies.
DISCUSSION
This study aimed to investigate the CI genotypes and antiviral protection phenotypes of newly described Wolbachia strains wPanMK and wPanCI in their natural D. pandora hosts.
Molecular basis of CI in D. pandora.
While the mechanisms involved in CI are not fully understood, experimental evidence suggests the involvement of cifA and cifB gene products in a two-by-one modification (CifA and CifB)-rescue (CifA) system (46–48). CI rescue can occur only between sufficiently related Wolbachia strains (66) where amino acid identities of both Cif proteins fall above the range of incompatibility (29 to 69%) (48). CifA and CifB homologs in wPanCI and wPanMK are highly similar type I homologs (Table 2). While cifB nucleotide sequences are highly similar between the two strains, the protein encoded by wPanMK is highly divergent due to the introduction of a frameshift mutation (Fig. 3). The degree of difference between the wPanCI and wPanMK CifB protein sequences falls within the range of incompatibility (Table 2). Contrastingly, the wPanMK cifA gene is highly conserved and similar to that in wPanCI, falling above the range of incompatibility. As wPanCI-induced CI is still rescued by wPanMK and the cifA homologs fall in the region of compatibility but the cifB homologs do not, this suggests that CifA may afford wPanMK the ability to rescue the CI induced by wPanCI and that CifB does not play a role in the rescue component of the CI phenotype. This is consistent with recent evidence that has shown that transgenic expression of wMel cifA in ovaries alone can rescue wMel-induced CI (47) and inhibit temperature-dependent growth defects caused by cifB expression in yeast systems (46). This is in contrast with another report which showed that wPip’s cif homologs were unable to transgenically rescue CI in Drosophila melanogaster (46); however, there are a number of possible reasons (discussed previously [47]) for the conflicting results of these two transgenic studies.
Putative domains of the CifA and CifB homologs have been previously described, but their functions remain to be investigated (46, 48). Limited functional analysis has shown that the Ulp1-like catalytic residue in the C-terminal deubiquitylase domain is integral to CifB-induced toxicity in yeast systems (46). However, this domain appears to be restricted to type I alleles, and as alleles of other types can still induce CI, it may not be an essential CI modification factor (76). This study revealed that this domain is intact in the wPanCI CifB homolog (Fig. 3A). Conversely, it is located within the truncated region of CifB encoded by wPanMK (Fig. 3B). The lack of this potentially important domain in the wPanMK CifB homolog combined with the apparently reduced expression levels suggests that this protein may be nonfunctional (Fig. 2 and 3). As wPanMK is unable to induce CI, these results are consistent with the theory that CifB is integral to CI induction and modification.
The overall results from this study provide evidence consistent with the involvement of CifA in CI rescue and of CifB in CI induction, providing more evidence to support the two-by-one model that is likely to be at work in the CI phenotype. Furthermore, other factors, yet to be identified, may be required for the complete CI phenotype. Additionally, Cif homologs that are not type I may be present and functioning in wPanCI and wPanMK. Comparative genomic analysis of wPanMK and wPanCI, as well as of other strains that can rescue but not induce CI, could help to further disentangle these contrasting models and provide further insight into the mechanistic functions of these Cif proteins in the CI phenotype of Wolbachia.
Wolbachia-mediated protection.
The CrPV model was used to investigate the protection phenotypes of wPanCI and wPanMK in the D. pandora-Wolbachia system. As antiviral protection is influenced by Wolbachia genetics (21, 64, 65, 80–83), it was hypothesized that wPanCI and wPanMK would provide protection, as the closely related strains wRi, wAu, wInn, and wMel are protective against host pathogens (31, 32, 65). Analysis showed that wPanMK provided protection against CrPV-induced mortality, while wPanCI enhanced CrPV-induced mortality (Fig. 4). Although unanticipated, these results reflect the high degree of variability that exists across the Wolbachia phylogeny in terms of the interactions of this symbiont with other host viruses (33, 64, 65, 81, 83–85). These effects on survival were shown not to be related to changes in viral accumulation (Fig. 4C). This suggests a complex picture of the effects of Wolbachia infection on the viral susceptibility of its hosts. It would be interesting to perform similar survival assays with a variety of other pathogens, including bacteria, to assess the spectrum of wPanMK-mediated protection.
As wPanCI and wPanMK are different strains of Wolbachia, genetic differences may exist that explain the observed differential protection; genetic components integral to antiviral protection may be present in wPanMK, while components that enhance viral infection may be present in wPanCI. Further genetic comparison of these strains may help elucidate any components that may be involved in the observed viral protection or enhancement. Wolbachia density is a genetically linked trait that has been shown to influence protection phenotypes (64, 65, 77, 80, 81, 86, 87). There were no differences observed between wPanCI and wPanMK densities in D. pandora hosts (Fig. 5), implying that whole-fly density is not a factor that explains their differential protection; however, tissue-specific Wolbachia density may play a role. Interestingly infection with wPanCI increased survival in pl-wPanCI flies (P = 0.0077 by the Cox mixed-effects model) (Fig. 5A), suggesting that the CI strain may have a benefit for its host separate from viral protection.
