Abstract
Indole-diterpenes (IDTs) such as the aflatrems, janthitrems, lolitrems, paspalitrems, penitrems, shearinines, sulpinines, and terpendoles are biogenetically related but structurally varied tremorgenic and neurotoxic mycotoxins produced by fungi. All these metabolites derive from the biosynthetic intermediate paspaline, a frequently occurring IDT on its own right. In this comprehensive review, we highlight the similarities and differences of the IDT biosynthetic pathways that lead to the generation of the main paspaline-derived IDT subgroups. We survey the taxonomic distribution and the regulation of IDT production in various fungi and compare the organization of the known IDT biosynthetic gene clusters. A detailed assessment of the highly diverse biological activities of these mycotoxins leads us to emphasize the significant losses that paspaline-derived IDTs cause in agriculture, and compels us to warn about the various hazards they represent towards human and livestock health. Conversely, we also describe the potential utility of these versatile molecules as lead compounds for pharmaceutical drug discovery, and examine the prospects for their industrial scale manufacture in genetically manipulated IDT producers or domesticated host microorganisms in synthetic biological production systems.
Keywords: Indole-diterpene, fungal secondary metabolite, biosynthesis, mycotoxin, food and feed safety, drug discovery, heterologous production
Introduction
Indole-diterpenes (IDTs) are small molecule secondary metabolite natural products biosynthesized by a select group of ascomycetous fungi including Aspergillus, Penicillium, Emericella, Eupenicillium, Claviceps, Epichloë, Escovopsis, Neotyphodium, Periglandula and Tolypocladium species, as well as the zygomycetous fungus Mucor irregularis (Saikia et al. 2008; Schardl et al. 2013; Gao et al. 2016; Dhodary et al. 2018; Supplementary Table S1). IDTs are important mycotoxins that provoke potent neurotoxic and tremorgenic symptoms in insects and mammals, at least partly due to their inhibition of potassium ion channels in the nervous system (Dowd et al. 1988; Uhlig et al. 2009; Imlach et al. 2011). In their native ecological contexts, IDTs defend the overwintering structures of the producing fungi, and serve as effector molecules for mutualistic interactions between these fungi and their plant hosts by deterring grazing by large animals and insects (Panaccione et al. 2006; di Menna et al. 2012; Thom et al. 2014). IDT toxicoses of livestock cause significant economic losses in agriculture (Botha et al. 1996; Cawdell-Smith et al. 2010; Philippe 2016). Conversely, IDTs have been considered for the development of pesticides or plant-protecting antifeedants, to be used as components of integrated pest management systems (Panaccione et al. 2014; Saikkonen et al. 2016). Due to their additional, potent and varied bioactivities, IDTs may also serve in the future as drug lead compounds for the development of human and veterinary medications.
IDTs are biosynthesized from geranylgeranyl diphosphate (GGPP) and an indole moiety that originates from a tryptophan precursor (Laws and Mantle 1989; Byrne et al. 2002). After the cloning of the paxilline biosynthetic gene cluster from Penicillium paxilli (Young et al. 2001), the biosynthesis of all the major IDT subgroups were also elucidated from diverse filamentous fungi (Zhang et al. 2004; Young et al. 2006; Motoyama et al. 2012; Nicholson et al. 2015; Kozák et al. 2018). These biosynthetic gene clusters share a common, conserved set of core genes, and are supplemented with additional genes that encode enzymes for various tailoring reactions responsible for the idiosyncratic structural elements of the IDT subgroups and the individual IDT congeners (Zhang et al. 2004; Young et al. 2005; Young et al. 2006; Nicholson et al. 2009). In addition, most IDT biosynthetic enzymes show various levels of substrate and product flexibilities, with even the core set of genes differing in their precise characteristics. Together, these variations create a metabolic grid responsible for the remarkable structural diversity of IDTs produced by fungi.
Our knowledge on the regulation of IDT biosynthesis is still limited. Co-regulation of aflatrem production with sclerotia development has been demonstrated in A. flavus (Ehrlich and Mack 2014), while a wide spectrum of environmental conditions including temperature, light, various carbon and nitrogen sources were shown to influence penitrem production in P. crustosum (Kalinina et al. 2017). Nevertheless, a deeper understanding of mycotoxin production, including the regulation of the biosynthesis of tremorgenic IDTs will be crucial to combat the agricultural threats posed by these fungi (Uhlig et al. 2009; Moyano et al. 2010; Lee et al. 2017). Moreover, further studies are necessary to estimate the real dimensions of the risks posed by these fungi and their harmful metabolites to consumers of agricultural products (Moldes-Anaya et al. 2012; Eriksen et al. 2013). In particular, the co-occurrence and potential synergistic effects of IDTs with other mycotoxins in foods and feeds warrants further investigations (EFSA Panel on Contaminants in the Food Chain (CONTAM) 2012).
Gaining a deeper insight into the evolution, organization and transcriptional regulation of IDT biosynthetic gene clusters may also provide us with valuable tools to control or even eliminate IDT production during industrial fermentations with fungal species such as Claviceps paspali, where the presence of these metabolites represents a safety risk (Kozák et al. 2018). Intriguingly, some paspaline-derived IDTs such as penitrem A may also be regarded as Janus-faced compounds with potential applications in anticancer chemotherapies, either as monotherapies or in combination with other antiproliferative drugs (Sallam et al. 2013a; Sallam et al. 2013b; Goda et al. 2018). Therefore, future metabolic engineering and fermentation optimization studies may need to target the improvement of the yields of these compounds under industrial fermentation conditions (Motoyama et al. 2012; Kalinina et al. 2017). The construction of various fungal synthetic biology platforms for the heterologous production of IDTs with potential biomedical significance is also on the agenda (Tagami et al. 2014; Liu et al. 2015; Tang et al. 2015; Oikawa et al. 2016; Liu et al. 2016).
To the best of our knowledge, this is the first comprehensive review that covers the genetic, biochemical, ecological, veterinary, medical and industrial aspects of the production of these important metabolites by fungi.
Detection and structural characterization of IDTs in biological samples
Although the connection between moldy food and some diseases such as ergotism was suspected for centuries, it was only in the 20th century that the occurrence of secondary metabolite mycotoxins in these foods was discovered to be the molecular basis for such illnesses (Uraguchi 1969). Recent developments of analytical techniques make it increasingly straightforward to detect, even in an untargeted fashion, a large variety of mycotoxins such as IDTs in complex matrices such as food and feed.
Paxilline and paspaline, the simplest IDT congeners, were first isolated half a century ago and characterized by elemental analysis and various spectroscopic methods such as infrared (IR), ultraviolet-visible (UV-Vis), and mass spectroscopy (MS) (Fehr and Acklin 1966; Cole et al. 1974). Although the first nuclear magnetic resonance (NMR) spectroscopy results about paspaline were published in 1977, the assignments of the signals had to be refined almost two decades later (Munday-Finch et al. 1996). Finally, the complete structures of these mycotoxins were elucidated in 1980 by X-ray crystallography (Springer and Clardy 1980).
The discovery of novel IDT subclasses was a relatively slow process in the 1980s, mainly because of the lack of versatile analytical techniques. Immunochemical methods like ELISA were considered the most selective and sensitive analytical tools of the era. However, the development of these methods required the very molecule that needed to be analyzed, making these techniques unsuitable for discovery studies. Thin layer chromatography (TLC) served as the most important tool for both the isolation of unknown molecules and the identification of known IDTs (El-Banna et al. 1987; Sanchis et al. 1988; Scuteri et al. 1992). In addition to TLC, high performance liquid chromatography (HPLC) became increasingly important in this decade for the analysis of organic compounds, including natural product mycotoxins (Maes et al. 1982; Frisvad 1987; Russell et al. 1989). By 1987 a standardized HPLC method was developed that allowed the detection of all the important groups of mycotoxins and many other fungal secondary metabolites, covering 182 compounds (Frisvad and Thrane 1987). However, the utility of HPLC separation combined with fluorescent detection is rather limited for paspaline-derived IDTs, since only the janthitrems exhibit significant fluorescence (Gallagher et al. 1980b; Lauren and Gallagher 1982). The most traditional mass spectrometric (MS) technique, electron ionization (EI) was also applied for the analysis of mycotoxins. However, this technique is best suited for volatile compounds (Fellows et al. 1981). Although X-ray crystallography provides detailed information about the structures of organic molecules, it has only been rarely applied to study IDTs (Gallagher et al. 1980a; Nozawa et al. 1987; Kawai and Nozawa 1989) since it requires special pretreatments and relatively larger amounts of the sample.
NMR spectroscopic techniques have gone through a remarkable development in the last few decades, resulting in applications such as 2D NMR spectroscopy (Aue et al. 1976) and magnetic resonance imaging (MRI) (Lauterbur 1973). Research towards the structural elucidation of novel IDTs benefited largely from the various 2D NMR techniques (De Jesus et al. 1981; Laakso et al. 1992; Wilkins et al. 1992; Belofsky et al. 1995; Munday-Finch et al. 1995). Although examples of HPLC-coupled NMR applications have been described (Sumarah et al. 2005), these methods have not gained widespread acceptance due to the high cost of the instruments and the lower sensitivity of detection as compared to MS.
The most versatile analytical method for the trace analysis of organic substances is mass spectrometry (MS). This is due to the high sensitivity of MS and its ability to provide structural information such as fragmentation patterns recorded in MS/MS experiments, and elemental composition determined via exact molecular mass measurement with high resolution MS instruments (Q-TOF MS, Orbitrap, etc.). Since the advent of liquid-phase MS interfaces, most importantly the electrospray ion sources, MS analysis can readily be coupled with HPLC allowing sensitive and selective analysis of complex mixtures by measuring directly the molecular mass (typically the mass/charge ratio [m/z] of protonated molecules), including those of new compounds (Naik et al. 1995). The capabilities of the liquid chromatography – mass spectrometry (LC-MS) technique are clearly demonstrated by the rapidly increasing number of target bacterial and fungal metabolites that can be analyzed in a single assay (Sulyok et al. 2006; Sulyok et al. 2007; Vishwanath et al. 2009; Malachova et al. 2014). In addition to multicomponent analysis, the most advanced high-resolution MS approaches allow researchers to perform untargeted LC-MS surveys, resulting in a complex dataset containing information about the molecular masses (normal MS spectra) and about the structures (fragmentation and isotopic patterns) represented in the analyte. The collected datasets can be retrospectively examined even years after the actual measurement, without the need to repeat the experiment (Andersen et al. 2016; Renaud and Sumarah 2016).
Biosynthesis of paspaline-derived IDTs in fungi
Biosynthesis of paspaline
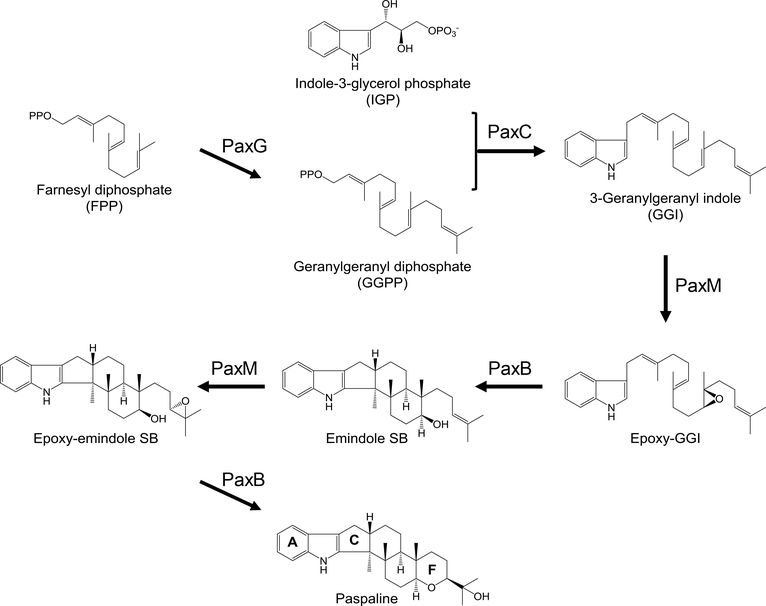
Paspaline is the founding member of the paspaline-derived IDTs that feature an angular hexacyclic ring system consisting of a tetracyclic diterpene fused to an indole moiety (Fig. 1). This scaffold is further elaborated by prenylation, oxidation, reduction, cyclization and chlorination in the various members of the group (Saikia et al. 2007; Tagami et al. 2013; Liu et al. 2016). Paspaline-derived IDTs have been classified in various ways, using historic and structural criteria. Parker et al. suggested nine subclasses: the penitrems, janthitrems, lolitrems, aflatrems, terpendoles, shearinines, sulpinines, paxilline and the paspaline/paspalinine/paspalitrems group (Parker and Scott 2005). Another classification by Sings et al. divides IDTs into six structural groups: paspalanes, aflatremanes, penitremanes, janthitremanes, lolitremanes and nodulisporanes (Sings and Singh 2003). Nodulisporanes are not derived from paspaline, and lack the F ring of the paspaline skeleton, so they are not discussed in this review.
Figure 1. Biosynthesis of paspaline in Penicillium paxilli.
The cyclic diterpene moiety of paspaline is derived from geranylgeranyl diphosphate (GGPP), biosynthesized from farnesyl diphosphate by the GGPP synthase PaxG in P. paxilli and its orthologues in other IDT producers (Fig. 1) (Tagami et al. 2013). Deletion of paxG results in the complete elimination of the production of the whole spectrum of IDTs in P. paxilli (Young et al. 2001; Saikia and Scott 2009). Orthologues of paxG were detected in all characterized IDT gene clusters except that of the terpendole K producer Tolypocladium album (Motoyama et al. 2012).
