Abstract
This study explored whether ASD phenotypes in the child were associated with a history of anxiety or depression in the mother. We hypothesized that an ASD profile in children characterized by mild delays and increased rates of dysregulation would be associated with preexisting maternal anxiety or depression. Participants were 672 preschool children with ASD and their mothers. Children were classified as ASD after a comprehensive developmental evaluation. Mothers reported whether a healthcare provider ever diagnosed them with anxiety or depression before the birth of their child. Four child ASD phenotypes were derived from latent class analysis: Mild Language Delay with Cognitive Rigidity (Type 1), Significant Developmental Delay with Repetitive Motor Behaviors (Type 2), General Developmental Delay (Type 3), and Mild Language and Motor Delay with Dysregulation (i.e., aggression, anxiety, depression, emotional reactivity, inattention, somatic complaints, and sleep problems) (Type 4). Type 2 ASD served as the referent category in statistical analyses. Results showed that 22.6% of mothers reported a diagnosis of anxiety or depression before the birth of their child. Maternal anxiety or depression was associated with 2.7 times the odds (95% confidence interval: 1.4, 5.3) of Type 4 or Dysregulated ASD in the child; maternal anxiety and depression was associated with 4.4 times the odds (95% confidence interval: 1.4, 14.0) of Type 4 or Dysregulated ASD in the child. Our findings suggest an association between Dysregulated ASD in the child and anxiety and depression in the mother. These findings can enhance screening methods and inform future research efforts.
Keywords: Autism, Phenotype, Maternal, Anxiety, Depression
Autism spectrum disorder (ASD) is a developmental condition defined by deficits in social communication and interaction and the presence of stereotyped behaviors and interests (American Psychiatric Association (APA) 2013). Symptoms of ASD persist throughout life although presentation may change with maturation (APA 2013). Understanding the complex etiologies associated with different ASD phenotypes in early childhood, and factors associated with these etiologies and phenotypes, may help alleviate debilitating symptoms. Twin and family studies suggest a strong genetic component to the development of ASD with heritability estimates of 50% and higher in population-based samples (Colvert et al. 2015; Sandin et al. 2014). However, genetic mechanisms are multifaceted and poorly understood, and could be a result of de novo mutations, copy number variations, multigene interactions, or a combination of these and other factors (De la Torre-Ubieta et al. 2016; Geschwind 2011). Consequently, ASD may share a variety of genetic and non-genetic origins with other associated conditions that differ in phenotypic expression (Daniels et al. 2008). Describing the relationship between associated conditions in the parents and ASD phenotypes in the child may strengthen ASD identification efforts and inform future research on underlying mechanisms that lead to the development of ASD.
Anxiety and depression are reported to be common among individuals with ASD (Joshi et al. 2010; Simonoff et al. 2008), and a number of studies have found an association between parental anxiety and depression and development of ASD in offspring (Bolton et al. 1998; Daniels et al. 2008; DeLong 2004; DeLong and Dwyer 1988; Jokiranta et al. 2013; Larsson et al. 2005; Piven and Palmer 1999; Sullivan et al. 2012). Moreover, some studies highlighted an association between maternal depression in particular and specific ASD phenotypes (Cohen and Tsiouris 2006; DeLong 2004; DeLong and Dwyer 1988; Lajiness-O’Neil and Menard 2008; Vasa et al. 2012; Wiggins et al. 2017). Cohen and Tsiouris (2006) found that recurrent depressive states in the mother were related to higher adaptive and cognitive functioning and increased rates of problem behaviors in the child with ASD. Similarly, Lajiness-O’Neil and Menard (2008) reported an association between maternal mood disorder and improved visuospatial functioning in ASD probands, and Vasa et al. (2012) reported higher odds of having a child with Asperger disorder versus autistic disorder among mothers who reported a diagnosis of depression. These studies suggest that maternal psychiatric conditions – depression in particular – may be predictive of ASD in the offspring with symptom profiles marked by milder developmental delays and increased behavioral and emotional problems.
A recent study by Wiggins et al. (2017) found four subtypes of preschool children with ASD enrolled in the Study to Explore Early Development (SEED) (Schendel et al. 2012): Mild Language Delay with Cognitive Rigidity (Type 1 ASD), Significant Developmental Delay with Repetitive Motor Behaviors (Type 2 ASD), General Developmental Delay (Type 3 ASD), and Mild Language and Motor Delay with Dysregulation (i.e., aggression, anxiety, depression, emotional reactivity, inattention, somatic complaints, and sleep problems) (Type 4 ASD). Characteristics of children defined as Type 4 ASD were similar to those described by Cohen and Tsiouris (2006) and Lajiness-O’Neil and Menard (2008) who were more likely to have a depressed mother: these children had average visual reception skills and increased rates of problem behaviors. The purpose of this study was to explore whether maternal anxiety or depression diagnosed prior to childbirth were associated with these child ASD phenotypes to inform more targeted identification and research frameworks. We hypothesized that mothers with preexisting anxiety or depression would be more likely than those without to have a child with Type 4 ASD compared to Type 2 ASD when Type 2 ASD was the referent category.
