Abstract
Total body irradiation causes acute and delayed toxicity to hematopoietic, pulmonary, cardiac, gastrointestinal, renal and other organ systems. Angiotensin converting enzyme (ACE) inhibitors mitigate many of the delayed injuries to these systems. The purpose of this study was to define echocardiographic features in rats at two times after irradiation, the first being before lethal radiation pneumonitis (50 days) and the second after recovery from pneumonitis but before lethal radiation nephropathy (100 days) and to determine the actions of the ACE inhibitor lisinopril. Four groups of female WAG/RijCmcr rats at 11–12 weeks of age were studied: nonirradiated, nonirradiated+lisinopril, 13 Gy partial body irradiation sparing one hind leg (leg-out PBI), 13 Gy leg-out PBI+lisinopril. Lisinopril was started 7 days after radiation. Echocardiograms were obtained at 50 and 100 days and cardiac histology was assessed after 100 days. Irradiation without lisinopril demonstrated echocardiographic transient pulmonary hypertension by 50 days, which was largely resolved by 100 days in survivors. Irradiated rats given lisinopril showed no increase in pulmonary artery pressures at 50 days, but exhibited left ventricular remodeling. By 100 days these rats showed some signs of pulmonary hypertension. Lisinopril alone had no impact of echocardiographic endpoints at either time point in nonirradiated rats. Mild increases in mast cells and fibrosis in the heart were observed after 100 days following 13 Gy leg-out PBI. These data demonstrate irradiation-induced pulmonary hypertension which was reversed in survivors of pneumonitis. Lisinopril modified cardiovascular remodeling to enhance survival in this model from 41% to 86% (p=0.0013).
Keywords: angiotensin converting enzyme inhibitors, delayed effects of acute radiation exposure (DEARE), echocardiography, pneumonitis
INTRODUCTION
Delayed effects of acute radiation exposure (DEARE) which may result from a nuclear attack or terrorist event include injury to the lungs, gastrointestinal (GI) tract, kidneys, brain and heart (DiCarlo et al. 2011; Williams and McBride 2011). Previous studies demonstrated that combined hydration, antibiotics and lisinopril together mitigate acute radiation syndrome (ARS) and DEARE to multiple organs in rats after 13 Gy partial body irradiation (PBI) with one hind leg outside the field (hereafter termed leg-out PBI). Substantial morbidity between 40 and 80 days was seen due to radiation pneumonitis and related cardiopulmonary complications (Fish et al. 2016). Survivors of radiation pneumonitis went on to develop lethal radiation nephropathy after 100 days post-irradiation (Fish et al. 2016; Medhora et al. 2015; Medhora et al. 2014). Nephropathy is quantified by the accepted biomarker, blood urea nitrogen (BUN) >120 mg dL−1, that has been defined to proceed to lethal renal failure in irradiated rats (Moulder et al. 2011). Echocardiographic abnormalities including pulmonary hypertension and congestive heart failure during pneumonitis in rats receiving 15 Gy radiation to the whole thorax only have been identified (Medhora et al. 2015). However, it is unknown whether these changes are observed in survivors that recover from pneumonitis. Also, in rats with multiple organs exposed to radiation such as 13 Gy leg-out PBI, there is little information on delayed effects to the heart. The purposes of this study were to define (i) delayed changes in echocardiographic features in the heart after 13 Gy leg-out PBI during pneumonitis, when multiple organs are included in the field, (ii) echocardiographic changes after recovery from radiation pneumonitis, and (iii) the effects of the ACE inhibitor lisinopril on delayed radiation-induced cardiac injury.
MATERIALS AND METHODS
Animal care and irradiation (leg-out PBI)
Animals were maintained in the Animal Care Facilities of the Medical College of Wisconsin, which are fully accredited by the American Association for Accreditation of Laboratory Animal Care. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC). Female WAG/RijCmcr rats (11–12 weeks old) were bred, irradiated and housed in an isolated facility until echocardiography was performed in an external location. Three independent sets of rats were used to measure echocardiographic parameters at 50 and/or 100 days. Rats were irradiated as previously described (Fish et al. 2016) with 13 Gy to the whole body, except for one hind limb that was carefully externalized from the chamber and shielded with a 0.25 inch lead block. All rats received supportive care consisting of Enrofloxacin (10 mg kg−1day−1) from days 2–14 and hydration by daily subcutaneous injection of saline (40 mL kg−1day−1) from days 3–7 (Fish et al. 2016). Rats that became morbid in the study as defined by the IACUC were euthanized and necropsy was performed.
Experiments were conducted in 3 sets. The first set of rats was randomized into 3 groups: (i) nonirradiated controls (n=8) that was given supportive care but no irradiation, (ii) irradiation-only rats that was given 13 Gy leg-out PBI with supportive care (n=8) and (iii) 13 Gy leg-out PBI irradiation and supportive care plus lisinopril (~24 mg m−2 day−1, n=8) added to the drinking water starting from day 7 after irradiation and continuing until the study was terminated (Fish et al. 2016). This first set of rats had echocardiography at 50 days after irradiation and followed for survival. The second set of rats were randomized in the same way as set one, (i) nonirradiated controls (n=6), (ii) irradiation-only (13 Gy leg-out PBI with supportive care, n=11) and (iii) 13 Gy leg-out PBI irradiation with supportive care plus lisinopril (n=8). This second set of rats had echocardiography at 100 days after irradiation and euthanized for histology soon after. The third set of rats were randomized into 4 groups: (i) nonirradiated controls (n=5) with supportive care, (ii) irradiation-only (13 Gy leg-out PBI with supportive care) (n=4) and (iii) 13 Gy leg-out PBI irradiation and supportive care plus lisinopril (n=5), (iv) nonirradiated controls plus lisinopril (n=5) with supportive care. The third set of rats had echocardiography at 50 and survivors at 100 days after irradiation and euthanized for histology after 100 days.
