Abstract
Gender is an important confounding variable in biomarker development that must be incorporated into biomarker discovery and validation. Additionally, understanding of gender as a biological variable is essential for effective translation of biomarkers in animal models to human populations. Towards these ends, we conducted high-throughput targeted metabolomics using LC-MS/MS and multiplexed immunoassay analyses using a Luminex-based system in both male and female mice in a model of total-body irradiation at a radiation dose consistent with the hematopoietic acute radiation syndrome. Metabolomic and immunoassay analyses identified metabolites and cytokines that were significantly different in plasma from naïve and irradiated C57BL/6 mice consisting of equal numbers of female and male mice at 3 days after 8.0 or 8.72 Gy, an approximate LD60–70/30 dose of total-body irradiation. An additional number of metabolites and cytokines had gender-specific responses after radiation. Analyses in sham-irradiated mice illustrate the presence of stress-related changes in several cytokines due simply to undergoing the irradiation procedure absent actual radiation exposure. Basal differences in metabolite levels between female and male were also identified as well as time-dependent changes in cytokines up to 9 days post-exposure. These studies provide data toward defining the influence of gender on plasma-based biomarker candidates in a well-defined mouse model of acute radiation syndrome.
Keywords: biological indicators, radiation damage, whole body irradiation, mice
INTRODUCTION
Acute, high-dose radiation exposure causes a well-characterized set of radiation dose-dependent and time-dependent organ malfunctions that can lead to severe morbidity and death. Due to its highly proliferative nature, bone marrow (BM) is the most sensitive tissue to radiation damage (Till and McCulloch 1961; Abramson et al. 1977; Visser et al. 1984; Jones et al. 1989; Jones et al. 1990; Keller and Snodgrass 1990; Hall 2000). Exposures of 2–10 Gy in mice result in the dose dependent hematopoietic (H) acute radiation syndrome (H-ARS) leading to death within weeks from infection and/or hemorrhage if untreated(Coleman et al. 2003; Dainiak et al. 2003). The growing threat of terrorist events involving radiation, as well as the potential for radiation accidents, underscores the need for effective MCM against radiation damage, and appropriate animal models for efficacy testing of such MCM (Chua et al. 2012; Plett et al. 2012; Plett et al. 2015; Unthank et al. 2015). Candidate MCM for ARS must follow the FDA’s “Animal Rule” approval pathway since human efficacy studies are unethical to perform (Crawford 2002; FDA 2015). Biomarkers are a key element required for the establishment of drug development tools (i.e., animal models) as well as drug development under the Animal Rule (FDA 2015; FDA 2014).
Radiation exposure triggers a complicated and diverse cascade of molecular responses both directly and indirectly related to metabolism (Coy et al. 2011; Jones et al. 2014a; Singh et al. 2016; Pannkuk et al. 2017). As such, endogenous metabolism is an effective readout of altered biochemical pathways that contribute to early and late effects of irradiation (IR) exposure (Brush et al. 2007; Jones et al. 2014a; Singh et al. 2016). Radiation metabolomics has shown promise in associating molecular signatures resulting from the biophysiological response to the radiation insult. Both discovery and targeted metabolomics has been used to characterize the utility of metabolite biomarkers to inform on radiation-induced tissue injury (Patterson et al. 2010; Coy et al. 2011; Jones et al. 2014a; Jones et al. 2015a; Jones et al. 2015b; Jones et al. 2017a; Jones et al. 2017b). However, gender differences in the plasma metabolome have been reported (Trabado et al. 2017). Gender differences in radiation damage and radiation-induced metabolic diseases have also been suggested but remain poorly defined (Tonorezos et al. 2012; Tatsukawa et al. 2013; Zhang et al. 2014). Additionally, gender differences in the field of radiobiology, including ARS as well as the delayed effects of acute radiation exposure (DEARE) remain under-studied.
In addition to the metabolome, the proteome, especially radiation-induced cytokines, has been proposed as a source of radiation biomarkers. Increased levels of inflammatory cytokines occur early after radiation exposure and can persist at varying degrees of severity for several years. This has been well illustrated in atomic bomb survivors, where chronically elevated levels of pro-inflammatory cytokines such as TNF-alpha, IFN-beta, IL-6, and IL-10 have been observed (Ridker et al. 2000; Stampfer et al. 2004; Hayashi et al. 2005; Kusunoki and Hayashi 2008). Radiation-induced inflammatory cytokines are produced by several cell types including alveolar macrophages (Hallahan et al. 1989; O’Brien-Ladner et al. 1993), fibroblasts (Brach et al. 1993), glioma cells (Yamanaka et al. 1993), keratinocytes (Kirnbauer et al. 1991; Kondo et al. 1993; Muller and Meineke 2007), peritoneal macrophages, splenocytes (Cheda et al. 2008), and endothelial cells (Li et al. 2000; Gaugler et al. 2001; Gaugler et al. 2005), and mesenchymal stem cells. Cytokine production is often regulated by autocrine or paracrine positive feedback loops, further perpetuating the inflammatory status (Shao and Sheng 2007; Ogura et al. 2008).
MATERIALS AND METHODS
All irradiation studies were conducted according to the authors’ published mouse model of H-ARS (Plett et al. 2012; Plett et al. 2014; Plett et al. 2015). Brief details or slight modifications are given below.
Mice and husbandry
Specific pathogen free C57BL/6 mice (50/50 male/female; Jackson Laboratory, Bar Harbor, Maine) were received at 10 weeks of age. Weights ranged from 15.0 to 21.5 gm for females and 19.0 to 28.0 gm for males. Mice were uniquely identified by ear punch and/or tail marks, and acclimated for 2 weeks prior to irradiation at 12 weeks of age. Husbandry and health status monitoring were carried out as previously described(Plett et al. 2012). Mice were housed in microisolator cages and provided with autoclaved acidified water in sipper tubes daily beginning on day 1 post-irradiation and daily wet feed beginning on day 4. No antibiotics were administered to the mice. All studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Irradiation and dosimetry
Mice were placed in single chambers of a Plexiglas irradiation apparatus and were exposed to a single uniform total-body dose of gamma radiation of 8.00 or 8.72 Gy (determined in pilot studies conducted in our facility to be the LD60/30 and LD70/30, respectively) from a 137Cs radiation source at 0.63–0.68 Gy min-1 (Nordion International, Ontario, Canada) or 0.97–1.03 Gy min-1 (Mark I Irradiator, JL Shepherd, San Fernando, CA, USA). For irradiations utilizing the GammaCell 40 irradiator, mice were exposed bilaterally on the dorsal and ventral sides, while in the Mark I irradiator, mice were rotated horizontally and exposed from a single source posteriorly and anteriorly. Plasma for metabolomics analyses were from mice exposed to the LD70/30 (872 cGy) and collected on day 3 post-exposure. Non-irradiated control samples were from sham-irradiated mice placed in the irradiation apparatus and irradiator for the same amount of time as irradiated mice, but the irradiator was not turned on. Mice were irradiated between 8:00 and 11:30 am to control for chronoradiosensitivity effects, and were not anesthetized during exposure.
In-house dosimetry verified that dose homogeneity in the exposure field of the mice was 0.0–4.3% of calculated central dose. To verify exposure doses, Landauer Inlight OSL nanodosimeters were placed in mouse phantoms and exposed along with the mice during all or select exposures in most studies. Nanodosimeters were read on a validated Landauer microStar reader calibrated with standard Dot dosimeters exposed with a NIST-traceable 137Cs source (Battelle Memorial Institute, WA). Reproducibility of individual dots was 3±1 % with accuracy of 4±2%. Dose output checks (using farmer-type ion chambers and a validated electrometer), and dose field uniformity checks by exposing film, are performed periodically by an onsite medical physicist.
Cytokine analyses
Mice were euthanized by CO2-inhalation, blood obtained by cardiac puncture, and serum or EDTA-plasma removed from cells after centrifugation at 9,600 g for 4 minutes. Samples were stored in ~100 uL aliquots at −80oC for batch analyses. Samples were thawed, centrifuged to remove particulates (16,300 g for 10 min), and analyzed for 32 cytokines/chemokines by Luminex xMAP technology as previously described(Nelson et al. 2014; Shinha et al. 2015; Dynlacht et al. 2017). Targets were detected using a premixed kit from Millipore (MILLIPLEX Mouse Cytokine/Chemokine Magnetic Bead Panel, Millipore, Billerica, MA) designed to quantitate the mouse proteins interleukin (IL)-1 alpha, IL-1 beta, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, interferon-gamma inducible protein (IP10), keratinocyte-derived chemokine (KC), eotaxin, monocyte chemoattractant protein 1 (MCP-1), monokine induced by gamma interferon (MIG), macrophage inflammatory protein-2 alpha (MIP-2 alpha), macrophage inflammatory protein-1 alpha (MIP-1 alpha), macrophage inflammatory protein-1 beta (MIP-1 beta), interferon-gamma (IFN-gamma), lipopolysaccharide-induced CXC chemokine (LIX), tumor necrosis factor-alpha (TNF-alpha), regulated upon activation normal T cell expressed and secreted (RANTES), leukemia inhibitory factor (LIF), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony- stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), vascular endothelial growth factor (VEGF). Samples were diluted 2-fold according to the manufacturer’s recommendations, assayed in duplicate using a Bio-Plex 200 System with High Throughput Fluidics (HTF) Multiplex Array System (Bio-Rad Laboratories, Hercules, CA) and analyzed using the Bio-Plex™ 6.0 Manager software (Bio-Rad, Hercules, CA). Cytokines were analyzed on days 3, 6, and 9 after radiation exposure with 2–12 mice per group as described in the figure legends and on figure 6 panel A.
High-Throughput, Targeted Metabolomics
Targeted, quantitative metabolomics was performed using Biocrates AbsoluteIDQ p180 kit (Biocrates, Life Science AG, Innsbruck, Austria). The AbsoluteIDQ p180 kit was prepared as described by the manufacturer. The kit is a combined flow injection (FIA) and liquid chromatography (LC) tandem mass spectrometry assay. The assay quantifies up to 188 metabolites from five metabolite classes: acylcarnitines, amino acids, biogenic amines, glycerophospholipids, sphingolipids, and hexose. Internal standards, analyte derivatization and metabolite extraction are integrated into a 96-well plate kit. Metabolite detection is done via pre-selected selected reaction monitoring (SRM) transitions.
For the sample preparation of plasma samples, 10 uL of plasma was placed directly onto the 96 well kit plate. Briefly, after the plasma sample was placed onto the plate 10 uL of internal standard cocktail was added. This step was followed by drying with nitrogen. Next, a 5% solution of phenylisothiocyanate in ethanol:water:pyridine (1:1:1, v/v/v) was added for derivatization of biogenic amines and amino acids. Metabolite extraction was then achieved with 5 mM ammonium acetate in methanol. The FIA and LC were preformed according to the manufacturer’s instructions on a tandem mass spectrometry platform that consisted of a Shimadzu Prominence UFLC XR high-performance liquid chromatograph (HPLC) (Shimadzu, Columbia, MD) coupled to an AB Sciex QTRAP® 5500 hybrid tandem quadrupole/linear ion trap mass spectrometer (AB Sciex, Framingham, MA). The MetIQ software (Biocrates) controlled the assay workflow including sample registration, calculation of metabolite concentrations, and assay validation.