The indole group of IDTs most likely derives from indole-3-glycerol phosphate (Fig. 1). Prenylation of C3 of the indole with the concomitant elimination of glyceraldehyde 3-phosphate yields 3-geranylgeranyl indole (GGI), a common intermediate for all IDTs. This reaction is catalyzed by the prenyl transferase PaxC in P. paxilli and its orthologues in other fungi. Although the preferred substrate for prenylation is indole-3-glycerol phosphate, tryptophan was also accepted by recombinant PaxC during in vitro studies with the purified enzyme (Tagami et al. 2013). Epoxidation of GGI by PaxM, a FAD-dependent monooxygenase (or its orthologues) yields 10(11)-epoxygeranylgeranyl indole. Cyclization of this intermediate by PaxB or its orthologues affords emindole SB. Finally, another epoxidation-cyclization sequence, catalyzed again by the PaxM - PaxB pair and their orthologues in other fungi, yields paspaline through the formation of the F ring of the IDT skeleton (Tagami et al. 2013; Van de Bittner et al. 2018).
Generation of chemical diversity in the paspaline-derived IDT families
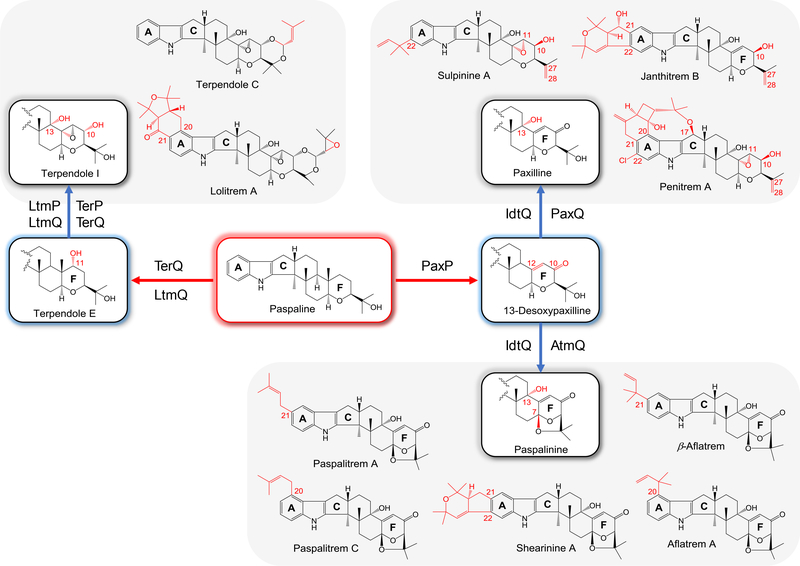
IDT biosynthesis diverges after paspaline, with further modifications of this common intermediate by various oxidations, reductions and prenylations leading to the amazing chemical diversity of the various IDT families (Fig. 2). The first divergent reaction is the oxidation of paspaline. On the first branch, TerQ-catalyzed hydroxylation of paspaline at C11 yields terpendole E in T. album (Motoyama et al. 2012). This intermediate is further oxidized by TerP to eliminate the pendant methyl group on C12, yielding 13-desoxyterpendole I that features a C11(12) epoxide and a C10 alcohol (Motoyama et al. 2012). The C11(12) epoxide is also present in lolitrem B produced by Neotyphodium lolii, suggesting that the TerQ orthologue LtmQ also possesses C11 hydroxylation activity (Gallagher et al. 1981; Philippe 2016). Correspondingly, the terpendole E-like compound lolicine was isolated from the lolitrem producer N. lolii (Munday-Finch et al. 1998). Next, TerQ catalyzes another oxidation, this time the hydroxylation of C13 to form terpendole I. This intermediate is O-prenylated at C27 by TerF or its orthologues such as LtmF of N. lolii or Epichloë festucae. Next, oxidative acetal ring formation by the cytochrome P450 TerK or LtmK gives rise to terpendole C. Additional diprenylation at C20 and C21 by LtmE and oxidative ring closure by the cytochrome P450 LtmJ of N. lolii provide the lolitremane IDTs in N. lolii/E. festucae (Young et al. 2006; Saikia et al. 2012).
Figure 2. Biosynthesis of the three main subgroups of paspaline-derived indole-diterpenes.
Only the main diversity-generating biosynthetic steps and the corresponding enzymes are shown.
On the other branch diverging from paspaline (Fig. 2), the cytochrome P450 monooxygenase PaxP and its orthologues catalyze the oxidative elimination of the pendant methyl group connected to C12 and the formation of the C10 ketone to yield 13-desoxypaxilline via the intermediate β-PC-M6 (McMillan et al. 2003). From 13-desoxypaxilline, the pathway again bifurcates. PaxQ-catalyzed hydroxylation of C13 in 13-desoxypaxilline yields paxilline en route to the pentirem/jantithrem/sulpinine families of IDTs (McMillan et al. 2003; Nicholson et al. 2015). On the other branch, AtmQ of A. flavus and its orthologues such as IdtQ of C. paspali also hydroxylate C13, but they oxidize C7 as well to form a cyclic acetal with the C27 alcohol to afford paspalinine. The order of the C13 vs. C7 oxidations is still unclear and may even be dissimilar in different fungi (Nicholson et al. 2009; Kozák et al. 2018). Thus, during the elucidation of the IDT product spectrum of C. paspali, paxilline and paspalinine were both detected in sclerotia extracts together with paspalicine, the 13-desoxy analogue of paspalinine. This indicates that IdtQ can accept 13-desoxypaxilline for either C7 or C13 oxidation in this fungus (Uhlig et al. 2014). On the other hand, paspalicine could not be isolated during the heterologous production of aflatrems using AtmQ of A. flavus, suggesting that C13 hydroxylation of 13-desoxypaxilline may precede the formation of the cyclic acetal with that enzyme (Nicholson et al. 2009). In any case, formation of paspalinine opens the way towards the biosynthesis of the aflatrems, paspalitrems and shearinines. Thus, the substrate and product specificities of the TerQ/PaxQ/AtmQ-like and the TerP/PaxP/AtmP-like cytochrome P450 enzymes in different fungi define the F ring architecture of the IDT skeleton, resulting in the terpendole/lolitrem, the penitrem/janthitrem/sulpinine, and the paspalitrem/aflatrem/shearinine subgroups of paspaline-derived IDTs.
Aflatrem, β-aflatrem, and the paspalitrems are the monoprenylated derivatives of paspalinine (Fig. 2). In the case of aflatrems, the dimethylallyl transferase AtmD catalyzes the γ-selective prenylation of paspalinine at the C20 or the C21 positions to afford aflatrem A and β-aflatrem, respectively. Interestingly, AtmD also accepts paxilline and paspaline as substrates (Liu et al. 2013). While paxilline is prenylated at C20 or C21 just as in paspalinine, paspaline is modified at the C21 or C22 positions. For paspalitrems, the IdtF dimethylallyl transferase of C. paspali conducts an α-selective prenylation of paspalinine at the C21 or the C20 positions to yield paspalitrem A or paspalitrem C, respectively. Paspalitrem A is then further oxidized at C32 giving rise to paspalitrem B. For shearinines, the JanD dimethylallyl transferase from P. janthinellum conducts two consecutive α-selective prenylations at C21 and C22 to yield shearinine K (Nicholson et al. 2015; Liu et al. 2016). This intermediate undergoes a two-step oxidative cyclization sequence catalyzed by the JanO FAD-dependent oxidase that yields a bicyclic ring system fused to the paspalinine core, affording shearinine A. Finally, an additional hydroxylation conducted by the cytochrome P450 JanJ gives rise to shearinine D.
At the paxilline branch of the biosynthetic pathway (Fig. 2), the biosynthesis of penitrems, the most elaborate paspaline-derived IDTs, requires 17 enzymes in P. simplicissimum. Paxilline is first reduced by PtmH at the C10 ketone to yield the corresponding alcohol, followed by α-selective prenylation at C20 by the dimethylallyl transferase PtmD. The prenylated analogue undergoes an acetylation-elimination sequence catalyzed by PtmV and PtmI to yield 20-prenylpenijanthine with a terminal olefin. The order of the PtmH-PtmD-PtmVI reactions seems to be somewhat flexible (Oikawa et al. 2016). Biosynthesis of janthitrems may follow a similar sequence, with the exception that a diprenylase-oxidative cyclase pair orthologous to JanD-JanO in shearinine biosynthesis is involved in the formation of the bicyclic system fused to the paxilline ring A. Indeed, the P. paxilli gene cluster contains the JanD and JanO orthologues PaxD and PaxO, respectively. Paxilline is an excellent substrate for recombinant PaxD that produces 21,22-diprenylpaxilline. While PaxD and PaxO are silent (or at least very weakly expressed) under normal fermentation conditions thus leaving the fungus to produce paxilline as its major IDT product, the P. paxilli gene cluster may nevertheless encode the biosynthesis of a janthitrem congener (Liu et al. 2016). Similarly, sulpinine biosynthesis may follow a sequence analogous to that of PtmH-PtmD-PtmVI for penitrems, with the relevant dimethylallyl transferase catalyzing a γ-selective prenylation at C22 on ring A.
For penitrems, γ-hydroxylation of the prenyl side chain by PtmO prefaces a head-to-head coupling with dimethylallyl diphosphate during a prenylation-initiated cationic cyclization event catalyzed by the PtmE dimethylallyl transferase. Oxidative ring expansion by the PtmK cytochrome P450 yields the bicyclo[4,2,0]octane system, followed by the formation of the 8-membered oxocane cyclic ether bridging to C17, catalyzed by another cytochrome P450 (PtmU). Epoxide formation at C11(12) by PtmL, chlorination at C22, and hydroxylation of the bicyclooctane by PtmJ finally affords penitrem A (Liu et al. 2015).
It is important to note that many of the IDT biosynthetic enzymes described here display substrate and product flexibility, leading to a significant metabolic crosstalk among the pathways leading to the three main biogenetic subgroups of IDTs. Consequently, many fungi use a single set of enzymes that form a complex biosynthetic grid with considerable plasticity, thus producing multiple paspaline-derived metabolites that belong to more than one IDT chemical families. Similarly, the structural classification of paspaline-derived IDTs is also somewhat arbitrary and depends on the structural elements or scaffold tailoring events that are considered the most important by the authors. For example, janthithremanes are often understood to include the janthitrems (derived from paxilline) and the shearinines (originating from paspalinine).
IDT production in various fungi
To date, more than 50 fungal species have been demonstrated to produce paspaline-derived IDTs (Supplementary Table S1). The great majority of IDT producer fungi belong to only two ascomycetous classes in the Pezizomycotina subphylum: the Eurotiomycetes (Aspergillus, Penicillium, Emericella and Eupenicillium species in the Aspergillaceae and Trichocomaceae families within the Eurotiales order) and the Sordariomycetes (Claviceps, Epichloë, Escovopsis, Neotyphodium, Periglandula and Tolypocladium species in the Clavicipitaceae, Hypocreaceae and Ophiocordycipitaceae families in the Hypocreales order).
In the order Eurotiales (class Eurotiomycetes), at least 24 Penicillium and Eupenicillium species have been shown to produce various paspaline-derived IDTs (Supplementary Table S1). The most common IDT in these fungi is penitrem A, a metabolite that is of paramount importance for the contamination of foodstuffs with mycotoxins. One of the most important model organisms used in IDT biosynthetic studies is the saprophytic species P. paxilli, which produces paxilline. A set of bioactive paxilline analogues were also isolated from Penicillium camemberti, a white mold that is widely used in cheese ripening.
Among the more than 10 Aspergillus and Emericella species that produce paspaline-derived IDTs, the notorious aflatoxin producer A. flavus is also capable of synthetizing aflatrem A and at least two additional aflatrem congeners. Aflatrem and paspaline were also detected in cultures of two other Aspergillus species, A. minisclerotigenes and A. parvisclerotigenus. Importantly, the koji mold Aspergillus. oryzae was shown to produce 13-desoxypaxilline, a common intermediate for aflatrem biosynthesis. Some other Aspergilli, including A. desertorum, A. foveolatus and A. striatus are verified paxilline producers while A. alliaceus synthesizes two paxilline-like IDTs in axenic cultures.
In the order Hypocreales (class Sordariomycetes), at least 13 species produce paspaline-derived IDTs (Supplementary Table S1). In the family Clavicipitaceae, C. paspali and C. cynodontis yield paspalitrems while the well-known ergot alkaloid producer C. purpurea synthesizes only less complex IDTs such as paspaline. Epichloë gansuensis produces paxilline, while the IDT spectra of two additional clavicipitaceous fungi, N. lolii and E. festucae are rather different. These two fungi form IDTs with the C11(12) epoxide, giving rise to terpendoles and the more elaborate lolitrems. Meanwhile, Periglandula ipomoeae strains, symbionts of morning glory, also produce terpendole analogues such as terpendoles C, K, and E (Schardl et al. 2013; Lee et al. 2017; Gardner et al. 2018). Fungi in two other hypocrealean families also produce paspaline-derived IDTs. Thus, T. album (basionym Chaunopycnis alba; family Ophiocordycipitaceae) is another important terpendole producer, while Escovopsis weberi (family Hypocreaceae) produces shearinines.
Interestingly, the zygomycetous fungus Mucor irregularis has recently been shown to yield penitrems and the related rhizovarins. M. irregularis (family Mucoraceae, order Mucorales, phylum Mucoromycota) is the only currently known fungus outside the Ascomycota phylum that produces paspaline-derived IDTs (Gao et al. 2016).
