Abstract
It has been speculated that the addition of antioxidants to diet could act as either radioprotectors or as mitigators of radiation injury. In preparation for studies of the mitigation efficacy of antioxidants, rats were placed on a modified version of AIN-76A, the diet typically used in such studies. This AIN-76A diet is refined and has no synthetic antioxidants or isoflavones. Compared to the natural-ingredient Teklad 8904 diet used in previous studies, use of the AIN-76A diet from 1–18 weeks after irradiation significantly reduced injury in a radiation nephropathy model. A confirmation study included an additional arm in which the AIN-76A diet was started 2 weeks prior to irradiation; again, the switch to AIN-76A post-irradiation mitigated radiation nephropathy (p<0.001), but switching to the AIN-76A diet pre-irradiation had no effect (p>0.2). The two diets do not differ in salt content, but the AIN-76A diet is somewhat lower in protein (18% vs. 24%). The protein source (primarily soy in Teklad 8904 versus casein in AIN-76A) might explain the effects. However, replacing the casein in AIN-76A with soy did not change the mitigation efficacy of the diet (p>0.2 for comparison of the different AIN-76A diets). A similar study in a rat radiation pneumonitis model also suggested mitigation by post-irradiation use of AIN-76A, although the effect was not statistically significant (p=0.07). In conclusion, base diet alone can have biologically-significant effects on organ radiosensitivity, but the mechanistic basis for the effect and its dependence of timing relative to irradiation are unclear.
Keywords: diet, radiation damage, kidneys, lungs
INTRODUCTION
It has been widely speculated that the addition of antioxidants to the diet could act as either radioprotectors and/or as mitigators of radiation injury (Brown et al. 2010, Kennedy and Wan 2011, Johnke et al. 2014, Singh et al. 2017). When studies of this approach are done in rodents they generally use purified diets (usually AIN-76A or AIN-93G) as their base diet (Reeves 1997). These refined diets lack phytochemicals such as isoflavones and have lower levels of many antioxidant nutrients. In preparation for studies of the mitigation efficacy of dietary antioxidants we placed rats on this type of diet, specifically a modified version of AIN-76A. In a radiation nephropathy model (Cohen et al. 2015) we found that, compared to our standard diet (Teklad 8904), use of the AIN-76A diet after irradiation significantly decreased the incidence and severity of radiation nephropathy. Further studies were then conducted to confirm this unexpected effect, to attempt to determine its mechanistic basis, and to test whether the effect also extended to radiation pneumonitis.
MATERIALS AND METHODS
Animal Models
The studies were performed in syngeneic WAG/RijCmcr rats that were bred and housed in a moderate security barrier. The animals were free of Mycoplasma pulmonis, Pseudomonas, and common rat viruses. Supportive care (antibiotics and/or fluids) was not required at the radiation doses used. The rats were maintained in a moderate-security barrier at the Biomedical Resource Center of the Medical College of Wisconsin, which is fully accredited by the American Association of Accreditation of Laboratory Animal Care. The animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC). Animals were monitored daily. Based on direction from the IACUC, rats were designated as morbid and euthanized if they met specified veterinary criteria.
We used a leg-out partial body irradiation (PBI) model of radiation nephropathy, as reported by Fish et al. (2016). In brief, unanesthetized 7–8-week-old male rats were confined in a plastic jig and irradiated with 320 kV x-rays to a mid-line dose of 11 Gy. One leg was externalized and shielded with a 0.25-inch lead block. Further details of the model and dosimetry are found in Fish et al. (2016). The renal injury endpoints were the development of azotemia (as blood urea nitrogen, BUN) and the interval to renal failure (defined at the time to reach a BUN of 120 mg/dl) (Moulder et al. 2011). BUN was measured at regular intervals for up to 38 weeks after irradiation, and animals that were morbid or whose blood urea nitrogen (BUN) exceeded 120 mg/dl (renal failure) were euthanized.