Blood was harvested from the jugular vein around 100 days and blood urea nitrogen (BUN) was assayed from serum as described previously (Medhora et al. 2015). Surviving rats were sacrificed shortly thereafter.
Echocardiography at 50 and 100 days
The 50-day time for echocardiography was selected based on previous results with this and other models (Boerma et al. 2005; Ghosh et al. 2009a; Medhora et al. 2015) in which most irradiated rats survived to this time before morbidity from lethal radiation pneumonitis. The 100 day time-point after irradiation was chosen to capture survivors that recovered from radiation pneumonitis but had no lethality from radiation nephropathy.
Transthoracic echocardiography was performed in anesthetized (2% isoflurane) animals 50 and 100 days after radiation or at the corresponding time in nonirradiated, age-matched controls as previously described (Medhora et al. 2015; Sonin et al. 2013). The numbers of rats used for echocardiography in each group and both time points are shown in Tables 2a and 2b.
Table 2a.
Rat body weights at baseline (@ radiation or sham) and 50 days in rats randomized to receive 0 Gy, 0Gy + lisinopril, 13 Gy-leg out or 13 Gy leg-out PBI + lisinopril. Data are expressed as medians with 20–80 percentiles. One irradiation only rat was morbid from acute radiation syndrome (ARS, not shown).
| 50 days (sets 1&3) Treatment | Median body weight (20–80%) @ irradiation [grams] | Median body weight (20–80%) @ 50 days [grams] | N |
|---|---|---|---|
| 0 Gy | 162 (146 to 168) |
180 (173 to 186) |
13 |
| 0 Gy, Lisinopril | 158 (150 to 161) |
175 (169 to 183) |
5 |
| 13 Gy | 159 (151 to 162) |
142 (134 to 166) |
12 |
| 13 Gy, Lisinopril | 153 (149 to 159) |
146 (138 to 154) |
13 |
Table 2b.
Rat body weights at baseline and 100 days in rats randomized to receive 0 Gy, 0Gy + lisinopril, 13 Gy-leg out or 13 Gy leg-out PBI + lisinopril. The table also shows major findings for morbidity at necropsy. Weights are expressed as medians with 20–80 percentiles. One irradiation only and one irradiation+lisinopril rat were morbid from acute radiation syndrome (ARS, not shown).
| 100 days (sets 2&3) Treatment | Median body weight (20–80%) @ irradiation [grams] | Median body weight (20–80%) @ 100 days [grams] | Days in study | N | Major findings |
|---|---|---|---|---|---|
| 0 Gy | 160 (155 to 170) |
185 (181 to 202) |
100 | 11 | Termination |
| 0 Gy, Lisinopril | 158 (150 to 161) |
182 (174 to 186) |
100 | 5 | Termination |
| 13 Gy | 162 (149 to 164) |
149 (136 to 153) |
55 | 1 | Mottled lungs, scarred liver |
| 55–74 | 6 | Pleural effusion, scarred liver | |||
| 100 | 8 | Termination | |||
| 13 Gy, Lisinopril | 156 (148 to 162) |
156 (145 to 158) |
64,66 | 2 | GI hemorrhage, bloat |
| 90,102 | 2 | Anesthesia death | |||
| 100 | 9 | Termination |
Histology of the heart
The heart was harvested, fixed and transverse sections cut across both ventricles at the papillary level. Whole mount sections of each heart were stained with tryptase (IMGENEX catalogue #IMG-80250, 1:150) or Masson’s trichrome and scanned as described previously (Gao et al. 2013). Histology was performed at the Children’s Research Institute Histology Core, Milwaukee. Quantitation was done in sections by a reviewer who was masked to the treatment groups. Mast cells were counted in a whole mount section including both left and right ventricle and the septum at ~papillary level under 5X lens magnification. Fibrosis was quantitated using Image J software in trichrome stained sections for the ratio of blue color (fibrotic tissues) and red color (nuclei) in 4 random fields/heart separating left and right ventricles.
Statistical analyses
Survival of different groups of rats was compared using a Peto-Peto Wilcoxin test. Rats with acute radiation syndrome (ARS) in the first 30 days after 13 Gy leg-out PBI (n=3) were censored because the focus of this study was manifestation and mechanism of mitigation of delayed effects to multiple organs. Data are presented as means ± standard deviations and compared by t-tests, or as medians with % ranges and compared by Mann–Whitney U tests on Ranks. For multi-group comparisons of echocardiogram parameters and histology, the significance of differences was assessed by ANOVA followed by the Holm-Sidak, Bonferroni/Dunn or Tukey methods for multiple comparison.
