Abstract
Autoimmune diseases, rejection of transplanted organs and grafts, chronic inflammatory diseases, and immune-mediated rejection of biologic drugs impact a large number of people across the globe. New understanding of immune function is revealing exciting opportunities to help tackle these challenges by harnessing – or correcting - the specificity of immune function. However, realizing this potential requires precision control over the interaction between regulatory immune cues, antigens attacked during inflammation, and the tissues where these processes occur. Engineered materials – such as polymeric and lipid particles, scaffolds, and inorganic materials – offer powerful features that could help selectively regulate immune function during disease without compromising healthy immune functions. In this review, we highlight some of the exciting developments to leverage biomaterials as carriers, depots, scaffolds – and even as agents with intrinsic immunomodulatory features – to promote immunological tolerance.
Keywords: biomaterial, immunology and autoimmunity, nanotechnology, microparticle and nanoparticle, vaccine and immunotherapy, tolerance, scaffold
Autoimmune disease, inflammatory conditions, and rejection of organs and tissue grafts are all underpinned by undesirable immune reactions at cell, tissue, and systemic levels. Biomaterials offer powerful opportunities to control interactions across these scales to promote immunological tolerance that selectively controls inflammation. This review summarizes emerging opportunities in this area that exploit biomaterials as carriers, as as scaffolds, and as intrinsic modulatory materials.
1. Introduction
Immune tolerance is the functional unresponsiveness of the immune system towards cells or tissues. During healthy immune function, tolerance against self-tissues is maintained. However, the breakdown of tolerance can lead to autoimmune disease, conditions in which the immune system mistakenly recognizes and attacks host tissues and cells. Tissue destruction in autoimmune disease is driven by T and B cells specific for self-antigens which become aberrantly activated. These cells also secrete proteins – antibodies and inflammatory cytokines – that play major roles in the inflammatory effects occurring during autoimmunity. Autoimmune disease affects men and women of all ages, with some of the most prevalent autoimmune diseases including multiple sclerosis (MS), type 1 diabetes (T1D), rheumatoid arthritis and lupus. Transplantation is another area where similar inflammatory processes occur, but in a different context. In particular, a lack of tolerance of a recipient to a donor can result in the destruction of allogenic transplants – such as organs – because the tissue is seen as foreign by the immune cells of the recipient[1]. Other areas where a lack of immune tolerance can negatively impact human health are allergic reactions and immune responses generated against recombinant protein drugs or viral vectors used for gene delivery[2]. In allergies, for example, an immune response against otherwise harmless substances can trigger excess inflammation. This pathology is generally driven by allergen-specific B cells that generate IgE antibodies. In the case of immune responses against protein drugs or viral vectors, repeated treatment with these drugs can raise neutralizing antibodies in the patient that bind the proteins and inhibit their function upon subsequent administration. Thus, there are significant opportunities to improve new therapies involving immune tolerance across several important disease areas.
The mechanisms maintaining tolerance are exquisitely complex: the immune system integrates multiple layers of signals, including cytokines, ligands for surface receptors, chemokines, stromal tissue features, and other soluble factors. These signals are present in healthy tissue, at sites of inflammation, and also in immune tissues such as the spleen and lymph nodes (LN); the latter are key sites in which adaptive immune responses develop. The types of signals encountered during the generation of an immune response define the specific features of the resulting immune response, ultimately determining whether the response will be inflammatory or tolerogenic.
To initiate an immune response, antigen presenting cells (APCs) such as dendritic cells (DCs) and macrophages survey the periphery in search of foreign pathogens. Once an APC encounters a pathogen, the pathogen is phagocytosed or otherwise sampled, followed by migration of the APC to LNs. At these sites, the pathogen is processed and antigens present in the pathogen are displayed on the surface of APCs within a protein complex called major histocompatibility complex (MHC). Presentation of antigen in MHC to T and B cells in the presence of inflammatory signals - including inflammatory cytokines and surface activation markers on APCs - promotes expansion of these lymphocytes and drives a pro-inflammatory response[3]. A key feature of this expansion is the molecular specificity that results in an enormous increase in the number of lymphocytes recognizing the peptide fragment displayed by the APC, while lymphocytes recognizing other antigens remain inactive. In contrast to activation, a lack of inflammatory signals or the presence of regulatory signals during antigen presentation can promote tolerogenic responses. These tolerogenic response span deletion, functional unresponsiveness of antigen specific T or B cells, and expansion of regulatory T cells (TREG) or other regulatory cell populations that suppress pro-immune reactions[4].
Historically, approaches to inhibit unwanted destruction of healthy cells have focused on the administration of immunosuppressants which broadly suppress inflammatory function in immune cells[5]. These include anti-inflammatory agents or corticosteroids. Recently more specific treatments have been applied, including recombinant cytokines and monoclonal antibodies. Antibody treatments function to deplete T or B cells, stop inflammatory function, or restrict migration of self-reactive immune cells. However, although antibody-based therapies are more specific, they are not selective in the sense that they bind their target receptor on all cells displaying these molecules. Thus, these therapies exert effects on both autoreactive immune cells and on immune cells exhibiting normal function. This lack of selectivity can leave patients immunocompromised or susceptible to specific types of infection. Further, existing treatments are not curative and must be repeatedly administered, often for life. These drawbacks highlight the potential impact of engineering more effective and safer therapies for to promote immune tolerance.
Polymers, lipids, quantum dots (QDs), metals and other biomaterials are emerging as tools to improve the design of vaccines and immunotherapies[6]. Biomaterials are commonly formulated as microparticles (MPs) or nanoparticles (NPs), organized into self-assembling structures, or formulated into scaffolds for implantation. These designs provide attractive features for immunotherapies. For example, polymeric or lipid particles can be used to provide controlled release or co-delivery of multiple immune signals, and can be modified with ligands to enhance targeting to APCs in LNs or the spleen. Additionally, NPs can passively drain through lymphatics to LNs, permitting accumulation of delivered immune signals in these tissues. Both NPs and MPs are internalized by APCs, which can enhance intracellular delivery of immunomodulators or self-antigens. Implantable scaffolds can function as depots for the localized delivery of immune signals and promote migration of immune cells. This allows the engineering of a localized tolerogenic microenvironment. Additionally, an emerging area of research is investigating how intrinsic immunogenic properties of biomaterials modulate immune cell function, and how these properties can be harnessed to promote tolerance. All of these features are creating new opportunities to not only effect large changes in immune response, but also to control specific features of immune function.
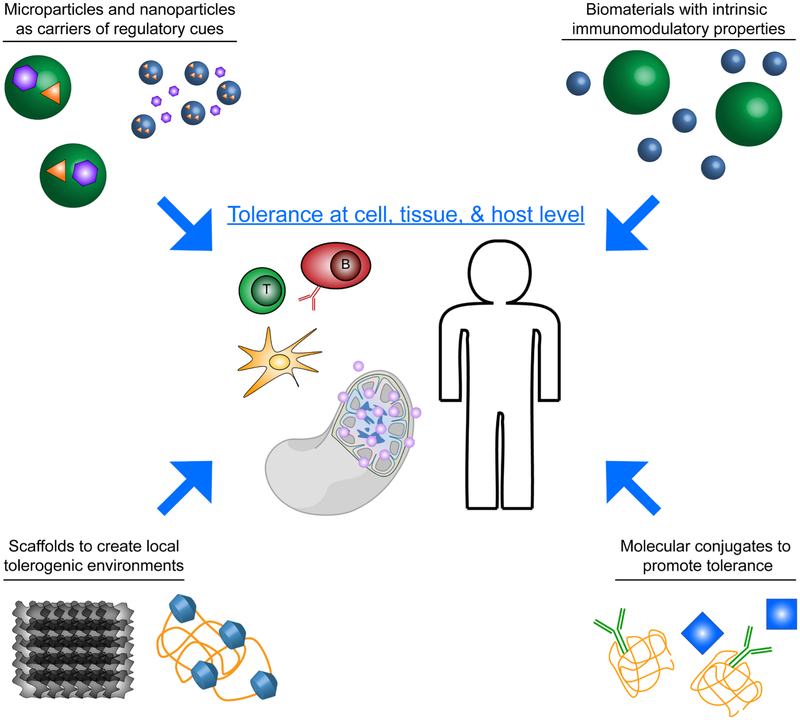
In this review we present key ways in which biomaterials are being investigated to promote tolerogenic immune function in autoimmune disease, transplantation, and allergies, as well as to tackle challenges associated with anti-drug antibodies. These changes are being effected across multiple scales, including specific cell populations, at the tissue level, and systemically throughout the host (Figure 1). We first discuss strategies employing NPs and MPs to deliver immunomodulatory molecules and self-antigens. Included in this discussion are examples using molecular conjugates instead of conventional solid NPs. Next we describe recent work to understand how the intrinsic physicochemical properties of biomaterials can promote tolerogenic function in immune cells. We conclude by presenting efforts using immunomodulatory scaffolds as tolerance-inducing platforms for disease, or as local immunomodulatory microenvironments to promote tolerance during transplantation. Key references illustrating each of these areas are summarized in Table 1.
Figure 1.
Graphical depiction of major areas in which biomaterials are being used to engineer immunological tolerance across scales of cells, tissues, and systemically throughout the host.
Table 1.
Materials-based strategies to promote immune tolerance
| Material | Approach for Tolerance | Reference |
|---|---|---|
| Microparticles or nanoparticles | Delivering immunomodulators to DCs | [8-10, 12-16, 18] |
| Tolerogenic presentation of immunomodulators | [23] | |
| Co-delivering self-antigen with regulatory cue using particulate carriers |
[24-31, 33-36] | |
| Self-assembling auto-antigen and regulatory cue into carrier free particles | [46, 52] | |
| Targeting autoantigens to tolerogenic zones in LNs, spleen and liver | [60-67, 70-71] | |
| Modifying presentation of autoantigens to lymphocytes | [72-82] | |
| Active targeting immunomodulators to DCs | [87, 127] | |
| Active targeting immunomodulators to T cells | [88-90] | |
| Active targeting immunomodulators to LNs | [91] | |
| Promoting local tolerogenic microenvironments | [11, 17-19, 100-104, 106-110, 128] | |
| Intrinsicly immunogenic materials | Harnessing intrinsic tolerogenic properties | [68-69, 111-114] |
| Molecularly designed auto-antigens | Active targeting to erythrocytes | [92-95] |
| Scaffolds | Localized tolerogenic vaccines | [118-120] |
| Localized tolerogenic environment for transplantation | [103, 122-124] |
2. NPs and MPs offer attractive features as carriers of signals that drive tolerance
2.1. Particulate delivery of immunomodulatory cues can promote tolerogenic DCs
The majority of research harnessing biomaterials to promote tolerance has focused on utilizing particles for the controlled delivery of immune signals. Most of these investigations have applied particles synthesized from degradable polymers or liposomes, although inorganic materials such as metals and QDs have also been investigated. One broad strategy applying particulate delivery for tolerance is to deliver immunomodulators to promote tolerogenic DCs. An increasing understanding of signaling pathways in DCs has led to the discovery of a number of new pathways which may be targeted to tune the inflammatory or tolerogenic function of DCs. Examples include signaling pathways involved in DC metabolism, sensing of immunogenic cell death, and pathways using toll like receptors (TLR), which DCs and other APCs use to sense “danger signals” present on pathogens[7] . Immunomodulators targeting these pathways can be small molecule drugs or biologics, including proteins, peptides, antibodies, lipids or nucleic acids. However, when these treatments are given as soluble systemic administrations they require frequent dosing due to short half-life; such regimens result in exposure of non-target tissues to the treatment. Since particulate materials drive more efficient uptake by APCs – and because biomaterials offer tunable release rates – these carriers have recently been harnessed to overcome the challenges above by providing controlled release of a number of tolerogenic immunomodulators, including immunosuppressants[8-11] metabolic modulators[12-14], TLR agonists[15-16], regulatory cytokines[17-18] and ligands[19] for receptors that promote tolerogenic function in DCs. However, the vast majority of these investigations have been in pre-clinical models. While particulate delivery vehicles – in particular, liposomes - have successfully been approved for enhancing the safety and efficacy of chemotherapeutics, investigation of these strategies for delivery of immunomodulators in clinical trials is needed.
