ABSTRACT
Background
Clinical, neurophysiological, and pathological evidence suggest an association between Parkinson's disease (PD) and peripheral neuropathy (PNP), with a possible causative role of levodopa metabolic products, such as homocysteine and methylmalonic acid.
Methods
We conducted a systematic review of studies reporting cases of PNP in l‐dopa‐treated PD patients indexed in PubMed between January 1990 and March 2018.
Results
We identified 38 articles reporting cases of PNP in PD patients treated with oral l‐dopa or with l‐dopa/carbidopa intestinal gel infusion (LCIG). Prevalence of PNP was 30.2% in the former group and 42.1% in the latter. Oral l‐dopa was mostly associated with slowly progressive PNP, whereas LCIG showed an acute or subacute onset in 35.7% of cases. In both groups, there was an association between PNP and higher l‐dopa doses, as well as with the following biochemical alterations: increased homocysteine; reduced vitamin B12; increased methylmalonic acid; and reduced vitamin B6. A skin biopsy was performed in 181 patients, showing signs of small fibers neuropathy in 169 (93.4%). Positive, yet preliminary, results were observed in patients receiving periodic vitamin supplementation.
Conclusions
Over one third of PD patients in treatment with l‐dopa may develop PNP, with a significantly higher prevalence of acute and subacute forms in those receiving LCIG. Pathogenic mechanisms remain unclear, but possibly related to a complex interplay between peripheral neurodegenerative processes and l‐dopa neurotoxic metabolites. Prospective, randomized, clinical trials are required to identify factors associated with the onset and progression of PD‐associated PNP and clarify the protective role of B‐group vitamin supplementation.
Keywords: Parkinson's disease, neuropathy, levodopa, LCIG, small fibers
In 2008, Toth and colleagues described, for the first time, an unexpectedly high prevalence of peripheral neuropathy (PNP) in patients with Parkinson's disease (PD).1, 2 Since then, several studies confirmed a pathological association between PNP, PD, and dopamine‐replacement therapies, estimating that up to 55% of patients treated with oral levodopa, and 75% of those treated with l‐dopa/carbidopa intestinal gel (LCIG) infusion, may develop clinical or subclinical signs of PNP during the course of the disease.2, 3
Although the pathogenesis of PD‐related PNP remains uncertain, at least two separate mechanisms seem to be involved: the iatrogenic effect of dopaminergic therapies, in particular l‐dopa, and the neurodegeneration of selected components of the peripheral nervous system, which frequently remain subclinical. Mechanisms linking l‐dopa intake and PNP are unclear,4, 5, 6 but potentially related to the metabolic pathway of l‐dopa, which may result in the accumulation of neurotoxins such as homocysteine (Hcy) and methylmalonic acid (MMA; Fig. 1). The complex interplay between l‐dopa catabolic pathways and PD‐specific neurodegenerative processes, also occurring in l‐dopa‐naïve patients,7 remains to be clarified.
Figure 1.