Each plasma cohort had 5 biological samples per dose per time point per gender: male naïve (sham-irradiated), n=5; female naïve (sham-irradiated), n=5; male irradiated 8.72 Gy assayed 3 days post-exposure, n=5; female irradiated 8.72 Gy assayed 3 days post-exposure, n=5.
Metabolomic nomenclature
Lipid notations listed as “Class_XX-Y” notate particular lipids indicating the XX total carbon number in the fatty acid chains and the Y number of double bonds. For example, PC_36–3 is phosphatidylcholine (PC) 36:3; i.e., a phosphatidylcholine with 36 total carbons among the two alkyl chains with 3 double bonds present. PC lipids listed as PC_36–3 are “PCa” where both moieties at the sn-1 and sn-2 position are fatty acids bound to the glycerol backbone via ester bonds. PC lipids listed as PC_O-36–3 are “PCe” where one of the moieties, either the sn-1 or sn-2 position, is a fatty alcohol bound via an ether bond. For sphingomyelins (SM), the number of double bonds or the presence of a hydroxyl group (OH) are indicated for the fatty acid in the amide bond with the assumption that the backbone is sphingosine (d18:1). SFA refers to a sum of PC lipids containing fully saturated fatty acids (SFA), MUFA refers to a sum of PC lipids containing monounsaturated fatty acids (MUFA), and PUFA refers to a sum of PC lipids containing polyunsaturated fatty acids (PUFA). Lipid species measurements include potential isobaric and isomeric species (Biocrates 2017).
Metabolomic Bioinformatics
Statistical analyses were performed using the MetaboAnalyst web-based statistical package (Xia et al. 2015; Xia and Wishart 2016) and GraphPad Prism (v 6.03, La Jolla, CA). The data generated from the AbsoluteIDQ p180 kit which included analyte name and calculated concentration were imported into MetaboAnalyst for multivariate analysis. The metabolite data were sum normalized, log transformed, and mean centered. Multivariate analysis included principal component analysis (PCA) and partial least square discriminate anlaysis (PLS-DA). The univariate analysis was performed in GraphPad Prism.
RESULTS
To define sex-related differences in the radiation responsiveness of biomarkers in H-ARS survivors, the metabolomic profile was established in 3-month old male and female H-ARS survivors and their age-matched non-irradiated (sham-irradiated) controls. We used a quantitative high-throughput, targeted metabolomics assay to quantify 232 individual metabolites (188), metabolite sums (17), and metabolite ratios (27). We also quantified serum cytokines via a multiplexed immunoassay as a function of gender and time after radiation dose. Since some cytokines/chemokines are considered stress hormones, and the irradiation procedure itself can be a stressful event to mice, levels of cytokines/chemokines can change in irradiated mice secondary to the stress of the irradiation procedure. It is therefore important that non-irradiated controls also undergo all steps of the irradiation procedure (minus the exposure), so that changes in cytokines/chemokines due to procedural stress can be separated from those due to the actual radiation exposure. For these reasons, all non-irradiated control samples in this study were obtained from sham-irradiated mice, which were placed in the irradiation apparatus in the irradiator for the same amount of time as the irradiated mice, but the irradiator was not turned on.
Plasma metabolites changed 3 days post-LD70/30 (8.72 Gy) total-body irradiation (TBI) radiation exposure
High-throughput targeted metabolomics was conducted on plasma collected 3 days post-exposure from cohorts of C57BL/6 mice (n=10) that were either naïve to irradiation (sham-irradiated) or exposed to 8.72 Gy TBI. Each n=10 cohort had n=5 female and n=5 male mice. Data for metabolite parameters measured for grouped cohorts comprised of equal numbers of females and males that were either naïve to irradiation or 3 days post-8.72 Gy TBI are provided in Supplemental Table 1. Multivariate analysis was conducted to identify metabolites that differed between naïve and irradiated mouse plasma (Fig. 1). In PLS-DA, the experimental groups are defined in an effort to identify features that best delineate the differences between groups. Here, PLS-DA analysis revealed that naïve and irraditated mouse plasma had distinct metabolomic profiles (R2=0.99, Q2=0.79) where the elliptical shaded areas represented the 95% confidence interval (Fig 1a). The volcano plot highlighted metabolites that reached significance thresholds of p<0.05, p<0.01, and p<0.001 (Fig. 1b). Sixteen metabolites were significantly different between naïve and irradiated plasma from cohorts consisting of equal numbers of female and male mice grouped together (Table 1). These metabolites were from a variety of classes including acylcarnitines (2), amino acids (3), biogenic amines (6), lysophospholipids (1), phosphatidylcholines (1), and sphingomyelins (3) (Fig. 1c). Of the metabolites identified as significantly changed in Table 1, 12 of these parameters decreased in response to radiation, while 4 of them increased. Metabolites reduced after radiation included amino acids citrulline (Cit) and citrulline-related ratios (Cit/Arg and Cit/Orn); biogenic amines spermidine, Ac-Orn, alpha-AAA, total DMA, and creatinine; lysophosphatidylcholine, LPC_20–3; acyl carnitines C0 (free carnitine) and C2 (acetylcarnitine); and the ratio of phosphatidylcholine lipids that contain monounsaturated and saturated fatty acids (MUFA_PC/SFA_PC). Metabolites that were increased after radiation included the ratio of spermine/spermidine and sphingomyelins, SM_36-2, SM_34-1, and SM_36-1. Among the metabolites that were significantly changed, we noted several trends that are represented in the select metabolites shown in Fig. 1d - Fig. 1f. Some metabolites displayed similar female/male naïve levels and showed significant differences between naïve and irradiated in the female and male grouped cohort as well as between naïve and irradiated in the female and male single-sex cohorts including Cit, Cit/Arg, Cit/Orn, Spermidine, Spermine/Spermidine, alpha-AAA, MUFA_PC/SFA_PC, and LPC_20–3 (Fig. 1d, Table 1). Cit is shown as representative of metabolites that are significantly changed in the female and male grouped cohort as well as in both female and male single-sex cohorts. Conversely, some metabolites with similar female/male naïve levels were changed between naïve and irradiated in the female and male grouped cohort but not changed between naïve and irradiated either the female or the male single-sex cohorts including C0, SM_36-1, and creatinine (Table 1). Some metabolites were changed between naïve and irradiated in female and male grouped cohort and changed between naïve and irradiated in female single-sex cohort but not changed in the male single-sex cohort including SM_36-2, Ac-Orn, total DMA, C2, and SM_34-1 (Fig. 1e, Table 1). SM_36-2 is shown as representative of metabolites that are significantly changed in female and male grouped cohort and changed in female single-sex cohort only. And lastly, some metabolites are significantly changed in the female and male grouped cohorts as well as in the female and male single-sex cohorts but have significant basal differences between naïve female and male levels including MUFA_PC/SFA_PC and LPC_20–3 (Fig. 1f, Table 1). LPC_20–3 is shown as representative of metabolites that are significantly changed after radiation but have significant basal differences between naïve female and male levels.
Fig. 1. Plasma metabolites changed at 3 days post-8.72 Gy TBI.
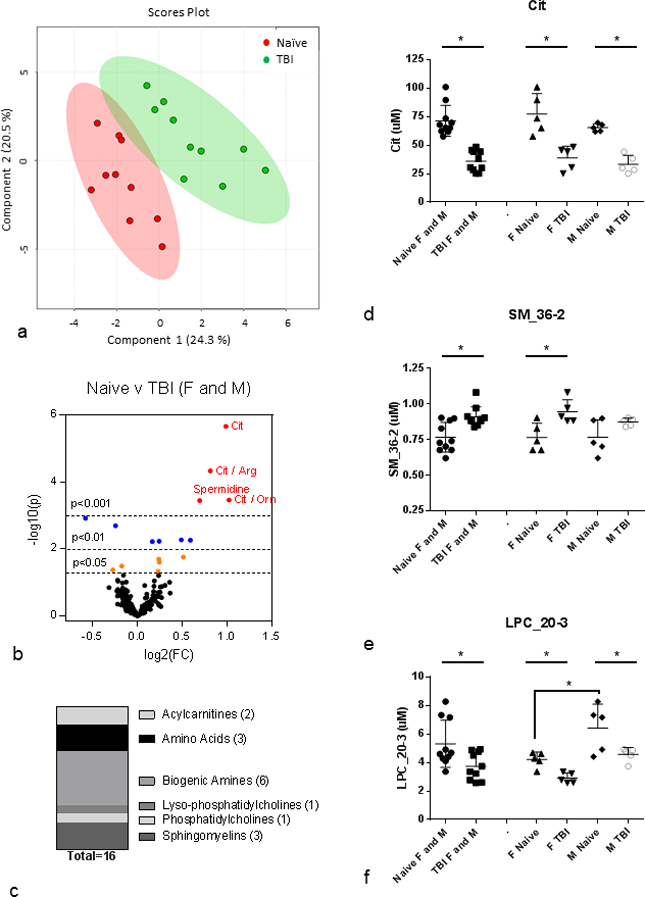
(a) PLS-DA plot: naïve (red) v TBI (green); n=10 per group; female and male are grouped together; R2=0.99, Q2=0.79. Each point represents a data set from an individual animal. The 95% confidence intervals are indicated by elliptical patterns per group. Data were sum normalized, log transformed, and mean centered. (b) Volcano Plot: naïve v TBI; n=10 per group; female and male are grouped together. FC=fold change, p=pvalue. Red dots are metabolites with p<0.001, blue dots are metabolites with p<0.01, and orange dots are metabolites with p<0.05. (c) Class distribution of significantly changed metabolites listed in Table 1 (p<0.05). (d) Cit as representative of metabolites that are significantly changed in female and male grouped cohort as well as in female and male single-sex cohorts. (e) SM_36-2 as rpresentative of metabolites that are significantly changed in female and male grouped cohort and in female single-sex cohort only. (f) LPC_20–3 as representative of metabolites that are significantly changed in female and male grouped cohort as well as in female and male single-sex cohorts but have significant basal differences between female and male. All plasma metabolites assayed from 3 month old C57BL/6 mice; n=10 for each female and male grouped cohort (Naïve, IR) where each n=10 cohort had n=5 each of female and male. Mean ± SD; *p<0.05. M=male, F=female, IR=3 d post-8.72 Gy, naïve = sham.
Table 1. Plasma metabolites significantly changed at 3 days post-8.72 Gy TBI.