2.2. Nanoparticles can be used to tune the presentation of immunomodulatory signals
The above body of work demonstrates particulate delivery of immunomodulators is an effective strategy to promote tolerogenic function in APCs in preclinical models. An emerging area of study that could speed translation of these therapies to the clinic focuses on understanding how the presentation of immunomodulatory signals on the surface of particles impacts immune response, and how this presentation can be tuned to promote specific immune function (e.g., tolerance). Parameters which can be varied include immune signal density or valency on the surface of particles, or the geometry of the particles the immunomodulators are delivered with. While these interactions have been more frequently investigated in the context of promoting pro-immune function [20-22], exploration of this area with the goal of promoting tolerance could provide unique benefits. One recent example examining the geometric presentation of an immunomodulatory ligand to generate immune tolerance involved synthesis of rod-shaped poly(Lactide-co-Glycolide) (PLGA) NPs encapsulating phosphatidyl serine (PS)[23]. PS can function as a tolerogenic signal when expressed on the surface of apoptotic cells. This mechanism exists to prevent potential autoimmune reactions against cells undergoing normal programmed cell death. Nanorods loaded with PS reduced the secretion of inflammatory cytokines and expression of surface activation markers in bone marrow derived DCs after stimulation with LPS, a common inflammatory signal. Interestingly, in a co-culture of DCs and transgenic T cells specific for myelin - the self-antigen attacked in MS, PS nanorods reduced inflammatory cytokine secretion compared to either liposomes or larger (1 μm) rods presenting that PS at equivalent doses. This result illustrates the principle that the properties of the particle (e.g., geometry, size) can impact the ability of cues such as PS to suppress inflammatory function. Additionally, administration of PS nanorods to mice induced with experimental autoimmune encephalomyelitis (EAE) – a mouse model of MS –significantly reduced paralysis caused by the disease relative to liposomes presenting PS, which provided no therapeutic benefit over untreated mice.
2.3. Particulate carriers enable co-delivery of self-antigen and regulatory cues to drive antigen-specific tolerance
The above examples focus on particulate delivery of immunomodulators alone. While these approaches can provide significant advantages over systemic bolus administration of immunosuppressants, they are not inherently antigen-specific. While inflammation during disease may result in release and presentation of self-antigens in tissues these therapies reach, many NP and MP strategies are now exploring co-delivery of antigens with regulatory cues as therapeutic vaccine that could induce antigen-specific tolerance. These treatments generally involve co-administration of self-antigens attacked during disease and a regulatory signal. The self-antigen provides the specificity of the response, while the regulatory cue redirects the response against this self-antigen by polarizing antigen-specific cells away from inflammatory phenotypes and toward regulatory function (i.e., tolerance). By co-encapsulating self-antigens and regulatory signals in particles, the likelihood that the target cell or tissue receives both signals can be significantly increased, an important criteria for driving antigen-specific tolerance. For example, co-localizing these signals within DCs presenting self-antigen to T cells in LNs recognizing this antigen could bias these cells toward TREG.
Leveraging this strategy, a number of recent preclinical studies have demonstrated particulate co-delivery of self-antigens and regulatory signals can effectively promote antigen-specific tolerance[24-28]. These studies have been conducted in multiple mouse models of autoimmune disease, and in settings aimed at preventing formation of anti-drug antibodies against recombinant protein drugs. NPs that passively drain to LNs after systemic injection have been tested in this capacity, as well as MPs, which are carried to LNs by APCs after phagocytosis at the site of injection. One specific application of this strategy has utilized PLGA NPs co-encapsulating self-antigens with rapamycin, a small molecule inhibitor of the mTOR pathway. Moldonado et al. demonstrated in a model of relapsing remitting MS that NPs co-encapsulating self-antigen and rapamycin promoted tolerance more effectively compared to NPs delivering rapamycin or self-antigen alone[29]. Additionally, this report demonstrated tolerance was antigen-specific. Another variation of this platform co-delivers rapamycin encapsulated in NPs with unencapsulated soluble self-antigens. While this does not necessarily ensure both signals will reach all of the same LNs after delivery, one advantage is complex or larger antigen sources which may be difficult to encapsulate in particles can be used. For example, Meliani et al. recently demonstrated tolerance against adeno-associated virus (AAV) gene delivery vectors could be promoted by co-administration of soluble AAVs with NPs encapsulating rapamycin[30]. Both of these approaches are modular, since the specificity of tolerance can be altered by changing the self-antigen co-delivered with rapamycin. For example, NPs co-delivering self-antigens and rapamycin have been used to effectively promote tolerance toward multiple recombinant protein drugs[31-34]. Excitingly, this strategy has been expanded to phase 2 clinical trials to tolerize and prevent formation of anti-drug antibodies against pegadricase, a front line treatment for gout.
Several other regulatory signals have been co-delivered with self-antigens to promote tolerance. The Quintana lab investigated a ligand for the aryl-hydrocarbon receptor, a transcription factor which has recently been shown to promote tolerogenic function in DCs and expand TREG.[35] The aryl hydrocarbon receptor ligand was co-adsorbed on the surface of gold NPs with myelin peptide, and these NPs were used to treat mice induced with EAE. In line with the previous example, only NP formulations co-delivering the ligand and myelin prevented disease. This finding indicates co-delivery of self-antigen and regulatory signal can provide a synergistic therapeutic effect, and that tolerance was antigen-specific. Recently this platform was expanded to promote tolerance in a T1D mouse model, where gold NPs were used to co-deliver the aryl hydrocarbon receptor ligand and an islet self-antigen attacked during T1D[36].
2.4. Carrier free particles can be engineered to promote tolerance
The above examples demonstrate the potential of particulate co-delivery of self-antigens and regulatory signals to promote antigen specific tolerance. All of these examples utilize a “carrier” to enable co-delivery by encapsulating, adsorbing, or conjugating immune signals. Although this strategy has demonstrated promising results, the addition of a delivery vehicle increases overall vaccine complexity which is a significant hurdle for translation. Further, a number of recent investigations have revealed that many common particulate carriers exhibit some degree of intrinsic immunogenicity[37-45]. This immunogenicity adds a confounding factor which complicates the understanding of the role of individual vaccine components. In particular for autoimmune and inflammatory disease, any excess inflammation caused by the carrier could exacerbate disease or limit the therapeutic efficacy.
Motivated by the challenges above, “carrier-free” tolerogenic vaccines and immunotherapies are also being explored by building nanostructured materials directly from immune signals. This approach mimics some of the attractive features of polymeric or other biomaterial carriers (i.e. particulate formulation, co-delivery, cargo protection), while simplifying the number of components and eliminating immunogenic carrier effects. One such strategy involves using electrostatic interaction between oppositely charged self-antigens and regulatory signals to form polyplex-like NPs. Importantly, this method eliminates the need for the use of harsh solvents or high energy mixing that is commonly used during synthesis of particulate vaccines formulated with polymeric carriers that can potentially damage active vaccine components. Further, while loading of immune cargo in most delivery systems involving polymer carriers is relatively low, this carrier-free method relying on self-assembly provides 100% loading since the particles are constructed entirely from the active immune components. Recent work from our lab has demonstrated this approach as an effective strategy to promote tolerance in a mouse model of EAE[46]. In this work, a myelin self-antigen (MOG) was designed to be cationic by appending arginine residues (R) to the antigenic MOG peptide sequence (MOGRX). GpG, a regulatory nucleic acid ligand of toll-like receptor 9 (TLR9) - an immunostimulatory receptor on APCs – served as both a regulatory cue and an anionic component. These self-assembled NPs were formed by admixing MOGRx and GpG. Administration to mice with EAE reduced the severity of paralysis and neuroinflammation.
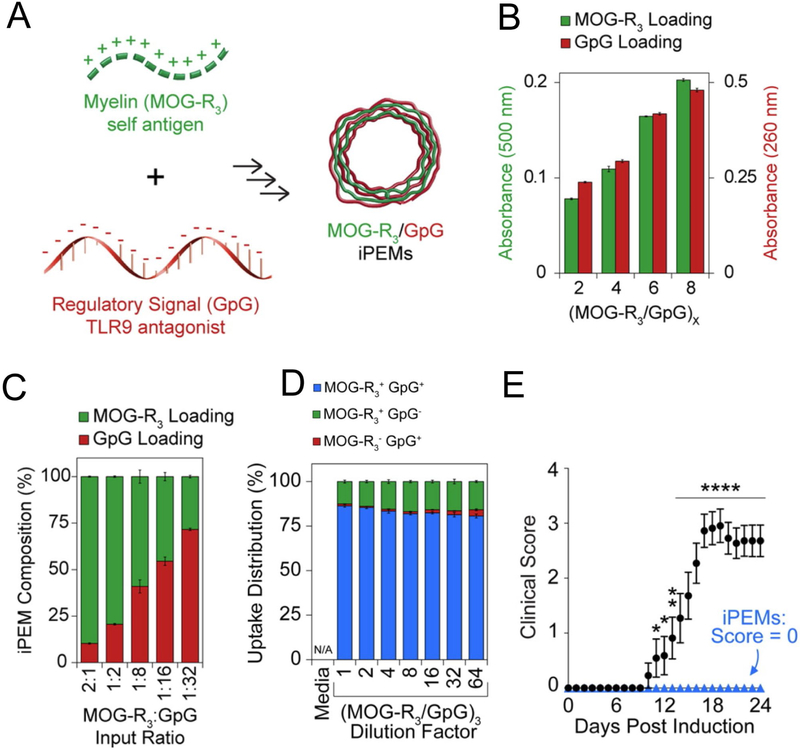
Expanding on this work, our lab recently developed a platform to assemble immune signals into immune polyelectrolyte multilayers (“iPEMS”) using an electrostatic layer-by-layer process[47-48]. In particular, iPEMs can be formed into hollow, carrier free microcapsules consisting entirely of immune signals[49-51]. Because this approach is modular, iPEM capsules consisting of MOGRX and GpG could be synthesized to promote tolerance using the same electrostatic assembly process (Figure 2A)[52]. Likewise, the absolute and relative loading of each component could be controlled by increasing the number of bilayers during iPEM assembly (Figure 2B), or by changing the ratio of MOGRX and GpG in the bilayers (Figure 2C). iPEMS were readily internalized by DCs, and importantly a high frequency of DCs internalizing at least one cargo internalized both MOG and GpG (Figure 2D). iPEM treatment of mice induced with EAE increased TREG frequency in spleens, and strikingly, completely prevented symptoms of EAE in 100% of mice (Figure 2E). These structures were also able to decrease myelin-specific inflammation in samples isolated from human MS patients, indicating possible clinical significance. Together these examples illustrate how self-assembly can be leveraged in designing modular systems to promote tolerance.
Figure 2.
A) Schematic for the assembly of carrier free iPEMs consisting of MOGRX and GpG. B) The total loading of iPEM components can be tuned by increasing the number of bilayers during synthesis. C) The relative loading levels of iPEM components can be tuned by varying the ratio of components in the bilayers. D) iPEMs co-deliver both GpG and MOGRX to the same DCs. E) iPEM treatment completely inhibits disease in EAE. Adapted from [52].
2.5. Biomaterials can be harnessed to alter the trafficking and processing of self-antigens
While the above studies demonstrate that co-delivery of self-antigens and regulatory signals can serve as tolerogenic vaccines, a recent area of investigation involves delivering self-antigen alone as a means of inducing antigen-specific tolerance. Two primary strategies have been investigated along these lines. In this section we will discuss the first strategy, which focuses on engineering delivery vehicles to alter APC processing of self-antigens by promoting localization of the antigens in tolerogenic scavenging domains located in the liver and lymphoid tissues (e.g., spleens, LNs). The second focuses on altering presentation or valency of self-antigens on the surface of particles or polymers to directly modulate interactions with lymphocytes. These examples are discussed in section 2.6.
Past pre-clinical studies involving infusion of antigens coupled to apoptotic splenocytes have revealed these treatments promote antigen-specific tolerance [53-57]. Mechanistically, this effect appears to arise from co-localization of antigen coupled splenocytes with specialized microdomains in the marginal zone of spleens and LNs. These microdomains serve to process apoptotic self-cells in a tolerogenic manner[58]. A key cell type involved in this process are macrophages expressing the scavenger receptor, macrophage receptor with collagenous structure (MARCO); this receptor plays a role in recognizing apoptotic cells, against which the host naturally desires tolerance[58]. Excitingly this strategy has previously been proven safe in phase I clinical trials for MS[59]. However, this strategy faces significant hurdles for translation since the harvesting and ex vivo processing of donor splenocytes is costly and complicated.
To leverage the underlying mechanism just described, while overcoming the challenges of cell-based therapies, one approach is to covalently couple self-antigens to polymeric NPs or MPs with sizes similar to apoptotic cell fragments (~500nm) [60-65]. This strategy has been applied to promote tolerance in preclinical models of MS, type 1 diabetes, bone marrow transplantation, islet transplantation, and allergic airway inflammation. Getts et al. demonstrated 500nm PS beads covalently linked with myelin self-antigens prevented disease in a relapsing remitting model of EAE (RR-EAE) after i.v. administration. These particles co-localize with MARCO expressing macrophages in the marginal zones of spleens after administration[66]. Hunter et al. demonstrated degradable PLGA NPs of similar size displaying myelin self-antigens on the surface promote antigen-specific tolerance in RR-EAE[67]. While the precise mechanism for tolerance is not fully understand, this study and others using PLGA NPs displaying self-antigens have demonstrated both size and charge play a role in efficacy; NPs approximately 500nm in diameter with negative surface charges display the highest level of co-localization with MARCO and efficacy in promoting tolerance. Recent studies, which are discussed in section 3.2, have also demonstrated that negatively charged particles delivered without self-antigens can drive tolerance [68-69].