Male-killing strains of Wolbachia have the potential to impose significant population-level costs, i.e., reduced effective population size, reduced genetic diversity, and possible population collapse (12). Furthermore, it has been suggested that infection dynamics of wPanCI and wPanMK coinfection in populations would be governed by the relative fitness of females, and consistent with this, wPanCI-infected individuals have higher fitness in caged lab populations (31). However, the context-dependent fitness benefits provided by Wolbachia infections have not been considered. Pathogen protection, for example, is an important feature of Wolbachia infection that can differentially affect infection dynamics under certain selective contexts, and it may allow strains that impose fitness costs to persist in host populations (53, 78). Despite recent screening of viruses that naturally infect Drosophila species (79, 88), viruses naturally infecting D. pandora remain unknown, as it is a species that has only been recently described (69). If pathogenic viral infections are present in D. pandora populations, then wPanCI and wPanMK may be differentially affected as a consequence of their differential protection phenotypes. If an infecting virus exists in a population, applying selection for wPanMK-mediated protection, wPanMK infection may have higher fitness than wPanCI infection and therefore may become more prevalent and compensate for other fitness costs. Furthermore, if wPanCI-mediated enhancement of viral pathology is observed in the field, this would negatively affect its prevalence within the population. However, in the absence of selection for antiviral protection, wPanMK infection may not be as advantageous, and the dynamics may then favor wPanCI. It should be noted, however, that laboratory-observed protection/enhancement phenotypes may not translate to wild populations, with recent data for another Wolbachia strain that provides viral protection (wMel in D. melanogaster) raising concerns around whether protection can be readily demonstrated under field conditions (89). The fact that wPanMK may have the potential to provide antiviral protection in wild populations and also maintains the ability to rescue the CI induced by wPanCI may explain how it is able to be maintained within natural populations coinfected by wPanCI. The overall selective context (e.g., presence and strength of selection for protection) needs to be considered in understanding how Wolbachia infection dynamics may be affected in wild populations (53).
Conclusion.
This study characterized the newly described strains wPanCI and wPanMK, which coexist in natural populations, by investigating viral projection and characterizing genes underlying CI. We show that only the MK strain, which exists at a low frequency in natural populations, has antiviral activity. We also show that this strain lacks intact genes leading to CI, unlike the other, more common CI strain, which has intact genes. Perhaps the MK Wolbachia strain gains a fitness advantage from antivirus protection in natural populations, which would help explain their persistence given that the MK infection is widespread geographically and likely stable in populations (31). It would be worthwhile to transfer this infection to mosquito disease vectors through microinjection to test its effects on viral blocking as well as its impact on host reproduction.
MATERIALS AND METHODS
Drosophila stock maintenance.
Lines w1118-wMelpop and Co-wAu were maintained in the Johnson lab on standard cornmeal agar medium (65). Four D. pandora lines that either contained Wolbachia (pl-wPanCI and C168-wPanMK) or were cured from Wolbachia infection by tetracycline treatment (pl-Tet and C168-Tet) were obtained from the Hoffmann lab and maintained on modified cornmeal-dextrose medium (Bloomington) as previously described (31). Note that C168-wPanMK flies were supplemented with males from the pl line at each generation, functionally homogenizing the nuclear background. All lines were kept at 25°C ± 0.5°C on 12-hour light-dark cycles. PCR was used to confirm the correct Wolbachia infection status of the fly lines. Virus-free lines were prepared by dechorionation as previously described (65), and RT-qPCR analysis confirmed the absence of preexisting infection with CrPV.
Dissections.
Ten female flies 11 to 14 days old, from three different cohorts of pl-wPanCI and C168-wPanMK flies, were dissected and their ovaries collected in 0.75% saline solution on ice. These 20 pairs of ovaries that were collected from each cohort were stored in 1 ml of TRIzol (Invitrogen), and either RNA was directly extracted from the samples or the samples were stored at −20°C before extraction.
Survival assays.
To account for physiological variation associated with age and sex, all survival assays used 4- to 7-day-old female flies from pl-wPanCI pl-Tet, C168-wPanMK, and C168-Tet. CrPV was prepared as described previously (90). Three biological replicates were conducted with 3 separate cohorts of flies. Within each biological replicate there were two treatment groups (PBS negative control and CrPV) that each contained three separate vials of at least 15 flies. Carbon dioxide-anesthetized flies were injected with 50.6 nl of 1 × 109 infectious units (IU)/ml of CrPV or with 50.6 nl of PBS for the negative-control treatment group. Injections were made to the upper lateral part of the abdomen using needles pulled from borosilicate glass capillaries (Drummond Scientific) and a Nanoject II microinjector (Drummond Scientific). Following injection, mortality was scored daily until all virus-infected flies died. Mortality on day one was considered needle stick associated and the data were rezeroed to account for this. Pools of five flies from each fly-Wolbachia pairing that were injected alongside each treatment group in each biological replicate used for the survival assay were collected at 0 and 2 days postinfection (dpi) following challenge with CrPV and used to assess the accumulation of CrPV RNA.