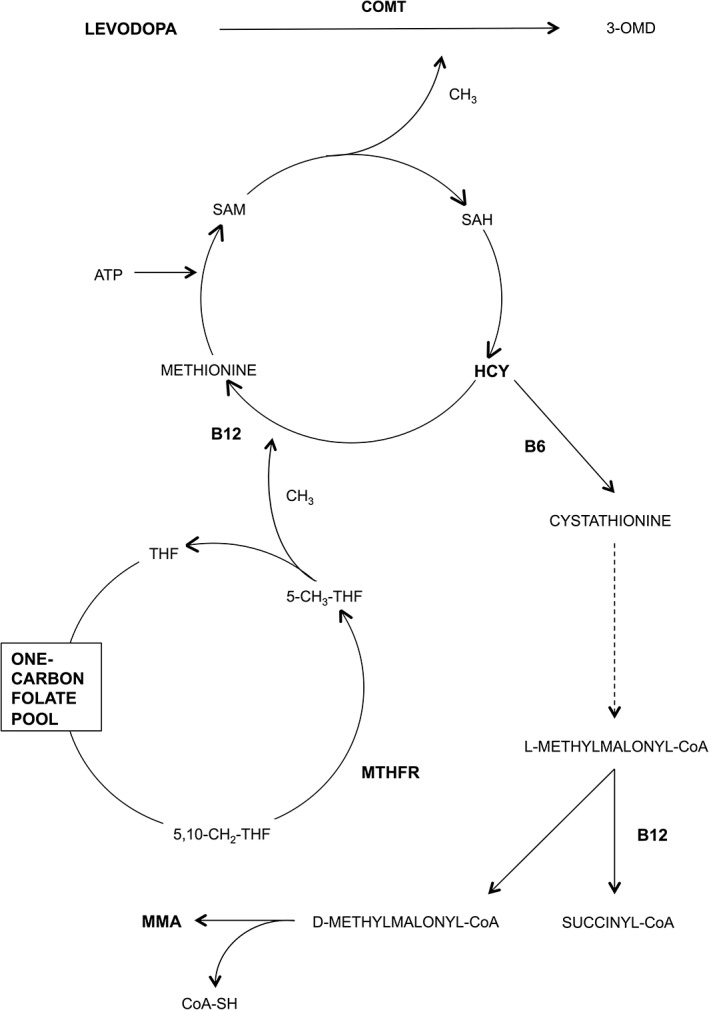
l‐dopa metabolic pathway. l‐dopa is methylated by COMT through donation of a methyl group by S‐adenosylmethionine (SAM). This reaction results in production of SAH and, then, Hcy, which is catabolized through two possible metabolic pathways: (1) remethylation to methionine, which requires 5‐methyltetrahydrofolate (5‐CH3‐THF) as a methyl donor and vitamin B12 (B12) as enzymatic cofactor; (2) trans‐sulfuration to cysteine, which requires vitamin B6 (B6) as a cofactor. This latter pathway results in the formation of L‐methylmalonyl‐CoA, which is metabolized to succinyl‐CoA or, in case of vitB12 deficiency, to MMA. As a final result, chronic intake of l‐dopa can lead to accumulation of Hcy and MMA, as well as to depletion of vitB6, vitB12, and folate. Abbreviations: 3‐OMD, 3‐O‐methyldopa; 5,10‐CH2‐THF, 5,10‐methylenetetrahydrofolate.
In this systematic review, we sought to critically analyze the association between PNP and l‐dopa, with particular attention to oral versus infusion l‐dopa therapy. In addition, we aimed to review pharmacological strategies for the prevention and treatment of l‐dopa‐associated PNP.
Materials and Methods
Literature Search Strategy
We systematically searched PubMed for human studies published in English between January 1990 and March 2018 using a combination of the following search terms: “levodopa,” “duodenal levodopa infusion,” “levodopa/carbidopa intestinal gel,” “LCIG,” “neuropathy,” “peripheral neuropathy,” “polyneuropathy,” “peripheral nervous system disease,” “vitamin B12,” “cobalamin,” “vitamin B6,” “pyridoxine,” “vitamin B9,” “folate,” “methylmalonic acid,” and “homocysteine.” No restrictions were applied to sex, age, disease duration, disease severity, or diagnostic criteria used for the diagnosis of PNP. Previously published literature reviews were excluded, as well as book chapters, letters to the editor, editorials not providing new data, and studies referring only to autonomic neuropathy.
Study selection
We reviewed abstracts for relevance and searched the reference lists for relevant articles not captured by the original searching strategy. Included studies were checked for duplicates or studies reporting data from the same population. In the latter case, the longer follow‐up available was used.
Data Extraction
The following data were extracted using a standardized form: study design, study sample, length of follow‐up (when present), clinical and electrophysiological methodology used for the diagnosis of PNP, prevalence of PNP, PNP type (large fiber vs. small fiber PNP), age, duration of l‐dopa treatment, l‐dopa equivalent daily dose, measures of statistical association between l‐dopa dose/duration and PNP, measures of statistical association between laboratory data (vitamin [vit]B6, vitB12, folate, MMA, and Hcy), and PNP.