In addition to using polymeric carriers to deliver self-antigens, other materials have also been investigated. Myelin self-peptides, for example, have been coupled to QDs to investigate how the density of self-antigen display impacts the therapeutic induction of tolerance in mouse models of MS[70]. QDs coated with defined MOG peptide densities on the surface were synthesized, ranging from 17 peptides per MOG-QD (17:1) to 52 peptides per MOG-QD (52:1); hydrodynamic diameter ranged from 15 to 20nm. MOG-QDs drained efficiently to LNs after s.c. injection and co-localized with cells expressing MARCO in these tissues. Interestingly, while treatment of mice with MOG-QDs protected mice from EAE, the level of efficacy was dependent on the density of MOG on the QD. In particular, at a fixed MOG dose, a low density of MOG peptide on a larger number of particles was more effective in driving tolerance than a higher density of MOG peptide on a smaller number of particles. This example demonstrates an important opportunity to leverage the precision of engineered materials to study immune function, which might ultimately lead to design rules for vaccines and immunotherapies. Another paper involving QDs investigated delivery of myelin peptide on negatively charged QDs coated with poly(maleic anhydride-alt-1-octadecene)[71]. After i.v. injection over 90% of the QDs accumulated in liver sinusoidal endothelial cells (LSECs); these cell specialize in processing apoptotic cells, similarly to macrophages displaying scavenger receptors in LNs and spleens discussed earlier. MOG QDs prevented disease in EAE compared to mice treated with unloaded QDs. Together these studies demonstrate particulate delivery can alter trafficking and processing of self-antigens by promoting co-localization with APCs in scavenging domains in lymphoid tissue and the liver. While these materials could be very useful as tools, a potential limitation for QD-based therapies is the potential for toxicity, which warrants continued investigation.
2.6. Biomaterials can be used to alter the presentation of self-antigens
The examples discussed above demonstrate how antigen-coupled NPs can effectively co-opt natural tolerogenic pathways through co-localization with tolerogenic domains in lymphoid organs. As alluded to in the previous section, a second strategy to drive tolerance involves tuning the valency or surface density of self-antigens presented on the surface of NPs or grafted to low molecular weight polymers. Unlike the previous examples using QDs delivering self-antigens these changes in antigen display directly alter interactions with antigen-specific lymphocytes to promote tolerance. Similar to strategies discussed in Section 2.2, these designs alter the presentation of ligands to tune immune cell function. One key distinction however is the designs discussed in this section display self-antigens rather than immunomodulators as ligands.
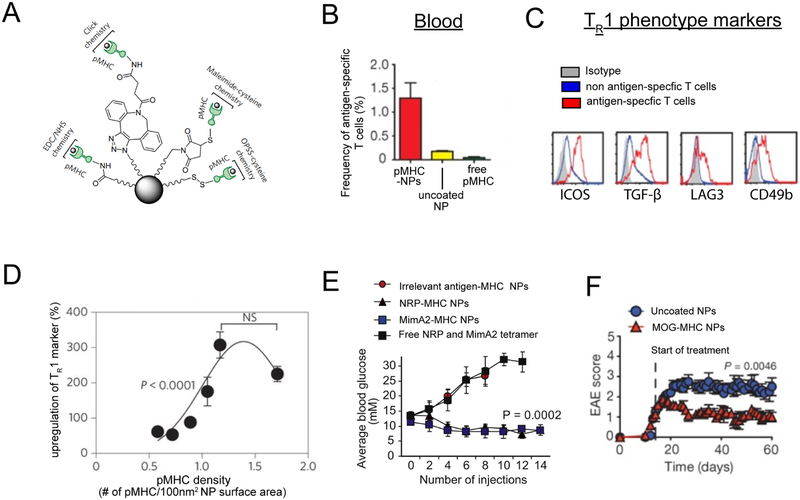
The Santamaria group has demonstrated iron oxide NPs functionalized with islet self-antigens displayed in MHC complexes (pMHC-NPs) can inhibit the development of T1D in a mouse model of spontaneous T1D [72-75]. These NPs can be covalently conjugated with pMHC self-antigen complexes using a variety of chemistries (Figure 3A). Mechanistically pMHC-NPs expand antigen specific T cells (Figure 3B) with a regulatory phenotype called TR1 (Figure 3C). Expansion of these regulatory type T cells is likely mediated through the high density of pMHC presented on the surface NPs. This is supported by the observation that pMHC-NPs must display pMHC above a certain density in order to optimally expand antigen specific regulatory T cells and prevent disease (Figure 3D). Interestingly, pMHC NPs displaying different T1D relevant antigens (MimA2 or NRP) could blunt disease (Figure 3E). In these studies, efficacy depended on NP conjugation, since administration of soluble pMHC did not expands antigen specific T cells or inhibit T1D.Tolerance induction could also be extended to other diseases, as pMHC-NPs with appropriate self-antigens could be prepared and used to generate tolerance in EAE (Figure 3F) and collagen-induced arthritis. Excitingly, the translational potential of this approach was demonstrated in a humanized mouse model of T1D. In this model pMHC NPs presenting human islet self-antigens effectively expanded antigen-specific regulatory T cells in immunodeficient NOD mice with immune systems reconstituted with peripheral blood mononuclear cells from diabetic human donors. One limitation to this strategy is the complexity of recombinantly expressing and displaying self-antigen in MHC.
Figure 3.
A) pMHC can be conjugated to NP surfaces with a variety of chemistries. B) pMHC treatment expands antigen specific T cells. C) Flow cytometry plots demonstrating T cells expanded by pMHC NP express surface markers characteristic of TR1 phenotype. D) A minimum threshold of pMHC density on NPs to optimally polarize antigen specific TR1. E) pMHC NPs formulated with multiple T1D self-antigens (NRP, MimA2 inhibit incidence of T1D. F) pMHC NPs formulated with myelin autoantigen (MOG) inhibit EAE. Adapted from [72-73, 75].
Another approach harnessing multivalent delivery of self-antigens to promote tolerance involves using low molecular weight polymers presenting self-antigens to directly modulate interactions with B cells, termed “soluble antigen arrays” [76-81]. B cells require antigen recognition of the B cell receptor to trigger B cell activation. However, repeated signaling through the B cell receptor (BCR) in the absence of a co-stimulatory signal can promote functional exhaustion or anergy. Materials can achieve multivalent presentation of self-antigens. This multi-valent display increases self-antigen avidity, or the combined functional affinity, towards autoreactive BCRs. Increased avidity can promote targeting and repeated binding of BCRs to promote anergy. The Berkland group has formulated hyaluronic acid (HA) polymers presenting multivalent arrays of another myelin self-antigen (PLP) and an intracellular adhesion molecule 1 peptide inhibitor (LABL), termed SAgAPLP:LABL. While PLP binds BCRs on self-reactive B cells during MS and provides antigen signal, the LABL serves to promote prolonged cellular adhesion. SAhAPLP:LABL displayed significant therapeutic efficacy in the mouse model of RR-EAE, and delivery of unconjugated, PLP and LABL mixed with HA provided no protection[76]. Interestingly SAgAPLP:LABL provided enhanced therapeutic efficacy when compared to PLGA NPs (500nm) with PLP and LABL grafted to the surface[77]. This may be due to differences in biodistribution, or to the conformational flexibility of SAgAPLP:LABL that could increase interaction with BCRs. Mechanistic studies revealed that SAgAPLP:LABL bound antigen specific B cells in vitro while blunting functional BCR signaling[78]. Presentation of both PLP and LABL on polymer arrays were necessary for enhanced cell binding and signaling disruption. In subsequent studies, Hartwell et al. synthesized non-degradable multivalent polymer arrays displaying PLP and LABL, which displayed even greater avidity and inhibition of BCR signaling, as well as enhanced therapeutic efficacy in EAE compared to degradable HA SAgAPLP:LABLs. [82]
2.7. Nanoparticles can be actively targeted to specific immune cells or lymphoid tissues to promote tolerance
While the examples thus far have involved passive drainage or relied on APCs to transport particles to LNs and the spleen, a key feature of biomaterials is the ability to actively target encapsulated cargo to specific immune cells or tissues. This capability can greatly enhance the efficacy of tolerogenic therapies. In the drug delivery field, this potential has been extensively leveraged by directly modifying carriers or drugs with antibodies or other targeting moieties to target these cargos to desired cells or tissues. Targeting provides significant advantages over systemic administration of free cargo, for example, by enabling dose sparing and minimizing exposure of drugs to non-target tissues. Further, targeting moieties can be utilized to target specific proteins expressed by a particular cell population of interest. This strategy has recently been applied to provide more precise control over the delivery of self-antigens and immunomodulators in the context of promoting immune tolerance.
As discussed in Section 1, MP and NP delivery provides a “passive” targeting effect to DCs, since they can be internalized at the injection site or after draining to LNs. However, to improve the efficiency of targeting to DCs, targeting ligands can be combined with particulate encapsulation of immunomodulatory cargo. Although active targeting of DCs with soluble immunomodulators might enhance tolerogenic immunotherapies, combining active targeting with biomaterial carriers has remained relatively unexplored in this area. One prevalent target for recent antibody-based targeting approaches is the endocytotic c-type lectin (DEC-205) receptor, which is primarily expressed by DCs. Self-antigen-antibody conjugates targeting DEC-205 have been exploited to promote tolerance in mouse models of colitis and MS [83-85]. One study explored PLGA MPs conjugated with targeting moieties for either DEC-205 or CD11c, an additional protein highly expressed on the surface of APCs. [86]. This study found MPs functionalized with antibodies specific for DEC-205 or CD11c, or a peptide (PD-2) that specifically binds CD11c enhanced in vitro uptake of MPs by DCs. These targeted MPs did not cause toxicity, and importantly, did not increase activation of DCs; the latter could potentially negate the effects of tolerogenic therapies or even exacerbate inflammation. When administered to mice by s.c. injection at the footpad, MPs conjugated with PD-2 enhanced internalization of MPs within DCs and macrophages in the draining LN over three days, compared to unmodified MPs. This result suggests that PD-2 targeting MPs may be an effective platform to deliver self-antigens and/or regulatory signals. However, further investigation is needed to demonstrate that particles actively targeting DCs can promote functional tolerance.
Another investigation utilized actively-targeted NPs to target DCs in Peyer’s patches, a specialized lymphoid tissue in the gut[87]. Since healthy immune systems maintain local tolerance toward the collection of microorganisms in the gut – the microbiome, the lymphoid tissue in the gut is predisposed toward tolerance. Therefore, targeting self-antigens toward Peyer’s patches might promote an antigen-specific tolerogenic response. In this study, a self-antigen (heat shock protein, H6P) attacked during T1D was encapsulated in chitosan NPs functionalized with two targeting ligands[87]. NPs were functionalized with mannose, which targets mannose receptors expressed on DCs, and arginylglycylaspartic acid (RGD) which targets M cells; M cells are concentrated in the epithelium of Peyer’s patches, and exhibit high levels of transcytosis – a process where macromolecules or particles can be transported across both sides of a cell. The authors reasoned these dual targeted NPs could aid in delivery through the gut endothelium – a major hurdle for oral delivery – and additionally serve to target the self-antigen to DCs. Oral administration of targeted NPs resulted in significantly enhanced accumulation of NPs in Peyer’s patches compared to untargeted NPs, ultimately preventing disease onset in a mouse model of T1D.
In addition to targeting DCs, T cells can be actively targeted to promote tolerance. These strategies aim to expand and maintain the suppressive function of TREG or to blunt expansion and effector function of inflammatory, self-reactive T cells. One study utilized PLGA NPs functionalized with antibodies specific for CD4 T cells to deliver the immunomodulatory cytokines, IL-2 and TGF-β [88]. While IL-2 and TGF-β polarize TREG differentiation, in vivo administration is hampered by toxicity that develops from exposure of these cytokines to off target tissues and cells. In cell culture, antibody-modified NPs bound CD4 T cells and increased TREG differentiation compared to treatment with equivalent doses of soluble IL-2 and TGF-β. In mice, i.p administration of CD4 targeted NPs increased TREG frequencies in the mesenteric LNs and spleens of mice, compared to untreated mice. Another study targeting CD4 T cells used a liponanogel encapsulating KN-93, an inhibitor of calcium/calmodulin-dependant protein kinase; this pathway is involved in production of the inflammatory cytokine, IL-17, a key driver of inflammation in many autoimmune diseases[89]. Compared to non-targeted liponanogels, weekly treatment with the CD4-targeted nanogels reduced clinical EAE scores and the number of IL-17 producing T cells in the central nervous system (CNS). A recent report applied the nanolipogel platform for the active targeting of a DNA-methlytransferase inhibitor, 5-azacytidine, to T cells[90]. Administration of nanolipogels targeting CD4 preferentially expanded TREG. Further, administration of nanolipogels targeting either CD4 or CD8 protected from symptoms of inflammation occurring during a mouse model of lupus.