Gene clusters for IDT biosynthesis
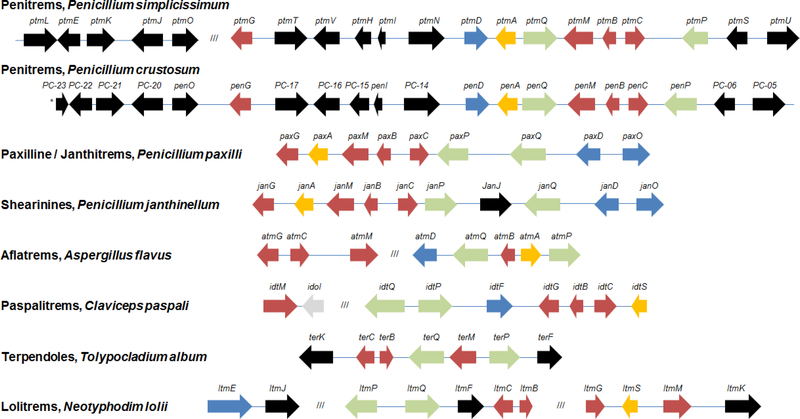
To date, ten gene clusters for the biosynthesis of the paspaline-derived IDTs have been identified (Fig. 3), including those for penitrems (P. crustosum and P. simplicissimum) (Liu et al. 2015; Nicholson et al. 2015), paxilline (P. paxilli) (Scott et al. 2013), shearinines (P. janthinellum) (Nicholson et al. 2015), aflatrems (A. flavus and A. oryzae) (Zhang et al. 2004; Nicholson et al. 2009), paspalitrems (C. paspali) (Kozák et al. 2018), terpendoles (T. album) (Motoyama et al. 2012), and lolitrems (N. lolli and its anamorph E. festucae) (Young et al. 2006; Saikia et al. 2012). All these gene clusters contain orthologues of the paxG, paxM, paxC and paxB genes whose enzyme products are collectively responsible for the biosynthesis of paspaline as described in section 3.1 (Fig. 1, Supplementary Table S2). The only exception is the terpendole K gene cluster of C. alba that is missing a GGPP synthase gene dedicated to IDT biosynthesis, indicating that this precursor is supplied by the primary metabolic GGPP synthase of the strain (Motoyama et al. 2012).
Figure 3. IDT biosynthetic gene clusters.
Genes encoding enzymes for: paspaline biosynthesis (red); oxidation of the paspaline rings E and F (green); prenylation of C20, C21 or C22 of paspaline (blue); unknown membrane-associated processes (yellow); and IDT group-specific tailoring reactions (black) are highlighted. See Supplementary Table S2 for protein similarities. Practically identical gene clusters for aflatrems in A. flavus and A. oryzae, and for lolitrems in N. lolii and E. festucae are represented by only one cluster each. *, End of the contig (P. crustosum).
In addition to the paxGMCB orthologues, all biosynthetic gene clusters for the paspaline-derived IDTs elucidated so far also contain genes similar to paxP and paxQ (Supplementary Table S2). The encoded cytochrome P450 monooxygenases are responsible for the oxidations that channel paspaline towards the paxilline, paspalinine or terpendole E-type cores of the various paspaline-derived IDT families (Fig. 2). All biosynthetic gene clusters for paspaline-derived IDTs also feature additional genes encoding enzymes for the subsequent modification of the angled hexacyclic skeleton (Fig. 3, Supplementary Table S2).
The known biosynthetic gene clusters for the different paspaline-derived IDT families show little synteny. The only exception to date is the pair of clusters for paxilline and the shearinines (Fig. 3), where the order and orientation of the genes are the same, except for the absence of a janJ orthologue in the paxilline gene cluster (Liu et al. 2016). In contrast, clusters that are derived from closely related species that produce metabolites belonging to the same IDT family are highly syntenic. For example, A. flavus and A. oryzae RIB40 contain aflatrem-type gene clusters with identical organizations and chromosomal locations, with the corresponding Atm enzymes of the two species showing at least 95% pairwise identities. Nevertheless, A. oryzae produces non-prenylated paspalenes due to a single nucleotide insertion into exon 7 that renders atmQ nonfunctional (Nicholson et al. 2009; Qiao et al. 2010; Rank et al. 2012).
Unusually for fungal secondary metabolite biosynthetic gene clusters, several of the IDT clusters seem to be distributed into multiple loci, although sequence closure of draft genomes may lead to the revision of this notion in some cases. Thus, the penitrem biosynthetic genes of P. simplicissimum, but not those of P. crustosum, are separated into two subclusters. The ptm2 and ptm1 subclusters of P. simplicissimum are syntenic with genes PC-23 - penO and penG - PC-05, respectively, within the penitrem cluster of P. crustosum. The aflatrem biosynthetic gene clusters of A. flavus and A. oryzae RIB40 are divided into two loci that reside on different chromosomes (the atm1 subcluster is located on chromosome 5, while atm2 is on chromosome 7) (Nicholson et al. 2009). The paspalitrem gene cluster also spans two separate contigs on the genome sequence assembly of C. paspali RRC-4128 (Schardl et al. 2013). These two contigs contain all the genes necessary for the biosynthesis of paspalitrems, except for the one that catalyzes C32 hydroxylation of paspalitrem A to yield the end product paspalitrem B (Kozák et al. 2018). Finally, the 11 known genes for lolitrem biosynthesis are dispersed into three ltm subclusters in N. lolli and E. festucae. Subcluster 1 is separated from subcluster 2 by a 35-kb genomic region, while subcluster 3 is located at 16 kb from subcluster 2. The interspersed genomic regions are rich in AT repeats and retrotransposon elements, indicating that the composite ltm1–3 cluster resides in a rapidly evolving region of the genome, such as a chromosomal sub-telomeric region. This capacity for rapid evolution is underlined by the observation that the ltm1–3 composite cluster may have been duplicated in its entirety in N. lolii strain Lp19, while subcluster 3 could not be detected in the very closely related strain Lp1. In contrast, E. festuceae F11 contains a single copy of the ltm1-3 composite cluster (Young et al. 2006).
Regulation of IDT production
The regulation of IDT production has received surprisingly little attention up till now. Thus, no cluster-specific regulator has been validated in any IDT gene cluster so far. Although the penitrem gene clusters of P. simplicissimum and P. crustosum feature putative regulatory genes (ptmS and PC-06), the function of these genes has not been determined experimentally (Liu et al. 2015). Similarly, the integration of IDT biosynthesis with other metabolic and morphogenetic processes also remains opaque. In A flavus, abundant aflatrem production is associated with sclerotia formation (Ehrlich and Mack 2014) while reduced aflatrem production results from the deletion of the ndsC gene encoding a global regulator of secondary metabolism and asexual development (Gilbert et al. 2016).
While N. lolii and E. festucae produce lolitrems in planta only (Young et al. 2006), other IDT producers also biosynthesize these compounds in axenic cultures (Motoyama et al. 2012; Nicholson et al. 2015; Kozák et al. 2018). A. oryzae produces the paxilline precursor 13-desoxypaxilline under specific growth conditions only (Rank et al. 2012; Fountain et al. 2016). In contrast, T. album produces large amounts of terpendoles in various media, suggesting that this biosynthetic pathway is not subject to a strict regulation in this strain (Motoyama et al. 2012). Similarly, P. crustosum strains isolated from different environments and substrates consistently produce penitrems (Frisvad and Filtenborg 1983; Yamaguchi et al. 1993; Sonjak et al. 2005). However, a variety of abiotic factors were still found to influence the production of these IDTs (Kalinina et al. 2017). Thus, cultivation in the dark at relatively low temperatures (22 °C); glucose or rhamnose as the carbon source; and supplementation of the medium with glutamate all increase penitrem production by P. crustosum. Interestingly, supplementation with tryptophan has the opposite effect in this fungus (Kalinina et al. 2017), in agreement with the notion that indole-3-glycerol phosphate and not tryptophan is the real precursor for IDT biosynthesis (Liu et al. 2015). In contrast, tryptophan serves as both a precursor and an inducer for ergot alkaloid biosynthesis in C. purpurea (Řeháček et al. 1971). In P. nigricans, penitrem production and sporulation are both induced by calcium chloride (Mantle et al. 1984), while CuSO4 increases penitrem production by P. crustosum (Kalinina et al. 2017).
Oxidative stress can also be an important factor for the regulation of tremorgenic IDT biosynthesis. Aflatrem biosynthesis is generally up-regulated in A. flavus by hydrogen peroxide, although different isolates react differently to varied concentrations of H2O2 (Fountain et al. 2016). In contrast, H2O2 has a strong inhibitory effect on penitrem biosynthesis by P. crustosum.
IDTs as threats to agriculture, public health, and the fermentation industries
Agricultural threats
Among the known IDTs, lolitrems and paspalitrems represent the most severe danger for livestock. These compounds cause a tremorgenic syndrome in grazing animals, referred to as “ryegrass stagger” in the case of lolitrem B ingestion (Fletcher and Harvey 1981; Gallagher et al. 1981), and “Paspalum stagger” or “Bermuda grass stagger” in the case of paspalitrem mycotoxicoses (Cole et al. 1977; Uhlig et al. 2009). Ryegrass stagger is typically caused by grazing on Lolium perenne (perennial ryegrass) infected by N. lolii (Gallagher et al. 1981), since lolitrem B is abundant in the N. lolii - L. perenne association (Philippe 2016). Ryegrass stagger is most frequently reported in New Zealand and Australia, and the affected animals include sheep, cattle and horses (di Menna et al. 2012). To manage ryegrass stagger, different endophyte strains with altered mycotoxin production spectra were isolated and tested. Amongst these, the endophytic fungus AR37 does not produce lolitrem B, but biosynthesizes epoxy-janthitrem in high concentrations. While epoxy-janthitrem is just as toxic to insects as lolitrem B, it does not cause a tremorgenic syndrome on grazing animals (Thom et al. 2013).
Outbreaks of Paspalum stagger is frequent in South Africa but case reports from the Americas, Europe, and New Zealand were also published (Mantle et al. 1978; Moyano et al. 2010; Cawdell-Smith et al. 2010). C. paspali infecting Paspalum dilatatum (Dallis grass) and C. cynodontis infecting Cynodon dactylon (Bermuda grass) produce similar paspalitrem IDT profiles (Uhlig et al. 2009). Ingestion of sclerotia containing these toxins causes a tremorgenic syndrome with very similar symptoms to that of ryegrass stagger (Moyano et al. 2010). Just as with lolitrem B intoxication, the affected animals usually recover rapidly after being removed from the infected pasture (Moyano et al. 2010; Cawdell-Smith et al. 2010).
Grazing on morning glories, most frequently on Ipomoea asarifolia and Ipomoea muelleri, may also cause a tremorgenic syndrome on livestock (Gardiner et al. 1965; Medeiros et al. 2003; Dorling et al. 2004; Carvalho de Lucena et al. 2014). This toxicosis is also associated with the presence of IDTs (Lee et al. 2017), produced by seed-transmitted endophytic fungi, most likely P. ipomoeae (Schardl et al. 2013; Lee et al. 2017). The main IDT congeners isolated from endophyte-infected I. asarifolia and I. muelleri are terpendole K, 11-hydroxy-12,13-epoxyterpendole K and 6,7-dehydroterpendole A (Lee et al. 2017).
A ryegrass stagger-like syndrome, “huecu’s disease” has been observed on sheep, horses, cattle and goats in Argentina. This is caused by the ingestion of the grass Poa huecu contaminated with penitrem A and B, produced by Penicillium species (Scuteri et al. 1992). Penitrem A may also contaminate the soil, and ingestion of such soil by grazing animals can also cause a tremorgenic syndrome (Patterson et al. 1979). However, most case reports of penitrem A intoxications involve the ingestion of moldy food by pets, most frequently dogs (Hayes et al. 1976; Richard et al. 1981; Hocking et al. 1988; Walter 2002). Symptoms on dogs include generalized convulsion, ataxia, vomiting, tremors, frequent urination and defecation, mydriasis (dilation of the pupil), polypnea (panting), and hyperthermia (Richard and Arp 1979; Richard et al. 1981). Typically, the affected animals recover within a few weeks or months, but ataxia may still remain observable even years later in severe cases of intoxication (Eriksen et al. 2010).
Contamination of animal feedstuffs by spoilage fungi that produce mycotoxins, including IDTs such as penitrem A (Stoev et al. 2010), represents another worldwide threat to the livestock industry. Such problems are independent of the geographical location or the origin of the feed from industrial or family-owned farms (EFSA Panel on Contaminants in the Food Chain (CONTAM) 2012).
Public health threats
Amongst IDTs, the toxic effects of only penitrem A and its analogues have been studied in depth, mainly in mice (Eriksen et al. 2013). Valuable information on the adverse physiological effects of these compounds on mammals also came from case reports on poisoned dogs that consumed various moldy foods infected by P. crustosum (Eriksen et al. 2010; Eriksen et al. 2013). Tremorgenic mycotoxicoses likely caused by penitrem A and/or other mycotoxins produced P. crustosum have only been reported very rarely in humans (Eriksen et al. 2013), and were connected to either the consumption of mold-contaminated food (Lewis et al. 2005) or drink (Cole et al. 1983), or resulted from exposure to moldy silage (Gordon 1993). It is important to note that food wastes from private households may contain high concentrations of tremorgenic mycotoxins, e.g. as high as 35–7,500 μg/kg of penitrem A (Rundberget et al. 2004)
The neurotoxic effects of penitrem A include tremors, convulsions, ataxia and nystagmus (involuntary eye movement) (Eriksen et al. 2013). In humans, symptoms affecting the gastrointestinal tract, such as nausea, vomiting, and bloody diarrhea have also been recorded (Cole et al. 1983). The toxicokinetic characteristics of this lipophilic molecule are relatively well-known and include (i) rapid absorption through biological membranes, (ii) rapid distribution within the body through the blood vessels to the liver, the kidneys and the central nervous system (penitrem A can penetrate the blood-brain barrier), (iii) metabolism in the liver to yield more hydrophilic compounds and (iv) excretion through the bile into the feces (Moldes-Anaya et al. 2009; Eriksen et al. 2010; Moldes-Anaya et al. 2012; Eriksen et al. 2013).
Penitrem A interferes with GABAergic neurotransmission in the central nervous system by inhibiting the GABA(A) receptors in the forebrain and in the cerebellum in a region-specific manner (Moldes-Anaya et al. 2011; Eriksen et al. 2013). In addition, this IDT is a potent antagonist of the high-conductance Ca2+-activated potassium channels (BK channels) in smooth muscles and peripheral tissues (Knaus et al. 1994; Eriksen et al. 2013). Importantly, lolitrem B and paxilline produced by N. lolii and E. festuce also inhibit these potassium channels, leading to ryegrass staggers in livestock (Dalziel et al. 2005; Imlach et al. 2008). Penitrem A-induced neurotoxicity may also be linked to the oxidative stress-inducing effect of this compound, as shown in cerebellar granule neurons in rats (Berntsen et al. 2017).