Metabolites assayed from 3 mo. old C57BL/6 mice; n=10 for each grouped cohort (Naïve, IR). Each n=10 cohort had n=5 each of female and male. p < 0.05 between grouped cohorts of naïve and IR.
|
Naive_F and M |
TBI_F and M |
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite | Mean | SD | SEM | %CV | N | Mean | SD | SEM | %CV | N | p value Naïv vs TBI |
Differentia Expression (up) |
* FDR <5% |
Significance of Naïve vs TBI in single sex cohorts |
Class | ||||||||||||||||||
| 1 | Cit | 71.37 | 13.61 | 4.31 | 19.1 | 10 | 36.04 | 9.09 | 2.88 | 25.2 | 10 | <0.0001 | Naïve | * | Both | AA | |||||||||||||||||
| 2 | Cit / Arg | 0.69 | 0.14 | 0.04 | 19.9 | 10 | 0.39 | 0.11 | 0.03 | 28.1 | 10 | <0.0001 | Naïve | * | Both | AA | |||||||||||||||||
| 3 | Cit / Orn | 1.27 | 0.41 | 0.13 | 32.1 | 10 | 0.63 | 0.22 | 0.07 | 34.8 | 10 | 0.0003 | Naïve | * | Both | AA | |||||||||||||||||
| 4 | Spermidine | 1.07 | 0.25 | 0.08 | 23.0 | 10 | 0.66 | 0.16 | 0.05 | 24.2 | 10 | 0.0004 | Naïve | * | Both | BA | |||||||||||||||||
| 5 | Spermine / Spermidine | 0.43 | 0.08 | 0.03 | 18.8 | 10 | 0.64 | 0.16 | 0.05 | 24.3 | 10 | 0.0012 | TBI | Both | BA | ||||||||||||||||||
| 6 | SM_36-2 | 0.77 | 0.10 | 0.03 | 13.6 | 10 | 0.91 | 0.07 | 0.02 | 7.7 | 10 | 0.0020 | TBI | F only | SM | ||||||||||||||||||
| 7 | Ac-Orn | 2.18 | 0.33 | 0.11 | 15.3 | 10 | 1.56 | 0.53 | 0.17 | 33.8 | 10 | 0.0053 | Naïve | F only | BA | ||||||||||||||||||
| 8 | alpha-AAA | 10.46 | 3.11 | 0.98 | 29.8 | 10 | 6.96 | 1.63 | 0.51 | 23.4 | 10 | 0.0055 | Naïve | Both | BA | ||||||||||||||||||
| 9 | MUFA_PC / SFA_PC | 5.38 | 0.60 | 0.19 | 11.2 | 10 | 4.55 | 0.58 | 0.18 | 12.7 | 10 | 0.0059 | Naïve | Both # | PC | ||||||||||||||||||
| 10 | total DMA | 0.59 | 0.05 | 0.02 | 8.3 | 10 | 0.52 | 0.04 | 0.01 | 7.8 | 10 | 0.0060 | Naïve | F only | BA | ||||||||||||||||||
| 11 | LPC_20-3 | 5.32 | 1.65 | 0.52 | 31.0 | 10 | 3.74 | 0.97 | 0.31 | 25.8 | 10 | 0.0173 | Naïve | Both # | LPC | ||||||||||||||||||
| 12 | C0 | 22.01 | 3.13 | 0.99 | 14.2 | 10 | 18.70 | 2.66 | 0.84 | 14.2 | 10 | 0.0202 | Naïve | Neither | AC | ||||||||||||||||||
| 13 | C2 | 15.40 | 2.58 | 0.82 | 16.7 | 10 | 13.02 | 1.66 | 0.53 | 12.8 | 10 | 0.0247 | Naïve | F only | AC | ||||||||||||||||||
| 14 | SM_34-1 | 17.47 | 2.83 | 0.89 | 16.2 | 10 | 19.76 | 1.28 | 0.41 | 6.5 | 10 | 0.0320 | TBI | F only | SM | ||||||||||||||||||
| 15 | SM_36-1 | 1.91 | 0.39 | 0.12 | 20.3 | 10 | 2.32 | 0.44 | 0.14 | 19.1 | 10 | 0.0424 | TBI | Neither | SM | ||||||||||||||||||
| 16 | Creatinine | 6.34 | 1.07 | 0.34 | 16.9 | 10 | 5.41 | 0.86 | 0.27 | 15.9 | 10 | 0.0463 | Naïve | Neither | BA | ||||||||||||||||||
p value < 0.05 for basal difference between naïve female and naïve male metabolite levels
Basal differences in metabolite levels between female and male mouse plasma
To investigate the basal differences between naïve female/male metabolite levels further, multivariate analysis was conducted on the plasma metabolite profile for female and male mice naïve to irradiation (sham irradiated) to determine basal metabolite parameters that differed according to gender (Fig. 2, Supplemental Table 2). Data for all the metabolite parameters measured for naïve cohorts of females and males are provided in Supplemental Table 3. PLS-DA analysis revealed that basal female and basal male metabolomics profiles are significantly different (R2=1.0, Q2=0.96) (Fig. 2a). The volcano plot highlights metabolites that reached significance thresholds of p<0.05, p<0.01, and p<0.001 (Fig. 2b). 92 metabolites significantly differed at the p<0.05 threshold and 71 metabolites also satisfied FDR<5% (Supplemental Table 2). Many of the basal differences between female and male were lipid species, predominantly PCs (75), but also LPCs (9) and SMs (6). PC species significantly different between naïve female and male were split between PCa (29) and PCe (37) individual species as well as 7 sums of PC species and 3 ratios (Supplemental Table 2). Most individual PCa and PCe species contained PUFA with 21 of the PCa and 25 of the PCe containing PUFA. PCa and PCe contained a smaller proportion of MUFA (5 and 7, respectively) and SFA (3 and 5, respectively). Four individual SM species were significantly different between naïve female and male as well as two sums and one ratio. LPC species that differed between naïve female and male were mostly saturated (4) but also were monounsaturated (3) and polyunsaturated (1) and included one sum (Fig. 2c, Supplemental Table 2).
Figure 2. Basal differences in plasma metabolites between naïve female and male C57BL/6 mice.
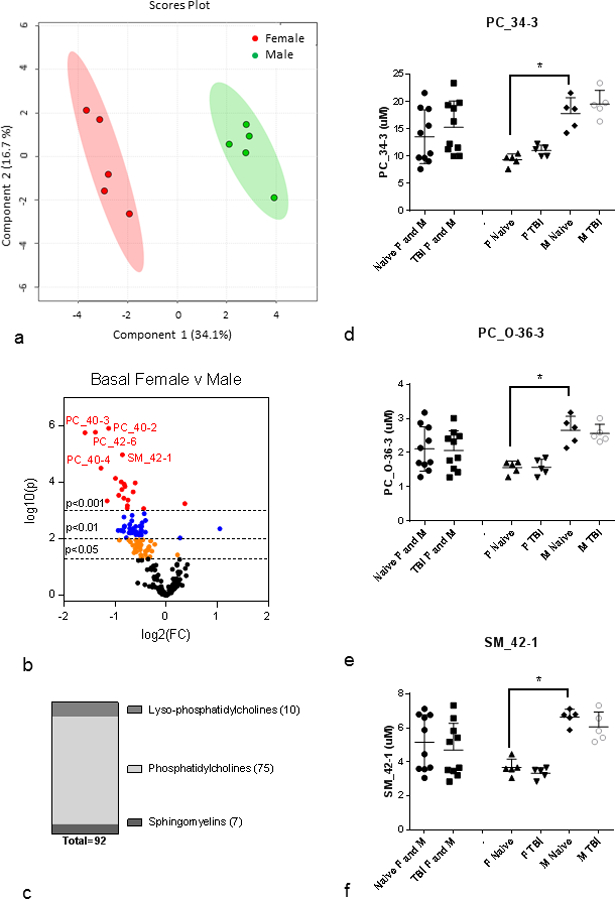
(a) PLS-DA plot: basal female (red) v male (green); n=5 per group; R2=1.0, Q2=0.96. Each point represents a data set from an individual animal. The 95% confidence intervals are indicated by elliptical patterns per group. Data were sum normalized, log transformed, and mean centered. (b) Volcano Plot: basal female v male; n=5 per group. FC=fold change, p=pvalue. Red dots are metabolites with p<0.001, blue dots are metabolites with p<0.01, and orange dots are metabolites with p<0.05. (c) Class distribution of significantly changed metabolites listed in Supplemental Table 2 (p<0.05). (d-f) PC_34–3, PC_O-38–3, and SM_42-1 as representative metabolites that are significantly different between female and male cohorts. All plasma metabolites assayed from 3 month old C57BL/6 mice; n=10 for each female and male grouped cohort (Naïve, IR) where each n=10 cohort had n=5 each of female and male. Mean ± SD; *p<0.05. M=male, F=female, IR=3 d post-8.72 Gy, naïve = sham.
Fig. 2d – Fig. 2f highlight three examples of metabolites that display significant basal differences between naïve female and male metabolite levels: PC_34–3, PC_O_36–3, and SM_42-1. In each case shown here, male metabolite levels were higher than female and both gender’s metabolite levels were insensitive to radiation. As shown in Fig. 1f, not all metabolites with basal differences were insensitive to radiation and as shown in Fig. 1e, responsiveness to radiation was not always the same in plasma metabolites in both genders. To investigate the differences in gender-specific responses to raditaion, we analyzed single-sex cohorts for differences in metabolite levels between naïve and irradiated within a given gender.
Differences in metabolite levels on day 3 post-LD70/30 in the female single-sex cohort
Multivariate analysis was conducted on the plasma metabolite profile from the female cohort to determine metabolites that differed between naïve (sham-irradiated) and irradiated (Fig. 3, Table 2). Data for all the metabolite parameters measured in the female single-sex cohort are provided in Supplemental Table 4. PLS-DA analysis revealed that the naïve and irradiated metabolite profiles differed significantly in the female single-sex cohort (R2=0.99, Q2=0.67) (Fig. 3a). The volcano plot highlights metabolites that reached significance thresholds of p<0.05 and p<0.01 (Fig. 3b). 26 metabolites were significantly different between naïve and irradiated in the female single-sex cohort (Table 2). These metabolites were from a similar variety of classes as the grouped female/male cohort, but differed in the number of species from each class: acylcarnitines (1), amino acids (4), biogenic amines (5), lysophosphatidylcholines (4), phosphatidylcholines (7) and sphingomyelins (5) (Fig. 3c). Among the PC species that differed significantly between naïve and irradiated in the female single-sex cohort were four PCa and two PCe. PCa predominantly contained PUFA (3) but also contained SFA (1); PCe contained PUFA (1) and SFA (1). LPC were polyunsaturated (1), monounsaturated (2), and saturated (1) while sphingomyelin species included three individual species. Of the 26 metabolites significantly changed after radiation in the female single-sex cohort, 13 species were also significantly changed in the female/male grouped cohort (Table 1, Table 2). The remaining 13 metabolites exhibited significant changes after radiation only within the female single-sex cohort (Table 2). Female-specific changes in lipids were all in the form of increases in metabolite levels after radiation with the exception of the ratio of SM-OH/Total SM-non-OH which decreased due to an increase in the Total SM-non-OH (Table 2). Fig. 3d–Fig. 3f highlights three metabolites among those that only change significantly in the female single-sex cohort: PC_26–0, LPC_26–1, SM_34-2. In these selected examples, it should be noted that male single-sex cohort metabolite values were insensitive to radiation and were also higher than female levels. The higher male levels that were observed were similar or higher than the significantly elevated levels in females after IR (Fig. 3d – Fig. 3f).