Method
SEED is a multisite, community-based study of children 30–68 months of age (born between September 1, 2003 and August 31, 2006) designed to describe ASD phenotypes and investigate how genetic and environmental risk factors are associated with ASD profiles. Children were eligible for SEED if they were born and continued to live in one of six catchment areas located in California, Colorado, Georgia, Maryland, North Carolina, and Pennsylvania; and lived with a caregiver who spoke English (or English or Spanish at the California and Colorado sites) and could provide legal consent to participate. Three groups of children were invited to participate: (1) those with known ASD were recruited from multiple education and healthcare providers that serve children with disabilities, (2) those with other developmental disorders were recruited from the same education and healthcare sources, and (3) children in the population comparison group (POP) were randomly sampled from state vital records. All enrolled families provided written informed consent for participation. This analysis was limited only to children who met the SEED ASD case definition since we were particularly interested in the association between maternal anxiety and/or depression and how ASD presents in preschool children.
All children were screened for ASD risk with the Social Communication Questionnaire – Current Version (SCQ) upon enrollment (Rutter et al. 2003). The SCQ is a parent-report questionnaire that evaluates social-communication deficits associated with ASD. The SCQ was developed for children at least 48 month of age but has been validated in younger children when the cutoff score is reduced from 15 points to 11 points (Allen et al. 2007; Corsello et al. 2007; Eaves et al. 2006; Snow and Lecavalier 2008; Wiggins et al. 2007). Children who had a SCQ score less than 11 points, and did not have a previous diagnosis of ASD, were seen in person for a general developmental assessment that consisted of the Mullen Scales of Early Learning (MSEL) Mullen 1995). All other children received a more comprehensive developmental assessment that included the MSEL, Autism Diagnostic Observation Schedule (ADOS) Lord et al. 1999; Lord et al. 2000; Gotham et al. 2007), Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994), and the Vineland Adaptive Behavior Scales – Second Edition (Sparrow et al. 2005). The ADOS – a child-based observation instrument – and the ADI-R – a parent-based interview – are considered gold-standard diagnostic instruments and are often used to classify and characterize children with ASD (Gray et al. 2008). Children with ASD were defined as those who met SEED case criteria based on results of the ADOS (utilizing ADOS-2 algorithms) and ADI-R (Wiggins et al. 2015). Clinicians who administered the ADOS and ADI-R demonstrated scoring reliability before data collection and throughout the study period (Schendel et al. 2012).
Additional information on child development, maternal health, and sociodemographic status of the family was obtained via a comprehensive caregiver interview and several self-administered questionnaires, including the Child Behavior Checklist-Preschool Form (CBCL-1.5-5) (Achenbach 1992), early development questionnaire, and child sleep habits questionnaire. Each component of data collection was subject to quality control procedures (Schendel et al. 2012). A detailed description of study methods can be found in Schendel et al. (2012). This study was approved by Institutional Review Boards at the Centers for Disease Control and Prevention and at each SEED site.
Maternal Psychiatric History
Maternal psychiatric data were obtained via self-report on a maternal medical history form created for the SEED study. Mothers were asked to provide information on whether they had ever received specific medical or psychiatric diagnoses from a doctor or other healthcare provider. If the respondent confirmed a particular diagnosis, she was asked to specify the age of onset and whether the condition was present during pregnancy. Maternal anxiety disorder was defined in this study as a confirmatory response that an anxiety disorder was diagnosed before the birth of the child. Maternal depressive disorder was defined similarly as a confirmatory response that depression was diagnosed before the birth of the child. Mothers who reported other psychiatric conditions were placed in the “no anxiety” or “no depression” group unless anxiety or depression was specifically endorsed by the mother. For instance, there were five mothers who endorsed a diagnosis of bipolar disorder without a diagnosis of depression; these five mothers were placed in the “no depression” group. Mothers who were diagnosed with anxiety and depression after the birth of the child were not considered to have anxiety or depression in this analysis in order to exclude conditions such as postpartum depression and anxiety or depression as a response to parenting a child with atypical development.
Maternal anxiety and depression are components of the broader autism phenotype (BAP) – a set of sub-clinical characteristics of ASD – and are associated with child ASD phenotypes (Rubenstein et al. 2018). Maternal BAP was measured with the Social Responsiveness Scale-Adult (SRS-A), a 65-item Likert scale questionnaire that measures ASD-related social deficits (Constantino and Todd 2005). Each parent was asked to have a friend, spouse, or relative complete the SRS-A on the parent and then return it to SEED staff. T-scores of 60 or greater represent mild risk for ASD and were used for this study to classify a parent as BAP positive (Constantino 2002).
Child Phenotype Data
Child phenotype was defined by a latent class analysis (LCA) described by Wiggins et al. (2017) on the same sample of children defined herein. LCA is a statistical procedure that assumes that responses on observed variables can be explained by membership in unmeasured latent classes (Vermunt 2010). Individuals are classified into subgroups based on similar patterns of observed data that reflect probability of class membership. Wiggins et al. (2017) performed a latent class analysis with the variables in Table 1. Results showed that a four-class model best fit the data with a high precision of classification (i.e., entropy = 0.92). Moreover, Lo-Mendell-Rubin test supported the four-class model (p = 0.001), as the five-class model did not provide significantly better fit than the four-class model; as did Bayesian information criterion (BIC) and sample adjusted BIC. Between-class differences elucidated characteristics that most defined children represented in each latent class. Twenty eight percent of children with ASD were classified as Mild Language Delay with Cognitive Rigidity (Type 1 ASD), 26% as Significant Developmental Delay with Repetitive Motor Behaviors (Type 2 ASD), 34% as General Developmental Delay (Type 3 ASD), and 12% as Mild Language and Motor Delay with Dysregulation (Type 4 ASD). Children described as Type 2 ASD had more developmental delays than those in other classes and children described as Type 1 ASD and Type 4 ASD had fewer delays than those in other classes. Children described as Type 4 ASD had more symptoms of dysregulation (i.e., aggression, anxiety, depression, emotional reactivity, inattention, somatic complaints, and sleep problems) than those in other classes. Further details on this LCA method and results are provided elsewhere (Wiggins et al. 2017).