We used a whole-thorax irradiation model of radiation pneumonitis developed by Medhora et al. (2012). Further details of the model and dosimetry are found in Medhora et al. (2012). In brief, unanesthetized 9–10-week-old female rats were confined in a plastic jig and irradiated with 320 kV x-rays to a mid-lung dose of 12.75 Gy. The endpoint was morbidity previously associated with radiation pneumonitis at this dose, requiring euthanasia after 6 weeks and prior to 11.5 weeks (Medhora et al. 2012).
Diets
Starting at weaning, rats were fed a standard natural-ingredient rodent diet, Teklad 8904, which has ~24% protein by weight. The primary protein source is soybean meal with other significant sources being fish meal, wheat and corn. Control animals remained on 8904, while other rats were switched to experimental purified diets before or after irradiation. The purified diets were two versions of AIN-76A that were modified to replace sucrose with dextrose, maltodextrin and additional corn starch. These AIN-76A diets had ~18% protein by weight and did not contain a synthetic antioxidant. Protein was provided by either casein (TD.120199) or isolated soy protein (TD.130709). For the sterilization required by the facility, diets were gamma-irradiated to a dose between 20 and 50 kGy. Table 1 shows the macronutrients in the diets plus selected other nutrients that might have effects on normal tissues radiosensitivity (Moulder et al. 2002, Brown et al. 2010, Kennedy and Wan 2011, Sieber et al. 2011, Johnke et al. 2014, Singh et al. 2017); further details on the diet composition can be obtained from Envigo, Madison, WI, USA, www.envigo.com.
TABLE 1:
DIET COMPARISONS
| Nutrient | T.8904 Teklad Rodent Diet |
TD.120199 AIN-76A (Casein) |
TD.130709 AIN-76A (Soy) |
|
|---|---|---|---|---|
| Protein (%) | 25 | 18 | 18 | |
| Carbohydrate (%) | 40 | 61 | 61 | |
| Fat (%) | 4.5 | 5.2 | 5.2 | |
| Saturated (%) | 0.9 | 0.7 | 0.7 | |
| Linoleic acids (%) | 1.9 | 2.9 | 2.9 | |
| Energy density (kcal/g) | 3.0 | 3.6 | 3.6 | |
| Sodium (%) | 0.3 | 0.1 | 0.3 | |
| Potassium (%) | 1.0 | 0.4 | 0.4 | |
| Phosphorus (%)a | 0.7 | 0.5 | 0.6 | |
| Calcium (%) | 1.3 | 0.5 | 0.6 | |
| Magnesium (%) | 0.3 | 0.05 | 0.06 | |
| Selenium (mg/kg) | 0.3 | 0.1 | 0.1 | |
| Zinc (mg/kg) | 80 | 34 | 45 | |
| Total iron (mg/kg)b | 300 | 45 | 70 | |
| Iron added (mg/kg) | 95 | 36 | 36 | |
| Copper (mg/kg) | 24 | 6 | 8 | |
| Vitamin A (IU/g) | 13 | 4 | 4 | |
| Vitamin B6 (mg/kg) | 13 | 6 | 6 | |
| Vitamin B12 (mg/kg) | 0.05 | 0.01 | 0.01 | |
| Vitamin D (mg/kg) | 2.4 | 1 | 1 | |
| Isoflavones (mg/kg) | ~500 | none | ~400 | |
| Cholesterol (mg/kg) | 50 | 40 | 0 |
available (non-phytate) phosphorus.
iron may be as much as 5X less available in T.8904 than in the AIN diets
Statistical Methods
Survival curves were done by the Kaplan-Meier technique and compared by the method of Lee and Desu (1972). Survival curves are not plotted beyond the time where the number of animals at risk dropped below two. Physiological parameters are shown as medians with 20–80% ranges and were compared by Mann-Whitney tests. Medians, ranges and non-parametric tests were used for analysis of physiological parameters because in groups with renal dysfunction these parameters are not always normally distributed, and because some animals were euthanized with renal failure prior to scheduled assays. For the purpose of calculating median values in groups where some animals (but less than 50%) had been euthanized with renal failure prior to the scheduled assay, the parameter for the euthanized animals was considered to be more extreme than that of any survivor. If more than 50% of the animals in a group had been euthanized with renal failure prior to the scheduled assay, the BUN point was plotted as “renal failure”.