Data Analysis
Results were summarized as follows: prevalence of PNP; clinical/demographic characteristics; association between l‐dopa exposure and PNP; association between biochemical alterations and PNP; therapeutic strategies used for the management of PD‐associated PNP. Data from large vs. small fibers neuropathy were reported separately. Studies reporting data from patients receiving oral versus infusion dopaminergic therapies were reported separately.
Results
Search Results
Of the 138 studies initially identified, 35 met the inclusion and not one of the exclusion criteria and were included in the analyses; three additional studies were identified through the screening of the reference lists. Thus, a total of 38 studies underwent data extraction (Supporting Information Table S1). Sixteen studies reported data from patients treated with oral l‐dopa, 14 from patients treated with LCIG, and three from both groups. Overall, 1,348 patients were considered, 451 of whom (33.5%) met the diagnostic criteria for PNP. Five studies (four from patients treated with oral l‐dopa and one from patients treated with LCIG), for a total of 181 patients, used skin biopsies to analyze the involvement of small unmyelinated peripheral nerve fibers, finding significant alterations in 93.4% (169 of 181) of patients.
PNP and Oral l‐dopa
The association between PNP and oral l‐dopa was evaluated in 19 studies,1, 2, 3 , 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 including a total of 1,137 patients. A total of 353 PNPs were described, with a large predominance of slowly progressive axonal neuropathy selectively involving the sensory fibers. Estimated PNP prevalence, after excluding case series, was 30.2% (339 of 1,123). All studies used nerve conduction studies in association with clinical symptoms and/or clinical validated scales for the diagnosis of PNP. The only exception was represented by the study from Andreasson and colleagues,10 who relied only on clinical scales for the diagnosis of PNP. After removing the results from this study, estimated prevalence of PNP decreased from 30.2% to 29.6% (323 of 1,090). Only 10 cases were classified as subacute15 (Supporting Information Table S1).
Association With the Dose of l‐dopa
The association between PNP and l‐dopa dosage was evaluated in 16 studies: 12 found an association between PNP and higher doses of l‐dopa,1, 2, 3, 8, 9, 13, 14, 15, 16, 18, 19, 23 whereas four showed negative results.10, 12, 17, 20 Three studies did not provide any measures of association between PNP and l‐dopa dosage.11, 21, 22
The association between PNP and duration of l‐dopa therapy was evaluated in eight studies, with positive results in 4 cases.1, 13, 15, 18 Ceravolo and colleagues18 described a 3.08‐fold increased risk of PNP in patients with long (more than 3 years) versus short (less than 3 years) exposure to l‐dopa.
Association With Biochemical Alterations
There were 15 studies testing the association between PNP and vitB12, five of which (33.3%) showed a correlation between low levels of vitB12 and PNP1, 18, 20, 21, 22 (Supporting Information Table S1); 13 studies tested the association between PNP and Hcy, 10 of which (77.0%) showed a correlation between high levels of Hcy and PNP1, 2, 3, 9, 10, 13, 15, 18, 21, 22 (Supporting Information Table S1); nine studies tested the association between PNP and folate, one of which (11.1%) showed a correlation between low levels of folate and PNP19 (Supporting Information Table S1); six studies tested the association between PNP and MMA, four of which (66.7%) showed a correlation between high levels of MMA and PNP1, 2, 8, 21 (Supporting Information Table S1); and one study tested the association between PNP and vitB6, reporting low vitB6 plasma levels in patients with PNP, as well as an association between low vitB6 plasma levels and high doses of l‐dopa.3
Small Fibers Neuropathy in PD Patients Treated With Oral l‐dopa
The association between oral l‐dopa and small fiber neuropathy was tested in four studies using skin biopsies, and assessing a total of 164 patients, 62 of whom (37.8%) l‐dopa‐naïve.7, 24, 25, 26 The researchers found a reduction in the density of Meissner corpuscles, myelinated fibers, and intraepidermal nerve fiber (IENFD) in the majority of cases. Two studies7, 24 described an association between higher l‐dopa doses and reduced density in the large fibers, but no significant differences in small fibers pathology related to l‐dopa intake. One study25 found an inverse correlation between the cumulative dose of l‐dopa and intraepidermal fiber density, whereas another study26 did not find any correlations between skin denervation and l‐dopa cumulative dose, but an inverse correlation between IENFD and disease duration, possibly reflecting an association between small fibers pathology and duration of l‐dopa treatment.