Table 1.
Latent class variables for children with autism spectrum disorder enrolled in the Study to Explore Early Development (SEED)
| SEED data source | Latent class variables | Scores used in latent class analysis |
|---|---|---|
| Autism Diagnostic Observation Schedule | Autism symptom severity | Total severity scores from 1 to 10 |
| Autism Diagnostic Interview – Revised | Age at verbal language development Age at walking History of regression Insistence on sameness Repetitive behavior with objects Repetitive motor mannerisms Restricted interests Self-injurious behaviors Unusual sensory response |
Item scores from 4 to 62 months Item scores from 7 to 43 months Item score dichotomized into yes (regression in either language or social domains reported) or no (regression in language or social domains not reported) Item scores representing compulsions/rituals, difficulties with minor changes in routines, and resistance to trivial changes in the environment dichotomized into yes (any reported) and no (not reported) Item score dichotomized into yes (reported) and no (not reported) Item scores representing hand and finger mannerisms and other complex mannerisms dichotomized into yes (any reported) and no (not reported) Item scores representing unusual preoccupations, circumscribed interests, and unusual attachment to objects dichotomized into yes (any reported) and no (not reported) Item score dichotomized into yes (self-injurious behavior reported) and no (no self-injurious behavior reported) Item scores representing unusual sensory interests, undue sensitivity to noise, and negative response to specific sensory stimuli dichotomized into yes (any reported) and no (not reported) |
| Caregiver Interview | Early recognition of epilepsy/seizure disorder | Item score dichotomized into yes (parent report of epilepsy/seizure disorder) or no (no parent report of epilepsy/seizure disorder) |
| Child Behavior Checklist-Preschool Form (1.5–5) | Aggressive behaviors, anxiety/depression, attention problems, emotional reactivity, somatic complaints, withdrawn behaviors | Domain t-scores from 50 to 100 |
| Child Sleep Habits Questionnaire | Sleep problems | Total problems scores from 0 to 91 |
| Early Development Questionnaire | Problems with age at first social smile | Item scores dichotomized into yes (delayed social smile) and no (typical social smile) |
| Mullen Scales of Early Learning | Expressive language skills Fine motor skills Receptive language skills Visual reception skills |
Age equivalent scores from 2 to 70 Age equivalent scores from 4 to 68 Age equivalent from 1 to 69 Age equivalent scores from 5 to 69 |
| Social Communication Questionnaire | Social communication abilities | Total scores from 1 to 35 |
Statistical Analyses
Multinomial logistic regression models were used to assess the association between maternal anxiety and depression prior to childbirth and child phenotypic class. Two parameterizations of exposure were respectively included in the models: (1) anxiety or depression and (2) anxiety and depression. We did not assess associations between anxiety only or depression only because there were very few mothers who had a diagnosis of anxiety only and a child with Type 1 ASD (N = 4) or Type 4 ASD (N = 6). We chose Significant Developmental Delay with Repetitive Motor Behavior (Type 2 ASD) as our referent category for all regression models because this class had more developmental delays compared to children in other latent classes (Wiggins et al. 2017). Results of regression analyses therefore represent the increased odds of having a mother with preexisting anxiety or depression among higher functioning children (based on previous research) compared to lower functioning children in the SEED sample.
Adjusted models included maternal BAP, child race/ethnicity, child sex, maternal age (at birth of the child), maternal education, and study site as covariates; these latter variables were collected via telephone interview with the mother. Child race/ethnicity was determined using an algorithm that incorporated both maternal and paternal race and ethnicity. If parental race/ethnicity was not reported in the telephone interview (1.0% of participants), information from the child’s birth certificate was used to determine child race/ethnicity.
Regressions were conducted using an alpha level of 0.05. The classification error from the LCA was accounted for in all regression models where the exposure (in the crude model) or the exposure and covariates (adjusted model) were regressed against phenotypic class. This method is shown to be less biased and more efficient when assessing associations between a covariate and latent classes (Vermunt 2010).
Analyses were conducted in SAS 9.3 (SAS Institute, Cary NC), MPlus, and R Studio version 1.0.143.
Results
There were 707 children who met the SEED ASD case criteria; the mothers of 672 (95.0%) children with ASD completed the SEED maternal medical history form. Of the 672 children in our sample, 152 (22.6%) had a mother who reported a diagnosis of anxiety or depression prior to childbirth. Of these, 73.5% were white non-Hispanic, 14.5% were black non-Hispanic, and 0.7% were Hispanic (Table 1). Of mothers without an anxiety or depression diagnosis prior to childbirth, 52.9% were white non-Hispanic, 21.2% were black non-Hispanic, and 3.5% were Hispanic. Other demographic covariates did not differ between the two groups, but the overall sample was highly educated (49.7% of mothers with anxiety or depression and 53.0% of mothers without anxiety or depression had ≥16 years of education), and were older than the national average age of childbirth of 26.3 years (31.4 years for mothers with anxiety or depression and 31.8 years for those without anxiety or depression).32 There were 39 mothers (5.8%) who reported a diagnosis of both anxiety and depression before the birth of their child with ASD (Table 2).