RESULTS
In preparation of studies of the efficacy of dietary antioxidants for mitigation of radiation nephropathy a group of irradiated animals (n=10) was switched from our standard natural-ingredient rodent diet, Teklad 8904, to a casein-based AIN-76A diet; other animals (n=8) remained on Teklad 8904. The switch was done at 1 week after irradiation, as that is our standard start time for assessing potential mitigators that might be used after a radiological terrorism event (Moulder 2014). Animals remained on that diet until 18 weeks after irradiation. At 17 weeks after irradiation, the BUN in the group that stayed on Teklad 8904 was 100 (82–117) mg/dl, while in the animals switched to casein-based AIN-76A diet the BUN was 18 (17–19) mg/dl, which is a normal level. The striking effect on BUN was paralleled by survival, which was a median of 18 weeks for the animals that stayed on Teklad 8904 and 33 weeks for the animals that were switched to the casein-based AIN-76A diet.
As soon as we found these effects on BUN, we started a confirmation group with an additional 4 animals that stayed on Teklad 8904 and four that were switched to casein-based AIN-76A diet at 1 week after irradiation. Also in this group were 8 animals that were switched to the casein-based AIN-76A diet two weeks prior to irradiation. Again, animals remained on the AIN-76A diet until 18 weeks after irradiation. The survival data for the combined groups is shown in Figure 1 and the combined BUN data is shown in Figure 2.
Fig. 1.
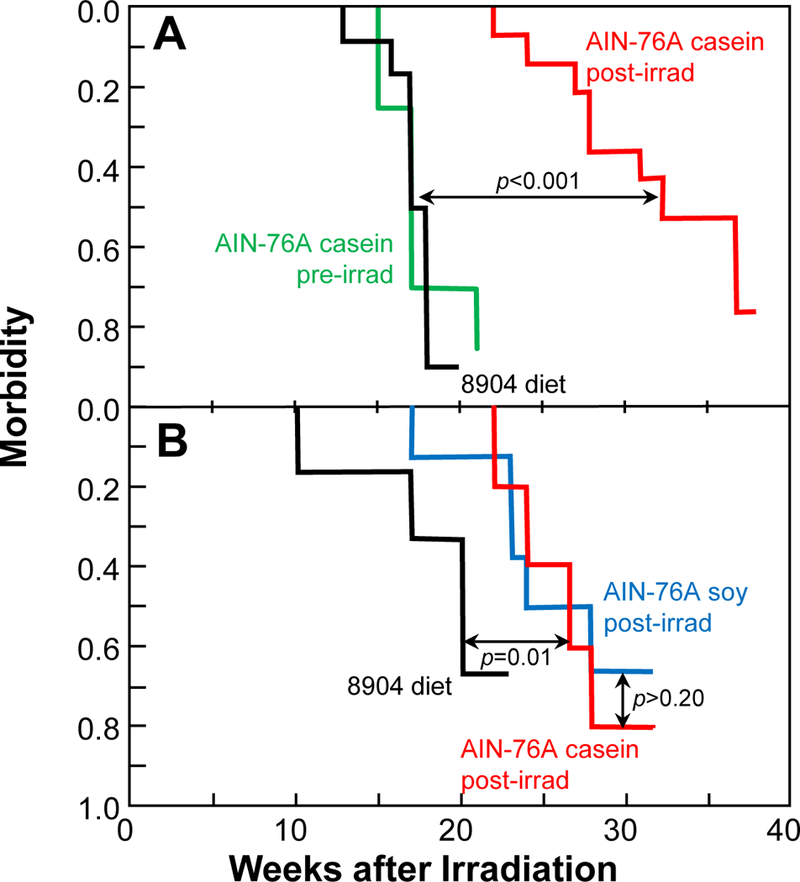
Kaplan-Meier morbidity curves as a function of diet for rats given 11 Gy single-dose PBI with one-hind limb shielded, where morbidity is defined as a BUN of 120 mg/dl. Panel A, Study 1: animals continuously fed a standard natural-ingredient rodent diet, Teklad 8904 (n=12, black); animals switched at 2 weeks prior to irradiation to a casein-based AIN-76A diet (n=8, green); animals switched at 1 week after irradiation to the same AIN-76A diet (n=14, red). Panel B, Study 2: animals continuously fed Teklad 8904 (n=6, black); animals switched at 1 week after irradiation to the casein-based AIN-76A diet (n=5, red); animals switched at 1 week after irradiation to a further modified AIN-76A diet in which the protein was switched from casein to soy (n=8, blue). In both studies, once the diet switch was made, animals stayed on the AIN-76A diet for until 18 weeks after irradiation when the diet was changed back to Teklad 8904.