Another potential strategy to promote tolerance is to actively target lymphoid tissue. In one example, poly-lactide (PLA) MPs encapsulating tacrolimus (MP-TAC), a small molecule mTOR inhibitor, were surface-conjugated with an antibody specific for peripheral lymph node addressin (PNAd), a glycoprotein expressed in regions of LNs where lymphocytes enter from the blood stream[91]. In this study the authors demonstrated mice receiving allogenic skingrafts displayed significantly increased PNAd expression in draining LNs compared to non-draining LNs. i.v. injection of PNAd-targeted MPs after allogenic skin transplantation led to co-localization of MPs with PNAd in transplant-draining LNs. The targeted MPs also accumulated at higher levels in draining LNs compared to non-draining LNs. This study also demonstrated the specificity of PNAd targeting, as administration of a PNAd-blocking antibody before MP injection reduced accumulation of targeted MPs in draining LNs to levels similar to those measured in mice injected with non-targeted MPs. To investigate the efficacy of LN-targeted MPs on prolonging graft survival, C57BL6 mice received a non-matched heart transplant from BalbC mice, followed by daily administration of targeted MPs. LN-targeted MP-TAC significantly prolonged graft survival while reducing serum Tacsirolimus levels compared to free tacsirolimus treatments. Together these studies indicate active targeting of LNs can localize the effects of immunomodulators in LNs to promote tolerance.
In addition to these examples of actively targeted particles, another tolerogenic strategy involves targeting self-antigens to apoptotic red blood cells (RBCs). RBCs are continually replaced in the body, where a large number of RBCs become apoptotic and are cleared in a tolerogenic manner by APCs in the spleen and liver. Kontos et. al conjugated the model antigen ovalbumin (OVA) to ERY1 (ERY1-OVA), a peptide which specifically targets glycophorin-A expressed on RBCs [92]. The authors demonstrated the conjugates specifically targeted RBCs, since ERY1-OVA bound to RBCs without binding to leukocytes after i.v. administration. Administration of allophycocanin-labeled ERY1 resulted in high levels of uptake in APCs in the spleen and in the liver. ERY1-OVA treatment reduced proliferation of antigen specific T cells and increased levels of apoptosis, indicating functional suppression of the T cell response. Similar results were obtained when an antibody fragment specific for glycophorin-A (TER119) was conjugated with SIINFEKL, a specific antigen peptide fragment from OVA protein. The ability of this platform to promote functional tolerance was tested in an adoptive transfer model of T1D, where activated T cells specific for the islet self-antigen P31 were infused into NOD mice. In mice treated with unmodified P31, transfer of P31 specific T cells induced diabetes within 7 days after cell transfer. In contrast, when P31 conjugated to TER was injected i.v, induction of diabetes was completely prevented, and all mice remained normoglycemic through the 62 day study. Grimm et al. demonstrated mechanistically in this system that polarizing TREG phenotype is involved in tolerance induction, and that long term tolerance is induced[93]. Another paper applied the in vivo RBC binding platform to effectively tolerize and prevent antibody formation against the therapeutic protein drug, Escherichia coli L-asparinase [94]. This could be beneficial since the efficacy of many biologic drugs is inhibited over time by antibodies raised against them. In addition to in vivo targeting of self-antigens to RBCs using antibodies, self-antigens have also been conjugated to RBCs ex vivo, then reinfused into animals. This approach has demonstrated efficacy and antigen-specific tolerance in mouse models of MS and spontaneous T1D[95].
2.8. Microparticles can function as controlled release depots to locally program tolerogenic microenvironments
In contrast to targeting cells or tissues with biomaterials, one new strategy to promote tolerance employs MPs as immunomodulatory depots to modulate the local microenvironment at the injection site. This can allow site specific modulation of the local microenvironment, directing the function of immune cells in these tissues, and ultimately generating changes in systemic immune function. Importantly, since these treatments can localize cargo at delivered sites, they provide the potential to promote tolerance while reducing systemic exposure of immunosuppressants. While most of the examples discussed in the previous section involve delivery of NPs or low molecular weight polymers, treatments to promote local tolerogenic environments using particles focus on larger MPs or scaffolds. One key aspect that differentiates delivery of MPs from NPs, is the differences in particle trafficking after injection. MPs are too large to enter lymphatics and therefore largely remain at the injection site after injection. While MPs are internalized and traffic to LNs through APCs, this process is not efficient. Therefore, MPs encapsulating immune signals can function as depots for immunomodulation of the local microenvironment.
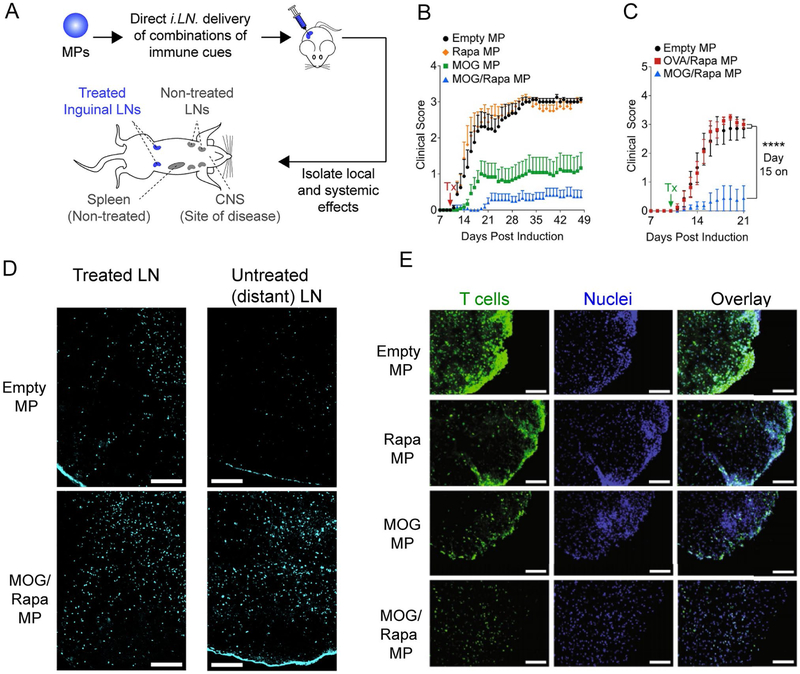
Previous work in our lab has combined immunomodulatory depots with direct LN injection to promote tolerance. This work is motivated by recent clinical trials for allergies, cancer, and diabetes involving direct, ultrasound-guided injection of lymph nodes with low, soluble doses of allergens, tumor-associated antigens, or self-antigens[96-98]. These injections are performed as outpatient procedures, and the studies highlight the exciting potential of LN injection to potently enhance both pro-immune and tolerogenic responses with striking dose sparing. However, these trials all utilized soluble immune signals, which rapidly drain from LNs after injection. By combining LN injection with PLGA MPs co-encapsulating antigens and immunostimulatory adjuvants, these cues can be retained in LNs because the particles are too large to drain from these sites. Instead, the MP depots slowly degrade to provide local controlled release of signals in the injected nodes[99]. Injecting antigen and molecular adjuvants in these depots, for example, greatly enhances antigen-specific T cell responses compared to LN injection of soluble vaccines or to MP vaccines injected at other non-LN sites. NPs exert a lower effect as well, because the smaller size allows more efficient drainage from the LN[99]. To apply this platform to promote tolerance, MPs co-encapsulating MOG and rapamycin were synthesized and injected intra-nodally (i.LN) in mice after induction of EAE [100](Figure 4A). A single LN injection inhibited symptoms of EAE compared to mice injected with empty MPs or MPs encapsulating rapa alone (Figure 4B). Interesting, while i.LN injection of MOG MPs provided a significant therapeutic effect, co-encapsulation of MOG and rapa provided a synergistic effect, since MOG/rapa MPs further decreased symptoms. This depot-mediated tolerance was antigen specific, as i.LN treatment with MPs encapsulating OVA/Rapa had no effect on EAE symptoms compared to empty MP treatment (Figure 4C). LN treatment with MOG/Rapa MPs promoted increased infilitration of TREG into treated LNs (Figure 4D). Interestingly, these local changes in the microenvironment of the treated LN also promoted systemic tolerogenic changes, as TREG frequencies were also elevated in distant, non-treated LNs (Figure 4D). Importantly, these effects were accompanied by decreased infiltration of T cells in the CNS of mice treated with MOG/Rapa MP depots (Figure 4E). Thus, local conditioning of the LN microenvironment allows generation of antigen-specific, tolerogenic cells that can migrate to other tissues to selectively control disease. In additional studies, mice received a single treatment with MOG/Rapa depots at the peak of EAE. This treatment resulted in a dramatic and permanent reversal of paralysis. While these data indicate that systemic, antigen-specific tolerance can be achieved through targeted reprogramming of the LN microenvironment, an important aspect of future research with clinical implications will be if these cured mice can mount functional immune responses against pathogens. This will help inform the clinical potential of the approach since existing therapies often leave patients susceptible to infection.
Figure 4.
A) Schematic for i.LN injection of MOG/Rapa MPs and analysis of their tolerogenic effects. B) Encapsulation of both MOG and rapamycin is required for the most potent tolerance. C) Tolerance induced by MOG/Rapa MPs is antigen specific. D) MOG/Rapa increases TREG in treated and untreated LNs. E) MOG/Rapa MPs reduce T cell proliferation in the CNS of mice induced with EAE. Adapted from [100].
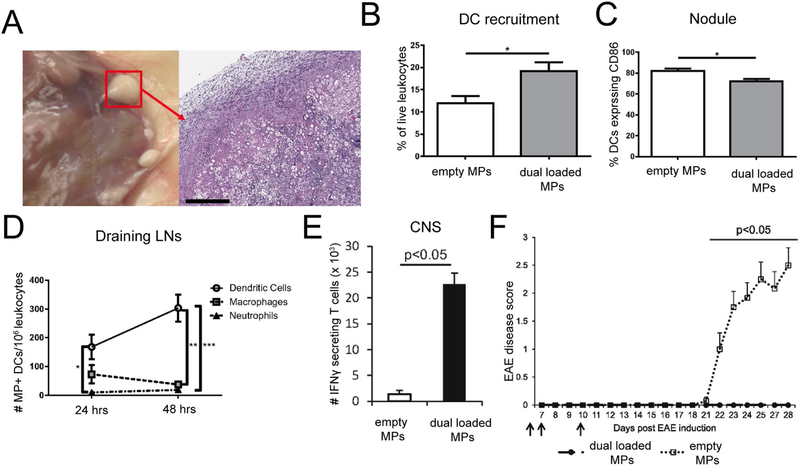
While the previous example harnessed size-restricted retention of MPs in LNs, another strategy focuses on using MPs to promote a tolerogenic microenvironment in peripheral tissue. In this strategy, depots are engineered to recruit and promote suppressive phenotypes in APCs, which then migrate to draining LNs to polarize tolerogenic immune response generated in these tissues. The Keselowski group designed an immunomodulatory depot system using dual sized microparticles encapsulating self-antigens and regulatory immune signals[101-104]. This system involves particles of two different sizes to direct the destination of different components of the therapy. First “small”, phagocytosible MPs were employed for intracellular delivery of antigens and immunomodulators active in the cytosol. Larger MPs (too large for phagocytosis) were used for extracellular delivery of immunomodulators which act on extracellular receptors. In this approach, the smaller MPs (~1μm) were synthesized to co-encapsulate a peptide insulin antigen attacked during T1D with vitamin D3, which acts as an immunosuppressant. These small MPs were then co-injected with large (~30 μm) MPs encapsulating GM-CSF, a chemoattractant which recruits DCs, and TGF-β, an immunoregulatory cytokine which promotes tolerogenic function in DCs. Administration of these depots to prediabetic NOD mice significantly decreased the incidence of spontaneous T1D[102]. To apply this platform to promote tolerance in MS models, a similar formulation was tested, but the insulin self-antigen was exchanged for MOG[101]. Subcutaneous administration of the immune signal loaded dual MPs resulted in the formation of visible nodules at the site of injection with infiltration of mononuclear cells, including DCs, and protein deposition (Figure 5A). DC infiltration into nodules increased after treatment with MPs loaded with antigens and immunomodulators, compared to empty MPs (Figure 5B). Importantly, DCs found in these nodules displayed reduced expression of the surface activation marker, CD86 (Figure 5C). DCs with internalized MPs were found within nodules and also in draining LNs. (Figure 5D). Additionally, when loaded dual MPs were administered to mice with EAE, DCs in draining LNs had a tolerogenic phenotype with reduced expression of MHCII and PD-L1 compared to empty MP treated mice. The authors then investigated how these changes in DCs polarized T cell phenotype and modulated inflammation in the CNS during EAE. MP treatment resulted in reduced pathogenic T cells expressing the inflammatory cytokines, IFNγ (Figure 5E) and IL-17, in the CNS. This reduction correlated with prevention of EAE symptoms over the 28 day study period when administered before manifestation of clinical symptoms (Figure 5F). Together, these results indicate MPs modulate the local microenvironment where DCs are recruited and adopt tolerogenic phenotypes. DCs then migrate to draining LNs and polarize T cell to phenotypes useful in controlling inflammation systemically and at sites of disease.