DNA extraction.
Pools of five 4- to 7-day-old female flies were collected from w1118-wMelpop, Co-wAu, pl-Tet, pl-wPanCI, C168-Tet, and C168-wPanMK and stored at −20°C (minimum, 1 hour). Flies were homogenized in 200 μl UltraPure water (Invitrogen) with three 3-mm glass beads using the TissueLyser II (Qiagen) for 90 s at 30 Hz. The homogenate was centrifuged for 5 min at 14,000 rpm, and DNA was extracted from 140 μl of the supernatant using the QIAamp viral RNA minikit (Qiagen), per the manufacturer’s instructions. Each centrifugation step was performed at 14,000 rpm, and a final elution of samples was done with 60 μl of UltraPure water. Samples were diluted 1:5 before being stored at −20°C until further processing.
PCR.
Conventional PCR was used to determine the presence of type I cif genes and to confirm the success of molecular cloning. Primers designed to conserved internal regions of cifA (604 bp) and cifB (431 bp) homologs were used to test the presence of these genes (Table 1, PCR primers for CifA and CifB). PCR mixtures were set up as follows: 1 µl of DNA template, 0.25 µl of Taq DNA polymerase (NEB), 0.5 µl of each 10 µM forward and reverse primers, 2.0 µl of 10× ThermoPol reaction buffer (NEB), 0.5 µl of 10 µM deoxynucleoside triphosphates (dNTPs), and 15.5 µl of UltraPure DNase- and RNase-free distilled water (Invitrogen), for a final reaction volume of 20 µl. All PCRs were run on an Applied Biosystems Veriti thermal cycler under the following condition: 94°C initial denaturing for 3 min and then 35 cycles of 94°C denaturing for 30 s, 52°C annealing for 1 min, and 68°C extension for 1 min. Amplification of samples was confirmed on 1% agarose gel.
RNA extraction and cDNA synthesis from Drosophila pandora flies and ovaries.
RNA was extracted from the pools of 20 dissected ovaries and pools of five flies at 0 dpi and 2 dpi with CrPV, collected as described above. Total RNA was extracted in 1 ml of TRIzol (Invitrogen) as per the manufacturer’s instructions, with the final RNA pellets resuspended in 12 μl of UltraPure water (Invitrogen). Two micrograms of RNA was treated with RQ1 RNase-free DNase (Promega), and the SuperScript III reverse transcriptase kit (Thermo Fisher Scientific Inc.) was used to synthesize cDNA, using random primers, both following the manufacturer’s protocol. Samples were run on a thermocycler following the following profile: 25°C for 5 min, 50°C for 60 min, and 70°C for 15 min. Samples were diluted 1:5 with UltraPure water (Invitrogen) before being used for RT-qPCR.
RT-qPCR.
RT-qPCR was used to measure the expression of cifA and cifB in ovary samples and accumulation of CrPV in fly samples. cDNAs from these samples were used as templates for RT-qPCR with the SYBR green qPCR SuperMix-UDG kit (Thermo Fisher). Previously validated primers for CrPV (Table 1, qPCR primers for CrPV) were used to determine the infection status of D. pandora. Viral accumulation of CrPV was analyzed by quantification of the amount of CrPV relative to that of actin88F reference gene RNA (91). Expression levels of the cif genes were measured relative to that of actin88F in ovary samples using previously validated primers (Table 1, qPCR primers for CifA and CifB). Two technical replicates of each sample were processed in a Rotor-gene thermal cycler (Corbett Life Sciences) under the following program: 50°C for 2 min, 95°C 2 min, 40 cycles of 95°C for 10 s and 60°C for 20 s (52°C for Wolbachia [Wsp] primers), and 72°C for 20 s. Specificity of amplification was confirmed by melting curve analysis between 65°C and 95°C. A sample of the DNase-treated RNA was analyzed to ensure that the DNase treatment had successfully removed all detectable DNA. The following controls were applied to the data: (i) cutoff values of 30 for the takeoff (threshold cycle [CT]), (ii) amplification values above 1.65, and (iii) standard deviation below 0.5 between at least two technical replicates.
qPCR.
Quantitative PCR (qPCR) used the same protocol and controls as for RT-qPCR, using genomic DNA as the template for the reaction instead of cDNA. qPCR was used to determine the relative abundance of Wolbachia and also to validate the primers used for both qPCR and RT-qPCR. All amplification results were compared to that for the host reference gene actin88F (92). For the estimation of Wolbachia density, the amplification of the Wolbachia surface protein (Wsp) gene was considered an appropriate proxy (91).
Long-amplification PCR.