Fig. 2.
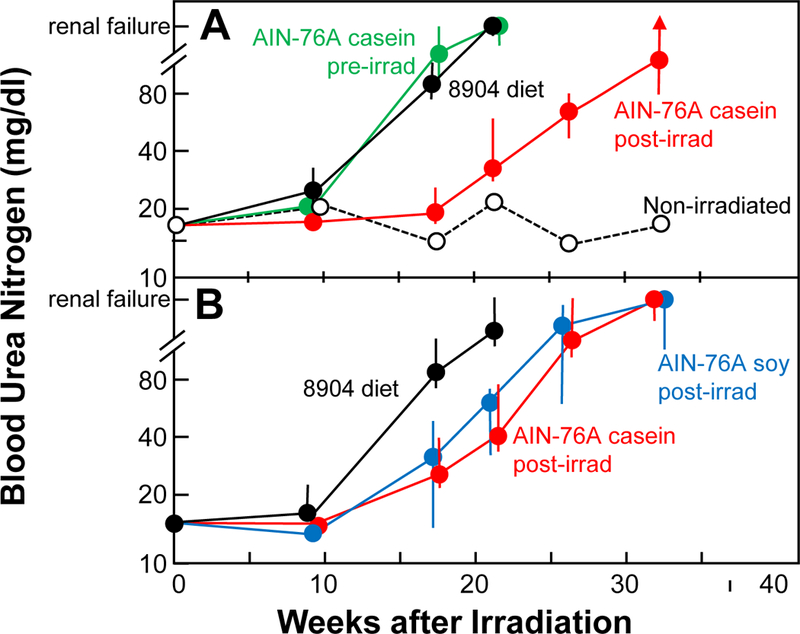
Azotemia (as blood urea nitrogen, BUN) as a function of diet for rats given 11 Gy single-dose PBI with one-hind limb shielded. These are the same animals shown in Figure 1. BUN values are medians with 20–80% confidence intervals; points are plotted as “renal failure” when more than 50% of the animals had been euthanized with BUN greater than 120 mg/dl, and upper confidence limits are replaced by an arrow when more than 20% of the animals had been euthanized with high BUN. Panel A, Study 1: animals continuously fed Teklad 8904 (black); animals switched at 2 weeks prior to irradiation to the casein-based AIN-76A (green); animals switched at 1 week after irradiation to the same AIN-76A diet (red). Also shown is data (open circles, dashed line) for concurrent sham-irradiated animals on the casein-based AIN-76A diet from 6 weeks of age (n=5). Panel B, Study 2: animals continuously fed Teklad 8904 (black); animals switched at 1 week after irradiation to the casein-based AIN-76A diet (n=5, red); animals switched at 1 week after irradiation to the soy-based AIN-76A diet (n=8, blue).
The switch to the casein-based AIN-76A diet at one week after irradiation dramatically increased survival (p<0.001, Fig 1, Panel A), but when the switch was made two weeks prior to irradiation there was no effect on survival (p>0.20, Fig. 1, Panel A). At 17 weeks and after, BUN levels were elevated (all p<0.002) in irradiated animals (vs. non-irradiated). BUN levels in animals on Teklad 8904 and those switched to AIN-76A 2 weeks prior to irradiation did not differ (all p>0.20, Fig. 2, Panel A). At 17 and 21 weeks after irradiation animals switched to AIN-76A at 1 week after irradiation had lower BUNs than that those that remained on Teklad 8904 (both p<0.0005, Fig. 2, Panel A).