Figure 5.
A) Nodules form at the site of injection of dual sized MPs loaded with immune signals. B) DCs migrate to and infiltrate nodules. C) The frequency of DCs within nodules expressing CD86 is lower in mice treated with loaded MPs. D) Increased frequencies of DCs internalized MPs are found in draining LNs of loaded MP treated mice. E) Dual loaded MPs decrease the number of IFNγ secreting T cells in the CNS of mice induced with EAE. F) Dual loaded MPs inhibit the onset of EAE. Adapted from [101].
Other approaches using MP depots focus on formulations delivering immunomodulators without antigen. While these designs do not function as tolerogenic vaccines, they can serve to modulate the phenotype of previously activated T cells, or T cells undergoing priming against self-antigens being presented as a result of ongoing inflammation. This approach has been applied to locally induce TREG by providing sustained concentrations of regulatory immune signals which favor TREG differentiation over inflammatory T cell phenotypes118, 105-106]. Another strategy involves administering MP depots to locally deliver chemoattractants which selectively recruit TREG to sites of inflammation[106-110]. These treatments can be applied to organ-specific or local autoimmune disease by administering depots at the site of inflammation to locally recruit TREG to suppress inflammation. The Little group, for example, has shown PLGA MPs encapsulating the chemokine CCL22 recruits TREG to injection sites and prevents inflammation in models of dry eye disease[106] and periodontal disease[108-110]. A key advantage of this strategy is that local recruitment and retention of TREG to sites of inflammation could provide site-targeted immunosuppression; this possibility is in contrast to systemic expansion of polyclonal TREG that could cause broad immunosuppression.
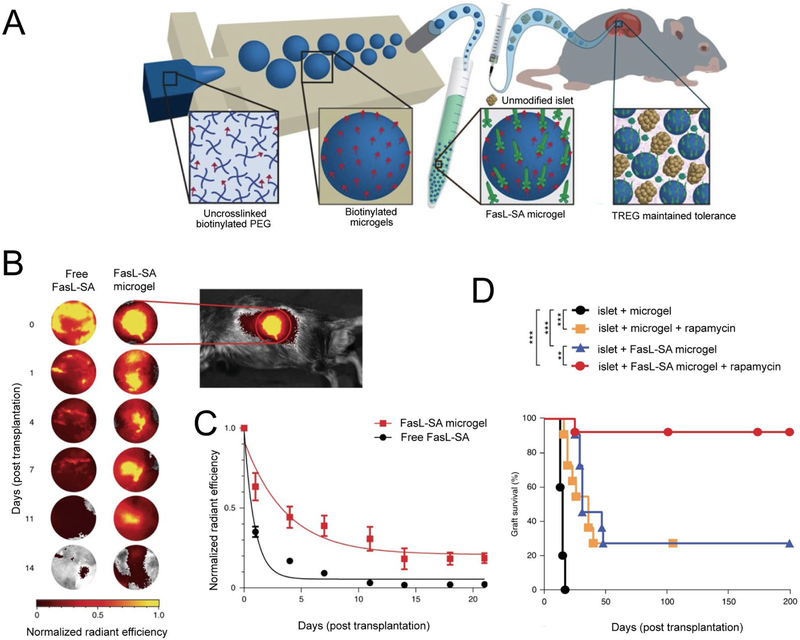
Another strategy uses co-deposition of MPs presenting Fas ligands with transplanted islets to induce tolerance. The Fas pathway is an immune checkpoint which functions to inhibit unrestrained inflammation. In particular, activated T cells upregulate Fas, and binding of this Fas ligand results in apoptosis. Headen et. al. applied this platform to promote tolerance to transplanted islets by synthesizing PEG crosslinked hydrogels (150μm) which covalently display biotin on the surface (Figure 6A)[19]. Streptavidin Fas ligands (SA-FasL) were then conjugated to the microgel surface (SA FasL microgels). After microgels conjugated with labeled Fas ligand were implanted in the kidney capsule of mice, Fas ligand was found to be presented at the implant site for over 20 days. In contrast Fas ligand signal was negligible by day 7 in mice receiving free Fas ligand (Figure 6B, 6C). To investigate how this prolonged presentation of Fas could enhance islet survival, SA-FasL microgels were co-transplanted with BalbC islets into the kidney capsules of diabetic C57BL6. Since the transplanted islets are from a different strain of mouse, the immune system of the mice receiving the transplant sees the islets as foreign. Mice receiving islet transplants and empty microgels rejected the transplants, with a median survival of 15 days. However, islets co-transplanted with SA-FasL microgels displayed significantly enhanced survival, exhibiting a median survival time of 31 days (Figure 6D). Strikingly, addition of a course of soluble rapamycin treatment (daily i.p injection for 15 days starting on the day of transplantation) to SA FasL microgels dramatically enhanced islet survival, where 90% of transplanted islets survived and functioned for over 200 days until the end of the study (Figure 6D). Importantly, this enhancement of survival was significantly higher compared to mice receiving islet transplants with rapamycin treatment alone, which displayed similar survival to islets co-transplanted with SA FasL microgels (without rapamycin). This result supports a synergistic effect of rapamycin treatment and SA FasL microgel treatment. Mechanistically, the authors demonstrated the effects of SA FasL microgels on promoting transplant tolerance were dependent on TREG, since depletion of TREG abrogated the efficacy of SA FasL microgels. These studies utilized a transgenic mouse model engineered to express TREG that can be depleted by injection of diphtheria toxin. In the studies, diabetes was chemically induced, then mice received transplants with SA-FasL microgels and rapamycin treatment. At day 50, mice were either left untreated or treated with diphtheria toxin to deplete TREG. All mice undergoing TREG depletion rejected islet transplants within 32 days, while the mice not receiving the TREG depletion maintained graft function until the end of the 200 day study. Finally, the authors demonstrated the SA-FasL microgel platform could be applied to promote survival of transplants in the epididymal fat pad, which is analogous to the omentum in humans and is a clinically relevant transplant site. Together this work demonstrates that materials can be harnessed to provide prolonged and localized presentation of FasL to promote tolerance towards transplanted islets. The SA FasL microgels potentially represents a simple off the shelf platform that could inhibit transplant rejection without the need for continual systemic immunosuppression, which is the current standard treatment for transplants. While this design demonstrated striking efficacy a short course of systemic immunosuppression was still required. Additional studies in large animal models are also necessary to further demonstrate the potential for clinical application.
Figure 6.
A) Schematic for the synthesis and implantation of SA FasL microgels. Conjugation of FasL to microgels increases FasL persistances at the site of implantation. Shown are B) representative images and C) summary data of detected SA FasL signal over time. D) SA FasL microgels prolong islet graft survival in diabetic mice when combined with a short course of systemic immunosuppression. Adapted from [19].
3. Biomaterials display intrinsic immunogenic material properties which can be harnessed to promote tolerance
The above sections describe tolerogenic strategies using materials as tools to deliver immune signals. An interesting area of investigation, however, focuses on the immunomodulatory effects of biomaterials themselves, in the absence of any explicit immune signals. As described above in Section 2.4, recent studies have demonstrated that biomaterials display intrinsic properties that can drive inflammatory or pro-immune function in immune cells. Other recent studies have described tolerogenic properties of various biomaterials, including synthetic polymers such as PLGA and PS[68-69], QDs[111-112], gold NPs[113] and cellulose nanofibers[114]. These intrinsic biomaterial properties are now starting to be explored in therapeutic contexts as tools to promote tolerance in preclinical models of autoimmune disease.
3.1. Nanoparticles can promote tolerogenic immune function through autophagy
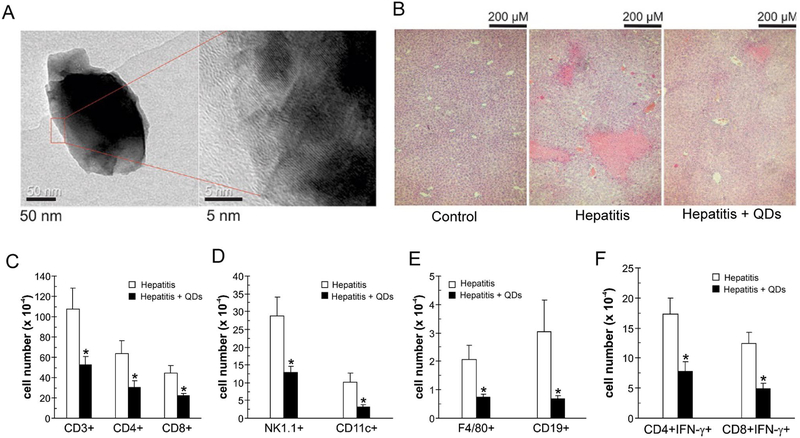
Recent studies demonstrate biomaterials can modulate immune cell function by enhancing or interfering with physiologic processes in cells. One example includes autophagy, a process through which cells recycle cellular components in response to stresses such as nutrient deprivation and oxidative stress. Autophagy is now known to be a regulator of immune cell functions, including proliferation, cytokine secretion, and survival/maintenance[115] . Damage from reactive oxygen species (ROS), which are produced by inflammatory innate immune cells and cytotoxic T cells can stimulate autophagy. Further, signaling through the mTOR pathway negatively regulates autophagy, and the ability of a variety of biomaterial NPs to regulate mTOR activity and authophagy in different cell types has been investigated[116]. However only recently have these effects been investigated and applied to immune cells for tolerogenic function. For example, recent work has evaluated the ability of graphene QDs to inhibit excess inflammation in a model of immune-mediated liver damage[111]. In particular, these anti-inflammatory effects of QDs have been linked to autophagy. Oval-shaped Graphene QDs were synthesized with lengths of approximately 40nm and heights of 3nm (Figure 7A) [111] In mice QDs accumulated preferentially in the liver after i.v. administration and reduced liver damage in an inducible model of immune mediated hepatitis. This was indicated by histology showing decreased tissue loss in QD treated mice, where red spots are visible in liver sections of mice induced with hepatitis are areas of damaged tissue (Figure 7B). In this model lower levels of hepatic enzymes alanine aminotransferase (ALT) and asparatate aminotransferase (AST) were detected in serum of QD treated mice. QD treatment significantly lowered infiltration of T cells (Figure 7C, CD3, CD4, CD8), NK cells (Figure 7D, NK1.1),dendritic cells (Figure 7D, CD11c), macrophages (Figure 7E, F4/80+) and B cells (Figure 7E, CD19) into the liver. Additionally, the number of IFNγ producing CD4 and CD8 T cells was reduced (Figure 7F), as was the expression of proteins involved in autophagy in the liver.
Figure 7.
A) TEM image of graphene QDs. B) QDs reduce liver damage in immune mediated hepatitis, as seen by reduced tissue damage (red spots). QDs reduce infiltration of C) T cells, D) NK cells (NK1.1) and DCs (CD11c), E) macrophages (F4/80) and B cells (CD19). F) QDs reduce the frequency of IFNγ producing CD4 and CD8 T cells. Adapted from [111].
Other studies have investigated QDs to drive autophagy as well[112] These studies demonstrated that graphene QDs reduced secretion of inflammatory cytokines, production of ROS and expression of CD40, CD80 and CD86 in DCs. These alterations in the inflammatory state of DCs resulted in reduced T cell proliferation and reduced frequency of T cells secreting IL-17 and IFNγ. At the same time, QDs increased the frequency of T cells secreting regulatory cytokines such as IL-10 and IL-4 during T cell co-cultures. Together, the results demonstrate graphene QDs can bias DCs toward functional, tolerogenic phenotypes. Mechanistically, these studies show QDs inhibited nuclear translation of NF-κB, which is a master regulator of DC activation, and inhibited mTOR which led to an increase in autophagy. Importantly, the critical role of autophagy was demonstrated by the finding that blocking autophagy in DCs using RNAi negated the effects of QDs on tolerogenic DC development. While these investigations confirm the ability of QDs to modulate autophagy, a limitation of existing knowledge is the lack of understanding with respect to what material properties influence the signaling of autophagy or other immune pathways. Understanding how physicochemical features – charge, size, geometry, hydrophobicity – and if relationships for specific materials are generalizable across classes of materials will aid in the rational design of biomaterial-based tolerogenic therapies.