RESULTS
Echocardiography
Fifty days following 13 Gy leg-out PBI, coincident with radiation pneumonitis in the irradiation only group (Fish et al. 2016; Ghosh et al. 2009b; Kma et al. 2012), rats exhibited increase in pulmonary artery systolic pressures (PASP) as determined by Doppler-derived parameters based on tricuspid regurgitation in 6/12 rats (Table 1a). Tricuspid regurgitation wasabsent in all nonirradiated rats. The PASP went from unmeasurable values in nonirradiated rats to a maximum of 98.03 mm Hg (mean 64.78 mm Hg, not shown in Table 1a) for the 6 rats with tricuspid regurgitation. Also, irradiated only rats had decreased pulmonary acceleration time/ejection time (PAT/ET) (Table 1a) consistent with higher pulmonary artery pressures. Left ventricular (LV) posterior wall (diastole, LVPWd) and isovolumic relaxation time (IVRT) were increased relative to nonirradiated controls in this group with marked decrease in stroke volume (Table 1a). Other changes between irradiated only and nonirradiated controls are marked (*) in Table 1a.
Table 1a.
Summary of echocardiographic and doppler measurements in 4 groups of rats: (nonirradiated (0 Gy), nonirradiated + lisinopril, irradiation only (13 Gy leg-out PBI) and irradiation+lisinopril, at 50 days.
| Echocardiogram Parameters | Mean ± SD | |||
|---|---|---|---|---|
| Days | 50 | 50 | 50 | 50 |
| Groups | 0 Gy n=13 |
0 Gy + Lis n=5 |
13 Gy only n=12 |
13 Gy + lis n=13 |
|
| ||||
| HR (beats/min) | 380±31 | 397±15 | 382±51 | 374±34 |
| SV (ml) | 0.48±0.07 | 0.48±0.05 | 0.31±0.16+* | 0.46±0.06# |
| RV width (cm) | 0.20±0.01 | 0.25±0.06 | 0.19±0.03# | |
| LVIDd (cm)& | 3.50±0.27 | 3.63±0.27 | 3.52±0.87 | 4.10±0.98 |
| EDV (ml)& | 3.22±0.42 | 3.40±0.46 | 2.37±1.33 | 4.02±0.52# |
| IVSD (cm)& | 0.85±0.13 | 0.82±0.05 | 1.17±0.36+* | 0.84±0.17# |
| LVPWd (cm)& | 0.78±0.13 | 0.70±0.08 | 1.10±0.18+* | 0.75±0.11# |
| ESV (ml)& | 0.52±0.21 | 0.63±0.10 | 0.27±0.23+* | 0.84+0.22*,# |
| LVIDs (cm)& | 1.82±0.33 | 1.99±0.12 | 1.55±0.56 | 2.50±0.24*,# |
| TAPSE | 0.20±0.05 | 0.16±0.04 | 0.21±0.03 | |
| PAT (ms) | 25.52±3.56 | 23.04±3.78 | 18.8±6.16* | 23.01±5.38 |
| ET (ms) | 62.32±3.74 | 61.88±6.05 | 68.56±6.48 | 63.66±6.67 |
| PAT/ET | 0.41±0.05 | 0.37±0.03 | 0.28±0.10* | 0.37±0.09 |
| IVRT (ms) | 12.72±2.31 | 10.39±1.89 | 19.59±7.75+* | 13.66±1.68# |
| RVOT (cm) | 2.47±0.45 | 2.14±0.32 | 2.31±0.71 | 2.52±0.44 |
| % EF | 83.8±6.4 | 81.2±2.0 | 89.9±4.1+* | 79.1±4.2# |
| % FS | 48.3±7.8 | 44.6±1.9 | 55.7±6.2+* | 42.7±4.1# |
| TR frequency | 0/13 | 0/5 | 6/12 | 0/13 |
HR-heart rate, SV-stroke volume, RV width-right ventricular width, LVIDd-left ventricular internal dimension (diastole), EDV-left end diastolic volume, IVSD-inter ventricular septum diastole, LVPWd-left ventricle posterior wall (diastole), ESV-left end systolic volume, LVIDs-left ventricular internal dimension (systole), TAPSE-tricuspid annular peak systolic excursion, PAT-pulmonary acceleration time, PAT/ET- pulmonary acceleration time/ejection time, ET-ejection time, IVRT-isovolumic relaxation time, RVOT-right ventricular outflow tract, EF-ejection fraction, FS-fractional shortening, TR-tricuspid regurgitation.
normalized for body weight (Kg−1);
p<0.0083 versus nonirradiated rats at 50 days;
p<0.0083 versus irradiation only at 50 days;
p<0.0083 versus nonirradiated with lisinopril rats at 50 days.
At 50 days, no 13 Gy leg-out PBI rats treated with lisinopril showed tricuspid regurgitation and the mean PAT/ET was not different from that of nonirradiated rats indicating normal pulmonary artery pressures. Compared to nonirradiated-only controls, 13 Gy leg-out PBI +lisinopril rats had increases in left end systolic volumes (ESV) and LV internal diameter at end systole (LVIDs) (Table 1a). Compared to the 13 Gy leg-out PBI rats the 13 Gy leg-out PBI +lisinopril rats had close to normal values for the 10 parameters that were altered by radiation-only. These included left end systolic and diastolic volumes (ESV, EDV), LV internal diameter at end systole (LVIDs), lower ejection fraction (EF) and fractional shortening (FS) (Table 1a).
By 100 days, tricuspid regurgitation and abnormalities in stroke volumes (SV and ESV), that were present in the 13 Gy leg-out PBI rats at 50 days were not observed in the survivors (Table 1b). Similarly, the indicator of pulmonary hypertension (e.g. PAT/ET) in the 13 Gy leg-out PBI rats was no longer present. However, right ventricular (RV) outflow tract (RVOT, an indicator of RV stress) which was not increased at 50 days, was above that of controls at 100 days. RV ejection time (ET) was also increased in this group, suggesting new RV dysfunction had developed (Table 1b).