Figure 3. Plasma metabolites changed at 3 days post-8.72 Gy TBI: female single-sex cohort.
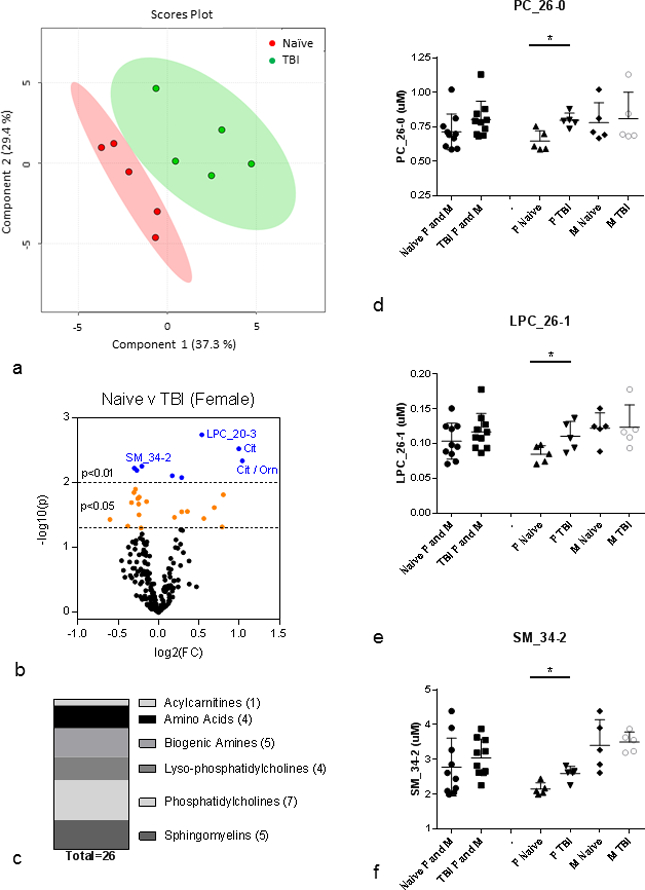
(a) PLS-DA plot: naïve (red) v TBI (green); n=5 per group; female only; R2=0.99, Q2=0.67. Each point represents a data set from an individual animal. The 95% confidence intervals are indicated by elliptical patterns per group. Data were sum normalized, log transformed, and mean centered. (b) Volcano Plot: naïve v TBI; n=10 per group; female only. FC=fold change, p=pvalue. Blue dots are metabolites with p<0.01, and orange dots are metabolites with p<0.05. (c) Class distribution of significantly changed metabolites listed in Table 2 (p<0.05). (d-f) PC_26–0, LPC_26–1, and SM_34-2 as representative metabolites that are significantly changed after IR in only the F cohort. All plasma metabolites assayed from 3 month old C57BL/6 mice; n=10 for each female and male grouped cohort (Naïve, IR) where each n=10 cohort had n=5 each of female and male. Mean ± SD; *p<0.05. M=male, F=female, IR=3 d post-8.72 Gy, naïve = sham.
Table 2. Plasma metabolites assayed at 3 days post-8.72 Gy TBI: female single-sex cohort.
Metabolites assayed from 3 mo. old C57BL/6 mice; n=5 for each cohort (Naïve, IR). p < 0.05 between female cohorts of naïve and IR.
| Female_Naive | Female_TBI | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite | mean | SD | SEM | %CV | N | mean | SD | SEM | %CV | N | P value Naïve vs. TBI |
Differential Expression (up) |
* FDR< 5% |
** also changed when grouped with male |
Class | |||||||||||||||||||
| 1 | LPC_20-3 | 4.22 | 0.53 | 0.24 | 12.50 | 5 | 2.91 | 0.36 | 0.16 | 12.51 | 5 | 0.0018 | Female_Naive | ** | LPC | |||||||||||||||||||
| 2 | Cit | 77.36 | 17.77 | 7.95 | 22.97 | 5 | 38.84 | 10.27 | 4.59 | 26.45 | 5 | 0.0030 | Female_Naive | ** | AA | |||||||||||||||||||
| 3 | Cit / Orn | 1.44 | 0.32 | 0.14 | 21.97 | 5 | 0.70 | 0.28 | 0.13 | 40.48 | 5 | 0.0046 | Female_Naive | ** | AA | |||||||||||||||||||
| 4 | LPC_28-1 | 0.15 | 0.01 | 0.01 | 8.48 | 5 | 0.18 | 0.01 | 0.00 | 3.06 | 5 | 0.0056 | Female_TBI | F only | LPC | |||||||||||||||||||
| 5 | PC_26-0 | 0.65 | 0.07 | 0.03 | 11.49 | 5 | 0.80 | 0.05 | 0.02 | 6.47 | 5 | 0.0060 | Female_TBI | F only | PC | |||||||||||||||||||
| 6 | SM_34-2 | 2.15 | 0.18 | 0.08 | 8.24 | 5 | 2.60 | 0.21 | 0.09 | 7.91 | 5 | 0.0065 | Female_TBI | F only | SM | |||||||||||||||||||
| 7 | Total SM-OH / Total SM-non OH | 0.14 | 0.01 | 0.00 | 5.73 | 5 | 0.12 | 0.01 | 0.00 | 4.71 | 5 | 0.0079 | Female_Naive | F only | SM | |||||||||||||||||||
| 8 | MUFA_PC / SFA_PC | 4.96 | 0.48 | 0.22 | 9.78 | 5 | 4.06 | 0.31 | 0.14 | 7.56 | 5 | 0.0084 | Female_Naive | ** | PC | |||||||||||||||||||
| 9 | SM_34-1 | 15.96 | 1.75 | 0.78 | 10.96 | 5 | 19.47 | 1.72 | 0.77 | 8.84 | 5 | 0.0126 | Female_TBI | ** | SM | |||||||||||||||||||
| 10 | SM_36-2 | 0.77 | 0.10 | 0.04 | 12.92 | 5 | 0.95 | 0.08 | 0.04 | 8.83 | 5 | 0.0143 | Female_TBI | ** | SM | |||||||||||||||||||
| 11 | Cit / Arg | 0.73 | 0.17 | 0.07 | 22.86 | 5 | 0.42 | 0.15 | 0.07 | 36.74 | 5 | 0.0154 | Female_Naive | ** | AA | |||||||||||||||||||
| 12 | Orn / Arg | 0.51 | 0.05 | 0.02 | 10.34 | 5 | 0.60 | 0.04 | 0.02 | 7.31 | 5 | 0.0167 | Female_TBI | F only | AA | |||||||||||||||||||
| 13 | PC_38-6 | 81.05 | 8.55 | 3.82 | 10.55 | 5 | 96.84 | 8.22 | 3.67 | 8.48 | 5 | 0.0177 | Female_TBI | F only | PC | |||||||||||||||||||
| 14 | PC_O-44-3 | 0.12 | 0.01 | 0.00 | 5.64 | 5 | 0.13 | 0.01 | 0.00 | 5.63 | 5 | 0.0197 | Female_TBI | F only | PC | |||||||||||||||||||
| 15 | LPC_26-0 | 0.17 | 0.01 | 0.00 | 3.05 | 5 | 0.22 | 0.04 | 0.02 | 15.98 | 5 | 0.0204 | Female_TBI | F only | LPC | |||||||||||||||||||
| 16 | PC_O-38-0 | 1.65 | 0.18 | 0.08 | 10.97 | 5 | 1.96 | 0.16 | 0.07 | 8.11 | 5 | 0.0215 | Female_TBI | F only | PC | |||||||||||||||||||
| 17 | Spermidine | 1.05 | 0.24 | 0.11 | 22.78 | 5 | 0.65 | 0.22 | 0.10 | 33.31 | 5 | 0.0243 | Female_Naive | ** | BA | |||||||||||||||||||
| 18 | PC_38-3 | 17.46 | 3.08 | 1.38 | 17.65 | 5 | 13.62 | 0.86 | 0.39 | 6.33 | 5 | 0.0278 | Female_Naive | F only | PC | |||||||||||||||||||
| 19 | C2 | 16.01 | 1.50 | 0.67 | 9.39 | 5 | 13.14 | 1.88 | 0.84 | 14.28 | 5 | 0.0282 | Female_Naive | ** | AC | |||||||||||||||||||
| 20 | PC_34-3 | 9.33 | 1.08 | 0.48 | 11.58 | 5 | 11.03 | 0.98 | 0.44 | 8.92 | 5 | 0.0314 | Female_TBI | F only | PC | |||||||||||||||||||
| 21 | total DMA | 0.61 | 0.04 | 0.02 | 7.30 | 5 | 0.53 | 0.05 | 0.02 | 9.75 | 5 | 0.0344 | Female_Naive | ** | BA | |||||||||||||||||||
| 22 | Ac-Orn | 2.38 | 0.34 | 0.15 | 14.18 | 5 | 1.61 | 0.59 | 0.26 | 36.58 | 5 | 0.0357 | Female_Naive | ** | BA | |||||||||||||||||||
| 23 | Spermine / Spermidine | 0.46 | 0.07 | 0.03 | 14.28 | 5 | 0.70 | 0.20 | 0.09 | 29.13 | 5 | 0.0370 | Female_TBI | ** | BA | |||||||||||||||||||
| 24 | LPC_26-1 | 0.08 | 0.01 | 0.01 | 14.52 | 5 | 0.11 | 0.02 | 0.01 | 19.21 | 5 | 0.0467 | Female_TBI | F only | LPC | |||||||||||||||||||
| 25 | alpha-AAA | 11.38 | 4.13 | 1.85 | 36.32 | 5 | 6.59 | 2.03 | 0.91 | 30.78 | 5 | 0.0483 | Female_Naive | ** | BA | |||||||||||||||||||
| 26 | Total SM-non OH | 33.56 | 4.36 | 1.95 | 13.00 | 5 | 39.02 | 3.00 | 1.34 | 7.68 | 5 | 0.0499 | Female_TBI | F only | SM | |||||||||||||||||||
Differences in metabolite levels 3 days post-LD70/30 in the male single-sex cohort
Multivariate analysis was conducted on the plasma metabolite profile from the male single-sex cohort to determine metabolites that differed between naïve (sham-irradiated) and irradiated (Fig. 4, Table 3). Data for all metabolite parameters measured for the male single-sex cohort are provided in Supplemental Table 5. PLS-DA analysis revealed that the naïve and irradiated metabolite profiles differed significantly in the male single-sex cohort (R2=0.98, Q2=0.65) (Fig. 4a). The volcano plot highlights metabolites that have reached the significance thresholds of p<0.05, p<0.01, and p<0.001 (Fig. 4b). Fifteen (15) metabolites were significantly different between naïve and irradiated in the male single-sex cohort (Table 3). These metabolites were from four different classes including amino acids (3), biogenic amines (4), lysophosphatidylcholines (1) and phosphatidylcholines (7) (Fig. 4c). Among the PC species that were significantly changed after radiation, two were PCa, three were PCe, all contained PUFA and decreased after radiation (Table 3). One LPC also decreased and was polyunsaturated. Among the 15 metabolites that were significantly changed after raqdiation in the male single-sex cohort, 8 were also changed in the grouped female/male cohort (Table 1, Table 3). The remaining 7 metabolites exhibited significant changes after radiation only in the male single-sex cohort. All male-specific changes in lipids were decreased except the ratio of PUFA_PC/MUFA_PC which increased due to a decrease in PUFA containing lipid species (Table 3). Fig. 4d – Fig. 4f highlights several metabolites that only changed in the male single-sex cohort: PC_40–2, PC_40–3, and PC_O-32–2. In these representative examples of male-specific changes, female metabolite levels were insensitive to IR and were lower than the radiation-induced decrease observed in male (Fig. 4d – Fig. 4f).