3.2. Intrinsic properties of biomaterials can alter targeting and trafficking of immune cells
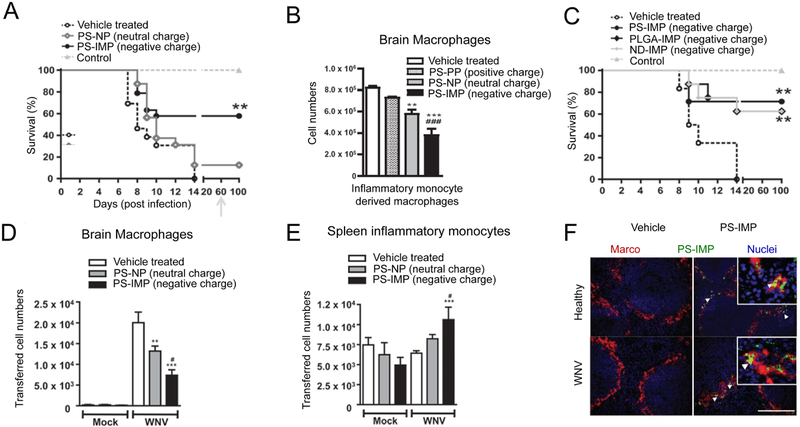
The strategies discussed in Section 2.5 demonstrate polymeric NPs functionalized with or encapsulating self-antigens can promote antigen-specific tolerance through delivery to tolerogenic zones in lymphoid organs. Interestingly, recent work has investigated the role of the NP itself in promoting tolerance – and toward the challenge just alluded to – probed which NP properties are involved. In one study, negatively charged polystyrene particles (500nm) were found to display tolerogenic properties [69]. These immune modifying particles (PS-IMP) were first investigated in a mouse model of west nile virus (WNV) encephalomyelitis. In this model, WNV brain infection leads to the infiltration of inflammatory monocytes, resulting in weight loss and morbidity. Mice were injected i.v. daily with PS-IMPs for 5 days following WNV infection. Treatment with PS-IMPs reduced symptoms and promoted 60% survival of infected mice, compared to 10% in untreated mice (Figure 8A). The authors reasoned the negative charge of the NPs played a role in inducing tolerance, since PS nanoparticles with a neutral (PS-NPs) did not increase survival compared to untreated mice. Additionally, an increase in survival correlated with a reduction in infiltration of inflammatory monocyte to the brain (Figure 8B). While positively charged PS-NPs (PS-PPs) did not decrease inflammatory monocyte infiltration compared to untreated mice, PS-NPs and PS-IMPs significantly decreased infiltration with PS-IMPs displaying the greatest effect. To further investigate the role of charge, WNV infected mice were infused with negatively charged PS-IMPs, negatively charged PLGA NPs (PLGA-IMPs), or negatively charged nanodiamonds (ND-IMPs). All three treatments protected mice from morbidity with similar efficacy (Figure 8C). Interestingly, PS-IMP treatment led to a significant increase in the number of inflammatory monocytes in the spleens of WNV-infected mice. To probe the role of PS-IMPs on modulating inflammatory monocyte trafficking, inflammatory monocytes were infused into WNV-infected mice, followed by infusion of PS-IMPs. In WNV-infected mice that did not receive PS-IMPs, high levels inflammatory monocytes migrated to the brain and differentiated into macrophages. In contrast, trafficking of monocytes into the brain was reduced (Figure 8D) and trafficking to the spleen was increased in mice treated with PS-IMPs (Figure 8E). Further, greater than 30% of monocytes in the spleen had internalized PS-IMPs. PS-IMPs were shown to co-localize predominantly with monocytes that expressed the MARCO scavenger receptor (Figure 8F). This indicated targeting of PS-IMPs to MARCO expressing monocytes in the spleen played a role in inhibiting inflammatory monocyte migration to sites of inflammation.
Figure 8.
A) negatively charged PS-IMPs protect mice from inflammation in WNV encephalomyelitis. B) Decreasing charge on PS NPs correlates with decreasing infiltration of macrophages into the brains in mice during WNV encephalomyelitis. C) PLGA, or NDs with similar charges to PS-IMPs confer similar efficacy to PS-IMPs in WNV encephalomyelitis. Inflammatory monocytes were injected i.v. into mice infected with WNV, and mice were treated with PS-IMPs. PS-IMPS D) decrease trafficking of inflammatory monocytes into the brain and differentiation into macrophages, while F) increasing migration to the spleen. G) PS-IMPs co-localize in domains expressing MARCO in the marginal zones of spleens. Adapted from [69].
To test if MARCO targeting was critical for PS-IMP efficacy, a mouse model of drug-induced peritoneal inflammation was used. PS-IMPs were infused into wild type or MARCO knockout mice and peritoneal inflammation was induced. While PS-IMPs inhibited migration of inflammatory macrophages into the peritoneum of wild type mice, inflammatory macrophage migration into the peritoneum was similar in MARCO knockout mice treated with vehicle or PS-IMPs. Further, PS-IMP uptake in macrophages was significantly lower in MARCO knockout mice. Together these results indicate that PS-IMPs target inflammatory monocytes in a MARCO-dependent manner, causing sequestration of monocytes in spleens instead of migration to sites of inflammation. Negative charge on the particle surface is critical for these effects and is generalizable across different biomaterial NPs. Excitingly, PS-IMPs were effective in preventing symptoms of inflammatory macrophage activation in sites of inflammation during several additional models, including EAE, cardiac and kidney reperfusion injury, and inflammatory bowel disease. An additional study demonstrated similarly sized negatively charged PLGA NPs could prevent inflammatory monocyte damage in a model of spinal cord injury[68]. These studies demonstrate that physiochemical properties of engineered tolerogenic therapies can have dramatic effects on the treatment efficacy and should be considered. Investigation of other material properties that potential modulate immune response will help inform the design criteria of materials engineered to promote tolerance.
4. Scaffolds can be engineered to promote tolerance
The examples above discuss the use of particles to promote tolerance. Another strategy involves implantation of macroscale 3D scaffolds, which typically are porous materials that can be engineered to mimic native ECM. Scaffolds have been heavily explored in tissue engineering since they provide a 3D structure for cells to facilitate cell adhesion and engraftment. Recently, scaffolds have been applied to engineer immune function and promote tolerance[117], where the ability of scaffolds to provide control over the spatiotemporal presentation of self-antigens and/or immunomodulators has been leveraged. Such immune cues can be incorporated within the bulk scaffold, or displayed on the scaffold surface. Additionally, immune cues can be incorporated in scaffolds after encapsulation in or adsorption on the surface of particles loaded in the scaffolds, providing the ability to finely-tune release kinetics. These ideas have been applied to induce antigen-specific tolerance in mouse models of autoimmune disease as well as allogenic transplants.
4.1. Scaffolds can be formulated as implantable tolerogenic vaccines for autoimmune disease.
One specific strategy utilizing scaffolds is to implant or inject scaffolds incorporating self-antigens and immunomodulatory cues to serve as a tolerogenic vaccine [103, 118-119]. Similar to the dual sized MP vaccine delivery systems discussed in Section 2.8, these designs aim to locally deliver immune cues and engineer a tolerogenic microenvironment which can then promote systemic tolerance. One potential advantage of utilizing a scaffold is the addition of a 3D structure which supports immune cell infiltration. Scaffolds provide a high level of control over the spaciotemporal presentation of self-antigens and regulatory cues, and this ability can aid in directing the phenotype of infiltrating cells. Further, scaffolds can be retrieved after implantation or injection, serving as a unique mechanistic tool to monitor local immune response[120-121]. The potential of harnessing scaffolds to engineer antigen-specific immune response have been previously demonstrated by the Mooney group. In these reports, immunomodulatory scaffolds delivering, tumor lysate, CpG – a TLRa, and GM-CSF functioned as an effective anti-tumor vaccine in preclinical models of melanoma. Excitingly, this strategy is currently being tested in phase 1 clinical trials (Clinical trial # NCT01753089). Recently, this strategy has been modified and applied to promote antigen specific tolerance in T1D. In this approach, injectable porous alginate scaffolds were synthesized with GM-CSF integrated in the constructs to serve as a chemoattractant for the recruitment of DCs into the [118-119]. prior to encapsulation within the scaffold GM-CSF was conjugated to gold NPs (AuNP + GM-CSF). AuNP conjugation provided sustained release of GM-CSF in vitro compared to scaffolds encapsulating unconjugated GM-CSF in the alginate. Scaffolds with AuNP + GM-CSF enhanced recruitment of DCs in vivo, where the total number of DCs which migrated into the scaffold was much higher over the course of 15 days compared to scaffolds delivering GM-CSF without AuNPs. Importantly, DCs within scaffolds containing AuNP + GM-CSF exhibited an immature phenotype, with reduced expression of surface activation markers compared to empty gels. This result indicates scaffolds may help drive tolerogenic adaptive immune functions. An additional report expanded this platform by formulating gels with an islet self-antigen (BDC) covalently-linked to the scaffold [118]. When mice were injected with these scaffolds, antigen-specific T cells migrated into scaffolds and differentiated into TREG, with over 60% of antigen specific T cells expressing the TREG marker FoxP3. Additionally, the frequency of BDC-specific TREG in islets was increased, which indicates local changes in the immune microenvironment of the scaffold also promoted systemic tolerance at sites of disease.
4.2. Scaffolds can promote a local tolorogenic environment to enhance transplant tolerance
In addition to serving as tolerogenic vaccines, scaffolds have also been applied to promote tolerance in islet transplantation [122-124]. More broadly in the diabetes field, scaffolds have been heavily investigated as a strategy to improve engraftment and survival of islet allografts; one scaffold for islet transplantation is currently being tested in clinical trials[125]. However, in this design, the scaffold functions primarily to support the adherence, survival and function of islets without actively promoting islet specific tolerance. In this study daily systemic immunosuppression is administered to prevent or delay immune mediated rejection of the islets within scaffolds. To overcome this challenge, recent pre-clinical and clinical investigations have focused on using biomaterials to encapsulate islets in microcapsules in order to isolate them from the immune system to prevent rejection. However, these features might also hinder islet function and cause difficulties for long term survival. Alternatively, promoting permanent tolerance toward islet allografts could negate the need for islet encapsulation.
Scaffolds which function to both aid in islet engraftment and promote tolerance have been engineered and tested in preclinical models. In one example, PLGA scaffolds were used to co-deliver islets and the immunomodulatory cytokine TGF-β, which inhibits activation and recruitment of immune cells and promotes TREG[124]. Inclusion of TGF-β within the scaffold reduced the infiltration of innate immune cells into the scaffold, including DCs, NK cells and macrophages. While this inhibition of leukocyte migration correlated which prolonged graft survival compared to unloaded scaffolds, all transplanted islets were eventually rejected. Another report investigated islet transplantation within scaffolds loaded with the immunomodulatory cytokine IL-33 [122]. In this study scaffolds delivering IL-33 enhanced TREG frequencies within the scaffold, and significantly enhanced graft survival compared to mice treated with scaffolds loaded with BSA. However < 15% of mice treated with scaffolds delivering IL-33 displayed long term survival. Incorporation of additional immunomodulators may enhance the efficacy of both of these examples.
In contrast to delivering immunomodulators within scaffolds, scaffolds can also be used as a vehicle to support co-delivery of islets and TREG [123]. PLGA scaffolds co-delivering TREG with islets prolonged survival after transplantation in diabetic NOD mice compared to mice receiving islet transplants in scaffolds without TREG. Additionally, protection was only observed in mice receiving TREG in the scaffold, as i.v. injection with an equivalent number of TREG had no therapeutic effect. Interestingly, it was observed that TREG from the host gradually infiltrate scaffolds and replace the adoptively transferred TREG over time. Together these results demonstrate scaffolds can be utilized to promote a tolerogenic microenvironment at the transplant site to inhibit immune mediated rejection of transplanted islets. Although scaffolds delivering TREG promoted long term survival in >50% of mice in the model tested, this strategy faces challenges associated with ex vivo expansion and culture of TREG. Further improvements in efficacy as well as testing in larger animal models are needed. Investigation of delivering other immunomodulators may enhance these designs.