Table 1b.
Echocardiography data from the same groups of rats 100 days after irradiation. Data are expressed as mean ± standard deviation (SD).
| Echocardiogram Parameters | Mean ± SD | |||
|---|---|---|---|---|
| Days | 100 | 100 | 100 | 100 |
| Groups | 0 Gy n=11 |
0 Gy + Lis n=5 |
13 Gy only n=8 |
13 Gy + lis n=9 |
|
| ||||
| HR (beats/min) | 392±44 | 407±50 | 338±58 | 385±45 |
| SV (ml) | 0.49±0.11 | 0.51±0.10 | 0.40±0.10 | 0.33±0.12* |
| RV width (cm) | 0.27±0.04 | 0.24±0.03 | 0.28±0.03 | |
| LVIDd (cm)& | 3.37±0.39 | 3.54±0.23 | 4.12±0.79* | 3.50±0.63 |
| EDV (ml)& | 3.23±0.85 | 3.41±0.68 | 3.35±1.05 | 2.48±1.11 |
| IVSD (cm)& | 0.77±0.09 | 0.80±0.17 | 1.12±0.20+* | 1.18±0.38* |
| LVPWd (cm)& | 0.80±0.19 | 0.65±0.09 | 1.04±0.09+* | 0.99±0.20* |
| ESV (ml)& | 0.62±0.30 | 0.63±0.20 | 0.49±0.25 | 0.31±0.36 |
| LVIDs (cm)& | 1.86±0.31 | 1.96±0.19 | 2.11±0.55 | 1.49±0.73 |
| TAPSE | 0.21±0.04 | 0.17±0.2 | 0.14±0.03* | |
| PAT (ms) | 27.05±4.22 | 21.34±4.57 | 24.95±8.05 | 16.53±6.72*# |
| ET (ms) | 65.05±6.68 | 62.07±4.93 | 83.7±12,46+* | 68.67±7.09# |
| PAT/ET | 0.42±0.05 | 0.34±0.05 | 0.30±0.11 | 0.24±0.10* |
| IVRT (ms) | 10.54±4.55 | 8.27±0.98 | 17.31±6.03+* | 15.59±6.01 |
| RVOT (cm) | 2.52±0.43 | 2.64±0.36 | 3.73±0.51+* | 2.66±0.46# |
| % EF | 81.0±5.3 | 81.7±2.5 | 85.4±5.4 | 90.0±8.1* |
| % FS | 44.8±5.3 | 45.2±2.6 | 49.6±5.4 | 59.1±15.2* |
| TR frequency | 0/11 | 0/5 | 0/8 | 3/9 |
HR-heart rate, SV-stroke volume, RV width-right ventricular width, LVIDd-left ventricular internal dimension (diastole), EDV-left end diastolic volume, IVSD-inter ventricular septum diastole, LVPWd-left ventricle posterior wall (diastole), ESV-left end systolic volume, LVIDs-left ventricular internal dimension (systole), TAPSE-tricuspid annular peak systolic excursion, PAT-pulmonary acceleration time, PAT/ET- pulmonary acceleration time/ejection time, ET-ejection time, IVRT-isovolumic relaxation time, RVOT-right ventricular outflow tract, EF-ejection fraction, FS-fractional shortening, TR-tricuspid regurgitation.
normalized to body weight (Kg−1);
p<0.0083 versus nonirradiated rats at 100 days;
p<0.0083 versus irradiation only at 100 days;
p<0.0083 versus nonirradiated with lisinopril rats at 100 days.
13 Gy leg-out PBI rats treated with lisinopril exhibited evidence of pulmonary artery hypertension at 100 days which was not present at 50 days based on tricuspid regurgitation in 3/9 rats and decreased tricuspid annular peak systolic excursion (TAPSE), PAT and PAT/ET (Table 1b). On the other hand, ESV and LVIDs that were increased at 50 days were not different from controls at 100 days in this group. Increases in IVSD, EF and FS were observed at 100 days though they were not present in this group at 50 days.
Nonirradiated rats treated with lisinopril alone had echocardiographic values that were not different from those of nonirradiated rats alone at 50 or 100 days after treatment.
DEARE in the groups of rats
There was no lethal pneumonitis in any group in the first 50 days. Survival by 100 days was diminished to ~41% in the 13 Gy leg-out PBI only group and to 86% in the 13 Gy leg-out PBI +lisinopril group (p=0.0013). The cause of morbidity in irradiation-only rats was radiation pneumonitis as determined by the lung injury and pleural effusion visible at necropsy (Table 2b). Rats were morbid from other causes in the irradiation+lisinopril group. The were no differences between sets for rats treated in the same manner. Body weights are shown for 50 days (Table 2a) and 100 days (Table 2b).
When bone marrow is spared, kidney injury becomes the dose-limiting factor with severe renal impairment when >7.8 Gy (Moulder et al. 2011) is delivered and enough time passes for injury to manifest. Accordingly, BUN (a marker of kidney injury) was measured to assess a potential contribution of renal dysfunction to cardiac injury in this model at 100 days. Median BUN was elevated to 54 (48–59) mg dL−1 in 13 Gy leg-out PBI rats only and 27 (25–32) mg dL−1 in 13 Gy leg-out PBI rats treated with lisinopril. Nonirradiated rats had BUN levels between 16–19 mg dL−1 and nonirradiated rats treated with lisinopril ranged from 18–22 mg dL−1. Thus even in untreated 13 Gy leg-out PBI rats, the deterioration in renal function based upon these indices is still mild and not a cause of lethality (BUN≥120 mg dL−1 (Fish et al. 2016).