Figure 4. Plasma metabolites changed at 3 days post-8.72 Gy TBI: male single-sex cohort.
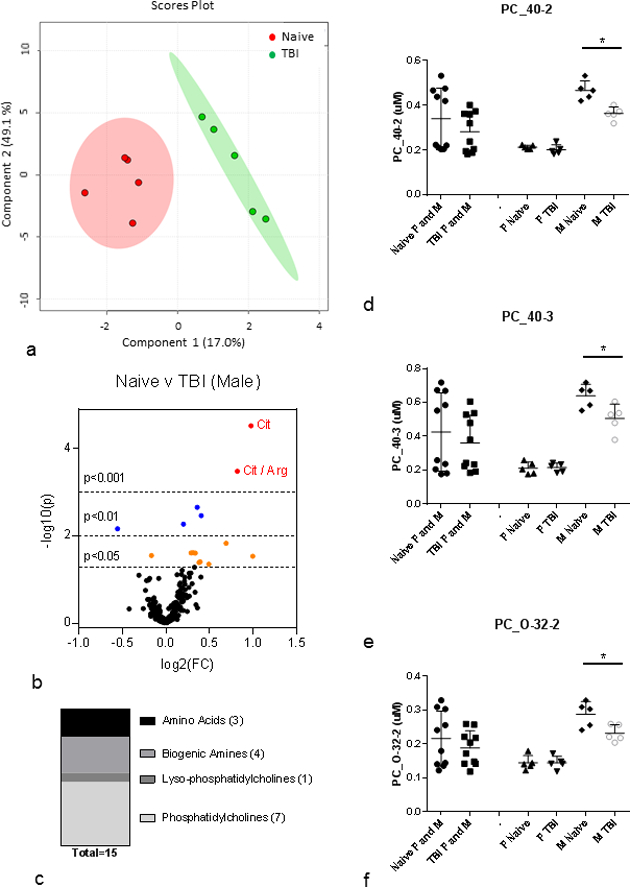
(a) PLS-DA plot: naïve (red) v TBI (green); n=5 per group; male only; R2=0.98, Q2=0.65. Each point represents a data set from an individual animal. The 95% confidence intervals are indicated by elliptical patterns per group. Data were sum normalized, log transformed, and mean centered. (b) Volcano Plot: naïve v TBI; n=5 per group; male only. FC=fold change, p=pvalue. Red dots are metabolites with p<0.001, blue dots are metabolites with p<0.01, and orange dots are metabolites with p<0.05, (c) Class distribution of significantly changed metabolites listed in Table 3 (p<0.05). (d-f) PC_42–0, PC_40–3, and PC_O-32–2 as representative metabolites that are significantly changed after IR in only the male cohort. All plasma metabolites assayed from 3 month old C57BL/6 mice; n=10 for each female and male grouped cohort (Naïve, IR) where each n=10 cohort had n=5 each of female and male. Mean ± SD; *p<0.05. M=male, F=female, IR=3 d post-8.72 Gy, naïve = sham.
Table 3. Plasma metabolites assayed at 3 days post-8.72 Gy TBI: male single-sex cohort.
Metabolites assayed from 3 mo. old C57BL/6 mice; n=5 for each cohort (Naïve, IR). p < 0.05 between male cohorts of naïve and IR.
| Male_Naive | Male_TBI | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite | mean | SD | SEM | %CV | N | mean | SD | SEM | %CV | N | P value Naïvevs TBI |
Differential Expression (Up) |
* FDR<5 % |
** also changed when grouped with female |
||||
| 1 | Cit | 65.38 | 3.41 | 1.52 | 5.21 | 5 | 33.24 | 7.80 | 3.49 | 23.48 | 5 | <0.0001 | Male_Naive | * | ** | |||
| 2 | Cit / Arg | 0.64 | 0.10 | 0.04 | 14.91 | 5 | 0.36 | 0.04 | 0.02 | 11.02 | 5 | 0.0003 | Male_Naive | * | ** | |||
| 3 | PC_40-2 | 0.47 | 0.04 | 0.02 | 9.13 | 5 | 0.36 | 0.03 | 0.01 | 8.16 | 5 | 0.0022 | Male_Naive | M only | ||||
| 4 | PC_O-34-3 | 1.46 | 0.18 | 0.08 | 12.59 | 5 | 1.10 | 0.06 | 0.03 | 5.91 | 5 | 0.0034 | Male_Naive | M only | ||||
| 5 | MUFA_PC / SFA_PC | 5.80 | 0.37 | 0.17 | 6.44 | 5 | 5.04 | 0.24 | 0.11 | 4.82 | 5 | 0.0053 | Male_Naive | ** | ||||
| 6 | Spermine / Spermidine | 0.40 | 0.09 | 0.04 | 22.42 | 5 | 0.59 | 0.07 | 0.03 | 12.54 | 5 | 0.0068 | Male_TBI | ** | ||||
| 7 | Spermidine | 1.08 | 0.28 | 0.12 | 25.78 | 5 | 0.67 | 0.10 | 0.05 | 15.15 | 5 | 0.0146 | Male_Naive | ** | ||||
| 8 | PC_O-32-2 | 0.29 | 0.04 | 0.02 | 13.01 | 5 | 0.23 | 0.02 | 0.01 | 10.72 | 5 | 0.0241 | Male_Naive | M only | ||||
| 9 | ADMA | 0.72 | 0.05 | 0.02 | 7.11 | 5 | 0.59 | 0.09 | 0.04 | 15.63 | 5 | 0.0242 | Male_Naive | M only | ||||
| 10 | PC_40-3 | 0.64 | 0.07 | 0.03 | 10.70 | 5 | 0.51 | 0.08 | 0.04 | 16.51 | 5 | 0.0246 | Male_Naive | M only | ||||
| 11 | PUFA_PC / MUFA_PC | 9.15 | 0.69 | 0.31 | 7.57 | 5 | 10.27 | 0.62 | 0.28 | 6.06 | 5 | 0.0279 | Male_TBI | M only | ||||
| 12 | Cit / Orn | 1.10 | 0.45 | 0.20 | 40.82 | 5 | 0.55 | 0.11 | 0.05 | 19.49 | 5 | 0.0289 | Male_Naive | ** | ||||
| 13 | PC_O-38-2 | 8.08 | 1.66 | 0.74 | 20.51 | 5 | 6.15 | 0.55 | 0.25 | 9.01 | 5 | 0.0388 | Male_Naive | M only | ||||
| 14 | alpha-AAA | 9.54 | 1.62 | 0.72 | 16.94 | 5 | 7.33 | 1.22 | 0.55 | 16.69 | 5 | 0.0403 | Male_Naive | ** | ||||
| 15 | LPC_20-3 | 6.43 | 1.67 | 0.75 | 25.99 | 5 | 4.57 | 0.49 | 0.22 | 10.75 | 5 | 0.0441 | Male_Naive | ** | ||||
Cytokines
Male / female differences in cytokine levels on day 3 post-LD70/30
Cytokines/chemokines were measured in peripheral blood samples from male and female mice on day 3 post-exposure and were categorized based on their changes relative to sham-irradiated controls (naïve).
Factors that significantly increased in both irradiated males and females compared with naïve mice (Fig. 5a) included IL-6, IP-10, Eotaxin, G-CSF, IL-5, KC, and MCP-1. Of these, levels of IL-6, IP-10, Eotaxin, and G-CSF were significantly higher in irradiated females compared to irradiated males. Cytokines/chemokines that were significantly decreased in either irradiated males or females compared with naïve (Fig. 5b) include IL-13, which was decreased in irradiated males only, IFN-gamma, which was decreased in irradiated females only, and MIG, which was decreased in irradiated males versus irradiated females but unchanged compared with naïve. Factors that trended toward an increase in irradiated females (albeit not significantly) but showed no change in irradiated males compared to naïve males, include IL-1 alpha, LIF, LIX, MIP-1 alpha, MIP-2 alpha, M-CSF, and TNF-alpha (Fig. 5c). Cytokines/chemokines that were not different between irradiated males and females, nor when compared to their respective sham-irradiated controls, include IL-1 beta, IL-2, IL-4, IL-7, IL-10, IL-12(p40), IL-12(p70), IL-15, IL-17, MIP-1 beta, GM-CSF, VEGF, and RANTES (Fig. 5d). IL-3 and IL-9 were not reliably detected in these samples (data not shown).
Fig. 5. Peripheral blood cytokine/chemokine levels in naïve and irradiated C57Bl/6 mice exposed to 8.0 or 8.72 Gy (LD60/30 or LD70/30, respectively) determined at day 3 post-exposure by bead-based Luminex xMAP technology.
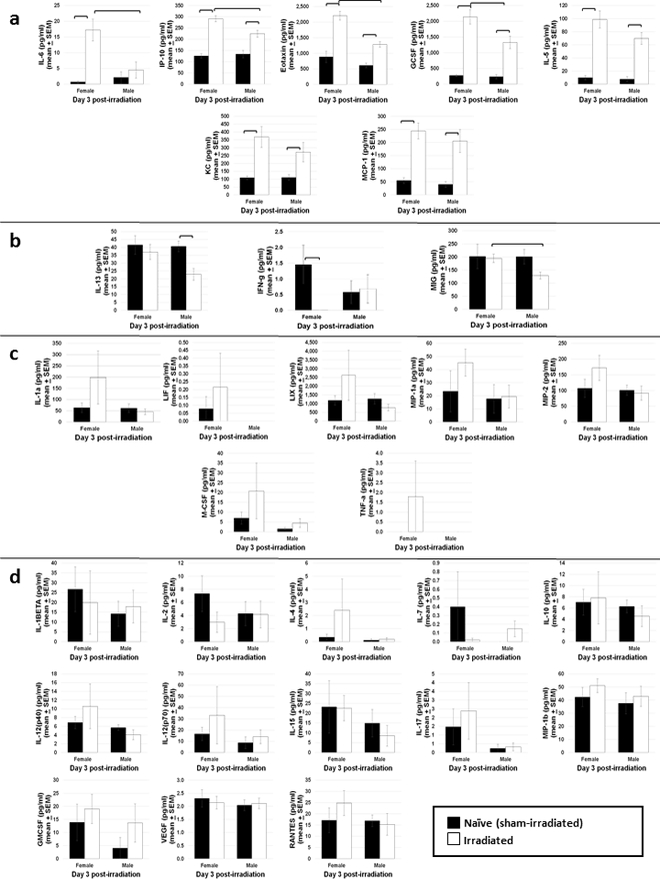
(a) shows factors that increased in both female and male irradiated mice compared to naïve, whereas factors that decreased in either irradiated males or irradiated females compared to naïve are shown in (b). (c) provides factors that increased in irradiated females (but not significantly) but did not change in irradiated males. Cytokines that did not change in either gender compared to naïve are given in (d). n=5–7 mice per treatment group. Bars indicate significant difference (p < 0.05).