5. Conclusion
The examples discussed in this review demonstrate the exciting potential for engineering biomaterials that promote tolerance that is safer, more selective, and more potent than current immunotherapies. While the majority of the work in this field has been investigated in preclinical models, a handful of these technologies have advanced toward clinical trials. Additionally, many of the studies discussed here have provided new mechanistic insight into how the delivery of immune signals can program tolerance and how material properties affect immune cell function. A greater understanding of these mechanisms will help the design of future biomaterial therapies. Finally, in the future biomaterials can be applied to emerging areas that are shedding new light on immune tolerance. These include immunometabolism, where exciting links between immune function and metabolism are being discovered [126], and studies demonstrating alterations in the gut microbiome can have a profound effect in autoimmune disease[127].
Acknowledgements
This work was supported in part by the United States Department of Veterans Affairs (Award # 1I01BX003690), the National Institutes of Health (Award # R01EB026896), the Damon Runyon Foundation (Award # DRR3415), and NSF CAREER Award # 1351688. J.M.G. is Pre-doctoral Fellow supported in part by the PhRMA Foundation Pharmaceutics Program.
Biography

Joshua M. Gammon is a PhD candidate in the Fischell Department of Bioengineering at the University of Maryland. His research at the interface of biomaterials with immunology aims to design new materials to modulate the immune response. Specifically he is interested in designing biomaterial based immunotherapies to promote safer and more effective treatments for autoimmune disease.

Christopher M. Jewell is an Associate Professor and Associate Chair in the Fischell Department of Bioengineering at the University of Maryland. Dr. Jewell also holds an appointment in the United States Department of Veterans Affairs at the Baltimore VA Medical Center. Dr. Jewell’s research spans engineering and immunology to study and manipulate immune function for therapeutic vaccines targeting autoimmunity and cancer. He earned his PhD in Chemical Engineering from the University of Wisconsin, then worked as a consultant at the Boston Consulting Group in the Healthcare Practice. Dr. Jewell completed his postdoctoral training at MIT and Harvard, launching his own lab 2012.
Footnotes
Disclosures
C.M.J. is an employee of the Baltimore VA Medical Center. The views reported in this paper do not reflect the views of the Department of Veterans Affairs or the United States Government. C.M.J. has an equity position in Cellth Systems, LLC.
Contributor Information
Joshua M. Gammon, Fischell Department of Bioengineering, University of Maryland, 8278 Paint Branch Drive RM 5110, College Park, MD 20742, USA
Prof. Christopher M. Jewell, Fischell Department of Bioengineering, University of Maryland, 8278 Paint Branch Drive RM 5110, College Park, MD 20742, USA cmjewell@umd.edu; Robert E. Fischell Institute for Biomedical Devices, 8278 Paint Branch Drive, College Park, MD 20742, USA; United States Department of Veterans Affairs, Baltimore VA Medical center, 10. N Green Street, Baltimore, Maryland 21201, USA; Department of Microbiology and Immunology, University of Maryland Medical School, 685 West Baltimore Street, HSF-I Suite 380, Baltimore, MD, 21201, USA; Marlene and Stewart Greenebaum Cancer Center, 22 South Greene Street, Baltimore, MD 21201, USA
References
- [1].Luo XR, Miller SD, Shea LD, Annu Rev Biomed Eng 2016, 18, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].De Groot AS, Scott DW, Trends Immunol 2007, 28, 482. [DOI] [PubMed] [Google Scholar]
- [3].Ganguly D, Haak S, Sisirak V, Reizis B, Nat Rev Immunol 2013, 13, 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dominguez-Villar M, Hafler DA, Nat Immunol 2018, 19, 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rosenblum MD, Gratz IK, Paw JS, Abbas AK, Sci Transl Med 2012, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM, Trends Immunol 2018, 39, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kawasaki T, Kawai T, Front Immunol 2014, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Look M, Saltzman WM, Craft J, Fahmy TM, Biomaterials 2014, 35, 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Look M, Stern E, Wang QA, DiPlacido LD, Kashgarian M, Craft J, Fahmy TM, J Clin Invest 2013, 123, 1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tang L, Azzi J, Kwon M, Mounayar M, Tong R, Yin Q, Moore R, Skartsis N, Fan TM, Abdi R, Cheng J, J Transplant 2012, 2012, 896141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ratay ML, Balmert SC, Bassin EJ, Little SR, Acta Biomater 2018, 71, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gammon JM, Adapa AR, Jewell CM, J Biomed Mater Res A 2017, 105, 2977. [DOI] [PubMed] [Google Scholar]
- [13].Gammon JM, Tostanoski LH, Adapa AR, Chiu YC, Jewell CM, J Control Release 2015, 210, 169. [DOI] [PubMed] [Google Scholar]
- [14].Gosselin EA, Tostanoski LH, Jewell CM, Aaps J 2017, 19, 1175. [DOI] [PubMed] [Google Scholar]
- [15].Ballester M, Jeanbart L, de Titta A, Nembrini C, Marsland BJ, Hubbell JA, Swartz MA, Sci Rep-Uk 2015, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Srivastava KD, Siefert A, Fahmy TM, Caplan MJ, Li XM, Sampson HA, J Allergy Clin Immun 2016, 138, 536. [DOI] [PubMed] [Google Scholar]
- [17].Jhunjhunwala S, Balmert SC, Raimondi G, Dons E, Nichols EE, Thomson AW, Little SR, J Control Release 2012, 159, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Balmert SC, Donahue C, Vu JR, Erdos G, Falo LD Jr., Little SR, J Control Release 2017, 261, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Headen DM, Woodward KB, Coronel MM, Shrestha P, Weaver JD, Zhao H, Tan M, Hunckler MD, Bowen WS, Johnson CT, Shea L, Yolcu ES, Garcia AJ, Shirwan H, Nat Mater 2018, 17, 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bandyopadhyay A, Fine RL, Demento S, Bockenstedt LK, Fahmy TM, Biomaterials 2011, 32, 3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, Buechler CR, Quinn KM, Smelkinson MG, Vanek O, Cawood R, Hills T, Vasalatiy O, Kastenmuller K, Francica JR, Stutts L, Tom JK, Ryu KA, Esser-Kahn AP, Etrych T, Fisher KD, Seymour LW, Seder RA, Nat Biotechnol 2015, 33, 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Andorko JI, Jewell CM, Bioeng Transl Med 2017, 2, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roberts RA, Eitas TK, Byrne JD, Johnson BM, Short PJ, McKinnon KP, Reisdorf S, Luft JC, DeSimone JM, Ting JP, Biomaterials 2015, 72, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Buyuktimkin B, Wang Q, Kiptoo P, Stewart JM, Berkland C, Siahaan TJ, Mol Pharm 2012, 9, 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen N, Kroger CJ, Tisch RM, Bachelder EM, Ainslie KM, Adv Healthc Mater 2018, 7, e1800341. [DOI] [PubMed] [Google Scholar]
- [26].Peine KJ, Guerau-de-Arellano M, Lee P, Kanthamneni N, Severin M, Probst GD, Peng H, Yang Y, Vangundy Z, Papenfuss TL, Lovett-Racke AE, Bachelder EM, Ainslie KM, Mol Pharm 2014, 11, 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Casey LM, Pearson RM, Hughes KR, Liu JMH, Rose JA, North MG, Wang LZ, Lei M, Miller SD, Shea LD, Bioconjug Chem 2018, 29, 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].LaMothe RA, Kolte PN, Vo T, Ferrari JD, Gelsinger TC, Wong J, Chan VT, Ahmed S, Srinivasan A, Deitemeyer P, Maldonado RA, Kishimoto TK, Front Immunol 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O'Neil C, Altreuter DH, Browning E, Johnston L, Farokhzad OC, Langer R, Scott DW, von Andrian UH, Kishimoto TK, P Natl Acad Sci USA 2015, 112, E156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Meliani A, Boisgerault F, Hardet R, Marmier S, Collaud F, Ronzitti G, Leborgne C, Verdera HC, Sola MS, Charles S, Vignaud A, van Wittenberghe L, Manni G, Christophe O, Fallarino F, Roy C, Michaud A, Ilyinskii P, Kishimoto TK, Mingozzi F, Nat Commun 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kishimoto TK, Ferrari JD, LaMothe RA, Kolte PN, Griset AP, O'Neil C, Chan V, Browning E, Chalishazar A, Kuhlman W, Fu FN, Viseux N, Altreuter DH, Johnston L, Maldonado RA, Nat Nanotechnol 2016, 11, 890. [DOI] [PubMed] [Google Scholar]
- [32].Lim HH, Yi HQ, Kishimoto TK, Gao FQ, Sun BD, Kishnani PS, Molec Genet Metab Re 2017, 13, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mazor R, King EM, Onda M, Cuburu N, Addissie S, Crown D, Liu XF, Kishimoto TK, Pastan I, P Natl Acad Sci USA 2018, 115, E733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang AH, Rossi RJ, Yoon J, Wang H, Scott DW, Cell Immunol 2016, 301, 74. [DOI] [PubMed] [Google Scholar]
- [35].Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ, Proc Natl Acad Sci U S A 2012, 109, 11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yeste A, Takenaka MC, Mascanfroni ID, Nadeau M, Kenison JE, Patel B, Tukpah AM, Babon JA, DeNicola M, Kent SC, Pozo D, Quintana FJ, Sci Signal 2016. 9, ra61. [DOI] [PubMed] [Google Scholar]
- [37].Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O'Hagan DT, Petrilli V, Tschopp J, O'Neill LAJ, Lavelle EC, P Natl Acad Sci USA 2009, 106, 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moyano DF, Goldsmith M, Solfiell DJ, Landesman-Milo D, Miranda OR, Peer D, Rotello VM, J Am Chem Soc 2012, 134, 3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y, Kawaguchi A, Hasegawa H, Kajino K, Ninomiya T, Ijiro K, Sawa H, Acs Nano 2013, 7, 3926. [DOI] [PubMed] [Google Scholar]
- [40].Sunshine JC, Perica K, Schneck JP, Green JJ, Biomaterials 2014, 35, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, Green JJ, Small 2015, 11, 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Andorko JI, Hess KL, Pineault KG, Jewell CM, Acta Biomater 2016, 32, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Andorko JI, Pineault KG, Jewell CM, J Biomed Mater Res A 2017, 105, 1219. [DOI] [PubMed] [Google Scholar]
- [44].Dold NM, Zeng Q, Zeng X, Jewell CM, Acta Biomater 2018, 68, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wibroe PP, Anselmo AC, Nilsson PH, Sarode A, Gupta V, Urbanics R, Szebeni J, Hunter AC, Mitragotri S, Mollnes TE, Moghimi SM, Nat Nanotechnol 2017. 12, 589. [DOI] [PubMed] [Google Scholar]
- [46].Hess KL, Andorko JI, Tostanoski LH, Jewell CM, Biomaterials 2017, 118, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang PP, Chiu YC, Tostanoski LH, Jewell CM, Acs Nano 2015, 9, 6465. [DOI] [PubMed] [Google Scholar]
- [48].Zhang P, Andorko JI, Jewell CM, Biotechnol Bioeng 2017, 114, 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chiu YC, Gammon JM, Andorko JI, Tostanoski LH, Jewell CM, Acs Biomater Sci Eng 2015, 1, 1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chiu YC, Gammon JM, Andorko JI, Tostanoski LH, Jewell CM, Acs Appl Mater Inter 2016, 8, 18722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bookstaver ML, Hess KL, Jewell CM, Small 2018, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tostanoski LH, Chiu YC, Andorko JI, Guo M, Zeng X, Zhang P, Royal W 3rd, Jewell CM, Acs Nano 2016, DOI: 10.1021/acsnano.6b04001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo XR, Terry RL, King NJC, Miller SD, J Immunol 2011, 187, 2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kheradmand T, Wang SS, Gibly RF, Zhang XM, Holland S, Tasch J, Graham JG, Kaufman DB, Miller SD, Shea LD, Luo XR, Biomaterials 2011, 32, 4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kheradmand T, Wang S, Bryant J, Tasch JJ, Lerret N, Pothoven KL, Houlihan JL, Miller SD, Zhang ZJ, Luo X, J Immunol 2012, 189, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Prasad S, Kohm AP, McMahon JS, Luo XR, Miller SD, J Autoimmun 2012, 39, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang S, Zhang X, Zhang L, Bryant J, Kheradmand T, Hering BJ, Miller SD, Luo X, Cell Transplant 2015, 24, 1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC, Blood 2011, 117, 5403. [DOI] [PubMed] [Google Scholar]