Additional harmful physiological effects of IDTs may include genotoxicity, as observed for paxillin in human lymphocytes (Sabater-Vilar et al. 2003). It is noteworthy that both P. crustosum extracts and penitrem A showed considerable cytotoxicity in vitro against human lung cancer, human hepatoma carcinoma, murine fibroblast and murine neuroblastoma cell lines (Bunger 2004). The physiological effects of IDTs might be even more varied and severe because toxigenic fungi typically produce a wide spectrum of harmful secondary metabolites (Bunger 2004; Andersen and Frisvad 2004; Santini et al. 2014; Prencipe et al. 2018). Unfortunately, the synergistic behavior of these compounds is a notoriously understudied area of mycotoxicology.
Paspalitrem IDTs are relatively rarely implicated in outbreaks of toxic syndromes in humans. One such case involved an outbreak of tremors in India in 1946. At that time, Paspalum scrobiculatum, a type of millet, was consumed in certain parts of India because of a shortage of rice. Tellingly, the paspalitrem producer C. paspali was isolated from the unwholesome grain (Aaronson 1988). Even today, another IDT producer, C. purpurea is often isolated from rye and barley, and paxilline can be detected in such specimen at the maximum concentration of 0.6 mg/kg (Bauer et al., 2017). C. purpurea sclerotia contain at least seven paspalenes, including paspaline (Uhlig et al. 2014). This raises the interesting possibility that these toxins may have played a role in the development of the feared symptoms of ergotism in the Middle Ages. Some authors, including Bauer and coworkers hypothesized that IDTs might have contributed to outbreaks of convulsive ergotism during history (Bauer et al. 2017).
Although the daily exposure of consumers to various IDTs is unknown, these toxic compounds have been detected in a number of food and drink products in highly variable concentrations, depending on the geographical location. Penitrem A and its producer, the food spoilage fungus P. crustosum have been detected in several agricultural products and foodstuff, such as beer, cheese, chestnut, meat products, vegetables, pudding, grape berries, honey, sausage, etc. (El-Banna and Leistner 1988; Overy et al. 2003; Sengun et al. 2008; Tancinova and Labuda 2009; Kacaniova et al. 2012; Santini et al. 2014; Camardo Leggieri et al. 2016; Olsen et al. 2017; Prencipe et al. 2018). Of course, penitrem A is not the only IDT that can be detected in contaminated foods. Paxilline and other IDTs were found in moldy tomatoes infected with Penicillium tularense (Andersen and Frisvad 2004). Lolitrem B and epoxy-janthitrem were observed in the fat and milk of growing and lactating animals that grazed on contaminated tall fescue (Miyazaki et al. 2004; Finch et al. 2012; Finch et al. 2013; Shimada et al. 2013; Zbib et al. 2015). The maximum concentration of these IDTs in cow milk reached 5 ng/ml and 109 ng/ml for lolitrem B and epoxy-janthitrem, respectively (Finch et al. 2013). Miyazaki et al. (2004) measured 210 ppb lolitrem B in the perirenal fat of Japanese Black cattle feeding on contaminated ryegrass (Miyazaki et al. 2004). However, the concentrations of these IDTs decrease rapidly in vivo when the animals are removed from the contaminated pasture, thus reducing the prevalence of food containing these two mycotoxins and limiting the threat to human health (Miyazaki et al. 2004; Finch et al. 2013).
Today, highly sensitive HPLC-MS methods are available to facilitate the detection of mycotoxin contaminants in food and drink samples (Rundberget and Wilkins 2002). A recent survey (Kalinina et al. 2018) revealed that 10% of cheese samples taken from the European single market contained penitrem A at an average concentration of 28.4 μg/kg and with a maximum concentration of 429 μg/kg. Considering such observations, it would be important to devise standardized analytical protocols for tremorgenic IDT mycotoxins. Even more importantly, it would be highly desirable for national and international regulatory agencies to define the maximum legally permitted levels of tremorgenic IDTs in human food and animal feed, similar to legal limits established for other mycotoxins such as aflatoxin (Medeiros et al. 2012; Oliveira et al. 2014).
Threats for the fermentations industries
C. paspali is used to produce ergot alkaloids in the pharmaceutical industries for the manufacture of drugs against migraine and for the treatment of Parkinson’s disease. IDT biosynthesis by C. paspali during industrial fermentations or agricultural production on infected grasses represents both an economic and a safety problem for the manufacturers. Precursors and cofactors that may be utilized for ergot production are depleted by IDT biosynthesis, reducing productivity. At the same time, IDTs are hazardous impurities that must be removed from ergot alkaloid products and must also be safely disposed (Kozák et al. 2018).
A. oryzae is widely used in biotechnology and the food industry. This fungus is the domesticated descendant of A. flavus. While the genotypes of A. oryzae and A. flavus are nearly identical, these fungi can still be distinguished by their morphological and physiological characteristics, and by their secondary metabolite profiles (Frisvad et al. 2018). While A. oryzae does not produce aflatoxin (Barbesgaard et al. 1992; Tao and Chung 2014), certain isolates of A. oryzae were demonstrated to produce tremorgenic paspaline-type IDTs such as 13-desoxypaxilline (Qiao et al. 2010; Rank et al. 2012). Production of these IDTs may represent an overlooked risk factor for fermented food products.
A possible solution may be to eliminate the production of IDT mycotoxins during various fermentations, for example by inactivating key IDT biosynthetic genes. This was demonstrated by the knockout of the idtCBGF paspalitrem biosynthetic genes in the industrially important ergot producer C. paspali. This led to the complete abrogation of IDT biosynthesis during fermentations, while the production of ergot alkaloids remained undisturbed with this strain (Kozák et al. 2018).
Future perspectives
Potential medical applications
Paspaline-derived IDTs are not in current medical use. Nevertheless, these metabolites show potent and wide-ranging bioactivities and have been considered for pharmaceutical development over the years. It is noteworthy that terpendole congeners were isolated in a systematic screening for acyl-CoA:cholesterol acyltransferase (ACAT) inhibitors of microbial origin (Huang et al. 1995). ACAT inhibitors are potential agents for the prevention of atherosclerosis (Lee et al. 1998). The most potent inhibitor is terpendole C (IC50: 2.1 μM) but terpendole D (IC50: 3.2 μM) was considered even more promising since that congener displayed relatively low cytotoxicity against J774 macrophages (Huang et al. 1995). After the discovery of two ACAT isozymes in mammals (Anderson et al. 1998; Cases et al. 1998), isozyme selectivity became a prerequisite for potential anti-atherosclerotic agents (Giovannoni et al. 2003). Testing the selectivity of a number of microbial ACAT inhibitors revealed that terpendole C inhibits both ACAT isozymes, reducing the enthusiasm for the further clinical development of these IDTs (Ohshiro et al. 2007).
Terpendoles were re-discovered in a systematic screening for specific M phase cell cycle inhibitors where terpendole E was found to specifically inhibit the human kinesin Eg5 (Nakazawa et al. 2003), a potential target for cancer therapy (Knight and Parrish 2008; Sarli and Giannis 2008). Terpendole E does not affect microtubules directly, but induces the formation of monopolar mitotic spindles in the M phase, similar to monastrol (Nakazawa et al. 2003; Tarui et al. 2014; Sheff et al. 2017). To increase the production of terpendole E that is an intermediate of terpendole K biosynthesis and is thus produced only in low amounts, the terP gene was inactivated in T. album leading to the accumulation of terpendole E (Motoyama et al. 2012). The same terP knockout strain also produces the novel shunt product 11-ketopaspaline (Tarui et al. 2014). Later studies revealed that terpendole E and 11-ketopaspaline are both potent inhibitors of the microtubule-stimulated ATPase activity of Eg5. Importantly, these terpendoles not only inhibit the wild type Eg5, but retain excellent activity against Eg5 variants that are resistant to the known Eg5 inhibitors S-trityl-L-cysteine and GSK-1 (Tarui et al. 2014).
Penitrems show in vitro antiproliferative, anti-haptotactic (cell migration inhibitory) and anti-invasive activities against human breast cancer cell lines. Penitrem A induces G1 cell cycle arrest and up-regulates the arrest protein p27 (Goda et al. 2018). The documented synergistic effects of penitrem A treatment with anti-HER drugs may have a significant impact on future development of breast cancer chemotherapies (Goda et al. 2018). Interestingly, the early biosynthetic intermediates paspaline and emindole SB also show noticeable antiproliferative and antimigratory activities, with the anti-haptotactic activity of paspaline almost equipotent to that of the more elaborate congener penitrem A (Sallam et al. 2013a; Sallam et al. 2013b). These notable activities of penitrem A are due, at least in part, to the inhibition of the Wnt/β-catenin pathway (Sallam et al. 2013a) that is a validated target of novel anticancer drugs (Lu et al. 2011). However, the BK channel (high-conductance Ca2+-activated potassium channel) inhibitory and tremorgenic activities of these IDTs present a formidable obstacle towards the development of penitrems as novel drugs (Sings and Singh 2003). Inhibition of the α-subunit of the BK channel interferes with neurotransmitter release mechanisms and neuroreceptors in the central and the peripheric nervous systems. To overcome these challenges, new penitrem analogues were synthesized. Some of these lack the BK channel inhibitory and tremorgenic activities while still repress β-catenin in human breast cancer cells (Sallam et al. 2013a). Another interesting approach is to mitigate the undesired effects of penitrems in a combination therapy with preventative agents such as astaxanthin or docosahexaenoic acid. Simultaneous application of penitrem A with these agents in rats significantly reduced toxicity and reversed the BK channel blockade associated with penitrem A alone (Goda et al. 2016).
While paspaline-derived IDTs are associated with tremorgenic activities, emindole SB-derived IDTs such as nodulisporic acids display highly potent insecticidal activities without observable adverse effects in mammals (Shoop et al. 2001). Thus, non-tremorgenic IDT analogues may find applications as pesticides in agriculture or even as anti-parasitic agents against insects feeding on humans.
Conversely, the potent BK channel inhibitory activity of penitrems may also be utilized in the future. The standard inhibitor for BK channels is iberiotoxin whose high price and membrane impermeability makes its use less than ideal for studies in organ models or whole animals. The higher membrane permeability, potency and efficacy of penitrem A may recommend this IDT as a good alternative to iberiotoxin for studying BK channels in vitro and in vivo (Stewart et al. 2012; Asano et al. 2012; Kyle et al. 2013; Needham et al. 2014).
Invasive fungal infections are an important medical problem, particularly in immunocompromised patients. However, treatment of invasive candidiasis is restricted to only a few drug families with a limited number of mechanisms of action (Tkacz and DiDomenico 2001). The toxicity of amphotericin B, and the emergence of resistance in the clinically relevant Candida albicans species against the azoles and the candins presents a clear demand for the development of new therapeutic strategies (Kathiravan et al. 2012). Importantly, shearinines D and E block the formation of biofilms by C. albicans (You et al. 2013). Biofilm formation makes the treatment of fungal infections very problematic, because biofilms constitute a barrier that can prevent antifungal drugs from reaching the fungal cells (Douglas 2003; Nett et al. 2010; Nett et al. 2011). C. albicans treated with shearinine D forms an irregular, sparse layer instead of a well-developed biofilm. Co-application of shearinine D or E synergistically enhanced the potency of amphotericin B against clinical Candida isolates (You et al. 2013).
Systematic screening for natural compounds active against the influenza A virus subtype H1N1 led to the isolation and characterization of an array of IDT congeners from P. camemberti. Three novel and six known paspaline- and paxilline-derived IDT analogues showed significant antiviral activity (IC50: 17.7 – 73.3 μM) (Fan et al. 2013), raising hopes that novel antiviral agents may be developed from these IDTs in the future.
Industrial scale production of IDTs using filamentous fungi
The industrial-scale production of paspaline-derived IDTs for future medical and agricultural applications requires careful consideration of the potential producer strains. For example, P. paxilli has been extensively used to clarify the individual steps of IDT production (Young et al. 2001). However, the pharmaceutical industry has yet to invest in the strain development and fermentation process optimization of this strain. Similarly, N. lolii may be difficult to adopt for the large-scale production of secondary metabolites due to its fastidious growth habits, genetic instability, and its requirement for a plant host for IDT production (Wiewióra et al. 2015).
Only a few studies investigated the production of IDTs in laboratory scale fermentations. Thus, P. nigricans produced 60 mg/L penitrem in a 60 L stirred fermentor in 5 days (Mantle et al. 1984). Engineered C. alba accumulated 36 mg/L terpendole E, an intermediate of terpendole K biosynthesis, in a 30 L fermentor in 2 days (Motoyama et al. 2012). Kalinina and coworkers demonstrated that the spectrum of penitrems produced by P. crustosum can also be controlled by varying the chemical and physical conditions of the fermentation (Kalinina et al. 2017). Encouragingly, C. paspali is currently used for ergot alkaloid production in the pharmaceutical industry. Although these fermentations have not been optimized for IDT production, the significant knowledge base available for the safe and economical industrial scale fermentation and the genetic manipulation of this fungus (Arcamone et al. 1960; Tudzynski et al. 2001; Kozák et al. 2018) may recommend C. paspali as a useful candidate to produce IDT congeners in the future.
Heterologous biosynthesis of IDTs
Domesticated microbial hosts for the heterologous expression of biosynthetic pathways are becoming increasingly important for the characterization of biosynthetic pathways and the clarification of reaction mechanisms. As a prominent example, the Oikawa group has been developing a heterologous production system for secondary metabolites. In this system, biosynthetic genes are cloned into multiple expression vectors and co-integrated into the genome of the domesticated filamentous fungus A. oryzae. In an influential set of publications, Oikawa and coworkers have used this expression system for the reconstitution of the biosynthesis of various IDTs to clarify the individual biosynthetic steps (Oikawa et al. 2016). Thus, the biosynthesis of paspaline was reconstituted in 2012 using A. oryzae transformants producing the P. paxilli IDT biosynthetic enzymes PaxG, PaxC, PaxM and PaxB (Liu et al. 2015). Adding the paxP and paxQ genes yielded paxilline in A. oryzae. In 2014, seven genes from the aflatrem biosynthetic locus of A. flavus were expressed in the A. oryzae host, leading to the heterologous production of aflatrem A and β-aflatrem in addition to paspaline and paspalinine (Tagami et al. 2014). In 2015, the same A. oryzae chassis was utilized to dissect the biosynthesis of penitrems by functionally analyzing 13 of the 17 genes encoded in the biosynthetic gene cluster of P. simplicissimum (Liu et al. 2015). The same A. oryzae heterologous expression system was also used to clarify the biosynthesis of shearinines from P. janthinellum (Liu et al. 2016).