Amplification of the cifA-cifB 5-kb locus involved reaction mixtures of 2 µl of genomic DNA, with 14.25 µl of UltraPure water (Invitrogen), 1 µl of LongAmp Taq DNA polymerase (NEB), 1 µl each of 10 µM forward and reverse primers (Table 1, PCR primers for CifA-CifB), 5 µl of 5× LongAmp Taq DNA polymerase buffer (NEB), and 0.75 µl of 10 µM dNTPs, for a final reaction mixture volume of 25 µl. Reactions were run on an Applied Biosystems Veriti thermal cycler under the following conditions: 94°C initial denaturing for 30 s; 30 cycles of 94°C denaturing for 30 s, 50°C annealing for 30 s, and 65°C extension for 4 min 30 s; and a final extension for 10 min at 65°C. The PCR products were purified from agarose gels using the Zymoclean gel DNA recovery kit (Zymo Research) following the manufacturer’s protocol.
Cloning and sequencing.
A-tailing of the gel-purified 5-kb long-amplification products was conducted by setting up reaction mixtures with 6 µl of the PCR product, 1 µl of 10× ThermoPol reaction buffer (NEB), 2 µl of 1 µM dATP, 0.2 µl Taq DNA polymerase (NEB), and 0.8 µl UltraPure DNase- and RNase-free distilled water (Invitrogen) and incubating for 20 min at 70°C. These A-tailed PCR products were then cloned into the pGEM-T Easy vector following the manufacturer’s protocol (Promega). The ligated vectors were transformed into competent XL1 Escherichia coli by heat shock. Successful transformations were identified by blue-white screening, and colony PCR confirmed the presence of the inserts. Purified DNA samples were sent to the Australian Genome Research Facility (Brisbane) for Sanger sequencing using sequencing primers 700 bp apart in both directions, providing full coverage of the entire 5-kb cifA-cifB loci. Alignment of the forward and reverse contig sequences for each of the clones showed 100% identity. To further confirm an observed frameshift mutation, PCR products of the 700-bp region surrounding the mutation were purified from a 1% agarose gel using the Zymoclean gel DNA recovery kit (Zymo Research) and directly sequenced.
Domain prediction for wPanCI and wPanMK Cif homologs.
The Cif protein sequences from wPanCI and wPanMK were analyzed for the presence of putative domains of type I homologs using the HHpred protein domain prediction tool (https://toolkit.tuebingen.mpg.de/#/tools/hhpred) (95) with default parameters and searching databases SCOPe95_2.06, SCOPe70_2.06, COG_KOG_v1.0, Pfam-A_v31.0, ECOD_F70_2010828, TIGRFAM_v15.0, NCBI_Conserved_Domain(CD)_v3.1, PRK_v6.9, and SMART_v6.0.
Statistical analysis.
The Cox proportional-hazard mixed-effects model (R package coxme) was used to compare the levels of survival of different treatment groups and determine the effect of Wolbachia infection on virus-induced mortality (19, 94). This model treats technical and biological replicates as random effects and virus treatment and Wolbachia infection status as fixed effects, with the assumption that the data have a Gaussian distribution. Results with P values of <0.05 were considered significant. All flies alive at the end of the experiment were treated as censored data. Survival curves were plotted using the survfit function in R studio version 1.0.153.
QGene software (Biotechniques) was used to analyze RT-qPCR and qPCR data by the ΔΔCT method (90, 93). Prism 7 (GraphPad Software, Inc.) was used to conduct Mann-Whitney unpaired nonparametric tests to investigate the significance of the difference between two groups for Wolbachia density and CrPV accumulation.
Accession number(s).
The nucleotide sequences for the cifA and cifB loci in wPanCI (accession number MG807657) and wPanMK (accession number MG807658) have been submitted to BankIt, GenBank.
ACKNOWLEDGMENTS
We thank Kelly Richardson for preparing and supplying flies, David Merritt for assistance with dissections, members of the Johnson lab and members of Sassan Asgari's lab for support and useful comments, and Simon Bloomberg for guidance on statistical analysis.
A.A.H. was supported by NIH grant R01GM104325 and NHMRC fellowship 1118640.