Table 2.
Differences in child, demographic, and study characteristics by presence of maternal anxiety or depression in the Study to Explore Early Development
| Maternal Anxiety or Depression |
No Maternal Anxiety or Depression |
X2 | p value | |||
|---|---|---|---|---|---|---|
|
N =152 |
N =520 |
|||||
| N | % | n | % | |||
| Child ASD Phenotypea | ||||||
| Type 1 | 47 | 30.9 | 145 | 27.9 | 10.8 | 0.01 |
| Type 2 | 29 | 19.1 | 148 | 28.5 | ||
| Type 3 | 49 | 32.2 | 176 | 33.8 | ||
| Type 4 | 27 | 17.8 | 51 | 9.80 | ||
| Child Race/Ethnicity | ||||||
| White | 112 | 73.7 | 275 | 52.9 | 29.4 | <0.01 |
| Black | 22 | 14.5 | 110 | 21.2 | ||
| Other | 17 | 11.2 | 116 | 22.3 | ||
| Hispanic | 1 | 0.70 | 18 | 3.50 | ||
| Missing | 1 | |||||
| Child Sex | ||||||
| Female | 21 | 13.8 | 99 | 19.0 | 2.20 | 0.10 |
| Male | 131 | 86.2 | 421 | 81.0 | ||
| Maternal Education (years) | ||||||
| <12 | 8 | 5.40 | 26 | 5.00 | 0.50 | 0.80 |
| 12 to <16 | 67 | 45.0 | 216 | 41.9 | ||
| >=16 | 74 | 49.7 | 273 | 53.0 | ||
| Missing | 3 | 5 | ||||
| Site | ||||||
| California | 18 | 11.8 | 91 | 17.5 | 6.50 | 0.30 |
| Colorado | 31 | 20.4 | 102 | 19.6 | ||
| Georgia | 30 | 19.7 | 101 | 19.4 | ||
| Maryland | 18 | 11.8 | 82 | 15.8 | ||
| North Carolina | 28 | 18.4 | 75 | 14.4 | ||
| Pennsylvania | 27 | 17.8 | 69 | 13.3 | ||
| Maternal age at child birth | ||||||
| Mean (sd) | 31.3 | 5.40 | 31.7 | 5.60 | 0.80 | 0.40 |
Phenotypic classes are defined as ASD with (1) Mild Language Delay with Cognitive Rigidity, (2) Significant Developmental Delay with Repetitive Motor Behaviors, (3) General Developmental Delay, and (4) Mild Language and Motor Delay with Dysregulation (e.g., anxiety/depression) and Ns are based on highest posterior probability of class membership
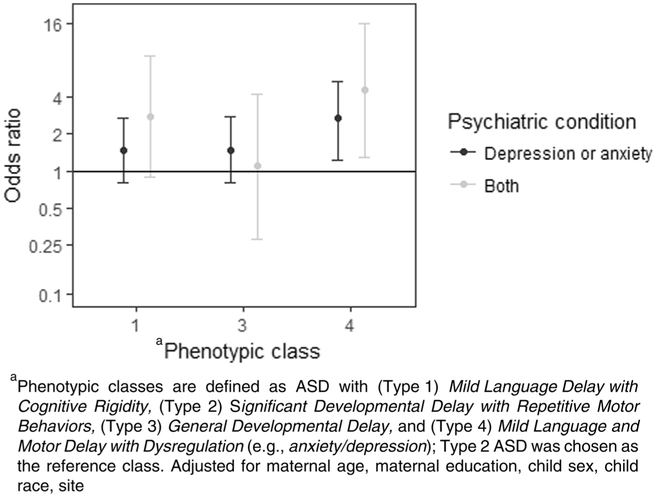
Crude and adjusted odds ratios (aOR) and 95% confidence intervals (CI) for the associations between maternal anxiety or depression prior to childbirth, and maternal anxiety and depression prior to childbirth, and child phenotypic class are presented in Table 3 and Fig. 1. In our adjusted model, children whose mothers reported a diagnosis of either anxiety or depression prior to childbirth had 2.7 (95% CI: 1.4, 5.3) times the odds of being in the Type 4 subtype as the Type 2 subtype. This result was slightly attenuated from the crude model (OR 2.9, 95% CI: 1.5, 5.4). The association remained significant when we assessed maternal diagnoses of both anxiety and depression prior to childbirth (aOR: 4.4, 95% CI: 1.4, 14.0). Odds ratios comparing maternal anxiety or depression in children with Type 1 or Type 3 phenotypic subtypes relative to the Type 2 subtype were not significant (Table 3).
Table 3.