Temporal changes in cytokine levels from day 3 to day 9 post-LD70/30
Temporal changes in circulating cytokines/chemokines in irradiated and sham-irradiated mice were examined on days 3, 6, and 9 post-exposure. Two cytokines, IL-5 and KC were increased on all three days in irradiated mice compared to naïve (Fig. 6a). MCP-1, MIP-2 alpha, Eotaxin, and TNF-alpha were increased in irradiated mice on day 3 only, and declined to levels observed in naïve mice thereafter (Fig. 6a). Circulating levels of cytokines in naïve mice shown in Fig. 6a (IL-5, KC, MCP-1, MIP-2 alpha, Eotaxin, and TNF-alpha) did not change over time, indicating that these factors are unaffected by the stress of the irradiation procedure. In contrast, factors shown in Fig. 6b (IL-1 alpha, IL-6, IP-10, LIX, G-CSF, and IL-15) increased over day 3 to day 9 in sham-irradiated mice. Of these cytokines, lethal radiation exposure resulted in further increases in IL-6, IP-10 and G-CSF, whereas circulating levels of IL-1 alpha and LIX were decreased following irradiation. The procedural stress of irradiation appeared to be transient for IL-1 beta, IL-2, IL-12(p70), IFN-gamma, M-CSF, and GM-CSF, with detectable levels of each of these factors observed on day 3 in both naïve and irradiated mice, followed by a decrease to non-detectable levels by days 6 and 9 (Fig. 6c). The following cytokines were not different between irradiated and sham-irradiated controls on any of the three days post-exposure, nor when observed over time: IL-7, IL-10, IL-13, MIG, MIP-1a, MPI-1b, VEGF, and RANTES (data not shown). Levels of IL-3, IL-9, IL-4, IL12 (p40), IL-17, and LIF were very low in the circulation and were not reliably detected in these samples (data not shown).
Fig. 6. Peripheral blood cytokine/chemokine levels in naïve and irradiated C57Bl/6 mice exposed to 8.0 or 8.72 Gy (LD60/30 or LD70/30, respectively) determined on days 3, 6, and 9 post-exposure by bead-based Luminex xMAP technology.
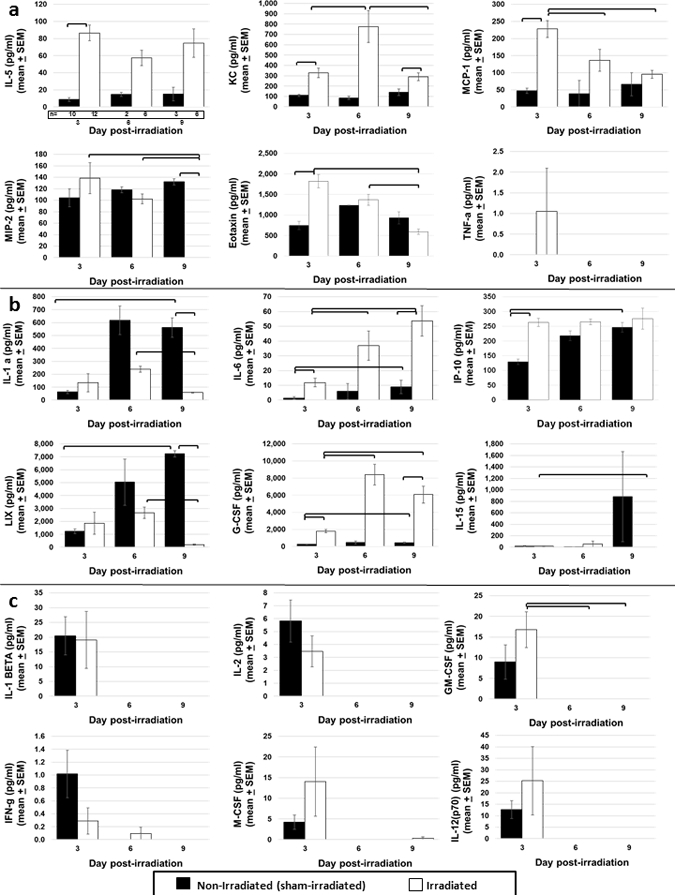
Some mice on days 6 and 9 served as controls in mechanistic studies and had been administered vehicle. (A) shows factors that increased in irradiated mice compared to naïve at one or more time points. (B) shows factors that increased over time in naïve mice, indicating a stress effect from the irradiation procedure. (C) shows factors with a transient increase in both naïve and irradiated mice, on day 3 only. The number of mice analyzed at each time point is given below the first graph in panel A. Statistics were not performed on day 6 data since n=2 for naïve samples. Bars indicate significant difference (p < 0.05).
DISCUSSION
High-throughput targeted metabolomics
High-throughput targeted metabolomics identified metabolites that had similar basal levels and were similarly responsive to radiation in both genders (Table 1). These metabolites would represent the highest priority for biomarker development and the highest potential for translation. Metabolites that had different basal levels, but a similar response to radiation could also prove useful, but would possibly require two separate reference ranges. Gender-specific metabolite response to radiation was observed in both female and male single-sex cohorts (Table 2, Table 3). Such gender-specific response makes these markers less useful for wide applicability with less potential as a biomarker. Gender specific-response in metabolites could, however, prove useful in understanding gender-based differences in biological mechanisms of injury and repair after radiation.
Notable gender-based differences in basal metabolite levels include mostly PC and ether-linked PC species. LPC_20–3 is an example of a metabolite that had a significant basal difference between female and male. However, a decrease in LPC_20–3 was observed in the grouped female/male cohort and both of the single-sex cohorts. LPC_20:3 contains dihomo-γ-linolenic acid which produces anti-inflammatory eicosanoid metabolites as well as metabolites that block arachidonic acid conversion to leukotrienes (Fan and Chapkin 1998; Sergeant et al. 2016). Gender-specific metabolite response to radiation was observed in both female and male single-sex cohorts. Female-specific metabolite changes contained a greater class diversity of species (LPC, PC, SM and AA) than the male-specific changes which were mostly PC species. Among the gender-specific changes, we observed female single-sex cohort changes in PC_38–3 which has previously also been observed to have a differential response according to gender after 10 Gy γ-irradiation in NHP (Pannkuk et al. 2016).
Citrulline is amino acid originating almost exclusively from small intestine enterocytes (Rabier and Kamoun 1995). Citrulline showed a robust response in both the female/male grouped cohort as well as significance in each single-sex cohort. The decreases in Cit/Arg and Cit/Orn that are observed are largely due to the marked decrease in citrulline. The degree of decrease observed in citrulline at 3 days post-8.72 Gy TBI is consistent with our previous observations in a mouse TBI model (Jones et al. 2015b) and our previous rigorous characterization of citrulline as a biomarker for the GI-ARS (Jones et al. 2014a; Jones et al. 2014b; Jones et al. 2015a; Jones et al. 2015b). In this work, citrulline was shown to have a similar response across species including mouse, minipig, and non-human primate (Rhesus macaques), dose- and time-dependent changes after radiation exposure in both TBI and partial-body irradiation (PBI) models, a jejunum-plasma correlation, and a correlation with crypt survival. Additional work defining citrulline’s tissue (jejunum)-plasma correlation is explored in another paper in this issue (Jones et al. 2018). Other groups have observed similar dose- and time-dependent changes as well as correlation with GI histopathology (Wang et al. 2015; Bujold et al. 2016; Pannkuk et al. 2016). In this study, there was no difference in basal citrulline levels in C57BL/6 mice according to gender consistent with reports of no sex differences between female and male citrulline levels in minipig and human (Bujold et al. 2016; Lentner 1981). Sex differences in Rhesus macaques NHPs have been reported where female levels were statistically lower than males with 29.2 ± 1.2 μM for female NHP compared to 33.7 ± 0.9 μM for male NHP (Bujold et al. 2016).
Sphingomyelin (SM) lipids are important for the organization of SM-rich domains known as lipid rafts that maintain the clustering of signaling molecules needed for signal transduction. SM species additionally serve as a source of signaling molecules via enzymatic breakdown of SM by sphingomyelinases (Chakraborty and Jiang 2013; Abe and Kobayashi 2014). We observed an elevation in three SM species: SM_36-2, SM_36-1, and SM_34-1. Two of these (SM_36-2 and SM_34-1) were significant in the female single-sex cohort but not the male single-sex cohort. Additional SM species were significantly elevated in the female single-sex cohort (SM_34-2 and Total SM-non OH). Ionizing radiation has been reported to stimulate sphingomyelinases, however, compensatory changes in sphingomyelinase activity have also been observed in response to chronic oxidative stress resulting in stabilization of membrane composition (Clement et al. 2009; Corre et al. 2013). The observed increases in SM here may indicate a beneficial response mounted toward oxidative stress concerning the maintenance of membrane structure and function. Associations between higher levels of plasma SM and healthy aging and longevity have been reported in a number of human studies with females having a steeper rate of increase in SM with age than males (Vaarhorst et al. 2011; Yu et al. 2012; Gonzalez-Covarrubias et al. 2013; Mielke et al. 2015; Trabado et al. 2017)
A decrease in spermidine and resultant change in the ratio of spermine/spermidine represent changes consistent with these polyamines’ function as quenchers of singlet molecular oxygen (Khan et al. 1992b). Spermidine and other polyamines protect replicating DNA from oxidative damage and have been proposed to provide protection against single-strand breaks induced by singlet oxygen (Khan et al. 1992a; Khan et al. 1992b). Depletion in spermidine as an essential polycation would also block translation inhibiting protein synthesis (Mandal et al. 2013).
Cytokines
Seven cytokines were significantly increased in both irradiated males and females on day 3 compared to naïve (IL-6, IP-10, Eotaxin, G-CSF, IL-5, KC, and MCP-1). Of these, IL-6, IP-10, Eotaxin, and G-CSF were significantly higher in irradiated females compared to irradiated males. MIG was also significantly increased in irradiated females compared with irradiated males, but with no change in naïve. Mechanisms behind these sex-related differences in radiation-induced cytokine release in this model are currently unknown, but it is tempting to speculate that sex hormones may be partly responsible. It has long been known that immunity differs in males and females in that females mount stronger immune responses and develop more autoimmune disorders than males, whereas males succumb to more infections and sepsis (Verthelyi 2001). Studies in both humans and experimental models suggest that these differences may be due to a relationship between sex hormones and levels of pro-inflammatory cytokines (Verthelyi 2001; Aulock et al. 2006; Ma et al. 2007; Robinson et al. 2011; Furman et al. 2014). While levels of cytokines do not appear to contribute to mortality in these studies since radiosensitivity in males and females is similar at this age (3 months old), such differences in cytokine levels may contribute to sex-related differences in radiosensitivity in mice of other ages, should they exist. In any case, these sex differences in acute cytokine response after irradiation may impact the development of strategies to use cytokines as biodosimeters of radiation exposure.