REFERENCES
- 1.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. 2008. How many species are infected with Wolbachia?—a statistical analysis of current data. FEMS Microbiol Lett 281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werren JH, Windsor DM. 2000. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc R Soc Lond B Biol Sci 267:1277–1285. doi: 10.1098/rspb.2000.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stouthamer R, Breeuwer JAJ, Hurst GDD. 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann AA, Turelli M, Harshman LG. 1990. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 126:933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werren JH, Zhang W, Guo LR. 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc R Soc Lond B 261:55–63. doi: 10.1098/rspb.1995.0117. [DOI] [PubMed] [Google Scholar]
- 6.Heath BD, Butcher RDJ, Whitfield WGF, Hubbard SF. 1999. Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Curr Biol 9:313–316. doi: 10.1016/S0960-9822(99)80139-0. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed MZ, Breinholt JW, Kawahara AY. 2016. Evidence for common horizontal transmission of Wolbachia among butterflies and moths. BMC Evol Biol 16. doi: 10.1186/s12862-12016-10660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werren J, Jaenike J. 1995. Wolbachia and cytoplasmic incompatibility in mycophagous Drosophila and their relatives. Heredity 75:320–326. doi: 10.1038/hdy.1995.140. [DOI] [PubMed] [Google Scholar]
- 9.Jaenike J, Dyer KA, Reed LK. 2003. Within-population structure of competition and the dynamics of male-killing Wolbachia. Evol Ecol Res 5:1023–1036. [Google Scholar]
- 10.Werren JH. 1997. Biology of Wolbachia. Annu Rev Entomol 42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 11.Hurst GDD, Jiggins FM, Pomiankowski A. 2002. Which way to manipulate host reproduction? Wolbachia that cause cytoplasmic incompatibility are easily invaded by sex ratio-distorting mutants. Am Nat 160:360–373. doi: 10.1086/341524. [DOI] [PubMed] [Google Scholar]
- 12.Dyer KA, Jaenike J. 2004. Evolutionarily stable infection by a male-killing endosymbiont in Drosophila innubila: molecular evidence from the host and parasite genomes. Genetics 168:1443–1455. doi: 10.1534/genetics.104.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlat S, Calmet C, Merçot H. 2001. On the mod resc model and the evolution of Wolbachia compatibility types. Genetics 159:1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann AA, Hercus M, Dagher H. 1998. Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 148:221–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiggins FM, Hurst GDD. 2011. Rapid insect evolution by symbiont transfer. Science 332:185–186. doi: 10.1126/science.1205386. [DOI] [PubMed] [Google Scholar]
- 16.Min K, Benzer S. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci U S A 94:10792–10796. doi: 10.1073/pnas.94.20.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moriyama M, Nikoh N, Hosokawa T, Fukatsu T, McFall NMJ. 2015. Riboflavin provisioning underlies Wolbachia’s fitness contribution to its insect host. mBio 6:e01732-15. doi: 10.1128/mBio.01732-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poinsot D, Merçot H. 1997. Wolbachia infection in Drosophila simulans: does the female host bear a physiological cost? Evolution 51:180–186. doi: 10.1111/j.1558-5646.1997.tb02399.x. [DOI] [PubMed] [Google Scholar]
- 19.Martinez J, Cogni R, Cao C, Smith S, Illingworth CJR, Jiggins FM. 2016. Addicted? Reduced host resistance in populations with defensive symbionts. Proc R Soc Lond B Biol Sci 283:20160778. doi: 10.1098/rspb.2016.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. 2007. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol 5:e114. doi: 10.1371/journal.pbio.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez J, Ok S, Smith S, Snoeck K, Day JP, Jiggins FM. 2015. Should symbionts be nice or selfish? Antiviral effects of Wolbachia are costly but reproductive parasitism is not. PLoS Pathog 11:e1005021. doi: 10.1371/journal.ppat.1005021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yen JH, Barr AR. 1971. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 232:657–658. doi: 10.1038/232657a0. [DOI] [PubMed] [Google Scholar]
- 23.Yen JH, Barr AR. 1973. Etiological agent of cytoplasmic incompatibility in Culex pipiens. J Invertebr Pathol 22:242–250. doi: 10.1016/0022-2011(73)90141-9. [DOI] [PubMed] [Google Scholar]
- 24.Hurst LD. 1991. The evolution of cytoplasmic incompatibility or when spite can be successful. J Theor Biol 148:269–277. doi: 10.1016/S0022-5193(05)80344-3. [DOI] [PubMed] [Google Scholar]
- 25.Hurst GDD, Jiggins FM. 2000. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg Infect Dis 6:329–336. doi: 10.3201/eid0604.000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurst LD. 1991. The incidences and evolution of cytoplasmic male killers. Proc R Soc Lond B Biol Sci 244:91–99. doi: 10.1098/rspb.1991.0056. [DOI] [Google Scholar]