Other hosts have also been used to express IDT biosynthetic gene clusters. For example, a versatile multigene expression system termed MIDAS was developed to express biosynthetic gene clusters in a P. paxilli strain with the entire paxilline biosynthetic cluster deleted (van Dolleweerd et al. 2018). This system was used to express key biosynthetic genes from Hypoxylon pulicicidum to produce nodulisporic acid intermediates and congeners (Van de Bittner et al. 2018). Although nodulisporic acid is not a paspaline-derived IDT and is thus not the subject of this review, the work still shows that strains derived from IDT producers can be useful hosts to produce other bioactive molecules. Meanwhile, nodulisporic acid derivatives are potent insecticides that lack observable adverse effects in mammals, including the tremorgenic activities associated with the paspaline-derived IDT core.
Combinatorial biosynthesis of novel, unnatural IDTs in synthetic biological platforms is another promising application. To design such a platform, the Tang group has used Saccharomyces cerevisiae as the chassis for the heterologous production of epoxy-geranylgeranyl indole (Tang et al. 2015). Co-expression of various IDT cyclases, some with previously unknown product specificities yielded not only paspaline (1.5 mg/L), but also various seco-IDTs and aflavinine- or anominine-type Markovnikov-derived cyclic scaffolds. This work demonstrates that diversity-oriented combinatorial biosynthesis with enzymes coopted from distinct and even orthogonal IDT biosynthetic pathways may be used to generate diverse, drug-like chemical matter towards the discovery of future medications. Presumably, such synthetic biological platforms will also be useful in the future for the large-scale and economical production of IDT congeners for medical or agricultural applications.
Supplementary Material
Acknowledgements
This work was supported by the European Union and the European Social Fund through the project EFOP-3.6.1-16-2016-00022 (to I. P.), the Higher Education Institutional Excellence Program of the Ministry of Human Capacities in Hungary (Biotechnology thematic program to I. P. and I. M.) and the U.S. National Institutes of Health (NIGMS 5R01GM114418 to I. M.).
Footnotes
Conflict of interest
I. P. declares no conflict of interests. I. M. has disclosed financial interests in Teva Pharmaceuticals Works Ltd., Hungary and DSM Nutritional Products, LLC, USA which are unrelated to the subject of the research presented here. L. K., Z. S., and L. T. are employees of Teva Pharmaceutical Works Ltd., Hungary. Responsibility for the conclusions drawn, and the opinions expressed in this article are solely those of the authors and are not shared by Teva Pharmaceutical Works Ltd.
Compliance with ethical standards
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the Authors.
References
- Aaronson S (1988) Paspalum spp. and Claviceps paspali in ancient and modern India. J Ethnopharmacol 24:345–348. [DOI] [PubMed] [Google Scholar]
- Andersen AJC, Hansen PJ, Joergensen K, Nielsen KF (2016) Dynamic cluster analysis: an unbiased method for identifying a + 2 element containing compounds in liquid chromatographic high-resolution time-of-flight mass spectrometric data. Anal Chem 88:12461–12469. doi: 10.1021/acs.analchem.6b03902 [DOI] [PubMed] [Google Scholar]
- Andersen B, Frisvad JC (2004) Natural occurrence of fungi and fungal metabolites in moldy tomatoes. J Agric Food Chem 52:7507–7513. doi: 10.1021/jf048727k [DOI] [PubMed] [Google Scholar]
- Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, Shelness GS, Rudel LL (1998) Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J Biol Chem 273:26747–26754. doi: 10.1074/jbc.273.41.26747 [DOI] [PubMed] [Google Scholar]
- Arcamone F, Bonino C, Chain EB, Ferretti A, Pennella P, Tonolo A, Vero L (1960) Production of lysergic acid derivatives by a strain of Claviceps paspali Stevens and Hall in submerged culture. Nature 187:238–239. doi: 10.1038/187238a0 [DOI] [PubMed] [Google Scholar]
- Asano S, Bratz IN, Berwick ZC, Fancher IS, Tune JD, Dick GM (2012) Penitrem A as a tool for understanding the role of large conductance Ca2+/voltage-sensitive K+ channels in vascular function. J Pharmacol Exp Ther 342:453–460. doi: 10.1124/jpet.111.191072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aue WP, Bartholdi E, Ernst RR (1976) Two-dimensional spectroscopy. Application to nuclear magnetic resonance. J Chem Phys 64:2229–2246. doi: 10.1063/1.432450 [DOI] [Google Scholar]
- Barbesgaard P, Heldt-Hansen H, Diderichsen B (1992) On the safety of Aspergillus oryzae: a review. Appl Microbiol Biotechnol 36:569–572. doi: 10.1007/BF00183230 [DOI] [PubMed] [Google Scholar]
- Bauer JI, Gross M, Cramer B, Wegner S, Hausmann H, Hamscher G, Usleber E (2017) Detection of the tremorgenic mycotoxin paxilline and its desoxy analog in ergot of rye and barley: a new class of mycotoxins added to an old problem. Anal Bioanal Chem 409:5101–5112. doi: 10.1007/s00216-017-0455-y [DOI] [PubMed] [Google Scholar]
- Belofsky GN, Gloer JB, Wicklow DT, Dowd PF (1995) Antiinsectan alkaloids: shearinines A-C and a new paxilline derivative from the ascostromata of Eupenicillium shearii. Tetrahedron 51:3959–3968. doi: 10.1016/0040-4020(95)00138-X [DOI] [Google Scholar]
- Berntsen HF, Bogen IL, Wigestrand MB, Fonnum F, Walaas SI, Moldes-Anaya A (2017) The fungal neurotoxin penitrem A induces the production of reactive oxygen species in human neutrophils at submicromolar concentrations. Toxicology 392:64–70. doi: 10.1016/j.tox.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Botha CJ, Kellerman TS, Fourie N (1996) A tremorgenic mycotoxicosis in cattle caused by Paspalum distichum (l.) infected by Claviceps paspali. J S Afr Vet Assoc 67:36–37. [PubMed] [Google Scholar]
- Bunger J (2004) Cytotoxicity of occupationally and environmentally relevant mycotoxins. Toxicology 202:199–211. doi: 10.1016/j.tox.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Byrne KM, Smith SK, Ondeyka JG (2002) Biosynthesis of nodulisporic acid A: precursor studies. J Am Chem Soc 124:7055–7060. doi: 10.1021/ja017183p [DOI] [PubMed] [Google Scholar]
- Camardo Leggieri M, Decontardi S, Bertuzzi T, Pietri A, Battilani P (2016) Modeling growth and toxin production of toxigenic fungi signaled in cheese under different temperature and water activity regimes. Toxins 9:e4. doi: 10.3390/toxins9010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho de Lucena KF, Rodrigues JMN, Campos EM, Dantas AFM, Pfister JA, Cook D, Medeiros RMT, Riet-Correa F (2014) Poisoning by Ipomoea asarifolia in lambs by the ingestion of milk from ewes that ingest the plant. Toxicon Off J Int Soc Toxinology 92:129–132. doi: 10.1016/j.toxicon.2014.10.019 [DOI] [PubMed] [Google Scholar]
- Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, Erickson SK, Farese RVJ (1998) ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem 273:26755–26764. doi: 10.1074/jbc.273.41.26755 [DOI] [PubMed] [Google Scholar]
- Cawdell-Smith AJ, Scrivener CJ, Bryden WL (2010) Staggers in horses grazing paspalum infected with Claviceps paspali. Aust Vet J 88:393–395. doi: 10.1111/j.1751-0813.2010.00624.x [DOI] [PubMed] [Google Scholar]
- Cole RJ, Dorner JW, Cox RH, Raymond LW (1983) Two classes of alkaloid mycotoxins produced by Penicillium crustosum Thom isolated from contaminated beer. J Agric Food Chem 31:655–657. doi: 10.1021/jf00117a045 [DOI] [PubMed] [Google Scholar]
- Cole RJ, Dorner JW, Lansden JA, Cox RH, Pape C, Cunfer B, Nicholson SS, Bedell DM (1977) Paspalum staggers: isolation and identification of tremorgenic metabolites from sclerotia of Claviceps paspali. J Agric Food Chem 25:1197–1201. doi: 10.1021/jf60213a061 [DOI] [PubMed] [Google Scholar]
- Cole RJ, Kirksey JW, Wells JM (1974) A new tremorgenic metabolite from Penicillium paxilli. Can J Microbiol 20:1159–1162. doi: 10.1139/m74-179 [DOI] [PubMed] [Google Scholar]
- Dalziel JE, Finch SC, Dunlop J (2005) The fungal neurotoxin lolitrem B inhibits the function of human large conductance calcium-activated potassium channels. Toxicol Lett 155:421–426. doi: 10.1016/j.toxlet.2004.11.011 [DOI] [PubMed] [Google Scholar]
- De Jesus AE, Steyn PS, Van Heerden FR, Vleggaar R, Wessels PL, Hull WE (1981) Structure and biosynthesis of the penitrems A-F, six novel tremorgenic mycotoxins from Penicillium crustosum. J Chem Soc Chem Commun 289–291. doi: 10.1039/C39810000289 [DOI] [Google Scholar]
- Dhodary B, Schilg M, Wirth R, Spiteller D (2018) Secondary metabolites from Escovopsis weberi and their role in attacking the garden fungus of leaf-cutting ants. Chem - Eur J 24:4445–4452. doi: 10.1002/chem.201706071 [DOI] [PubMed] [Google Scholar]
- di Menna ME, Finch SC, Popay AJ, Smith BL (2012) A review of the Neotyphodium lolii / Lolium perenne symbiosis and its associated effects on animal and plant health, with particular emphasis on ryegrass staggers. N Z Vet J 60:315–328. doi: 10.1080/00480169.2012.697429 [DOI] [PubMed] [Google Scholar]
- Dorling PR, Colegate SM, Allen JG, Nickels R, Mitchell AA, Main DC, Madin B (2004) Calystegines isolated from Ipomoea spp. possibly associated with an ataxia syndrome in cattle in North Western Australia In: Acamovic T, Stewart CS, Pennycott TW (eds) Poisonous plants and related toxins. CABI, Wallingford, pp 140–145. [Google Scholar]
- Douglas LJ (2003) Candida biofilms and their role in infection. Trends Microbiol 11:30–36. doi: 10.1016/S0966-842X(02)00002-1 [DOI] [PubMed] [Google Scholar]
- Dowd PF, Cole RJ, Vesonder RF (1988) Toxicity of selected tremorgenic mycotoxins and related compounds to Spodoptera frugiperda and Heliothis zea. J Antibiot 41:1868–1872. doi: 10.7164/antibiotics.41.1868 [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) (2012) Scientific opinion on the risks for public and animal health related to the presence of citrinin in food and feed: Citrinin in food and feed. EFSA J 10:2605. doi: 10.2903/j.efsa.2012.2605 [DOI] [Google Scholar]
- Ehrlich K, Mack B (2014) Comparison of expression of secondary metabolite biosynthesis cluster genes in Aspergillus flavus, A. parasiticus, and A. oryzae. Toxins 6:1916–1928. doi: 10.3390/toxins6061916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Banna AA, Leistner L (1988) Production of penitrem A by Penicillium crustosum isolated from foodstuffs. Int J Food Microbiol 7:9–17. doi: 10.1016/0168-1605(88)90067-0 [DOI] [PubMed] [Google Scholar]
- El-banna AA, Pitt JI, Leistner L (1987) Production of mycotoxins by Penicillium species. Syst Appl Microbiol 10:42–46. doi: 10.1016/S0723-2020(87)80008-5 [DOI] [Google Scholar]
- Eriksen GS, Jaderlund KH, Moldes-Anaya A, Schonheit J, Bernhoft A, Jaeger G, Rundberget T, Skaar I (2010) Poisoning of dogs with tremorgenic Penicillium toxins. Med Mycol 48:188–196. doi: 10.3109/13693780903225821 [DOI] [PubMed] [Google Scholar]
- Eriksen GS, Moldes-Anaya A, Fæste CK (2013) Penitrem A and analogues: toxicokinetics, toxicodynamics including mechanism of action and clinical significance. World Mycotoxin J 6:263–272. doi: 10.3920/WMJ2013.1574 [DOI] [Google Scholar]
- Fan Y, Wang Y, Liu P, Fu P, Zhu T, Wang W, Zhu W (2013) Indole-diterpenoids with anti-H1N1 activity from the aciduric fungus Penicillium camemberti OUCMDZ-1492. J Nat Prod 76:1328–1336. doi: 10.1021/np400304q [DOI] [PubMed] [Google Scholar]
- Fehr T, Acklin W (1966) Isolation of 2 new indole derivatives from the mycelia of Claviceps paspali. Helv Chim Acta 49:1907–1910. [Google Scholar]
- Fellows PA, Kyriakidis N, Mantle PG, Waight ES (1981) Electron impact mass spectra of penitrem A, some derivatives and its analogs. Org Mass Spectrom 16:403–404. doi: 10.1002/oms.1210160909 [DOI] [Google Scholar]
- Finch S, Fletcher L, Babu J (2012) The evaluation of endophyte toxin residues in sheep fat. N Z Vet J 60:56–60. doi: 10.1080/00480169.2011.634746 [DOI] [PubMed] [Google Scholar]
- Finch SC, Thom ER, Babu JV, Hawkes AD, Waugh CD (2013) The evaluation of fungal endophyte toxin residues in milk. N Z Vet J 61:11–17. doi: 10.1080/00480169.2012.704626 [DOI] [PubMed] [Google Scholar]