Data from a colleague working with a different rat nephropathy model (Mattson et al. 2005) suggested that the casein in the diet might be responsible for the beneficial effect of the AIN-76A diet, so we tested a version of AIN-76A where the protein source was soy. Animals were switched to the AIN-76A diets 1 week after irradiation and remained on them until 18 weeks after irradiation. Based on morbidity (Fig. 1, Panel B), the switch to AIN-76A was beneficial for both formulations of the AIN-76A diet (both p=0.01), and there was no difference between the two formulations (both p>0.20). At 17 and 21 weeks after irradiation animals switched to the AIN-76A diets had lower BUNs than those that remained on Teklad 8904 (all p<0.02), and BUN values for animals on the two AIN-76A diets did not differ (all p>0.20). This second study also established that it was not the lack of isoflavones in the casein-based AIN-76A diet that affected radiation nephropathy, as the soy-based AIN-76A diet had isoflavone levels similar to that of Teklad 8904 (Table 1).
Next, we performed a study to see if the mitigation efficacy of switching animals to the AIN-76A diet at 1 week after irradiation would extend to radiation pneumonitis. The switch to the AIN-76A diet reduced morbidity from radiation pneumonitis from 61% to 31% (p=0.07 vs animals that remained on Teklad 8904, Fig. 3). This suggests that the mitigation efficacy of the AIN-76A diet may extend to other organ systems.
Fig. 3.
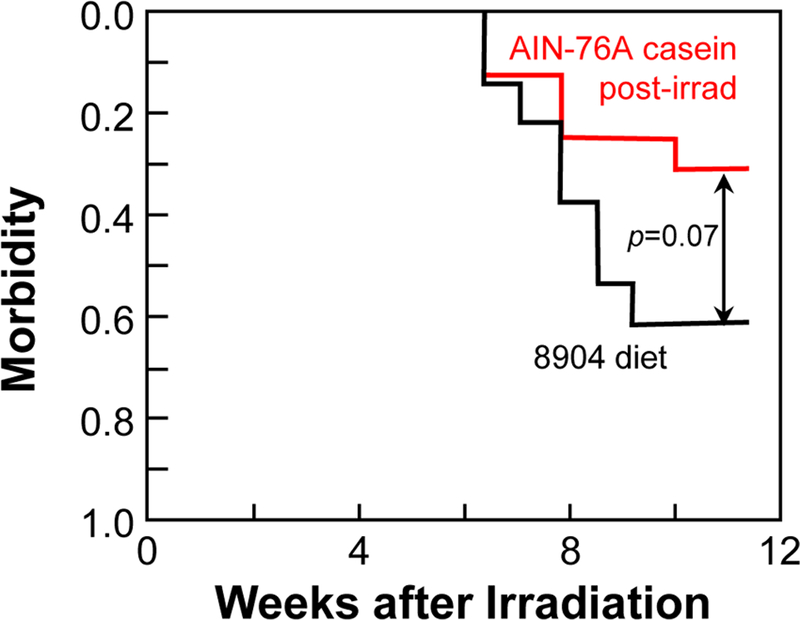
Kaplan-Meier morbidity curves as a function of diet for rats given 12.75 Gy whole-thorax irradiation. Follow-up ended at 11.5 weeks. Animals were continuously fed our standard diet, Teklad 8904 (n=12, black) or were switched at 1 week after irradiation to a casein-based AIN-76A diet (n=16, red). Once the diet switch was made, animals stayed on the AIN-76A diet for the duration of the study.