Odds ratios comparing child autism spectrum disorder phenotype by presence of maternal anxiety or depression in the Study to Explore Early Development
| Child ASD Phenotypea |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type 1 |
Type 3 |
Type 4 |
|||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Maternal anxiety or depression | |||||||||
| Unadjusted | 1.74 | 1.01, 3.00 | 0.05 | 1.49 | 0.85, 2.59 | 0.20 | 2.89 | 1.52, 5.51 | <0.01 |
| Adjusted | 1.48 | 0.81, 2.71 | 0.20 | 1.46 | 0.79, 2.71 | 0.20 | 2.59 | 1.30, 5.15 | <0.01 |
| Maternal anxiety and depression | |||||||||
| Unadjusted | 2.67 | 0.96, 7.43 | 0.06 | 1.11 | 0.34, 3.67 | 0.90 | 5.16 | 1.71, 15.6 | <0.01 |
| Adjusted | 2.77 | 0.89, 8.86 | 0.10 | 1.07 | 0.27, 4.23 | 0.90 | 4.71 | 1.39, 15.98 | 0.01 |
Bold values indicate statistically significant associations of at least p <0.05
Phenotypic classes are defined as ASD with (1) Mild Language Delay with Cognitive Rigidity, (2) Significant Developmental Delay with Repetitive Motor Behaviors, (3) General Developmental Delay, and (4) Mild Language and Motor Delay with Dysregulation (e.g., anxiety/depression)
Fig. 1.
Odds ratios comparing child autism spectrum disorder phenotype by presence of maternal anxiety or depression in the Study to Explore Early Development
Discussion
We observed marked variability in child ASD phenotype based on maternal report of anxiety or depression diagnosed before childbirth. Mothers who had a diagnosis of anxiety or depression had between two and three times the odds of having a child with ASD and Mild Language and Motor Delay with Dysregulation (Type 4 ASD) than ASD and Significant Developmental Delay with Repetitive Motor Behaviors (Type 2 ASD). Mothers who had a diagnosis of both anxiety and depression had four times the odds of having a child with Type 4 ASD than Type 2 ASD. These results suggest that a dysregulated developmental profile in preschool children with ASD is significantly associated with anxiety and depression in the mother. Future research should examine whether postnatal maternal anxiety or depression mitigates this association to determine impact of timing of maternal features on child ASD phenotypes.
Ours is not the first study to report an association between an ASD phenotype defined by higher rather than lower cognitive abilities in the child and anxiety and depression in the mother (Cohen and Tsiouris 2006; Lajiness-O’Neil and Menard 2008; Vasa et al. 2012). The benefit of our analysis is the careful phenotypic characterization of a large number of preschool children with ASD (n = 707), and demonstration that maternal anxiety and depression prior to the birth of the affected child are related to the expression of ASD in early stages of development. Healthcare providers might use these results to encourage screening for symptoms of dysregulation in addition to symptoms of ASD in young children. The Ages and Stages Questionnaire: Social-Emotional Form (Squires et al. 2005), Brief Infant-Toddler Social-Emotional Assessment (Briggs-Gowan et al. 2004), and CBCL-1.5-5 are examples of tools that screen for dysregulation in the preschool years. Children who have anxious or depressed mothers and symptoms of dysregulation but not ASD may need additional monitoring for ASD symptoms over time. Conversely, children who have anxious or depressed mothers and ASD may need additional monitoring for dysregulation and mental health supports over time. This type of screening and monitoring approach may lead to enhanced identification of children with Type 4 ASD and development of individualized treatment plans that address the unique needs of children in this population.
A developmental model for Dysregulated or Type 4 ASD may suggest that Type 4 ASD shares common risk factors with anxiety and depression that disrupt social, emotional, and behavior regulation more than nonverbal cognitive abilities (Bolton et al. 1998; DeLong and Dwyer 1988). These risk factors could be biologic/genetic, environmental, or both biologic/genetic and environmental. A detailed review of the myriad of potential biological risk factors for ASD, anxiety, and depression is beyond the scope of this paper. However, a brief review could help explain our findings. Numerous studies have implicated the regulation of serotonin in the development of ASD, anxiety disorders, and major depression (DeLong 2004; Mathews and Hamilton 2016; Nordquist and Oreland 2010). Individuals with all three disorders are often responsive to the same pharmacologic treatments, especially selective serotonin reuptake inhibitors (SSRI), which could suggest analogous receptor characteristics (DeLong 2004; Matthews and Hamilton 2016). Moreover, neuroimaging studies show that both ASD and depression involve altered synthesis of serotonin in the brain (DeLong 2004; Mathews and Hamilton 2016). Polymorphisms in the transporter gene 5HTT and monoamine oxidase A (MAOA) are likely candidates for differences in serotonin activity among those with ASD and have become subjects of investigation of pharmacological treatments for symptoms of ASD (Sucksmith et al. 2011). Another potential risk factor for numerous developmental and psychiatric disorders is variations in calcium-channel activity genes (Nakai et al. 2017).
Shared social environment and/or the impact of maternal psychiatric disorder on the management of a child with ASD may also play a role in the expression of behavioral and emotional disturbance in the child. For instance, mothers who are anxious or depressed may expose their children to a different prenatal and postnatal environment than mothers who are not anxious or depressed, and may have different parenting styles and/or levels of parenting stress that influence dysregulation in the child. Previous research shows that lack of warmth or over-involvement among the parent (s) leads to anxiety and depression in children (Yap et al. 2014). Other symptoms of dysregulation – such as somatic complaints and sleep problems – have less empirical support that links these symptoms to parent behaviors. Moreover, there are no identified studies on how parent behaviors influence the development of dysregulation among children with ASD. Future research is needed to elucidate how specific risk factors create susceptibility to increased behavioral and emotional problems in adults and children with and without an ASD diagnosis.