Heart histology
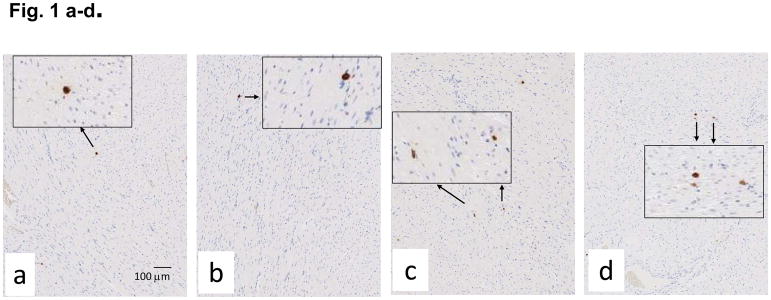
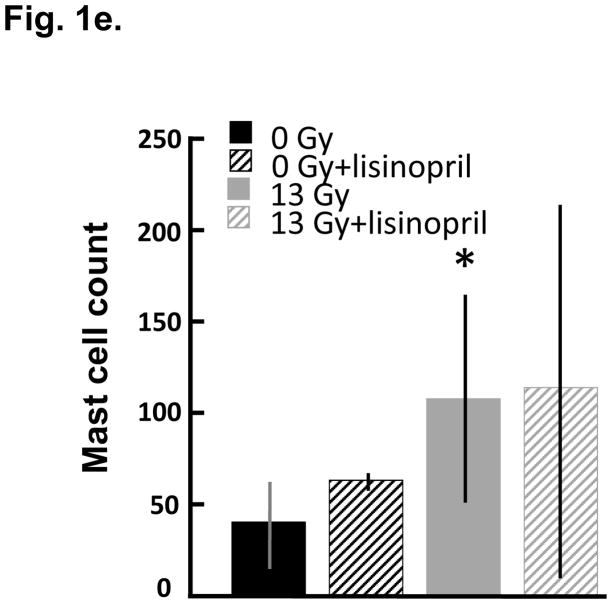
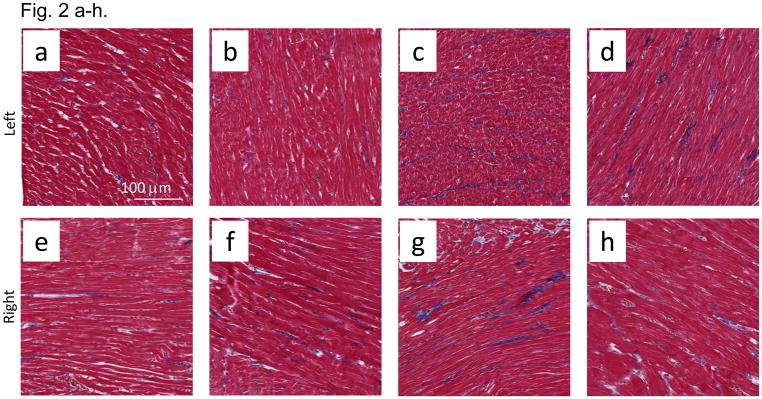
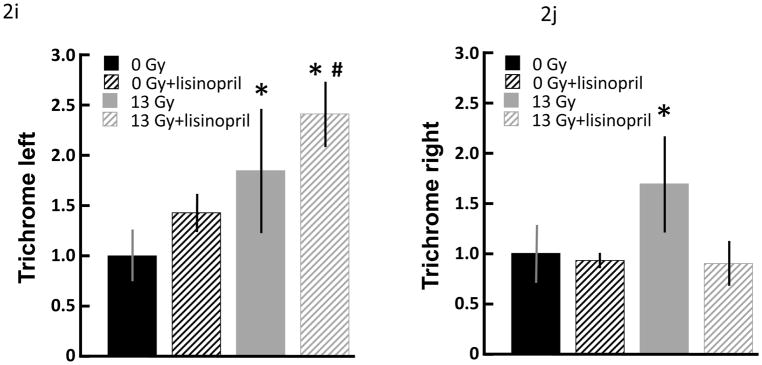
Mast cells infiltrates were minimal in both control and irradiated rats, though mast cells were increased at 100 days (Fig. 1a–d) after irradiation in a manner which was not impacted by lisinopril treatment (Fig. 1e). Fibrosis by radiation after 100 days was also evaluated. Trichrome blue stain (used to quantify fibrotic tissue) was increased in both left and right ventricles at 100 days in irradiated only rats (Fig. 2a–h). Lisinopril treatment protected from fibrosis in the right ventricle, but not in the left ventricle (Fig. 2i,j).
Fig. 1.