DISCUSSION
Several dietary components are known to affect renal radiosensitivity including, protein (Yatvin et al. 1984, Robbins et al. 1993), sodium (Moulder et al. 2002) and selenium (Sieber et al. 2011). Sodium content is ruled out as a factor in this study, as the soy-based AIN-76A and the Teklad 8904 have identical sodium levels (Table 1). While the casein-based AIN-76A did have a lower sodium content, previous work in this nephropathy model showed that it was the high- not the low-sodium diet that was beneficial (Moulder et al. 2002). Similarly, the decreased selenium content in the AIN-76A diets (Table 1) seems an unlikely factor as it is excess selenium that is beneficial in the nephropathy model (Sieber et al. 2011).
It is possible that the lower iron content of the AIN-76A diets (Table 1) were responsible for their mitigating benefit, as iron has been implicated in the pathogenesis of progressive chronic renal failure (e.g., Alfrey et al. 1989). However, the bioavailability of iron in natural-ingredient diets like Teklad 8904 is lower, perhaps by a factor of five, compared to refined diets like AIN-76A (Hubbard et al. 2013).
Protein content might be a factor (24% in Teklad 8904 vs. 18% in AIN-76A) because reducing the protein content from 20% to 4% in rats (Yatvin et al. 1984) and from 16% to 4% in pigs (Robbins et al. 1993) had a modest effect on radiation nephropathy. In addition, there are reports that decreasing dietary protein from the common 23–24% to the 12–14% range can increase survival (Barrows and Kokkonen 1975, Rao 2002) and decrease chronic renal problems (Rao 2002) in normal (that is, non-irradiated) rats. However, the low sodium (TD.94025) and normal sodium (TD.96036) diets that were studied in this radiation nephropathy model (Moulder et al. 2002) had protein levels (19%) very close to AIN-76A (18%), but had no noticeable effects on renal radiosensitivity.
Another factor we examined was the possibility that somewhat higher energy density of the AIN-76A diet might matter (Table 1); but at 11 weeks after irradiation the weights of the animals on Teklad 8904 were not significantly (p>0.20) different from those on casein-based AIN-76A, namely 219 (202–234) g on Teklad 8904 versus 220 (215–231) g on AIN-76A. Even at 17 weeks after irradiation, when the animals on Teklad 8904 were developing nephropathy, the weight difference was small although statistically significant (p<0.05), namely 219 (202–234) g on Teklad 8904 and 225 (118–240) g on casein-based AIN-76A.
One speculation that we have not investigated is a vascular nitric oxide synthase (NOS) related connection. Spradley et al. (2012) showed that switching Dahl salt-sensitive rats from AIN-76A to Teklad 8604 (the non-irradiated analog to 8904) “leads to small arterial NOS dysfunction and reduced NOS signaling, predisposing Dahl salt-sensitive rats to vascular disease.” In our rat radiation nephropathy model, there is a drop in renal nitric oxide activity after irradiation (Cohen et al. 1996), and there is indirect evidence that this drop plays a role in the pathogenesis of radiation nephropathy (Moulder et al. 2003). If the AIN-76A diet mitigates this drop in renal nitric oxide activity it might explain at least part of its benefit.
It is clear from these studies that base diet changes alone can have biologically significant effects on organ radiosensitivity. While the mechanistic basis for the effect and its dependence of timing relative to irradiation are unclear, these unexpected effects may point the way toward new approaches to the mitigation of radiation injuries.
Acknowledgements --
These studies were supported by the Department of Radiation Oncology at the Medical College of Wisconsin and by NIH grants AI107305 and AI101898. Eric Cohen’s work on this manuscript was supported with resources and the use of facilities at the Baltimore VA Medical Center.