Our findings also contribute to the discussion of diagnostic boundaries between ASD, anxiety, depression, and other developmental disorders and psychiatric illnesses. This discussion is especially relevant in light of another SEED analysis that found a significant association between parental broader autism phenotype (BAP) and Type 4 or Dysregulated ASD (Rubenstein et al. 2018). Parental BAP is a set of sub-clinical characteristics of ASD that includes anxiety, cognitive rigidity, poor social skills, and problems with pragmatic language Sucksmith et al. 2011). BAP – like ASD, anxiety, and depression – is highly heritable and prone to co-occur with other mental health problems (Bora et al. 2016; Lyall et al. 2014; Sasson et al. 2013). Together, the results of Wiggins et al. (2017), Rubenstein et al. (2018), and those described here imply shared etiologic risk among several conditions that blurs diagnostic boundaries and highlights the importance of diagnostic qualifiers that capture an array of social, emotional, and psychiatric disturbances.
There are several limitations to our analysis that deserve consideration. First, maternal diagnosed anxiety and depression were obtained via self-report and not confirmed with medical records or other sources of information. We did not have detailed descriptions of features, symptoms or severity of these conditions. Moreover, mothers who are anxious or depressed may have differential response patterns on the ADI-R and other parent-report measures compared to mothers who are not anxious or depressed (Chilcoat and Breslau 1997; De Los Reyes and Kazdin 2005; Gartstein et al. 2009). Second, some studies suggest that many women with clinical anxiety or depression are not diagnosed; we therefore may have mothers with undiagnosed anxiety and depression in our “unexposed” group, which may bias results toward the null (Ko et al. 2012). Conversely, mothers may have over-reported symptoms of anxiety or depression during pregnancy or falsely remembered a professional diagnosis that could explain variations in mood during the pregnancy period. Another consideration is that some studies describe over-reporting of psychiatric conditions in children of mothers with mood disorders, which could also influence our findings (De Los Reyes and Kazdin 2005; Verweij et al. 2011)). Third, our results were restricted to preschool children with ASD who live in specific catchment areas and are not necessarily generalizable to older children or to those living in other geographic settings. Fourth, due to small numbers of participants in certain subgroups, we could not investigate the associations between child ASD phenotypes and: (1) maternal anxiety only and depression only, (2) maternal anxiety and depression diagnosed after the birth of the child, or (3) paternal anxiety and depression. Finally, we did not examine biological or environmental factors that might contribute to the observed associations. We will continue to expand on these analyses as the SEED sample grows in subsequent phases.
It is also important to note that the prevalence of ASD and Significant Developmental Delay with Repetitive Motor Behaviors (Type 2 ASD) was lower among children with a mother who reported a diagnosis of anxiety or depression compared to the other groups. The higher prevalence in Dysregulated or Type 4 ASD among children with a mother who reported a diagnosis of anxiety or depression, coupled with the lower prevalence in Type 2 ASD, both lead to the increased odds ratios that we observed.
The strengths of our analysis supersede the limitations. We utilized a large sample of children with ASD, from multiple, diverse geographic locations, who were characterized with extensive phenotypic data subject to quality control procedures. Mothers were asked about their history of anxiety and depression in a standardized format that specified a healthcare provider must have provided the diagnosis. Children with ASD were subtyped according to a data-generated technique with a high degree of precision in latent classification. Findings from the latent classification approach are aligned with prior hypotheses regarding the relationship of subtypes of ASD to maternal anxiety and depression (Cohen and Tsiouris 2006; DeLong 2004), and supports the importance of examining ASD phenotypes when considering shared etiologic factors with co-occurring psychopathology.
In conclusion, we found that an ASD phenotype – named here as Dysregulated or Type 4 ASD and characterized by average visual reception skills, mild language and motor delays, and increased rates of dysregulation in preschool children – is significantly related to anxiety and depression in the mother. These findings can be used to enhance screening efforts for young children with anxious or depressed mothers that may lead to improved identification of both ASD and symptoms of dysregulation. These findings can also highlight the importance of diagnostic qualifiers that capture symptoms present in numerous psychiatric and developmental disorders that could help identify shared causes and effective treatment options.
Acknowledgments
The investigators acknowledge the contributions made to this study by project staff and enrolled families. Other author contributions were as follows: study concept (Lisa Wiggins), study design and methods (all authors), statistical plan (Eric Rubenstein, Lisa Wiggins, and Lin Tian), statistical analysis (Eric Rubenstein, Lin Tian, and Katherine Sabourin), statistical review and interpretation (all authors), manuscript preparation and/or review (all authors). This publication was supported by six cooperative agreements from the Centers for Disease Control and Prevention (CDC): Cooperative Agreement Number U10DD000180, Colorado Department of Public Health; Cooperative Agreement Number U10DD000181, Kaiser Foundation Research Institute (CA); Cooperative Agreement Number U10DD000182, University of Pennsylvania; Cooperative Agreement Number U10DD000183, Johns Hopkins University; Cooperative Agreement Number U10DD000184, University of North Carolina at Chapel Hill; and Cooperative Agreement Number U10DD000498, Michigan State University and the Health Services and Resources Administration (HRSA) Maternal Child Health Bureau, Leadership Education in Neurodevelopmental Disabilities (LEND) Grant Award #T73MC11044. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
Conflict of Interest There are no conflicts of interest to report.
Ethical Approval This research was reviewed and approved by Institutional Review Boards at the Centers for Disease Control and Prevention (CDC) and each study site.
>Informed Consent Informed consent was obtained by all families that participated in the study.