- 27.Schilthuizen M, Stouthamer R. 1997. Horizontal transmission of parthenogenesis-inducing microbes in Trichogramma wasps. Proc R Soc Lond B Biol Sci 264:361–366. doi: 10.1098/rspb.1997.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stouthamer R, Breeuwer JAJ, Luck RF, Werren JH. 1993. Molecular identification of microorganisms associated with parthenogenesis. Nature 361:66–68. doi: 10.1038/361066a0. [DOI] [PubMed] [Google Scholar]
- 29.Kageyama D, Nishimura G, Hoshizaki S, Ishikawa Y. 2002. Feminizing Wolbachia in an insect, Ostrinia furnacalis (Lepidoptera: Crambidae). Heredity 88:444–448. doi: 10.1038/sj.hdy.6800077. [DOI] [PubMed] [Google Scholar]
- 30.Rousset F, Bouchon D, Pintureau B, Juchault P, Solignac M. 1992. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc R Soc Lond B Biol Sci 250:91–98. doi: 10.1098/rspb.1992.0135. [DOI] [PubMed] [Google Scholar]
- 31.Richardson KM, Schiffer M, Griffin PC, Lee SF, Hoffmann AA. 2016. Tropical Drosophila pandora carry Wolbachia infections causing cytoplasmic incompatibility or male killing. Evolution 70:1791–1802. doi: 10.1111/evo.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unckless RL, Jaenike J. 2012. Maintenance of a male-killing Wolbachia in Drosophila innubila by male-killing dependent and male-killing independent mechanisms. Evolution 66:678–689. doi: 10.1111/j.1558-5646.2011.01485.x. [DOI] [PubMed] [Google Scholar]
- 33.Longdon B, Fabian DK, Hurst GDD, Jiggins FM. 2012. Male-killing Wolbachia do not protect Drosophila bifasciata against viral infection. BMC Microbiol 12:S8. doi: 10.1186/1471-2180-12-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurst GDD, Johnson AP, Schulenburg JHG, Fuyama Y. 2000. Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics 156:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werren JH. 1987. The coevolution of autosomal and cytoplasmic sex ratio factors. J Theor Biol 124:317–334. doi: 10.1016/S0022-5193(87)80119-4. [DOI] [Google Scholar]
- 36.Presgraves DC. 2000. A genetic test of the mechanism of Wolbachia-induced cytoplasmic incompatibility in Drosophila. Genetics 154:771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Neill SL, Karr TL. 1990. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature 348:178–180. doi: 10.1038/348178a0. [DOI] [PubMed] [Google Scholar]
- 38.Landmann F, Orsi GA, Loppin B, Sullivan W. 2009. Wolbachia-mediated cytoplasmic incompatibility is associated with impaired histone deposition in the male pronucleus. PLoS Pathog 5:e1000343. doi: 10.1371/journal.ppat.1000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lassy CW, Karr TL. 1996. Cytological analysis of fertilization and early embryonic development in incompatible crosses of Drosophila simulans. Mech Dev 57:47–58. doi: 10.1016/0925-4773(96)00527-8. [DOI] [PubMed] [Google Scholar]
- 40.Reed KM, Werren JH. 1995. Induction of paternal genome loss by the paternal‐sex‐ratio chromosome and cytoplasmic incompatibility bacteria (Wolbachia): a comparative study of early embryonic events. Mol Reprod Dev 40:408–418. doi: 10.1002/mrd.1080400404. [DOI] [PubMed] [Google Scholar]
- 41.Callaini G, Riparbelli MG, Giordano R, Dallai R. 1996. Mitotic defects associated with cytoplasmic incompatibility in Drosophila simulans. J Invertebr Pathol 67:55–64. doi: 10.1006/jipa.1996.0009. [DOI] [Google Scholar]
- 42.Binnington KC, Hoffmann AA. 1989. Wolbachia-like organisms and cytoplasmic incompatibility in Drosophila simulans. J Invertebr Pathol 54:344–352. doi: 10.1016/0022-2011(89)90118-3. [DOI] [Google Scholar]
- 43.Hoffmann AA, Clancy DJ, Merton E. 1994. Cytoplasmic incompatibility in Australian populations of Drosophila melanogaster. Genetics 136:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann AA, Turelli MJ. 1997. Cytoplasmic incompatibility in insects, p 42–80. In O’Neill SL, Hoffmann AA, Werren JH (ed), Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford, England. [Google Scholar]
- 45.Turelli M, Cooper BS, Richardson KM, Ginsberg PS, Peckenpaugh B, Antelope CX, Kim KJ, May MR, Abrieux A, Wilson DA, Bronski MJ, Moore BR, Gao J-J, Eisen MB, Chiu JC, Conner WR, Hoffmann AA. 2018. Rapid global spread of wRi-like Wolbachia across multiple Drosophila. Curr Biol 28:963–971.e968. doi: 10.1016/j.cub.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beckmann JF, Ronau JA, Hochstrasser M. 2017. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat Microbiol 2. doi: 10.1038/nmicrobiol.2017.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shropshire JD, On J, Layton EM, Zhou H, Bordenstein SR. 2018. One prophage WO gene rescues cytoplasmic incompatibility in Drosophila melanogaster. Proc Natl Acad Sci U S A 115:4987–4991. doi: 10.1073/pnas.1800650115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LePage DP, Metcalf JA, Bordenstein SR, On J, Perlmutter JI, Shropshire JD, Layton EM, Funkhouser-Jones LJ, Beckmann JF, Bordenstein SR. 2017. Prophage WO genes recapitulate and enchance Wolbachia-induced cytoplasmic incompatibility. Nature 543:243–247. doi: 10.1038/nature21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan W, O’Neill SL. 2017. Manipulation of the manipulators. Nature 543:182–183. doi: 10.1038/nature21509. [DOI] [PubMed] [Google Scholar]