Mast cell infiltration in nonirradiated and irradiated cardiac tissue. Mast cells were assessed by staining for tryptase (in brown, see arrows) after 100 days in nonirradiated control (n=9) (a); nonirradiated + lisinopril (n=5) (b); age-matched rats given 13 Gy leg-out PBI only (n=6) (c) and 13 Gy leg-out PBI + lisinopril (n=4) (d). Low magnification images are shown to provide an overall impression of the histology; insets show high magnification to identify cellular detail. Mast cells were counted in whole mount heart sections including left and right ventricles and cell counts presented as mean±SD in (e). Bars represent nonirradiated and irradiated groups without and with lisinopril as marked. Though mast cells were increased (*p<0.05) in the irradiation only group as compared to corresponding nonirradiated control hearts, mast cell infiltration was minimal in rat hearts with or without irradiation as compared to lungs of irradiated rats with pneumonitis (Szabo et al. 2010). Lisinopril did not protect from mast cell infiltratation after irradiation.
Fig. 2.
Fibrosis in nonirradiated and irradiated cardiac tissue. Cardiac fibrosis was assessed by blue color in trichrome stained sections of hearts after 100 days. Representative sections of the left ventricles (a–d) and right ventricles (e–h) are shown separately. Cardiac myocytes are shown from nonirradiated (a,b,e,f) and irradiated (c,d,g,h) hearts as follows: a) left ventricle from nonirrradiated heart; b) left ventricle from lisinopril-treated, nonirrradiated heart; c) left ventricle from irradiated (13 Gy leg-out PBI) heart; d) left ventricle from lisinopril-treated, irradiated heart; e) right ventricle from nonirradiated heart; f) right ventricle from lisinopril-treated, nonirradiated heart; g) right ventricle from irradiated heart; h) right ventricle from lisinopril-treated, irradiated heart. Blue color was quantitated by measuring the ratio of blue:red color separately and presented as means±SD (Fig 2i and 2j). Bars represent nonirradiated and irradiated groups without and with lisinopril as marked. Though interstitial fibrosis was increased in ventricles after irradiation (*p<0.05, n=9 nonirradiated; n=5 nonirradited+lisinopril; n=6 irradiated only; n=4 irradiated+lisinopril) as compared to corresponding nonirradiated ventricles or irradiated ventricles with lisinopril, fibrosis was minimal in rat hearts with or without irradiation or drug, and not expected to contribute significantly to cardiac dysfunction or remodeling.
DISCUSSION
Our data describe developing and resolving echocardiographic abnormalities in rats receiving 13 Gy leg-out PBI following exposure. Significant morbidity with pleural effusion was observed between 55–74 days in these rats (Table 1b) and the BUN was increased from control values at 100 days, strongly suggesting that the first-time point (50 days) corresponded to early radiation pneumonitis, and the second (100 days) an early stage of radiation nephropathy. These results are supported by organ injuries that have been previously reported in the same model (Fish et al. 2016). Fifty days following irradiation, rats exhibited increased pulmonary artery pressure as indicated by PASP, PAT/ET and tricuspid regurgitation. The LV dimensions, volumes and function were preserved, but there was evidence of LV hypertrophy based on the increase in LV posterior-wall thickness. In the irradiation+lisinopril group, the increases in pulmonary artery pressure and LV thickness by 50 days were not observed. However, lisinopril treatment was accompanied by increases in LV volume and internal dimension in systole. By 100 days in survivors, pulmonary artery pressures and other changes in the irradiation only rats observed at 50 days had normalized. In contrast, echocardiography from the irradiation+lisinopril group at 100 days exhibited elevated tricuspid regurgitation in 3 rats, and increases in TAPSE and PAT/ET, consistent with pulmonary hypertension. Nonirradiated rats given lisinopril alone had echocardiographic measurements indistinguishable from those of control rats, suggesting that differences in cardiac function of irradiated rats treated with lisinopril (versus no lisinopril) represent a capacity of this ACE inhibitor to modify remodeling evoked by irradiation, rather than a primary effect of this drug.
Lung injury is increased in rats irradiated with 13 Gy leg-out PBI and 10–13 Gy whole thorax irradiation (WTI) in a manner that peaked at 42–56 days and was resolved in surviving rats (Fish et al. 2016; Ghosh et al. 2009a; Kma et al. 2012; Zhang et al. 2008). These data support the hypothesis that 50 day post-irradiation changes in cardiac structure and function reflect a response to lung injury (e.g. hypoxemia and tachypnea) during pneumonitis, which resolves in survivors (Medhora et al. 2015). Lung histology of irradiation only rats after 10–13 Gy WTI also showed resolution at delayed times following pneumonitis (Kma et al. 2012; Zhang et al. 2008). Lungs of rats irradiated with 10 Gy WTI had increased pulmonary vascular resistance with right ventricular hypertrophy at 56 days that resolved by 150 days after recovery from pneumonitis (Ghosh et al. 2009a). Pulmonary vessels however, had decreased distensibility and increased vascular dropout during radiation pneumonitis, which did not recover even by one year (Ghosh et al. 2009a) and may contribute to cardiac injury and remodeling at 100 days.
Echocardiographic features at the peak of pneumonitis in rats receiving 15 Gy WTI have been previously reported (Medhora et al. 2015). Some features of cardiac dysfunction appear similar to the 13 Gy leg-out PBI and 15 Gy WTI models in that both show evidence of RV abnormalities [Table 3 and (Medhora et al. 2015)]. These data are consistent with increased pulmonary vascular resistance, which were have previously reported for WTI (Medhora et al. 2015; Molthen et al. 2012). On the other hand, there was substantially more evidence of right heart dysfunction (see RV widths) after 15 Gy WTI, a dose that has 100% cardiopulmonary morbidity within 70 days. There was more severe pulmonary arterial hypertension in the 15 Gy WTI relative to the leg-out PBI model based on PAT, PAT/ET, e′ and IVRT, but the pulmonary artery remodelling in the 15 Gy WTI model is probably underestimated based on decreased stroke volume and cardiac output in an overtly failing right heart (Table 3).