These data also provide strong evidence for acute stress-related changes in several cytokines due simply to undergoing the irradiation procedure (i.e., confinement in the radiation apparatus, placement in the darkened chambers of the irradiator, handling, fluctuations in temperature/humidity, etc.) absent actual radiation exposure. These stress-related responses are evidenced by significant increases in several cytokines in the sham-irradiated mice over time from day 3 to day 9 (i.e., IL-1a, IP-10, LIX, G-CSF, IL-6), or which spiked on day 3 in both sham-irradiated and irradiated mice, then decreased on days 6 and 9 (albeit not significantly). Many of these cytokines have been reported to increase in stress conditions in other experimental settings (Goshen and Yirmiya 2009; Yamakawa et al. 2009; Carpenter et al. 2010; Rohleder 2014; Cheng et al. 2015; Belay et al. 2017), but this is the first report, to our knowledge, of procedural stress from irradiation resulting in changes in circulating cytokines. For these reasons, it is important that any study examining mechanisms of radiation injury or recovery at early time points after exposure, include sham-irradiated controls, since stress responses in these mice due to the stress of undergoing the radiation procedure may effect changes in key endpoints. In such cases, it would be unknown whether changes attributed to the effects of radiation are due to the radiation exposure, or merely the stress of undergoing the irradiation procedure. While stress from euthanasia may also impact cytokine levels, mice were euthanized in these studies by CO2 inhalation following procedures shown to elicit the lowest levels of euthanasia stress as possible (as measured by serum cortisone levels)(Powell et al. 2016). Since all animals were euthanized in the same way, any effect of euthanasia stress on cytokine levels would have been similar for all mice, and unavoidable to our knowledge.
In these studies, decreased levels of citrullline and increased levels of IL-6 were observed in the circulation of irradiated mice compared to naive controls. An inverse relationship in the concentration of these two targets has been reported in inflammatory conditions and sepsis, both of which are likely to exist in the post-irradiation in vivo environment (van Waardenburg et al. 2007; Asgeirsson et al. 2011). Citrulline supplementation in a sepsis model resulted in significantly lower levels of serum IL-6, suggesting a possible role for decreased citrullline in controlling inflammatory responses (Asgeirsson et al. 2011).
CONCLUSION
Gender is an important confounding variable in biomarker development which must be incorporated into biomarker discovery and validation. Understanding of gender as a biological variable is essential for effective translation of biomarkers in animal models to human populations. Further study to more completely define the biokinetic profile, tissue specificity, species translation, dose dependency and confounding factors influencing variability in the measurement of these molecules after radiation exposure will be required to move these potential biomarkers forward toward validation and functional utility.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the members of the Medical Countermeasures Against Radiological Threats (MCART) consortium for their dedication, support, and guidance, and the Multiplex Analysis Core at the Indiana University Melvin and Bren Simon Cancer Center for providing support in analyzing samples and interpretation of data. Additionally, we acknowledge and thank the members of the Kane laboratory.
Funding Source:
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN266200500043, HHSN272201000046C and HHSN272201500013I, and grant 1U01AI107340-01. Additional support was provided by the University of Maryland School of Pharmacy Mass Spectrometry Center (SOP1841-IQB2014).
Footnotes
Conflicts of Interest:
Authors have no conflicts of interest to declare
REFERENCES
- Abe M, Kobayashi T. Imaging local sphingomyelin-rich domains in the plasma membrane using specific probes and advanced microscopy. Biochim Biophys Acta 1841: 720–6; 2014. [DOI] [PubMed] [Google Scholar]
- Abramson S, Miller RG, Phillips RA. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med 145: 1567; 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgeirsson T, Zhang S, Nunoo R, Mascarenas C, Dujovny N, Luchtefeld M, Cavey GS, Senagore A. Citrulline: a potential immunomodulator in sepsis. Surgery 150: 744–51; 2011. [DOI] [PubMed] [Google Scholar]
- Aulock SV, Deininger S, Draing C, Gueinzius K, Dehus O, Hermann C. Gender difference in cytokine secretion on immune stimulation with LPS and LTA. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 26: 887–92; 2006. [DOI] [PubMed] [Google Scholar]
- Belay T, Woart A, Graffeo V. Effect of cold water-induced stress on immune response, pathology and fertility in mice during Chlamydia muridarum genital infection. Pathogens and disease 75; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biocrates. Annotation of potantial isobaric and isomeric lipid species measured with the AbsoluteIDQ p180 Kit (and p150 Kit) 2: 35017; 2017. [Google Scholar]
- Brach MA, Gruss HJ, Kaisho T, Asano Y, Hirano T, Herrmann F. Ionizing radiation induces expression of interleukin 6 by human fibroblasts involving activation of nuclear factor-kappa B. J Biol Chem 268: 8466–72; 1993. [PubMed] [Google Scholar]
- Brush J, Lipnick SL, Phillips T, Sitko J, McDonald JT, McBride WH. Molecular mechanisms of late normal tissue injury. Semin Radiat Oncol 17: 121–30; 2007. [DOI] [PubMed] [Google Scholar]
- Bujold K, Hauer-Jensen M, Donini O, Rumage A, Hartman D, Hendrickson HP, Stamatopoulos J, Naraghi H, Pouliot M, Ascah A, Sebastian M, Pugsley MK, Wong K, Authier S. Citrulline as a Biomarker for Gastrointestinal-Acute Radiation Syndrome: Species Differences and Experimental Condition Effects. Radiat Res 186: 71–8; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between Plasma IL-6 Response to Acute Stress and Early-Life Adversity in Healthy Adults. Neuropsychopharmacology 35: 2617–2623; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M, Jiang XC. Sphingomyelin and its role in cellular signaling. Adv Exp Med Biol 991: 1–14; 2013. [DOI] [PubMed] [Google Scholar]
- Cheda A, Nowosielska EM, Wrembel-Wargocka J, Janiak MK. Production of cytokines by peritoneal macrophages and splenocytes after exposures of mice to low doses of X-rays. Radiat Environ Biophys 47: 275–83; 2008. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Jope RS, Beurel E. A pre-conditioning stress accelerates increases in mouse plasma inflammatory cytokines induced by stress. BMC neuroscience 16: 31; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HL, Plett PA, Sampson CH, Joshi M, Tabbey R, Katz BP, MacVittie TJ, Orschell CM. Long-term hematopoietic stem cell damage in a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys 103: 356–66; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AB, Gamerdinger M, Tamboli IY, Lutjohann D, Walter J, Greeve I, Gimpl G, Behl C. Adaptation of neuronal cells to chronic oxidative stress is associated with altered cholesterol and sphingolipid homeostasis and lysosomal function. J Neurochem 111: 669–82; 2009. [DOI] [PubMed] [Google Scholar]
- Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, Moulder JE, Preston RJ, Seed TM, Stone HB, Tofilon PJ, Wong RS. Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17–18, 2001. Radiat Res 159: 812–34; 2003. [DOI] [PubMed] [Google Scholar]
- Corre I, Guillonneau M, Paris F. Membrane signaling induced by high doses of ionizing radiation in the endothelial compartment. Relevance in radiation toxicity. Int J Mol Sci 14: 22678–96; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy SL, Cheema AK, Tyburski JB, Laiakis EC, Collins SP, Fornace A Jr. Radiation metabolomics and its potential in biodosimetry. Int J Radiat Biol 87: 802–23; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L New drug and biological drug products; Evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. 21 CFR parts 314 and 601, FDA, HHS; ACTION: Final Rule: 37988–37998; 2002. [PubMed] [Google Scholar]
- Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ, Farese AM. The hematologist and radiation casualties. Hematology Am Soc Hematol Educ Program: 473–96; 2003. [DOI] [PubMed] [Google Scholar]
- Dynlacht JR, Garrett J, Joel R, Lane K, Mendonca MS, Orschell CM. Further Characterization of the Mitigation of Radiation Lethality by Protective Wounding. Radiation Research 187: 732–742; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YY, Chapkin RS. Importance of dietary gamma-linolenic acid in human health and nutrition. J Nutr 128: 1411–4; 1998. [DOI] [PubMed] [Google Scholar]
- FDA. Product Development Under the Animal Rule: Guidance for Industry. [online] 2015. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm399217.pdf.
- FDA. Qualification Process for Drug Development Tools [online]. 2014. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm230597.pdf.
- Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proceedings of the National Academy of Sciences of the United States of America 111: 869–74; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler MH, Squiban C, Mouthon MA, Gourmelon P, van der Meeren A. Irradiation enhances the support of haemopoietic cell transmigration, proliferation and differentiation by endothelial cells. Br J Haematol 113: 940–50; 2001. [DOI] [PubMed] [Google Scholar]
- Gaugler MH, Vereycken-Holler V, Squiban C, Vandamme M, Vozenin-Brotons MC, Benderitter M. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res 163: 479–87; 2005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Covarrubias V, Beekman M, Uh HW, Dane A, Troost J, Paliukhovich I, van der Kloet FM, Houwing-Duistermaat J, Vreeken RJ, Hankemeier T, Slagboom EP. Lipidomics of familial longevity. Aging Cell 12: 426–34; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Frontiers in neuroendocrinology 30: 30–45; 2009. [DOI] [PubMed] [Google Scholar]
- Hall E Acute effects of total-body irradiation. In: Halls E ed. Radiobiolgy for the radiologist Philadelphia, PA: Lippincott Williams & Wilkins; 2000; 124–135. [Google Scholar]
- Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A 86: 10104–7; 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Morishita Y, Kubo Y, Kusunoki Y, Hayashi I, Kasagi F, Hakoda M, Kyoizumi S, Nakachi K. Long-term effects of radiation dose on inflammatory markers in atomic bomb survivors. Am J Med 118: 83–6; 2005. [DOI] [PubMed] [Google Scholar]
- Jones JW, Bennett A, Carter CL, Tudor G, Hankey KG, Farese AM, Booth C, MacVittie TJ, Kane MA. Citrulline as a Biomarker in the Non-human Primate Total- and Partial-body Irradiation Models: Correlation of Circulating Citrulline to Acute and Prolonged Gastrointestinal Injury. Health Phys 109: 440–51; 2015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Carter CL, Li F, Yu J, Pierzchalski K, Jackson IL, Vujaskovic Z, Kane MA. Ultraperformance convergence chromatography-high resolution tandem mass spectrometry for lipid biomarker profiling and identification. Biomed Chromatogr 31; 2017a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Clifford Z, Li F, Tudor G, Farese AM, Booth C, MacVittie TJ, Kane MA. Targeted metabolomics reveals metabolomic signatures correlating gastrointestinal tissue to plasma in a mouse total-body irradiation model. Health physics; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Jackson IL, Vujaskovic Z, Kaytor MD, Kane MA. Targeted metabolomics identifies pharmacodynamic biomarkers for BIO 300 mitigation of radiation-induced lung injury Pharmaceutical Research; 2017b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Scott AJ, Tudor G, Xu PT, Jackson IL, Vujaskovic Z, Booth C, MacVittie TJ, Ernst RK, Kane MA. Identification and quantitation of biomarkers for radiation-induced injury via mass spectrometry. Health Phys 106: 106–19; 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Tudor G, Bennett A, Farese AM, Moroni M, Booth C, MacVittie TJ, Kane MA. Development and validation of a LC-MS/MS assay for quantitation of plasma citrulline for application to animal models of the acute radiation syndrome across multiple species. Anal Bioanal Chem 406: 4663–75; 2014b. [DOI] [PubMed] [Google Scholar]
- Jones JW, Tudor G, Li F, Tong Y, Katz B, Farese AM, MacVittie TJ, Booth C, Kane MA. Citrulline as a Biomarker in the Murine Total-Body Irradiation Model: Correlation of Circulating and Tissue Citrulline to Small Intestine Epithelial Histopathology. Health Phys 109: 452–65; 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RJ, Celano P, Sharkis SJ, Sensenbrenner LL. Two phases of engraftment established by serial bone marrow transplantation in mice. Blood 73: 397–401; 1989. [PubMed] [Google Scholar]
- Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming units. Nature 347: 188–189; 1990. [DOI] [PubMed] [Google Scholar]
- Keller G, Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med 171: 1407–18; 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AU, Di Mascio P, Medeiros MH, Wilson T. Spermine and spermidine protection of plasmid DNA against single-strand breaks induced by singlet oxygen. Proc Natl Acad Sci U S A 89: 11428–30; 1992a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AU, Mei YH, Wilson T. A proposed function for spermine and spermidine: protection of replicating DNA against damage by singlet oxygen. Proc Natl Acad Sci U S A 89: 11426–7; 1992b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Kock A, Neuner P, Forster E, Krutmann J, Urbanski A, Schauer E, Ansel JC, Schwarz T, Luger TA. Regulation of epidermal cell interleukin-6 production by UV light and corticosteroids. J Invest Dermatol 96: 484–9; 1991. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kono T, Sauder DN, McKenzie RC. IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J Invest Dermatol 101: 690–4; 1993. [DOI] [PubMed] [Google Scholar]
- Kusunoki Y, Hayashi T. Long-lasting alterations of the immune system by ionizing radiation exposure: implications for disease development among atomic bomb survivors. Int J Radiat Biol 84: 1–14; 2008. [DOI] [PubMed] [Google Scholar]
- Lentner C Geigy scientific tables . 8th ed. Basel, Switzerland: Ciba-Geigy; 1981. [Google Scholar]
- Li WM, Huang WQ, Huang YH, Jiang DZ, Wang QR. Positive and negative hematopoietic cytokines produced by bone marrow endothelial cells. Cytokine 12: 1017–23; 2000. [DOI] [PubMed] [Google Scholar]
- Ma LJ, Guzman EA, DeGuzman A, Muller HK, Walker AM, Owen LB. Local cytokine levels associated with delayed-type hypersensitivity responses: modulation by gender, ovariectomy, and estrogen replacement. The Journal of endocrinology 193: 291–7; 2007. [DOI] [PubMed] [Google Scholar]
- Mandal S, Mandal A, Johansson HE, Orjalo AV, Park MH. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc Natl Acad Sci U S A 110: 2169–74; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Bandaru VV, Han D, An Y, Resnick SM, Ferrucci L, Haughey NJ. Factors affecting longitudinal trajectories of plasma sphingomyelins: the Baltimore Longitudinal Study of Aging. Aging Cell 14: 112–21; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K, Meineke V. Radiation-induced alterations in cytokine production by skin cells. Exp Hematol 35: 96–104; 2007. [DOI] [PubMed] [Google Scholar]
- Nelson RP Jr., Khawaja MR, Perkins SM, Elmore L, Mumaw CL, Orschell C, Paczesny S. Prognostic Biomarkers for Acute Graft-Versus-Host Disease Risk after Cyclophosphamide-Fludarabine Nonmyeloablative Allotransplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien-Ladner A, Nelson ME, Kimler BF, Wesselius LJ. Release of interleukin-1 by human alveolar macrophages after in vitro irradiation. Radiat Res 136: 37–41; 1993. [PubMed] [Google Scholar]
- Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29: 628–36; 2008. [DOI] [PubMed] [Google Scholar]
- Pannkuk EL, Fornace AJ Jr., Laiakis EC. Metabolomic applications in radiation biodosimetry: exploring radiation effects through small molecules. Int J Radiat Biol 93: 1151–1176; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Authier S, Wong K, Fornace AJ Jr. Targeted Metabolomics of Nonhuman Primate Serum after Exposure to Ionizing Radiation: Potential Tools for High-throughput Biodosimetry. RSC Adv 6: 51192–51202; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Lanz C, Gonzalez FJ, Idle JR. The role of mass spectrometry-based metabolomics in medical countermeasures against radiation. Mass Spectrom Rev 29: 503–21; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett PA, Chua HL, Sampson CH, Katz BP, Fam CM, Anderson LJ, Cox GN, Orschell CM. PEGylated G-CSF (BBT-015), GM-CSF (BBT-007), and IL-11 (BBT-059) analogs enhance survival and hematopoietic cell recovery in a mouse model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys 106: 7–20; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett PA, Sampson CH, Chua HL, Jackson W, Vemula S, Sellamuthu R, Fisher A, Feng H, Wu T, MacVittie TJ, Orschell CM. The H-ARS Dose Response Relationship (DRR): Validation and Variables. Health Phys 109: 391–8; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, Johnson CS, Katz BP, Farese AM, Parker J, MacVittie TJ, Orschell CM. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys 103: 343–55; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K, Ethun K, Taylor DK. The effect of light level, CO2 flow rate, and anesthesia on the stress response of mice during CO2 euthanasia. Lab Anim (NY) 45: 386–95; 2016. [DOI] [PubMed] [Google Scholar]
- Rabier D, Kamoun P. Metabolism of citrulline in man. Amino Acids 9: 299–316; 1995. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101: 1767–72; 2000. [DOI] [PubMed] [Google Scholar]
- Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17beta-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS pathogens 7: e1002149; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosomatic medicine 76: 181–9; 2014. [DOI] [PubMed] [Google Scholar]
- Sergeant S, Rahbar E, Chilton FH. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur J Pharmacol 785: 77–86; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Sheng H. Prostaglandin E2 induces the expression of IL-1alpha in colon cancer cells. J Immunol 178: 4097–103; 2007. [DOI] [PubMed] [Google Scholar]
- Shinha T, Mi D, Liu Z, Orschell CM, Lederman MM, Gupta SK. Relationships Between Renal Parameters and Serum and Urine Markers of Inflammation in Those With and Without HIV Infection. AIDS Research and Human Retroviruses 31: 375–383; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Newman VL, Romaine PL, Hauer-Jensen M, Pollard HB. Use of biomarkers for assessing radiation injury and efficacy of countermeasures. Expert Rev Mol Diagn 16: 65–81; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MJ, Ridker PM, Dzau VJ. Risk factor criteria. Circulation 109: IV3–5; 2004. [DOI] [PubMed] [Google Scholar]
- Tatsukawa Y, Misumi M, Yamada M, Masunari N, Oyama H, Nakanishi S, Fukunaga M, Fujiwara S. Alterations of body mass index and body composition in atomic bomb survivors. International journal of obesity (2005) 37: 1123–8; 2013. [DOI] [PubMed] [Google Scholar]
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation Research 14: 213; 1961. [PubMed] [Google Scholar]
- Tonorezos ES, Vega GL, Sklar CA, Chou JF, Moskowitz CS, Mo Q, Church TS, Ross R, Janiszewski PM, Oeffinger KC. Adipokines, body fatness, and insulin resistance among survivors of childhood leukemia. Pediatric blood & cancer 58: 31–6; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabado S, Al-Salameh A, Croixmarie V, Masson P, Corruble E, Feve B, Colle R, Ripoll L, Walther B, Boursier-Neyret C, Werner E, Becquemont L, Chanson P. The human plasma-metabolome: Reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. PLoS One 12: e0173615; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unthank JL, Miller SJ, Quickery AK, Ferguson EL, Wang M, Sampson CH, Chua HL, DiStasi MR, Feng H, Fisher A, Katz BP, Plett PA, Sandusky GE, Sellamuthu R, Vemula S, Cohen EP, MacVittie TJ, Orschell CM. Delayed Effects of Acute Radiation Exposure in a Murine Model of the H-ARS: Multiple-Organ Injury Consequent to <10 Gy Total Body Irradiation. Health Phys 109: 511–21; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaarhorst AA, Beekman M, Suchiman EH, van Heemst D, Houwing-Duistermaat JJ, Westendorp RG, Slagboom PE, Heijmans BT, Leiden Longevity Study G. Lipid metabolism in long-lived families: the Leiden Longevity Study. Age (Dordr) 33: 219–27; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Waardenburg DA, de Betue CT, Luiking YC, Engel M, Deutz NE. Plasma arginine and citrulline concentrations in critically ill children: strong relation with inflammation. The American journal of clinical nutrition 86: 1438–44; 2007. [DOI] [PubMed] [Google Scholar]
- Verthelyi D Sex hormones as immunomodulators in health and disease. International immunopharmacology 1: 983–93; 2001. [DOI] [PubMed] [Google Scholar]
- Visser JWM, Bauman JGJ, Mulder AH, Eliason JF, DeLeeuw AM. Isolation of murine pluripotent hematopoietic stem cells. J Exp Med 159: 1576; 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shao L, Hendrickson HP, Liu L, Chang J, Luo Y, Seng J, Pouliot M, Authier S, Zhou D, Allaben W, Hauer-Jensen M. Total Body Irradiation in the “Hematopoietic” Dose Range Induces Substantial Intestinal Injury in Non-Human Primates. Radiat Res 184: 545–53; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res 43: W251–7; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Wishart DS. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Current protocols in bioinformatics 55: 14.10.1–14.10.91; 2016. [DOI] [PubMed] [Google Scholar]
- Yamakawa K, Matsunaga M, Isowa T, Kimura K, Kasugai K, Yoneda M, Kaneko H, Ohira H. Transient responses of inflammatory cytokines in acute stress. Biological psychology 82: 25–32; 2009. [DOI] [PubMed] [Google Scholar]
- Yamanaka R, Tanaka R, Yoshida S. Effects of irradiation on cytokine production in glioma cell lines. Neurol Med Chir (Tokyo) 33: 744–8; 1993. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhai G, Singmann P, He Y, Xu T, Prehn C, Romisch-Margl W, Lattka E, Gieger C, Soranzo N, Heinrich J, Standl M, Thiering E, Mittelstrass K, Wichmann HE, Peters A, Suhre K, Li Y, Adamski J, Spector TD, Illig T, Wang-Sattler R. Human serum metabolic profiles are age dependent. Aging Cell 11: 960–7; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FF, Kelly MJ, Saltzman E, Must A, Roberts SB, Parsons SK. Obesity in pediatric ALL survivors: a meta-analysis. Pediatrics 133: e704–15; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