- 50.Ishmael N, Hotopp JCD, Ioannidis P, Biber S, Sakamoto J, Siozios S, Nene V, Werren J, Bourtzis K, Bordenstein SR, Tettelin H. 2009. Extensive genomic diversity of closely related Wolbachia strains. Microbiology 155:2211–2222. doi: 10.1099/mic.0.027581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, McGraw EA, Martin W, Esser C, Ahmadinejad N. 2004. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol 2:E69. doi: 10.1371/journal.pbio.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prout T. 1994. Some evolutionary possibilities for a microbe that causes incompatibility in its host. Evolution 48:909–911. doi: 10.1111/j.1558-5646.1994.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 53.Fenton A, Johnson KN, Brownlie JC, Hurst GDD. 2011. Solving the Wolbachia paradox: modeling the tripartite interaction between host, Wolbachia, and a natural enemy. Am Nat 178:333–342. doi: 10.1086/661247. [DOI] [PubMed] [Google Scholar]
- 54.Brownlie JC, Cass BN, Riegler M, Witsenburg JJ, Iturbe-Ormaetxe I, McGraw EA, O’Neill SL. 2009. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress (Wolbachia and iron stress). PLoS Pathogens 5. doi: 10.1371/journal.ppat.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brownlie JC, Johnson KN. 2009. Symbiont-mediated protection in insect hosts. Trends Microbiol 17:348–354. doi: 10.1016/j.tim.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Kremer N, Voronin D, Charif D, Mavingui P, Mollereau B, Vavre F. 2009. Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog 5:e1000630. doi: 10.1371/journal.ppat.1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teixeira L, Ferreira Á, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6:e1000002. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hedges LM, Brownlie JC, O’Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 59.Rainey SM, Shah P, Kohl A, Dietrich I. 2014. Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: progress and challenges. J Gen Virol 95:517–530. doi: 10.1099/vir.0.057422-0. [DOI] [PubMed] [Google Scholar]
- 60.Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, Moreira LA, Bossin HC, Moretti R, Baton LA, Hughes GL, Mavingui P, Gilles JR. 2014. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop 132:S150–S163. doi: 10.1016/j.actatropica.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Johnson KN. 2015. Bacteria and antiviral immunity in insects. Curr Opin Insect Sci 8:97–103. doi: 10.1016/j.cois.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 62.Rohrscheib CE, Frentiu FD, Horn E, Ritchie FK, van Swinderen B, Weible MW, O’Neill SL, Brownlie JC. 2016. Intensity of mutualism breakdown is determined by temperature not amplification of Wolbachia genes. PLoS Pathog 12:e1005888. doi: 10.1371/journal.ppat.1005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chrostek E, Teixeira L. 2015. Mutualism breakdown by amplification of Wolbachia genes. PLoS Biol 13:e1002065. doi: 10.1371/journal.pbio.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, Teixeira L. 2013. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet 9:e1003896. doi: 10.1371/journal.pgen.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osborne SE, Leong YS, O’Neill SL, Johnson KN. 2009. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog 5:e1000656. doi: 10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bourtzis K, Dobson SL, Braig HR, O’Neill SL. 1998. Rescuing Wolbachia have been overlooked. Nature 391:852–853. doi: 10.1038/36017. [DOI] [PubMed] [Google Scholar]
- 67.Turelli M, Hoffmann A. 1995. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140:1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charlat S, Le Chat L, Merçot H. 2003. Characterization of non-cytoplasmic incompatibility inducing Wolbachia in two continental African populations of Drosophila simulans. Heredity 90:49–55. doi: 10.1038/sj.hdy.6800177. [DOI] [PubMed] [Google Scholar]
- 69.McEvey SF, Schiffer M. 2015. New species in the Drosophila ananassae subgroup from northern Australia, New Guinea and the South Pacific (Diptera: Drosophilidae), with historical overview. Rec Aust Mus 67:129–161. doi: 10.3853/j.2201-4349.67.2015.1651. [DOI] [Google Scholar]
- 70.Baldo L, Dunning Hotopp JC, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, Hayashi C, Maiden MC, Tettelin H, Werren JH. 2006. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boyle L, O’Neill SL, Robertson H, Karr T. 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260:1796–1799. doi: 10.1126/science.8511587. [DOI] [PubMed] [Google Scholar]
- 72.Corbin C, Heyworth ER, Ferrari J, Hurst GD. 2017. Heritable symbionts in a world of varying temperature. Heredity (Edinb) 118:10–20. doi: 10.1038/hdy.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clancy DJ, Hoffmann AA. 1998. Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia‐infected Drosophila simulans. Entomol Exp Appl 86:13–24. doi: 10.1046/j.1570-7458.1998.00261.x. [DOI] [Google Scholar]