Table 3.
Comparison of key echocardiographic parameters between 15 Gy whole thorax irradiation [WTI, (Medhora et al. 2015)] and 13 Gy leg-out partial body irradiation (PBI). Data are expressed as mean ± standard deviation (SD).
| Echocardiogram Parameters | 15 Gy WTI (42 days) n=6, Mean ± SD |
13 Gy PBI (50 days) n=12, Mean ± SD |
Implication differences in the two models |
|---|---|---|---|
| RV width (cm) | 0.44±0.06 | 0.25±0.06* | Higher PAH in 15 Gy |
| RV free wall (cm) | 0.14±0.05 | 0.10±0.05 | |
| CO (ml/min) | 54.7±30.8 | 116.9±78.2 | Worse heart function in 15 Gy |
| PAT (ms) | 13.0±2.14 | 18.8±6.16* | Higher PAH in 15 Gy |
| PAT/ET | 0.19±0.04 | 0.28±0.10* | Higher PAH in 15 Gy |
| IVRT (ms) | 39.6±7.65 | 19.6±7.75* | Worse heart function in 15 Gy |
| e′ (m/s) | 0.03±0.01 | 0.05±0.02* | Higher PAH in 15 Gy |
RV-right ventricular, CO-cardiac output, PAT-pulmonary acceleration time, PAT/ET-pulmonary acceleration time/ejection time, IVRT-isovolumic relaxation time, e′-early diastolic mitral annulus velocity, PAH-pulmonary artery hypertension.
p<0.05 versus 15 Gy WTI.
Cardiac histology from irradiated rats shows increased but mild inflammation by mast cells. Mast cells are known mediators of cardiac remodeling and pro-fibrotic activity (Levick et al. 2011). In correlation with this finding, increased fibrosis was detected in hearts of irradiation only versus nonirradiated rats. Infiltration of cardiac muscle with mast cells is described at 6 months (~180 days) following 18 Gy single dose irradiation to the heart of rats (Boerma et al. 2005). Mast cell deficient rats exhibited more severe deterioration of cardiac function than their sufficient litter mates in that study (Boerma et al. 2005), consistent with a protective effect of mast cell products. Taken in context, there is a narrow range of mast cell activity that is necessary for protective physiologic remodeling in that too little activity as seen in the mast cell deficient rats or too much activity as seen with radiation is detrimental. Our data with trichrome stains of the heart show increased fibrosis after irradiation in both the left and right ventricle. Only right ventricular fibrosis was mitigated by lisinopril, suggesting that fibrosis of the RV may be related to lung pathology, whereas LV fibrosis more likely reflects direct but mild radiation injury to the heart itself.
The ACE inhibitor captopril is reported to attenuate radiation-induced cardiopulmonary damage when the heart was present in the field of radiation (van der Veen et al. 2015). Lisinopril increased survival of rats receiving 13 Gy leg-out PBI by 120 days (Fish et al. 2016). ACE inhibitors protected from radiation pneumonitis after WTI (Fish et al. 2016; Ghosh et al. 2009b), diminished pulmonary fibrosis 7 months after WTI (Kma et al. 2012) and protected from renal injury (Fish et al. 2016). This class of drugs is well reported to diminish cardiac remodeling after myocardial ischemia (Cohn et al. 2000) by initiating molecular and cellular changes that modify heart shape and function. The reason for the increased LV diameters in irradiation+lisinopril rats at 50 days in our studies is unknown. However, it is not attributable to lisinopril alone; echocardiographic data from this group were not different from those of control rats. It is also probably not in response to pneumonitis (as there is little evidence of this process at 50 days in the irradiation+lisinopril group), so it more likely represents cardiac remodeling. By 100 days, lisinopril-treated rats exhibited some signs of pulmonary hypertension. Again, the reason(s) for this shift is not clear, based on the data (Table 2). ACE inhibitors may delay radiation evoked pneumonitis so it is possible that unresolved pneumonitis contributes to increased pulmonary artery (PA) pressures. However, lack of pleural effusions and protection from irradiation-induced elevated ET and RVOT observed in irradiation only rats at 100 days suggest that rats treated with lisinopril do not simply recapitulate the pathophysiology of 13 Gy rats at 50 days. Loss of >50% of the rats in the irradiation only group introduces a confounding variable in that data from the surviving rats reflect those selected for survival, while only 14% of irradiation+lisinopril rats were morbid. The answers to these and other such interesting questions will need further studies.
CONCLUSION
Rats that survive 13 Gy leg-out PBI develop transient pulmonary hypertension which is apparent by 50 days and largely resolved by 100 days in survivors. Rats treated with lisinopril after receiving 13 Gy leg-out PBI escape the change in pulmonary artery pressures at 50 days, but exhibit compensatory LV remodeling. Most indices of pulmonary hypertension are resolved by 100 days in the surviving irradiation-only rats. However, by 100 days, the lisinopril treated 13 Gy leg-out PBI rats show echocardiographic signs of pulmonary hypertension though survival is doubled at 86% versus 41% in the 13 Gy leg-out PBI only rats.