- [59].Lutterotti A, Yousef S, Sputtek A, Stumer KH, Stellmann JP, Breiden P, Reinhardt S, Schulze C, Bester M, Heesen C, Schippling S, Miller SD, Sospedra M, Martin R, Sci Transl Med 2013, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Smarr CB, Yap WT, Neef TP, Pearson RM, Hunter ZN, Ifergan I, Getts DR, Bryce PJ, Shea LD, Miller SD, P Natl Acad Sci USA 2016, 113, 5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pearson RM, Casey LM, Hughes KR, Wang LZ, North MG, Getts DR, Miller SD, Shea LD, Mol Ther 2017, 25, 1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bryant J, Hlavaty KA, Zhang X, Yap WT, Zhang L, Shea LD, Luo XR, Biomaterials 2014, 35, 8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kuo R, Saito E, Miller SD, Shea LD, Molecular Therapy 2017, 25, 1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hlavaty KA, McCarthy DP, Saito E, Yap WT, Miller SD, Shea LD, Biomaterials 2016, 76, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Prasad S, Neef T, Xu D, Podojil JR, Getts DR, Shea LD, Miller SD, J Autoimmun 2018, 89, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo XR, King NJC, Shea LD, Miller SD, Nature Biotechnology 2012, 30, 1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, Miller SD, Acs Nano 2014, 8, 2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jeong SJ, Cooper JG, Ifergan I, McGuire TL, Xu D, Hunter Z, Sharma S, McCarthy D, Miller SD, Kessler JA, Neurobiol Dis 2017, 108, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Getts DR, Terry RL, Getts MT, Deffrasnes C, Muller M, van Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu HL, Ma J, Martin A, Shae LD, Witting P, Kansas GS, Kuhn J, Hafezi W, Campbell IL, Reilly D, Say J, Brown L, White MY, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJC, Sci Transl Med 2014, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hess KL, Oh E, Tostanoski LH, Andorko JI, Susumu K, Deschamps JR, Medintz IL, Jewell CM, Adv Funct Mater 2017, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Carambia A, Freund B, Schwinge D, Bruns OT, Salmen SC, Ittrich H, Reimer R, Heine M, Huber S, Waurisch C, Eychmuller A, Wraith DC, Korn T, Nielsen P, Weller H, Schramm C, Luth S, Lohse AW, Heeren J, Herkel J, J Hepatol 2015, 62, 1349. [DOI] [PubMed] [Google Scholar]
- [72].Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang JG, Garabatos N, Izquierdo C, Agrawal S, Keough MB, Yong W, James E, Moore A, Yang Y, Stratmann T, Serra P, Santamaria P, Nature 2016, 530, 434. [DOI] [PubMed] [Google Scholar]
- [73].Singha S, Shao K, Yang Y, Clemente-Casares X, Sole P, Clemente A, Blanco J, Dai Q, Song FY, Liu SW, Yamanouchi J, Umeshappa CS, Nanjundappa RH, Detampel P, Amrein M, Fandos C, Tanguay R, Newbigging S, Serra P, Khadra A, Chan WCW, Santamaria P, Nat Nanotechnol 2017, 12, 701. [DOI] [PubMed] [Google Scholar]
- [74].Sugarman J, Tsai S, Santamaria P, Khadra A, Immunol Cell Biol 2013, 91, 350. [DOI] [PubMed] [Google Scholar]
- [75].Tsai SE, Shameli A, Yamanouchi J, Clemente-Casares X, Wang JG, Serra P, Yang Y, Medarova Z, Moore A, Santamaria P, Immunity 2010, 32, 568. [DOI] [PubMed] [Google Scholar]
- [76].Northrup L, Sestak JO, Sullivan BP, Thati S, Hartwell BL, Siahaan TJ, Vines CM, Berkland C, Aaps J 2014, 16, 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chittasupho C, Sestak J, Shannon L, Siahaan TJ, Vines CM, Berkland C, Mol Pharmaceut 2014, 11, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hartwell BL, Hall AS, Swafford D, Sullivan BP, Garza A, Sestak JO, Northrup L, Berkland C, Mol Pharmaceut 2016, 13, 330. [DOI] [PubMed] [Google Scholar]
- [79].Kuehl C, Thati S, Sullivan B, Sestak J, Thompson M, Siahaan T, Berkland C, J Pharm Sci-Us 2017, 106, 3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Thati S, Kuehl C, Hartwell B, Sestak J, Siahaan T, Forrest ML, Berkland C, J Pharm Sci-Us 2015, 104, 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hartwell BL, Pickens CJ, Leon M, Northrup L, Christopher MA, Griffin JD, Martinez-Becerra F, Berkland C, J Autoimmun 2018, 93, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hartwell BL, Pickens CJ, Leon M, Berkland C, Biomacromolecules 2017, 18, 1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Spiering R, Margry B, Keijzer C, Petzold C, Hoek A, Wagenaar-Hilbers J, van der Zee R, van Eden W, Kretschmer K, Broere F, J Immunol 2015, 194, 4804. [DOI] [PubMed] [Google Scholar]
- [84].Wadwa M, Klopfleisch R, Buer J, Westendorf AM, Eur J Microbiol Immunol (Bp) 2016, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tabansky I, Keskin DB, Watts D, Petzold C, Funaro M, Sands W, Wright P, Yunis EJ, Najjar S, Diamond B, Cao Y, Mooney D, Kretschmer K, Stern JNH, Mol Med 2018, 24, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lewis JS, Zaveri TD, Crooks CP, Keselowsky BG, Biomaterials 2012, 33, 7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Chen YL, Wu J, Wang JJ, Zhang WJ, Xu BH, Xu XJ, Zong L, Diabetologia 2018, 61, 1384. [DOI] [PubMed] [Google Scholar]
- [88].McHugh MD, Park J, Uhrich R, Gao W, Horwitz DA, Fahmy TM, Biomaterials 2015, 59, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Otomo K, Koga T, Mizui M, Yoshida N, Kriegel C, Bickerton S, Fahmy TM, Tsokos GC, J Immunol 2015, 195, 5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Li H, Tsokos MG, Bickerton S, Sharabi A, Li Y, Moulton VR, Kong P, Fahmy TM, Tsokos GC, Jci Insight 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Azzi J, Yin Q, Uehara M, Ohori S, Tang L, Cai KM, Ichimura T, McGrath M, Maarouf O, Kefaloyianni E, Loughhead S, Petr J, Sun Q, Kwon M, Tullius S, von Andrian UH, Cheng JJ, Abdi R, Cell Rep 2016, 15, 1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kontos S, Kourtis IC, Dane KY, Hubbell JA, P Natl Acad Sci USA 2013, 110, E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Grimm AJ, Kontos S, Diaceri G, Quaglia-Thermes X, Hubbell JA, Sci Rep-Uk 2015, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lorentz KM, Kontos S, Diaceri G, Henry H, Hubbell JA, Sci Adv 2015, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Pishesha N, Bilate AM, Wibowo MC, Huang NJ, Li ZY, Dhesycka R, Bousbaine D, Li HJ, Patterson HC, Dougan SK, Maruyama T, Lodish HF, Ploegh HL, P Natl Acad Sci USA 2017, 114, 3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ludvigsson J, Wahlberg J, Casas R, N Engl J Med 2017, 376, 697. [DOI] [PubMed] [Google Scholar]
- [97].Witten M, Malling HJ, Blom L, Poulsen BC, Poulsen LK, J Allergy Clin Immunol 2013, 132, 1248. [DOI] [PubMed] [Google Scholar]
- [98].Ribas A, Weber JS, Chmielowski B, Comin-Anduix B, Lu D, Douek M, Ragavendra N, Raman S, Seja E, Rosario D, Miles S, Diamond DC, Qiu Z, Obrocea M, Bot A, Clin Cancer Res 2011, 17, 2987. [DOI] [PubMed] [Google Scholar]
- [99].Jewell CM, Lopez SCB, Irvine DJ, P Natl Acad Sci USA 2011, 108, 15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tostanoski LH, Chiu YC, Gammon JM, Simon T, Andorko JI, Bromberg JS, Jewell CM, Cell Rep 2016, 16, 2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cho JJ, Stewart JM, Drashansky TT, Brusko MA, Zuniga AN, Lorentsen KJ, Keselowsky BG, Avram D, Biomaterials 2017, 143, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lewis JS, Dolgova NV, Zhang Y, Xia CQ, Wasserfall CH, Atkinson MA, Clare-Salzler MJ, Keselowsky BG, Clin Immunol 2015, 160, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yoon YM, Lewis JS, Carstens MR, Campbell-Thompson M, Wasserfall CH, Atkinson MA, Keselowsky BG, Sci Rep-Uk 2015, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Lewis JS, Roche C, Zhang Y, Brusko TM, Wasserfall CH, Atkinson M, Clare-Salzler MJ, Keselowsky BG, J Mater Chem B 2014, 2, 2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ratay ML, Balmert SC, Bassin EJ, Little SR, Acta Biomaterialia 2018, 71, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ratay ML, Balmert SC, Acharya AP, Greene AC, Meyyappan T, Little SR, Sci Rep-Uk 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Jhunjhunwala S, Raimondi G, Glowacki AJ, Hall SJ, Maskarinec D, Thorne SH, Thomson AW, Little SR, Adv Mater 2012, 24, 4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Francisconi CF, Vieira AE, Biguetti CC, Glowacki AJ, Trombone APF, Letra A, Silva RM, Sfeir CS, Little SR, Garlet GP, J Endodont 2016, 42, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Araujo-Pires AC, Vieira AE, Francisconi CF, Biguetti CC, Glowacki A, Yoshizawa S, Campanelli AP, Trombone APF, Sfeir CS, Little SR, Garlet GP, J Bone Miner Res 2015, 30, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Glowacki AJ, Yoshizawa S, Jhunjhunwala S, Vieira AE, Garlet GP, Sfeir C, Little SR, P Natl Acad Sci USA 2013, 110, 18525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Volarevic V, Paunovic V, Markovic Z, Markovic BS, Misirkic-Marjanovic M, Todorovic-Markovic B, Bojic S, Vucicevic L, Jovanovic S, Arsenijevic N, Holclajtner-Antunovic I, Milosavljevic M, Dramicanin M, Kravic-Stevovic T, Ciric D, Lukic ML, Trajkovic V, Acs Nano 2014, 8, 12098. [DOI] [PubMed] [Google Scholar]
- [112].Tomic S, Janjetovic K, Mihajlovic D, Milenkovic M, Kravic-Stevovic T, Markovic Z, Todorovic-Markovic B, Spitalsky Z, Micusik M, Vucevic D, Colic M, Trajkovic V, Biomaterials 2017, 146, 13. [DOI] [PubMed] [Google Scholar]
- [113].Yang H, Fung SY, Xu SY, Sutherland DP, Kollmann TR, Liu MY, Turvey SE, Acs Nano 2015, 9, 6774. [DOI] [PubMed] [Google Scholar]
- [114].Tomic S, Kokol V, Mihajlovic D, Mircic A, Colic M, Sci Rep-Uk 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Levine B, Mizushima N, Virgin HW, Nature 2011, 469, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hulea L, Markovic Z, Topisirovic I, Simmet T, Trajkovic V, Trends Biotechnol 2016, 34, 349. [DOI] [PubMed] [Google Scholar]
- [117].Gosselin EA, Eppler HB, Bromberg JS, Jewell CM, Nat Mater 2018, 17, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Verbeke CS, Mooney DJ, Adv Healthc Mater 2015, 4, 2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Verbeke CS, Gordo S, Schubert DA, Lewin SA, Desai RM, Dobbins J, Wucherpfennig KW, Mooney DJ, Advanced Healthcare Materials 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Thelin MA, Kissler S, Vigneault F, Watters AL, White D, Koshy ST, Vermillion SA, Mooney DJ, Serwold T, Ali OA, Diabetes 2017, 66, 2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Azarin SM, Yi J, Gower M, Aguado BA, Sullivan ME, Goodman AG, Jiang EJ, Rao SS, Ren YY, Tucker SL, Backman V, Jeruss JS, Shea LD, Nat Commun 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Liu JMH, Zhang X, Joe S, Luo X, Shea LD, J Immunol Regen Med 2018, 1, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Graham JG, Zhang XM, Goodman A, Pothoven K, Houlihan J, Wang SS, Gower RM, Luo XR, Shea LD, Tissue Eng Pt A 2013, 19, 1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Liu JMH, Zhang J, Zhang XM, Hlavaty KA, Ricci CF, Leonard JN, Shea LD, Gower RM, Biomaterials 2016, 80, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Baidal DA, Ricordi C, Berman DM, Alvarez A, Padilla N, Ciancio G, Linetsky E, Pileggi A, Alejandro R, New Engl J Med 2017, 376, 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Hotamisligil GS, Immunity 2017, 47, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Li B, Selmi C, Tang R, Gershwin ME, Ma X, Cell Mol Immunol 2018, 15, 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ratay ML, Glowacki AJ, Balmert SC, Acharya AP, Polat J, Andrews LP, Fedorchak MV, Schuman JS, Vignali DAA, Little SR, J Control Release 2017, 258, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]