- Fletcher LR, Harvey IC (1981) An association of a Lolium endophyte with ryegrass staggers. N Z Vet J 29:185–186. doi: 10.1080/00480169.1981.34839 [DOI] [PubMed] [Google Scholar]
- Fountain JC, Bajaj P, Pandey M, Nayak SN, Yang L, Kumar V, Jayale AS, Chitikineni A, Zhuang W, Scully BT, Lee RD, Kemerait RC, Varshney RK, Guo B (2016) Oxidative stress and carbon metabolism influence Aspergillus flavus transcriptome composition and secondary metabolite production. Sci Rep 6:38747. doi: 10.1038/srep38747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad JC (1987) High-performance liquid chromatographic determination of profiles of mycotoxines and other secondary metabolites. J Chromatogr A 392:333–347. doi: 10.1016/S0021-9673(01)94277-3 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Filtenborg O (1983) Classification of terverticillate penicillia based on profiles of mycotoxins and other secondary metabolites. Appl Environ Microbiol 46:1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad JC, Møller LLH, Larsen TO, Kumar R, Arnau J (2018) Safety of the fungal workhorses of industrial biotechnology: update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei. Appl Microbiol Biotechnol. 102:9481–9515 doi: 10.1007/s00253-018-9354-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad JC, Thrane U (1987) Standardized high-performance liquid chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone retention indices and UV-VIS spectra (diode array detection). J Chromatogr 404:195–214. doi: 10.1016/S0021-9673(01)86850-3 [DOI] [PubMed] [Google Scholar]
- Gallagher RT, Finer J, Clardy J, Leutwiler A, Weibel F, Acklin W, Arigoni D (1980a) Paspalinine, a tremorgenic metabolite from Claviceps paspali Stevens et Hall. Tetrahedron Lett 21:235–238. doi: 10.1016/S0040-4039(00)71177-4 [DOI] [Google Scholar]
- Gallagher RT, Latch CM, Keogh RG (1980b) The janthitrems: fluorescent tremorgenic toxins produced by Penicillium janthinellum isolates from ryegrass pastures. Appl Environ Microbiol 39:272–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher RT, White EP, Mortimer PH (1981) Ryegrass staggers: isolation of potent neurotoxins lolitrem A and lolitrem B from staggers-producing pastures. N Z Vet J 29:189–190. doi: 10.1080/00480169.1981.34843 [DOI] [PubMed] [Google Scholar]
- Gardiner MR, Royce R, Oldroyd B (1965) Ipomoea muelleri intoxication of sheep in Western Australia. Br Vet J 121:272–277. doi: 10.1016/S0007-1935(17)41154-7 [DOI] [Google Scholar]
- Gardner DR, Welch KD, Lee ST, Cook D, Riet-Correa F (2018) Tremorgenic indole diterpenes from Ipomoea asarifolia and Ipomoea muelleri and the identification of 6,7-dehydro-11-hydroxy-12,13-epoxyterpendole A. J Nat Prod 81:1682–1686. doi: 10.1021/acs.jnatprod.8b00257 [DOI] [PubMed] [Google Scholar]
- Gao S-S, Li X-M, Williams K, Proksch P, Ji N-Y, Wang B-G (2016) Rhizovarins A–F, indole-diterpenes from the mangrove-derived endophytic fungus Mucor irregularis QEN-189. J Nat Prod 79:2066–2074. doi: 10.1021/acs.jnatprod.6b00403 [DOI] [PubMed] [Google Scholar]
- Gilbert MK, Mack BM, Wei Q, Bland JM, Bhatnagar D, Cary JW (2016) RNA sequencing of an nsdC mutant reveals global regulation of secondary metabolic gene clusters in Aspergillus flavus. Microbiol Res 182:150–161. doi: 10.1016/j.micres.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Giovannoni M, Piaz V, Vergelli C, Barlocco D (2003) Selective ACAT inhibitors as promising antihyperlipidemic, antiatherosclerotic and anti-Alzheimer drugs. Mini-Rev Med Chem 3:576–584. doi: 10.2174/1389557033487890 [DOI] [PubMed] [Google Scholar]
- Goda AA, Siddique A, Mohyeldin M, Ayoub N, El Sayed K (2018) The Maxi-K (BK) channel antagonist penitrem A as a novel breast cancer-targeted therapeutic. Mar Drugs 16: e157. doi: 10.3390/md16050157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda AA, Naguib KM, Mohamed MM, Amra HA, Nada SA, Abdel-Ghaffar A-RB, Gissendanner CR, El Sayed KA (2016) Astaxanthin and docosahexaenoic acid reverse the toxicity of the Maxi-K (BK) channel antagonist mycotoxin penitrem A. Mar Drugs 14:e208 doi: 10.3390/md14110208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KE (1993) Tremorgenic encephalopathy: a role of mycotoxins in the production of CNS disease in humans? Can J Neurol Sci J Can Sci Neurol 20:237–239. doi: 10.1017/S0317167100048010 [DOI] [PubMed] [Google Scholar]
- Hayes AW, Presley DB, Neville JA (1976) Acute toxicity of penitrem A in dogs. Toxicol Appl Pharmacol 35:311–320 doi: 10.1016/0041-008X(76)90290-8 [DOI] [PubMed] [Google Scholar]
- Hocking AD, Holds K, Tobin NF (1988) Intoxication by tremorgenic mycotoxin (penitrem A) in a dog. Aust Vet J 65:82–85 doi: 10.1111/j.1751-0813.1988.tb07366.x. [DOI] [PubMed] [Google Scholar]
- Huang XH, Nishida H, Tomoda H, Tabata N, Shiomi K, Yang DJ, Takayanagi H, Omura S (1995) Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. II. Structure elucidation of terpendoles A, B, C and D. J Antibiot 48:5–11 doi: 10.1002/chin.199529248 [DOI] [PubMed] [Google Scholar]
- Imlach WL, Finch SC, Dunlop J, Meredith AL, Aldrich RW, Dalziel JE (2008) The molecular mechanism of “ryegrass staggers,” a neurological disorder of K+ channels. J Pharmacol Exp Ther 327:657–664. doi: 10.1124/jpet.108.143933 [DOI] [PubMed] [Google Scholar]
- Imlach WL, Finch SC, Zhang Y, Dunlop J, Dalziel JE (2011) Mechanism of action of lolitrem B, a fungal endophyte derived toxin that inhibits BK large conductance Ca2+-activated K+ channels. Toxicon 57:686–694. doi: 10.1016/j.toxicon.2011.01.013 [DOI] [PubMed] [Google Scholar]
- Kacaniova M, Knazovicka V, Felsociova S, Rovna K (2012) Microscopic fungi recovered from honey and their toxinogenity. J Environ Sci Health Part A Tox Hazard Subst Environ Eng 47:1659–1664. doi: 10.1080/10934529.2012.687242 [DOI] [PubMed] [Google Scholar]
- Kalinina SA, Jagels A, Cramer B, Geisen R, Humpf H-U (2017) Influence of environmental factors on the production of penitrems A-F by Penicillium crustosum. Toxins 9:e210 doi: 10.3390/toxins9070210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina SA, Jagels A, Hickert S, Mauriz Marques LM, Cramer B, Humpf H-U (2018) Detection of the cytotoxic penitrems A–F in cheese from the European Single Market by HPLC-MS/MS. J Agric Food Chem 66:1264–1269. doi: 10.1021/acs.jafc.7b06001 [DOI] [PubMed] [Google Scholar]
- Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, Gadhwe S (2012) The biology and chemistry of antifungal agents: A review. Bioorg Med Chem 20:5678–5698. doi: 10.1016/j.bmc.2012.04.045 [DOI] [PubMed] [Google Scholar]
- Kawai K, Nozawa K (1989) Novel biologically active compounds from Emericella species. Bioact Mol 10:205–212. [Google Scholar]
- Knaus H-G, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LMH, Sanchez M, Giangiacomo K, Reuben JP (1994) Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry (Moscow) 33:5819–5828. doi: 10.1021/bi00185a021 [DOI] [PubMed] [Google Scholar]
- Knight SD, Parrish CA (2008) Recent progress in the identification and clinical evaluation of inhibitors of the mitotic kinesin KSP. Curr Top Med Chem 8:888–904 doi: 10.2174/156802608784911626 [DOI] [PubMed] [Google Scholar]
- Kozák L, Szilágyi Z, Vágó B, Kakuk A, Tóth L, Molnár I, Pócsi I (2018) Inactivation of the indole-diterpene biosynthetic gene cluster of Claviceps paspali by Agrobacterium-mediated gene replacement. Appl Microbiol Biotechnol 102:3255–3266. doi: 10.1007/s00253-018-8807-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle BD, Bradley E, Large R, Sergeant GP, McHale NG, Thornbury KD, Hollywood MA (2013) Mechanisms underlying activation of transient BK current in rabbit urethral smooth muscle cells and its modulation by IP3-generating agonists. Am J Physiol-Cell Physiol 305:609–622. doi: 10.1152/ajpcell.00025.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso JA, Gloer JB, Wicklow DT, Dowd PF (1992) Sulpinines A-C and secopenitrem B: new antiinsectan metabolites from the sclerotia of Aspergillus sulphureus. J Org Chem 57:2066–2071. doi: 10.1021/jo00033a030 [DOI] [Google Scholar]
- Lauren DR, Gallagher RT (1982) High-performance liquid chromatography of the janthitrems: fluorescent tremorgenic mycotoxins produced by Penicillium janthinellum. J Chromatogr 248:150–154. doi: 10.1016/S0021-9673(00)83747-4 [DOI] [Google Scholar]
- Lauterbur PC (1973) Image formation by induced local interactions. Examples employing nuclear magnetic resonance. Nature 242:190–191. doi: 10.1038/242190a0 [DOI] [PubMed] [Google Scholar]
- Laws I, Mantle PG (1989) Experimental constraints in the study of the biosynthesis of indole alkaloids in fungi. Microbiology 135:2679–2692. doi: 10.1099/00221287-135-10-2679 [DOI] [Google Scholar]
- Lee H, Roark W, Picard J, Sliskovic D, Roth B, Stanfield R, Hamelehle K, Bousley R, Krause B. (1998) Inhibitors of Acyl-CoA:Cholesterol O-acyltransferase (ACAT) as hypocholesterolemic agents: synthesis and structure-activity relationships of novel series of sulfonamides, acylphosphonamides and acylphosphoramidates. Bioorg Med Chem Lett 8:289–294. doi: 10.1016/S0960-894X(98)00011-0 [DOI] [PubMed] [Google Scholar]
- Lee ST, Gardner DR, Cook D (2017) Identification of indole diterpenes in Ipomoea asarifolia and Ipomoea muelleri, plants tremorgenic to livestock. J Agric Food Chem 65:5266–5277. doi: 10.1021/acs.jafc.7b01834 [DOI] [PubMed] [Google Scholar]
- Lewis PR, Donoghue MB, Hocking AD, Cook L, Granger LV (2005) Tremor syndrome associated with a fungal toxin: sequelae of food contamination. Med J Aust 182:582–584. [DOI] [PubMed] [Google Scholar]
- Liu C, Minami A, Dairi T, Gomi K, Scott B, Oikawa H (2016) Biosynthesis of shearinine: diversification of a tandem prenyl moiety of fungal indole diterpenes. Org Lett 18:5026–5029. doi: 10.1021/acs.orglett.6b02482 [DOI] [PubMed] [Google Scholar]
- Liu C, Minami A, Noike M, Toshima H, Oikawa H, Dairi T (2013) Regiospecificities and prenylation mode specificities of the fungal indole diterpene prenyltransferases AtmD and PaxD. Appl Environ Microbiol 79:7298–7304. doi: 10.1128/AEM.02496-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Tagami K, Minami A, Matsumoto T, Frisvad JC, Suzuki H, Ishikawa J, Gomi K, Oikawa H (2015) Reconstitution of biosynthetic machinery for the synthesis of the highly elaborated indole diterpene penitrem. Angewandte Chemie International Edition 54:5748–5752. doi: 10.1002/anie.201501072 [DOI] [PubMed] [Google Scholar]
- Lu W, Lin C, Roberts MJ, Waud WR, Piazza GA, Li Y (2011) Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/β-catenin pathway. PLoS ONE 6:e29290. doi: 10.1371/journal.pone.0029290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes CM, Steyn PS, Van Heerden FR (1982) High-performance liquid chromatography and thin-layer chromatography of penitrems A–F, tremorgenic mycotoxins from Penicillium crustosum. J Chromatogr A 234:489–493. doi: 10.1016/S0021-9673(00)81893-2 [DOI] [Google Scholar]
- Malachova A, Sulyok M, Beltran E, Berthiller F, Krska R (2014) Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J Chromatogr A 1362:145–156. doi: 10.1016/j.chroma.2014.08.037 [DOI] [PubMed] [Google Scholar]
- Mantle PG, Laws I, Tan MJ, Tizard M (1984) A novel process for the production of penitrem mycotoxins by submerged fermentation of Penicillium nigricans. J Gen Microbiol 130:1293–1298. doi: 10.1099/00221287-130-5-1293 [DOI] [PubMed] [Google Scholar]
- Mantle PG, Mortimer PH, White EP (1978) Mycotoxic tremorgens of Claviceps paspali and Penicillium cyclopium: a comparative study of effects on sheep and cattle in relation to natural staggers syndromes. Res Vet Sci 24:49–56. [PubMed] [Google Scholar]
- McMillan LK, Carr RL, Young CA, Astin JW, Lowe RGT, Parker EJ, Jameson GB, Finch SC, Miles CO, McManus OB, Schmalhofer WA, Garcia ML, Kaczorowski GJ, Goetz M, Tkacz JS, Scott B (2003) Molecular analysis of two cytochrome P450 monooxygenase genes required for paxilline biosynthesis in Penicillium paxilli, and effects of paxilline intermediates on mammalian maxi-K ion channels. Mol Genet Genomics 270:9–23. doi: 10.1007/s00438-003-0887-2 [DOI] [PubMed] [Google Scholar]