Acknowledgments
The authors wish to thank Marylou Mäder for the excellent technical support and animal care. Tissues were paraffin embedded, sectioned and stained with trichrome by the Children’s Research Institute Histology Core, Milwaukee. Yvonne Morauski assisted with preparing this manuscript. Andrea DiCarlo Cohen and Lanyn Talioferro at NIAID for the helpful discussions and input at U01 meetings. The studies were funded by U01AI107305 and revisions (Medhora), U01AI133594, (Medhora), R01AI101898 (Medhora), 1I01BX002256-01A1 (Jacobs), BX001681 (Jacobs), HL116530 (Jacobs), K08HL11148 (Strande), R01134932 (Strande), Institutional Research Grant 86-004-26 from the American Cancer Society (Bergom), Mary Kay Foundation Award Grant No. 017-29 (Bergom), Office of the Director of the NIH through Grant 8KL2TR000056 (Bergom), the CardioOncology Signature Pre-PPG award (Medhora, Jacobs, Strande and Bergom), Cardiovascular Center and Cancer Center (Medhora, Jacobs, Strande and Bergom), Medical College Wisconsin.
Footnotes
Conflicts of Interest and Source of Funding: The authors declare no conflicts of interest.
References
- Boerma M, Wang J, Wondergem J, Joseph J, Qiu X, Kennedy RH, Hauer-Jensen M. Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res. 2005;65:3100–3107. doi: 10.1158/0008-5472.CAN-04-4333. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, Bader JL, Coleman CN, Weinstock DM. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5(Suppl 1):S32–44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish BL, Gao F, Narayanan J, Bergom C, Jacobs ER, Cohen EP, Moulder JE, Orschell CM, Medhora M. Combined Hydration and Antibiotics with Lisinopril to Mitigate Acute and Delayed High-dose Radiation Injuries to Multiple Organs. Health Phys. 2016;111:410–419. doi: 10.1097/HP.0000000000000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Narayanan J, Joneikis C, Fish BL, Szabo A, Moulder JE, Molthen RC, Jacobs ER, Rao RN, Medhora M. Enalapril mitigates focal alveolar lesions, a histological marker of late pulmonary injury by radiation to the lung. Radiat Res. 2013;179:465–474. doi: 10.1667/RR3127.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SN, Wu Q, Mader M, Fish BL, Moulder JE, Jacobs ER, Medhora M, Molthen RC. Vascular injury after whole thoracic x-ray irradiation in the rat. Int J Radiat Oncol Biol Phys. 2009a;74:192–199. doi: 10.1016/j.ijrobp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, Jacobs ER, Medhora M. Renin-Angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2009b;75:1528–1536. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kma L, Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. Angiotensin converting enzyme inhibitors mitigate collagen synthesis induced by a single dose of radiation to the whole thorax. J Radiat Res. 2012;53:10–17. doi: 10.1269/jrr.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res. 2011;89:12–19. doi: 10.1093/cvr/cvq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Gao F, Glisch C, Narayanan J, Sharma A, Harmann LM, Lawlor MW, Snyder LA, Fish BL, Down JD, Moulder JE, Strande JL, Jacobs ER. Whole-thorax irradiation induces hypoxic respiratory failure, pleural effusions and cardiac remodeling. J Radiat Res. 2015;56:248–260. doi: 10.1093/jrr/rru095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Gao F, Wu Q, Molthen RC, Jacobs ER, Moulder JE, Fish BL. Model development and use of ACE inhibitors for preclinical mitigation of radiation-induced injury to multiple organs. Radiat Res. 2014;182:545–555. doi: 10.1667/RR13425.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molthen RC, Wu Q, Fish BL, Moulder JE, Jacobs ER, Medhora MM. Mitigation of radiation induced pulmonary vascular injury by delayed treatment with captopril. Respirology. 2012;17:1261–1268. doi: 10.1111/j.1440-1843.2012.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total-body irradiation. Radiat Res. 2011;175:29–36. doi: 10.1667/RR2400.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonin DL, Wakatsuki T, Routhu KV, Harmann LM, Petersen M, Meyer J, Strande JL. Protease-activated receptor 1 inhibition by SCH79797 attenuates left ventricular remodeling and profibrotic activities of cardiac fibroblasts. J Cardiovasc Pharmacol Ther. 2013;18:460–475. doi: 10.1177/1074248413485434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S, Ghosh SN, Fish BL, Bodiga S, Tomic R, Kumar G, Morrow NV, Moulder JE, Jacobs ER, Medhora M. Cellular inflammatory infiltrate in pneumonitis induced by a single moderate dose of thoracic x radiation in rats. Radiat Res. 2010;173:545–556. doi: 10.1667/RR1753.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen SJ, Ghobadi G, de Boer RA, Faber H, Cannon MV, Nagle PW, Brandenburg S, Langendijk JA, van Luijk P, Coppes RP. ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol. 2015;114:96–103. doi: 10.1016/j.radonc.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Williams JP, McBride WH. After the bomb drops: a new look at radiation-induced multiple organ dysfunction syndrome (MODS) Int J Radiat Biol. 2011;87:851–868. doi: 10.3109/09553002.2011.560996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Ghosh SN, Zhu D, North PE, Fish BL, Morrow NV, Lowry T, Nanchal R, Jacobs ER, Moulder JE, Medhora M. Structural and functional alterations in the rat lung following whole thoracic irradiation with moderate doses: injury and recovery. Int J Radiat Biol. 2008;84:487–497. doi: 10.1080/09553000802078396. [DOI] [PMC free article] [PubMed] [Google Scholar]