REFERENCES
- Alfrey AC, Froment DH, Hammond WS. Role of iron in the tubulo-interstitial injury in nephrotoxic serum nephritis. Kidney Int 36: 753–9; 1989. [DOI] [PubMed] [Google Scholar]
- Barrows CH Jr., Kokkonen G Protein synthesis, development, growth and life span. Growth 39: 525–33; 1975. [PubMed] [Google Scholar]
- Brown SL, Kolozsvary A, Liu J, Jenrow KA, Ryu S, Kim JH. Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality. Radiat Res 173: 462–468; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EP, Fish BL, Imig JD, Moulder JE. Mitigation of normal tissue radiation injury: Evidence from rat radiation nephropathy models. J Radiat Oncol 3: 1–8; 2015. [Google Scholar]
- Cohen EP, Fish BL, Moulder JE. The role of nitric oxide in radiation nephropathy. Arch Physiol Biochem 104: 200–206; 1996. [DOI] [PubMed] [Google Scholar]
- Fish BL, Gao F, Narayanan J, Bergom C, Jacobs ER, Cohen EP, Moulder JE, Orschell CM, Medhora M. Combined hydration and antibiotics with lisinopril to mitigate acute and delayed high-dose radiation injuries to multiple organs. Health Phys 111: 410–419; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard AC, Bandyopadhyay S, Wojczyk BS, Spitalnik SL, Hod EA, Prestia KA. Effect of dietary iron on fetal growth in pregnant mice. Comp Med 63: 127–35; 2013. [PMC free article] [PubMed] [Google Scholar]
- Johnke RM, Sattler JA, Allison RR. Radioprotective agents for radiation therapy: future trends. Future Oncol 10: 2345–2357; 2014. [DOI] [PubMed] [Google Scholar]
- Kennedy AR, Wan XS. Countermeasures for space radiation induced adverse biologic effects. Adv Space Res 48: 1460–1479; 2011. [Google Scholar]
- Lee ET, Desu MM. A computer program for comparing K samples with right-censored data. Comp Prog Biomed 2: 315–321; 1972. [DOI] [PubMed] [Google Scholar]
- Mattson DL, Meister CJ, Marcelle ML. Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 45: 736–741; 2005. [DOI] [PubMed] [Google Scholar]
- Medhora M, Gao F, Fish BL, Jacobs ER, Moulder JE, Szabo A. Dose-modifying factor for captopril for mitigation of radiation injury to normal lung. J Rad Res 53: 633–640; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE. The 2013 Dade W. Moeller Lecture: Medical countermeasures against radiological terrorism. Health Phys 170: 164–171; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total body irradiation. Radiat Res 175: 29–36; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE, Fish BL, Cohen EP. Dietary sodium modification and experimental radiation nephropathy. Int J Radiat Biol 79: 903–911; 2002. [DOI] [PubMed] [Google Scholar]
- Moulder JE, Fish BL, Cohen EP. ACE inhibitors and AII receptor antagonists in the treatment and prevention of bone marrow transplant nephropathy. Curr Pharm Design 9: 737–749; 2003. [DOI] [PubMed] [Google Scholar]
- Rao GN. Diet and kidney diseases in rats. Toxicol Pathol 30: 651–6; 2002. [DOI] [PubMed] [Google Scholar]
- Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr 127: 838S–841S; 1997. [DOI] [PubMed] [Google Scholar]
- Robbins MEC, Bywaters T, Jaenke RS, Rezvani M, Golding SJ, Whitehouse E, Hopewell JW. Influence of a low protein diet on radiation nephropathy in the pig. Int J Radiat Biol 64: 407–416; 1993. [DOI] [PubMed] [Google Scholar]
- Sieber F, Muir SA, Cohen EP, Fish BL, Mäder M, Schock AM, Althouse BJ, Moulder JE. Dietary selenium for the mitigation of radiation injury: effects of selenium dose escalation and timing of supplementation. Radiat Res 176: 366–374; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Hanlon BK, Santiago PT, Seed TM. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part III. Countermeasures under early stages of development along with ‘standard of care’ medicinal and procedures not requiring regulatory approval for use. Int J Radiat Biol 93: 885–906; 2017. [DOI] [PubMed] [Google Scholar]
- Spradley FT, Ho DH, Kang KT, Pollock DM, Pollock JS. Changing standard chow diet promotes vascular NOS dysfunction in Dahl S rats. Am J Physiol 302: R150–158; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatvin MB, Oberley TD, Mahler PA. The beneficial effect of dietary protein restriction on radiation nephropathy. Strahlentherapie 160: 707–714; 1984. [PubMed] [Google Scholar]