- Medeiros FHV de, Martins SJ, Zucchi TD, Melo IS de, Batista LR, Machado J da C (2012) Biological control of mycotoxin-producing molds. Ciênc E Agrotecnologia 36:483–497. doi: 10.1590/S1413-70542012000500001 [DOI] [Google Scholar]
- Medeiros RMT, Barbosa RC, Riet-Correa F, Lima EF, Tabosa IM, de Barros SS, Gardner DR, Molyneux RJ (2003) Tremorgenic syndrome in goats caused by Ipomoea asarifolia in Northeastern Brazil. Toxicon Off J Int Soc Toxinology 41:933–935 doi: 10.1016/S0041-0101(03)00044-8 [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Ishizaki I, Ishizaka M, Kanbara T, Ishiguro-Takeda Y (2004) Lolitrem B residue in fat tissues of cattle consuming endophyte-infected perennial ryegrass straw. J Vet Diagn Invest 16:340–342. doi: 10.1177/104063870401600416 [DOI] [PubMed] [Google Scholar]
- Moldes-Anaya A, Rundberget T, Faeste CK, Eriksen GS, Bernhoft A (2012) Neurotoxicity of Penicillium crustosum secondary metabolites: tremorgenic activity of orally administered penitrem A and thomitrem A and E in mice. Toxicon Off J Int Soc Toxinology 60:1428–1435. doi: 10.1016/j.toxicon.2012.10.007 [DOI] [PubMed] [Google Scholar]
- Moldes-Anaya A, Wilkins AL, Rundberget T, Fæste CK (2009) In vitro and in vivo hepatic metabolism of the fungal neurotoxin penitrem A. Drug Chem Toxicol 32:26–37. doi: 10.1080/01480540802416232 [DOI] [PubMed] [Google Scholar]
- Moldes-Anaya AS, Fonnum F, Eriksen GS, Rundberget T, Walaas SI, Wigestrand MB (2011) In vitro neuropharmacological evaluation of penitrem-induced tremorgenic syndromes: Importance of the GABAergic system. Neurochem Int 59:1074–1081. doi: 10.1016/j.neuint.2011.08.014 [DOI] [PubMed] [Google Scholar]
- Motoyama T, Hayashi T, Hirota H, Ueki M, Osada H (2012) Terpendole E, a kinesin Eg5 inhibitor, is a key biosynthetic intermediate of indole-diterpenes in the producing fungus Chaunopycnis alba. Chem Biol 19:1611–1619. doi: 10.1016/j.chembiol.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Moyano M, Molina A, Lora A, Mendez J, Rueda A (2010) Tremorgenic mycotoxicosis caused by Paspalum paspaloides (Michx.) Scribner infected by Claviceps paspali: a case report. Veterinární Medicína 55:336–338. doi: 10.17221/2964-VETMED [DOI] [Google Scholar]
- Munday-Finch SC, Miles CO, Wilkins AL, Hawkes AD (1995) Isolation and structure elucidation of lolitrem A, a tremorgenic mycotoxin from perennial ryegrass infected with Acremonium lolii. J Agric Food Chem 43:1283–1288. doi: 10.1021/jf00053a029 [DOI] [Google Scholar]
- Munday-Finch SC, Wilkins AL, Miles CO (1996) Isolation of paspaline B, an indole-diterpenoid from Penicillium paxilli. Phytochemistry 41:327–32. doi: 10.1016/0031-9422(95)00515-3 [DOI] [Google Scholar]
- Munday-Finch SC, Wilkins AL, Miles CO (1998) Isolation of lolicine A, lolicine B, lolitriol, and lolitrem N from Lolium perenne infected with Neotyphodium lolii and evidence for the natural occurrence of 31-epilolitrem N and 31-epilolitrem F. J Agric Food Chem 46:590–598. doi: 10.1021/jf9706787 [DOI] [PubMed] [Google Scholar]
- Naik JT, Mantle PG, Sheppard RN, Waight ES (1995) Penitremones A-C, Penicillium metabolites containing an oxidized penitrem carbon skeleton giving insight into structure-tremorgenic relationships. J Chem Soc Perkin Trans 1 Org Bio-Org Chem 1121–1125. doi: 10.1039/P19950001121 [DOI] [Google Scholar]
- Nakazawa J, Yajima J, Usui T, Ueki M, Takatsuki A, Imoto M, Toyoshima YY, Osada H (2003) A novel action of terpendole E on the motor activity of mitotic Kinesin Eg5. Chem Biol 10:131–137. doi: 10.1016/S1074-5521(03)00020-6 [DOI] [PubMed] [Google Scholar]
- Needham M, McGahon MK, Bankhead P, Gardiner TA, Scholfield CN, Curtis TM, McGeown JG (2014) The role of K + and Cl − channels in the regulation of retinal arteriolar tone and blood flow. Investig Opthalmology Vis Sci 55:2157–2165. doi: 10.1167/iovs.13-12948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett JE, Sanchez H, Cain MT, Andes DR (2010) Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J Infect Dis 202:171–175. doi: 10.1086/651200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett JE, Sanchez H, Cain MT, Ross KM, Andes DR (2011) Interface of Candida albicans biofilm matrix-associated drug resistance and cell wall integrity regulation. Eukaryot Cell 10:1660–1669. doi: 10.1128/EC.05126-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson MJ, Eaton CJ, Starkel C, Tapper BA, Cox MP, Scott B (2015) Molecular cloning and functional analysis of gene clusters for the biosynthesis of indole-diterpenes in Penicillium crustosum and P. janthinellum. Toxins 7:2701–2722. doi: 10.3390/toxins7082701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson MJ, Koulman A, Monahan BJ, Pritchard BL, Payne GA, Scott B (2009) Identification of two aflatrem biosynthesis gene loci in Aspergillus flavus and metabolic engineering of Penicillium paxilli to elucidate their function. Appl Environ Microbiol 75:7469–7481. doi: 10.1128/AEM.02146-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa K, Nakajima S, Kawai K, Udagawa S, Horie Y, Yamazaki M (1987) Novel indoloditerpenes, emindoles, and their related compounds from Emericella spp. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 29:637–643. [Google Scholar]
- Ohshiro T, Rudel LL, Omura S, Tomoda H (2007) Selectivity of microbial acyl-CoA: cholesterol acyltransferase inhibitors toward isozymes. J Antibiot 60:43–51. doi: 10.1038/ja.2007.6 [DOI] [PubMed] [Google Scholar]
- Oikawa H, Minami A, Liu C (2016) Total biosynthesis of fungal indole diterpenes using cell factories. Heterocycles 92:397–421. doi: 10.3987/REV-15-830 [DOI] [Google Scholar]
- Oliveira PM, Zannini E, Arendt EK (2014) Cereal fungal infection, mycotoxins, and lactic acid bacteria mediated bioprotection: from crop farming to cereal products. Food Microbiol 37:78–95. doi: 10.1016/j.fm.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Olsen M, Gidlund A, Sulyok M (2017) Experimental mould growth and mycotoxin diffusion in different food items. World Mycotoxin J 10:153–161. doi: 10.3920/WMJ2016.2163 [DOI] [Google Scholar]
- Overy DP, Seifert KA, Savard ME, Frisvad JC (2003) Spoilage fungi and their mycotoxins in commercially marketed chestnuts. Int J Food Microbiol 88:69–77. doi: 10.1016/S0168-1605(03)00086-2 [DOI] [PubMed] [Google Scholar]
- Panaccione DG, Beaulieu WT, Cook D (2014) Bioactive alkaloids in vertically transmitted fungal endophytes. Funct Ecol 28:299–314. doi: 10.1111/1365-2435.12076 [DOI] [Google Scholar]
- Panaccione DG, Cipoletti JR, Sedlock AB, Blemings KP, Schardl CL, Machado C, Seidel GE (2006) Effects of ergot alkaloids on food preference and satiety in rabbits, as assessed with gene-knockout endophytes in perennial ryegrass (Lolium perenne). J Agric Food Chem 54:4582–4587. doi: 10.1021/jf060626u [DOI] [PubMed] [Google Scholar]
- Parker EJ, Scott BD (2005) Indole-diterpene biosynthesis in ascomycetous fungi In: An Z (ed) Handbook of Industrial Mycology, Vol 22 Marcel Dekker, New York, pp. 405–426 [Google Scholar]
- Patterson DS, Roberts BA, Shreeve BJ, MacDonald SM, Hayes AW (1979) Tremorgenic toxins produced by soil fungi. Appl Environ Microbiol 37:172–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe G (2016) Lolitrem B and indole diterpene alkaloids produced by endophytic fungi of the genus Epichloe and their toxic effects in livestock. Toxins 8:e47. doi: 10.3390/toxins8020047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prencipe S, Siciliano I, Gatti C, Garibaldi A, Gullino ML, Botta R, Spadaro D (2018) Several species of Penicillium isolated from chestnut flour processing are pathogenic on fresh chestnuts and produce mycotoxins. Food Microbiol 76:396–404. doi: 10.1016/j.fm.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Qiao M-F, Ji N-Y, Liu X-H, Li K, Zhu Q-M, Xue Q-Z (2010) Indoloditerpenes from an algicolous isolate of Aspergillus oryzae. Bioorg Med Chem Lett 20:5677–5680. doi: 10.1016/j.bmcl.2010.08.024 [DOI] [PubMed] [Google Scholar]
- Rank C, Klejnstrup ML, Petersen LM, Kildgaard S, Frisvad JC, Held Gotfredsen C, Ostenfeld Larsen T (2012) Comparative chemistry of Aspergillus oryzae (RIB40) and A. flavus (NRRL 3357). Metabolites 2:39–56. doi: 10.3390/metabo2010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Řeháček Z, Kozová J, Řičicová A, Kašlík J, Sajdl P, Švarc S, Basappa SC (1971) Role of endogenous tryptophan during submerged fermentation of ergot alkaloids. Folia Microbiol (Praha) 16:35–40. doi: 10.1007/BF02887333 [DOI] [PubMed] [Google Scholar]
- Renaud JB, Sumarah MW (2016) Data independent acquisition-digital archiving mass spectrometry: application to single kernel mycotoxin analysis of Fusarium graminearum infected maize. Anal Bioanal Chem 408:3083–3091. doi: 10.1007/s00216-016-9391-5 [DOI] [PubMed] [Google Scholar]
- Richard JL, Arp LH (1979) Natural occurrence of the mycotoxin penitrem A in moldy cream cheese. Mycopathologia 67:107–109. doi: 10.1007/BF00440681 [DOI] [PubMed] [Google Scholar]
- Richard JL, Bacchetti P, Arp LH (1981) Moldy walnut toxicosis in a dog, caused by the mycotoxin, penitrem A. Mycopathologia 76:55–58. doi: 10.1007/BF00761899 [DOI] [PubMed] [Google Scholar]
- Rundberget T, Skaar I, Flåøyen A (2004) The presence of Penicillium and Penicillium mycotoxins in food wastes. Int J Food Microbiol 90:181–188. doi: 10.1016/S0168-1605(03)00291-5 [DOI] [PubMed] [Google Scholar]
- Rundberget T, Wilkins AL (2002) Determination of Penicillium mycotoxins in foods and feeds using liquid chromatography–mass spectrometry. Journal of Chromatography A 964:189–197. doi: 10.1016/S0021-9673(02)00698-2 [DOI] [PubMed] [Google Scholar]
- Russell R, Paterson M, Kemmelmeier C (1989) Gradient high-performance liquid chromatography using alkylphenone retention indices of insecticidal extracts of Penicillium strains. J Chromatogr A 483:153–168. doi: 10.1016/S0021-9673(01)93118-8 [DOI] [PubMed] [Google Scholar]
- Sabater-Vilar M, Nijmeijer S, Fink-Gremmels J (2003) Genotoxicity assessment of five tremorgenic mycotoxins (fumitremorgen B, paxilline, penitrem A, verruculogen, and verrucosidin) produced by molds isolated from fermented meats. J Food Prot 66:2123–2129. doi: 10.4315/0362-028X-66.11.2123 [DOI] [PubMed] [Google Scholar]
- Saikia S, Nicholson MJ, Young C, Parker EJ, Scott B (2008) The genetic basis for indole-diterpene chemical diversity in filamentous fungi. Mycol Res 112:184–199. doi: 10.1016/j.mycres.2007.06.015 [DOI] [PubMed] [Google Scholar]
- Saikia S, Parker EJ, Koulman A, Scott B (2007) Defining paxilline biosynthesis in Penicillium paxilli: functional characterization of two cytochrome P450 monooxygenases. J Biol Chem 282:16829–16837. doi: 10.1074/jbc.M701626200 [DOI] [PubMed] [Google Scholar]
- Saikia S, Scott B (2009) Functional analysis and subcellular localization of two geranylgeranyl diphosphate synthases from Penicillium paxilli. Mol Genet Genomics 282:257–271. doi: 10.1007/s00438-009-0463-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia S, Takemoto D, Tapper BA, Lane GA, Fraser K, Scott B (2012) Functional analysis of an indole-diterpene gene cluster for lolitrem B biosynthesis in the grass endosymbiont Epichloë festucae. FEBS Lett 586:2563–2569. doi: 10.1016/j.febslet.2012.06.035 [DOI] [PubMed] [Google Scholar]
- Saikkonen K, Young CA, Helander M, Schardl CL (2016) Endophytic Epichloë species and their grass hosts: from evolution to applications. Plant Mol Biol 90:665–675. doi: 10.1007/s11103-015-0399-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam AA, Ayoub NM, Foudah AI, Gissendanner CR, Meyer SA, El Sayed KA (2013a) Indole diterpene alkaloids as novel inhibitors of the Wnt/β-catenin pathway in breast cancer cells. Eur J Med Chem 70:594–606. doi: 10.1016/j.ejmech.2013.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam AA, Houssen WE, Gissendanner CR, Orabi KY, Foudah AI, El Sayed KA (2013b) Bioguided discovery and pharmacophore modeling of the mycotoxic indole diterpene alkaloids penitrems as breast cancer proliferation, migration, and invasion inhibitors. MedChemComm 4:1360–1369. doi: 10.1039/C3MD00198A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis V, Scott PM, Farber JM (1988) Mycotoxin-producing potential of fungi isolated from red kidney beans. Mycopathologia 104:157–162. doi: 10.1007/BF00437431 [DOI] [PubMed] [Google Scholar]