- 74.Merçot H, Poinsot D. 1998. Rescuing Wolbachia have been overlooked and discovered on Mount Kilimanjaro. Nature 391:853. doi: 10.1038/36021. [DOI] [PubMed] [Google Scholar]
- 75.Johnson KN, Christian PD. 1996. A molecular taxonomy for cricket paralysis virus including two new isolates from Australian populations of Drosophila (Diptera: Drosophilidae). Arch Virol 141:1509–1522. doi: 10.1007/BF01718251. [DOI] [PubMed] [Google Scholar]
- 76.Lindsey ARI, Rice DW, Bordenstein SR, Brooks AW, Bordenstein SR, Newton ILG. 2018. Evolutionary genetics of cytoplasmic incompatibility genes cifA and cifB in prophage WO of Wolbachia. Genome Biol Evol doi: 10.1093/gbe/evy012:evy012-evy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frentiu FD, Robinson J, Young PR, McGraw EA, O’Neill SL. 2010. Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS One 5:e13398. doi: 10.1371/journal.pone.0013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oliver KM, Campos J, Moran NA, Hunter MS. 2008. Population dynamics of defensive symbionts in aphids. Proc R Soc Lond B Biol Sci 275:293–299. doi: 10.1098/rspb.2007.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Webster CL, Waldron FM, Robertson S, Crowson D, Ferrari G, Quintana JF, Brouqui J-M, Bayne EH, Longdon B, Buck AH, Lazzaro BP, Akorli J, Haddrill PR, Obbard DJ. 2015. The discovery, distribution, and evolution of viruses associated with Drosophila melanogaster. PLoS Biol 13:e1002210. doi: 10.1371/journal.pbio.1002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Osborne SE, Iturbe-Ormaetxe I, Brownlie JC, O’Neill SL, Johnson KN. 2012. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Appl Environ Microbiol 78:6922–6929. doi: 10.1128/AEM.01727-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez J, Longdon B, Bauer S, Chan YS, Miller WJ, Bourtzis K, Teixeira L, Jiggins FM. 2014. Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathog 10:e1004369. doi: 10.1371/journal.ppat.1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cattel J, Martinez J, Jiggins F, Mouton L, Gibert P. 2016. Wolbachia-mediated protection against viruses in the invasive pest Drosophila suzukii. Insect Mol Biol 25:595–603. doi: 10.1111/imb.12245. [DOI] [PubMed] [Google Scholar]
- 83.Blagrove MSC, Arias-Goeta C, Failloux A-B, Sinkins SP. 2012. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci U S A 109:255–260. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amuzu HE, Tsyganov K, Koh C, Herbert RI, Powell DR, McGraw EA. 2018. Wolbachia enhances insect‐specific flavivirus infection in Aedes aegypti mosquitoes. Ecol Evol 8:5441–5454. doi: 10.1002/ece3.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hussain M, Lu G, Torres S, Edmonds JH, Kay BH, Khromykh AA, Asgari S. 2013. Effect of Wolbachia on replication of West Nile virus in a mosquito cell line and adult mosquitoes. J Virol 87:851–858. doi: 10.1128/JVI.01837-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adams KL, Daley DO, Qiu YL, Whelan J, Palmer JD. 2000. Repeated, recent and diverse transfers of a mitochondrial gene to the nucleus in flowering plants. Nature 408:354–357. doi: 10.1038/35042567. [DOI] [PubMed] [Google Scholar]
- 87.Chrostek E, Marialva MSP, Yamada R, O’Neill SL, Teixeira L. 2014. High anti-viral protection without immune upregulation after interspecies wolbachia transfer. PLoS One 9:e99025. doi: 10.1371/journal.pone.0099025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Webster CL, Longdon B, Lewis SH, Obbard DJ. 2016. Twenty-five new viruses associated with the Drosophilidae (Diptera). Evol Bioinform Online 12:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.White V, John-Sebastian E, Hoffmann A, Holmes E. 2018. No detectable effect of Wolbachia wMel on the prevalence and abundance of the RNA virome of Drosophila melanogaster. Proc R Soc Lond B Biol Sci 285:20181165–20181165. doi: 10.1098/rspb.2018.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hedges LM, Johnson KN. 2008. Induction of host defence responses by Drosophila C virus. J Gen Virol 89:1497–1501. doi: 10.1099/vir.0.83684-0. [DOI] [PubMed] [Google Scholar]
- 91.Braig HR, Zhou W, Dobson SL, O’Neill SL. 1998. Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J Bacteriol 180:2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McMeniman CJ, Lane AM, Fong AW, Voronin DA, Iturbe-Ormaetxe I, Yamada R, McGraw EA, O’Neill SL. 2008. Host adaptation of a Wolbachia strain after long-term serial passage in mosquito cell lines. Appl Environ Microbiol 74:6963–6969. doi: 10.1128/AEM.01038-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuan JS, Reed A, Chen F, Stewart CN Jr. 2006. Statistical analysis of real-time PCR data. BMC Bioinformatics 7:85–97. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Therneau TM, Grambsch PM, Pankratz VS. 2003. Penalized survival models and frailty. J Comput Graph Stat 12:156–175. doi: 10.1198/1061860031365. [DOI] [Google Scholar]
- 95.Zimmermann L, Stephens A, Nam SZ, Rau D, Kübler J, Lozajic M, Gabler F, Söding J, Lupas AN, Alva V. 2018. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J Mol Biol 430:2237–2243. doi: 10.1016/j.jmb.2017.12.007. [DOI] [PubMed] [Google Scholar]