References
- Achenbach T (1992). Child behavior checklist. Burlington: Achenbach System of Empirically Based Assessment. [Google Scholar]
- Allen CW, Silove N, Williams K, & Hutchins P (2007). Validity of the social communication questionnaire in assessing risk of autism in preschool children with developmental problems. Journal of Autism and Developmental Disorders, 37(7), 1272–1278. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington: American Psychiatric Publishing. [Google Scholar]
- Bolton PF, Pickles A, Murphy M, & Rutter M (1998). Autism, affective, and other psychiatric disorder: Patterns of familial aggregation. Psychiatric Medicine, 28, 385–395. [DOI] [PubMed] [Google Scholar]
- Bora E, Aydin A, Sarac T, et al. (2016). Heterogeneity of subclinical autistic traits among parents of children with autism spectrum disorder: Identifying the broader autism phenotype with a data-driven method. Autism Research, 10, 321–326. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Irwin JR, Wachtel K, & Cicchetti DV (2004). The brief infant –toddler social and emotional assessment: Screening for social-emotional problems and delays in competence. Journal of Pediatric Psychology, 29(2), 143–155. [DOI] [PubMed] [Google Scholar]
- Chilcoat HD, & Breslau N (1997). Does psychiatric history bias mothers’ reports? An application of a new analytic approach. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 971–979. [DOI] [PubMed] [Google Scholar]
- Cohen IL, & Tsiouris JA (2006). Maternal recurrent mood disorders and high-functioning autism. Journal of Autism and Developmental Disorders, 36, 1077–1088. [DOI] [PubMed] [Google Scholar]
- Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E, Gillan N, Hallett V, Lietz S, Garnett T, Ronald A, Plomin R, Rijsdijk F, Happé F, & Bolton P (2015). Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry, 72(5), 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN (2002). Social Responsiveness Scale. Los Angeles: Western Psychological Services. [Google Scholar]
- Constantino JN, & Todd RD (2005). Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry, 57, 655–660. [DOI] [PubMed] [Google Scholar]
- Corsello C, Hus V, Pickles A, Risi S, Cook EH, Leventhal BL, & Lord C (2007). Between a ROC and a hard place: Decision making and making decisions about using the SCQ. Journal of Child Psychology and Psychiatry, 48(9), 932–940. [DOI] [PubMed] [Google Scholar]
- Daniels J, Forssen U, Hultman CM, Cnattingius S, Savitz D, Feychting M, et al. (2008). Parental psychiatric disorder associated with autism spectrum disorders in the offspring. Pediatrics, 121(5), e1357–e1362. [DOI] [PubMed] [Google Scholar]
- De la Torre-Ubieta L, Won H, Stein JL, & Geshwind D (2016). Advancing the understanding of autism disease mechanisms through genetics. Nature Medicine, 22, 345–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Reyes A, & Kazdin AE (2005). Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychological Bulletin, 131, 483–509. [DOI] [PubMed] [Google Scholar]
- DeLong RG (2004). Autism and familial major mood disorder: Are they related? The Journal of Neuropsychiatry and Clinical Neurosciences, 16(2), 199–213. [DOI] [PubMed] [Google Scholar]
- DeLong RG, & Dwyer JT (1988). Correlation of family history with specific autistic subgroups: Asperger’s syndrome and bipolar affective disease. Journal of Autism and Developmental Disorders, 18, 593–600. [DOI] [PubMed] [Google Scholar]
- Eaves LC, Wingert HD, Ho HH, & Mickelson EC (2006). Screening for autism spectrum disorders with the social communication questionnaire. Journal of Developmental & Behavioral Pediatrics, 27(2), S95–S103. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Bridgett DJ, Dishion TJ, & Kaufman NK (2009). Depressed mood and maternal report of child behavior problems: Another look at the depression-distortion hypothesis. Journal of Applied Developmental Psychology, 30(2), 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind D (2011). Genetics of autism spectrum disorders. Trends in Cognitive Sciences, 15(9), 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, & Lord C (2007). The autism diagnostic observation schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders, 37, 613–627. [DOI] [PubMed] [Google Scholar]
- Gray K, Tonge B, & Sweeney D (2008). Using the autism diagnostic interview-revised and the autism diagnostic observation schedule with young children with developmental delay: Evaluating diagnostic validity. Journal of Autism and Developmental Disorders, 38, 657–667. [DOI] [PubMed] [Google Scholar]
- Jokiranta E, Brown AS, Heinimaa M, Cheslack-Postava P, Suominen A, & Sourander A (2013). Parental psychiatric disorders and autism spectrum disorders. Psychiatry Research, 207,203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, Kotarski M, Walls S, Biederman J (2010). The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: A large comparative study of a psychiatrically referred population. Journal of Autism and Developmental Disorders, 40, 1361–1370. [DOI] [PubMed] [Google Scholar]
- Ko JY, Farr SL, Dietz PM, & Robbnis CL (2012). Depression and treatment among U.S. pregnant and nonpregnant women of reproductive age, 2005-2009. Journal of Womens Health, 21(8), 830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajiness-O’Neil R, & Menard P (2008). Brief report: An autistic spectrum subtype revealed through familial psychopathology coupled with cognition in ASD. Journal of Autism and Developmental Disorders, 38, 982–987. [DOI] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Vingaard Olesen A, Agerbo E, et al. (2005). Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology, 161, 916–925. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur AL (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, & Risi S (1999). Autism diagnostic observation schedule. Los Angeles: Western Psychological Services. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. (2000). The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the Spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- Lyall K, Constantino JN, Weisskopf MG, Roberts AL, Ascherio A, & Santangelo SL (2014). Parental social responsiveness and risk of autism spectrum disorder in offspring. JAMA Psychiatry, 71, 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TJ & Hamilton BE (2016) Mean age of mothers is on the rise: United States, 2000–2014 NCHS Data Brief, no 232. Hyattsville: National Center for Health Statistics. [PubMed] [Google Scholar]