- Santini A, Mikušová P, Sulyok M, Krska R, Labuda R, Šrobárová A (2014) Penicillium strains isolated from Slovak grape berries taxonomy assessment by secondary metabolite profile. Mycotoxin Res 30:213–220. doi: 10.1007/s12550-014-0205-3 [DOI] [PubMed] [Google Scholar]
- Sarli V, Giannis A (2008) Targeting the kinesin spindle protein: basic principles and clinical implications. Clin Cancer Res Off J Am Assoc Cancer Res 14:7583–7587. doi: 10.1158/1078-0432.CCR-08-0120 [DOI] [PubMed] [Google Scholar]
- Schardl CL, Young CA, Hesse U, Amyotte SG, Andreeva K, Calie PJ, Fleetwood DJ, Haws DC, Moore N, Oeser B, Panaccione DG, Schweri KK, Voisey CR, Farman ML, Jaromczyk JW, Roe BA, O’Sullivan DM, Scott B, Tudzynski P, An Z, Arnaoudova EG, Bullock CT, Charlton ND, Chen L, Cox M, Dinkins RD, Florea S, Glenn AE, Gordon A, Güldener U, Harris DR, Hollin W, Jaromczyk J, Johnson RD, Khan AK, Leistner E, Leuchtmann A, Li C, Liu J, Liu J, Liu M, Mace W, Machado C, Nagabhyru P, Pan J, Schmid J, Sugawara K, Steiner U, Takach JE, Tanaka E, Webb JS, Wilson EV, Wiseman JL, Yoshida R, Zeng Z (2013a) Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet 9:e1003323. doi: 10.1371/journal.pgen.1003323. doi: 10.1371/journal.pgen.1003323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B, Young CA, Saikia S, McMillan LK, Monahan BJ, Koulman A, Astin J, Eaton CJ, Bryant A, Wrenn RE, Finch SC, Tapper BA, Parker EJ, Jameson GB (2013) Deletion and gene expression analyses define the paxilline biosynthetic gene cluster in Penicillium paxilli. Toxins 5:1422–1446. doi: 10.3390/toxins5081422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri M, Sala de Miguel MA, Viera JB, de Banchero EP (1992) Tremorgenic mycotoxins produced by strains of Penicillium spp. isolated from toxic Poa huecu parodi. Mycopathologia 120:177–182. doi: 10.1007/BF00436396 [DOI] [PubMed] [Google Scholar]
- Sengun I, Yaman D, Gonul S (2008) Mycotoxins and mould contamination in cheese: a review. World Mycotoxin J 1:291–298. doi: 10.3920/WMJ2008.x041 [DOI] [Google Scholar]
- Sheff JG, Farshidfar F, Bathe OF, Kopciuk K, Gentile F, Tuszynski J, Barakat K, Schriemer DC (2017) Novel allosteric pathway of Eg5 regulation identified through multivariate statistical analysis of hydrogen-exchange mass spectrometry (HX-MS) ligand screening data. Mol Cell Proteomics 16:428–437. doi: 10.1074/mcp.M116.064246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada N, Yoshioka M, Mikami O, Tanimura N, Yamanaka N, Hanazumi M, Kojima F, Miyazaki S (2013) Toxicological evaluation and bioaccumulation potential of lolitrem B, endophyte mycotoxin in Japanese black steers. Food Addit Contam Part Chem Anal Control Expo Risk Assess 30:1402–1406. doi: 10.1080/19440049.2013.790090 [DOI] [PubMed] [Google Scholar]
- Shoop WL, Gregory LM, Zakson-Aiken M, Michael BF, Haines HW, Ondeyka JG, Meinke PT, Schmatz DM (2001) Systematic efficacy of nodulisporic acid against fleas on dogs. J Parasitol 87:419–423. doi: 10.1645/0022-3395(2001)087[0419:SEONAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sings H, Singh S (2003) Tremorgenic and nontremorgenic 2,3-fused indole diterpenoids. Alkaloids Chem Biol 60:51–163. doi: 10.1016/S0099-9598(03)60002-7 [DOI] [PubMed] [Google Scholar]
- Sonjak S, Frisvad JC, Gunde-Cimerman N (2005) Comparison of secondary metabolite production by Penicillium crustosum strains, isolated from Arctic and other various ecological niches. FEMS Microbiol Ecol 53:51–60. doi: 10.1016/j.femsec.2004.10.014 [DOI] [PubMed] [Google Scholar]
- Springer JP, Clardy J (1980) Paspaline and paspalicine, two indole-mevalonate metabolites from Claviceps paspali. Tetrahedron Lett 21:231–234. doi: 10.1016/S0040-4039(00)71176-2 [DOI] [Google Scholar]
- Stewart M, Needham M, Bankhead P, Gardiner TA, Scholfield CN, Curtis TM, McGeown JG (2012) Feedback via Ca 2+ -activated ion channels modulates endothelin 1 signaling in retinal arteriolar smooth muscle. Investig Opthalmology Vis Sci 53:3059–3066. doi: 10.1167/iovs.11-9192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoev SD, Dutton MF, Njobeh PB, Mosonik JS, Steenkamp PA (2010) Mycotoxic nephropathy in Bulgarian pigs and chickens: complex aetiology and similarity to Balkan endemic nephropathy. Food Addit Contam 27:72–88 doi: 10.1080/02652030903207227 [DOI] [PubMed] [Google Scholar]
- Sulyok M, Berthiller F, Krska R, Schuhmacher R (2006) Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun Mass Spectrom 20:2649–2659. doi: 10.1002/rcm.2640 [DOI] [PubMed] [Google Scholar]
- Sulyok M, Krska R, Schuhmacher R (2007) A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal Bioanal Chem 389:1505–1523. doi: 10.1007/s00216-007-1542-2 [DOI] [PubMed] [Google Scholar]
- Sumarah MW, Miller JD, Blackwell BA (2005) Isolation and metabolite production by Penicillium roqueforti, P. paneum and P. crustosum isolated in Canada. Mycopathologia 159:571–577. doi: 10.1007/s11046-005-5257-7 [DOI] [PubMed] [Google Scholar]
- Tagami K, Liu C, Minami A, Noike M, Isaka T, Fueki S, Shichijo Y, Toshima H, Gomi K, Dairi T, Oikawa H (2013) Reconstitution of biosynthetic machinery for indole-diterpene paxilline in Aspergillus oryzae. J Am Chem Soc 135:1260–1263. doi: 10.1021/ja3116636 [DOI] [PubMed] [Google Scholar]
- Tagami K, Minami A, Fujii R, Liu C, Tanaka M, Gomi K, Dairi T, Oikawa H (2014) Rapid reconstitution of biosynthetic machinery for fungal metabolites in Aspergillus oryzae: total biosynthesis of aflatrem. ChemBioChem 15:2076–2080. doi: 10.1002/cbic.201402195 [DOI] [PubMed] [Google Scholar]
- Tancinova D, Labuda R (2009) Fungi on wheat bran and their toxinogenity. Ann Agric Environ Med 16:325–331. [PubMed] [Google Scholar]
- Tang M-C, Lin H-C, Li D, Zou Y, Li J, Xu W, Cacho RA, Hillenmeyer ME, Garg NK, Tang Y (2015) Discovery of unclustered fungal indole diterpene biosynthetic pathways through combinatorial pathway reassembly in engineered yeast. J Am Chem Soc 137:13724–13727. doi: 10.1021/jacs.5b06108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Chung SH (2014) Non-aflatoxigenicity of commercial Aspergillus oryzae strains due to genetic defects compared to aflatoxigenic Aspergillus flavus. J Microbiol Biotechnol 24:1081–1087. doi: 10.4014/jmb.1311.11011 [DOI] [PubMed] [Google Scholar]
- Tarui Y, Chinen T, Nagumo Y, Motoyama T, Hayashi T, Hirota H, Muroi M, Ishii Y, Kondo H, Osada H, Usui T (2014) Terpendole E and its derivative inhibit STLC- and GSK-1-resistant Eg5. ChemBioChem Eur J Chem Biol 15:934–938. doi: 10.1002/cbic.201300808 [DOI] [PubMed] [Google Scholar]
- Thom ER, Popay AJ, Waugh CD, Minneé EMK (2014) Impact of novel endophytes in perennial ryegrass on herbage production and insect pests from pastures under dairy cow grazing in northern New Zealand. Grass Forage Sci 69:191–204. doi: 10.1111/gfs.12040 [DOI] [Google Scholar]
- Thom ER, Waugh CD, Minnee EMK, Waghorn GC (2013) Effects of novel and wild-type endophytes in perennial ryegrass on cow health and production. N Z Vet J 61:87–97. doi: 10.1080/00480169.2012.715379 [DOI] [PubMed] [Google Scholar]
- Tkacz JS, DiDomenico B (2001) Antifungals: what’s in the pipeline. Curr Opin Microbiol 4:540–545. doi: 10.1016/S1369-5274(00)00248-4 [DOI] [PubMed] [Google Scholar]
- Tudzynski P, Correia T, Keller U (2001) Biotechnology and genetics of ergot alkaloids. Appl Microbiol Biotechnol 57:593–605. doi: 10.1007/s002530100801 [DOI] [PubMed] [Google Scholar]
- Uhlig S, Botha CJ, Vralstad T, Rolen E, Miles CO (2009) Indole-diterpenes and ergot alkaloids in Cynodon dactylon (Bermuda grass) infected with Claviceps cynodontis from an outbreak of tremors in cattle. J Agric Food Chem 57:11112–11119. doi: 10.1021/jf902208w [DOI] [PubMed] [Google Scholar]
- Uhlig S, Egge-Jacobsen W, Vralstad T, Miles CO (2014) Indole-diterpenoid profiles of Claviceps paspali and Claviceps purpurea from high-resolution Fourier transform Orbitrap mass spectrometry. Rapid Commun Mass Spectrom 28:1621–1634. doi: 10.1002/rcm.6938 [DOI] [PubMed] [Google Scholar]
- Uraguchi K (1969) Mycotoxic origin of cardiac beriberi. J Stored Prod Res 5:227–236. doi: 10.1016/0022-474X(69)90037-X [DOI] [Google Scholar]
- Van de Bittner KC, Nicholson MJ, Bustamante LY, Kessans SA, Ram A, van Dolleweerd CJ, Scott B, Parker EJ (2018) Heterologous biosynthesis of nodulisporic acid F. J Am Chem Soc 140:582–585. doi: 10.1021/jacs.7b10909 [DOI] [PubMed] [Google Scholar]
- van Dolleweerd CJ, Kessans SA, Van de Bittner KC, Bustamante LY, Bundela R, Scott B, Nicholson MJ, Parker EJ (2018) MIDAS: A modular DNA assembly system for synthetic biology. ACS Synth Biol 7:1018–1029. doi: 10.1021/acssynbio.7b00363 [DOI] [PubMed] [Google Scholar]
- Vishwanath V, Sulyok M, Labuda R, Bicker W, Krska R (2009) Simultaneous determination of 186 fungal and bacterial metabolites in indoor matrices by liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem 395:1355–1372. doi: 10.1007/s00216-009-2995-2 [DOI] [PubMed] [Google Scholar]
- Walter SL (2002) Acute penitrem A and roquefortine poisoning in a dog. Can Vet J Rev Veterinaire Can 43:372–374. doi: 10.1016/0041-008X(76)90290-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiewióra B, Żurek G, Pańka D (2015) Is the vertical transmission of Neotyphodium lolii in perennial ryegrass the only possible way to the spread of endophytes? PLOS ONE 10:e0117231. doi: 10.1371/journal.pone.0117231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins AL, Miles CO, Ede RM, Gallagher RT, Munday SC (1992) Structure elucidation of janthitrem B, a tremorgenic metabolite of Penicillium janthinellum, and relative configuration of the A and B rings of janthitrems B, E, and F. J Agric Food Chem 40:1307–1309. doi: 10.1021/jf00020a002 [DOI] [Google Scholar]
- Yamaguchi T, Nozawa K, Hosoe T, Nakajima S, Kawai K (1993) Indoloditerpenes related to tremorgenic mycotoxins, penitrems, from Penicillium crustosum. Phytochemistry 32:1177–1181. doi: 10.1016/S0031-9422(00)95087-8 [DOI] [Google Scholar]
- You J, Du L, King JB, Hall BE, Cichewicz RH (2013) Small-molecule suppressors of Candida albicans biofilm formation synergistically enhance the antifungal activity of amphotericin B against clinical Candida isolates. ACS Chem Biol 8:840–848. doi: 10.1021/cb400009f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, McMillan L, Telfer E, Scott B (2001) Molecular cloning and genetic analysis of an indole-diterpene gene cluster from Penicillium paxilli. Mol Microbiol 39:754–764. doi: 10.1046/j.1365-2958.2001.02265.x [DOI] [PubMed] [Google Scholar]
- Young CA, Bryant MK, Christensen MJ, Tapper BA, Bryan GT, Scott B (2005) Molecular cloning and genetic analysis of a symbiosis-expressed gene cluster for lolitrem biosynthesis from a mutualistic endophyte of perennial ryegrass. Mol Genet Genomics 274:13–29. doi: 10.1007/s00438-005-1130-0 [DOI] [PubMed] [Google Scholar]
- Young CA, Felitti S, Shields K, Spangenberg G, Johnson RD, Bryan GT, Saikia S, Scott B (2006) A complex gene cluster for indole-diterpene biosynthesis in the grass endophyte Neotyphodium lolii. Fungal Genet Biol 43:679–693. doi: 10.1016/j.fgb.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Zbib N, Repussard C, Tardieu D, Priymenko N, Domange C, Guerre P (2015) Toxicity of endophyte-infected ryegrass hay containing high ergovaline level in lactating ewes. J Anim Sci 93:4098–4109. doi: 10.2527/jas.2014-8848 [DOI] [PubMed] [Google Scholar]
- Zhang S, Monahan BJ, Tkacz JS, Scott B (2004) Indole-diterpene gene cluster from Aspergillus flavus. Appl Environ Microbiol 70:6875–6883. doi: 10.1128/AEM.70.11.6875-6883.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.