- Mullen E (1995). Mullen Scales of Early Learning. San Antonio: Pearson. [Google Scholar]
- Nakai N, Nagano M, Saitow F, Watanabe Y, Kawamura Y, Kawamoto A, Tamada K, Mizuma H, Onoe H, Watanabe Y, Monai H, Hirase H, Nakatani J, Inagaki H, Kawada T, Miyazaki T, Watanabe M, Sato Y, Okabe S, Kitamura K, Kano M, Hashimoto K, Suzuki H, & Takumi T (2017). Serotonin rebalances cortical tuning and behavior linked to autism symptoms in 15q11-13 CNV mice. Science Advances, 3(6), e1603001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist N, & Oreland L (2010). Serotonin, genetic variability, behavior, and psychiatric disorders – A review. Upsala Journal of Medical Sciences, 115, 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, & Palmer P (1999). Psychiatric disorder and the broad autism phenotype: Evidence from a family study of multiple-incidence autism families. American Journal of Psychiatry, 156, 557–563. [DOI] [PubMed] [Google Scholar]
- Rubenstein E, Wiggins LD, Schieve L, Bradley C, DiGuiseppi C, Moody E, et al. (2018). Associations between parental broader autism phenotype and child autism spectrum disorder phenotype in the study to explore early development. Autism. 10.1177/1362361317753563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter MA, Bailey A, & Lord C (2003). The social communication questionnaire. Los Angeles: Western Psychological Services. [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larssen H, Hutman C, & Reichenberg A (2014). The familial risk of autism. JAMA, 311(17), 1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Lam KS, Parlier M, Daniels J, & Piven J (2013). Autism and the broad autism phenotype: Familial patterns and intergenerational transmission. Journal of Neurodevelopmental Disorders, 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel D, DiGuiseppi C, Croen L, Fallin MD, Reed P, Schieve L, et al. (2012). The study to explore early development (SEED): A multi-site epidemiologic study of autism by the centers for autism and developmental disabilities research and epidemiology (CADDRE) network. Journal of Autism and Developmental Disorders, 42, 2121–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 921–929. [DOI] [PubMed] [Google Scholar]
- Snow AV, & Lecavalier L (2008). Sensitivity and specificity of the modified checklist for autism in toddlers and the social communication questionnaire in preschoolers suspected of having pervasive developmental disorders. Autism, 12(6), 627–644. [DOI] [PubMed] [Google Scholar]
- Sparrow S, Balla D, & Cicchetti D (2005). Vineland Adaptive Behavior Scales (2nd ed.). San Antonio: Pearson. [Google Scholar]
- Squires J, Bricker D, & Twombly E (2005). Ages and stages questionnaires: Social-emotional. Baltimore: Brookes Publishing. [Google Scholar]
- Sucksmith E, Roth I, & Hoekstra RA (2011). Autistic traits below the clinical threshold: Re-examining the broader autism phenotype in the 21st century. Neuropsyhology Review., 21, 360–389. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Magnusson C, Reichenberg A, Boman M, Dalman C, Davidson M, Fruchter E, Hultman CM, Lundberg M, Långström N, Weiser M, Svensson AC, & Lichtenstein P (2012). Family history of schizophrenia and bipolar disorder as risk factors for autism. Archives of General Psychiatry, 69(11), 1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa RA, Anderson C, Marvin AR, Rosenberg RE, Law JA, Thorn J, et al. (2012). Mood disorder in mothers of children on the autism spectrum are associated with higher functioning autism. Autism Research and Treatment, 2012, 1–8. 10.1155/2012/435646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt JK (2010). Latent class modeling with covariates: Two improved three-step approaches. Political Analysis, 18, 450–469. [Google Scholar]
- Verweij KH, Derks EM, Hendriks EJ, & Cahn W (2011). The influence of informant characteristics on the reliability of family history interviews. Twin Research and Human Genetics, 14, 217–220. [DOI] [PubMed] [Google Scholar]
- Wiggins LD, Bakeman R, Adamson L, & Robins D (2007). The utility of the social communication questionnaire in screening for autism in children referred for early intervention. Focus on Autism and Other Developmental Disabilities, 22(1), 33–38. [Google Scholar]
- Wiggins LD, Reynolds A, Rice C, Moody EJ, Bernal P, Blaskey L, et al. (2015). Using standardized diagnostic instruments to classify children with autism in the study to explore early development. Journal of Autism and Developmental Disorders, 45, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins LD, Tian L, Levy S, Rice C, Lee L-C, Schieve L, et al. (2017). Homogeneous subgroups of young children with autism improve phenotypic characterization in the study to explore early development. Journal of Autism and Developmental Disorders, 47, 3634–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MB, Pilkington PD, Ryan SM, & Jorm AF (2014). Parental factors associated with depression and anxiety in young people: A systematic review and meta-analysis. Journal of Affective Disorders, 156, 8–23. [DOI] [PubMed] [Google Scholar]