Clinical Management of PNP in Patients Receiving Oral l‐dopa Therapy
There was only one 3‐year prospective study1 analyzing the management of PNP in patients receiving oral l‐dopa therapy. The researchers found that a therapeutic regimen based on monthly vitB12 intramuscular injections might normalize plasma levels of Hcy and MMA and stabilize peripheral nervous system electrophysiological parameters in patients with cobalamin deficiency.
PNP and LCIG
The association between PNP and LCIG was evaluated in 17 studies,3, 5, 15, 16, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 including a total of 211 patients. A total of 98 PNPs were described, with an acute or subacute onset in 35.7% of cases (n = 35). The vast majority of cases (90.8%; n = 89) showed a sensory axonal PNP, whereas 9.2% (n = 9) showed demyelinating features15, 28, 32, 39 (Supporting Information Table S1). Estimated PNP prevalence, after excluding case reports and case series, was 42.1% (82 of 195).
Association With the Dose of LCIG
The association between PNP and LCIG dose was evaluated in five studies: four found an association between PNP and higher doses of LCIG.3, 15, 16, 28 The only study that did not find any correlations between PNP and LCIG dosage27 included participants that preventively received supplementation with vitB2, vitB6, vitB12, and folate. Twelve studies did not provide any measures of association between PNP and LCIG doses.5, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39
Association With Biochemical Alterations
There were 14 studies testing the association between PNP and vitB12, 10 of which (71.4%) showed a correlation between low levels of vitB12 and PNP3, 5, 29, 31, 32, 33, 34, 35, 36, 38 (Supporting Information Table S1); 11 studies tested the association between PNP and Hcy, nine of which (81.8%) showed a correlation between high Hcy and PNP3, 15, 28, 31, 32, 33, 34, 36, 38 (Supporting Information Table S1); eight studies tested the association between PNP and folate, four of which (50.0%) showed a correlation between low levels of folate and PNP3, 31, 33, 34 (Supporting Information Table S1); six studies tested the association between PNP and vitB6 reporting, in all cases, a correlation between low levels of vitB6 and PNP3, 15, 29, 32, 34, 36 (Supporting Information Table S1); and two studies tested the association between PNP and MMA, reporting high levels of MMA in patients with PNP36, 38 (Supporting Information Table S1).
Small Fibers Neuropathy in PD Patients Treated With LCIG
The association between LCIG and small fiber neuropathy was tested in one study using skin biopsies40 (Supporting Information Table S1). Five LCIG patients were evaluated during a 12‐months follow‐up, showing a severe IENFD reduction after 3, 6, and 12 months of treatment. The researchers did not provide any measures of association between small fiber neuropathy and LCIG doses.
Clinical Management of PNP in Patients Receiving LCIG
No cases of LCIG discontinuation were reported in patients developing slowly progressive, chronic PNP (n = 63). In 38 cases (60.3%), the therapeutic approach was based on vitamin supplementation, in particular vitB12. No data were reported in the remaining 25 cases (39.7%).
LCIG was discontinued in 22 of the 35 cases (62.9%) developing an acute or subacute PNP. In addition, 6 cases (17.1%) underwent intravenous immunoglobulin (IVIg) therapy, 3 (8.6%) intravenous high dose steroids, 2 (5.7%) plasma exchange (PE), and 1 (2.9%) received a treatment with IVIg, steroids, and PE. All patients developing an acute or subacute PNP received multivitamin supplementation. Interestingly, Rispoli and colleagues27 found that patients preventively treated with high‐doses multivitamin supplementation had a low incidence of LCIG‐induced PNP.
Discussion
This systematic review showed a 30.2% prevalence of PNP in the population of PD patients treated with oral l‐dopa and 42.1% in those treated with LCIG. Cases of PNP associated with oral l‐dopa were mostly characterized by a slowly progressive axonal neuropathy selectively involving the sensory fibers, whereas PNP cases associated with LCIG showed an acute or subacute onset in 35.7% of cases, and frequently involved both the motor and the sensory components of the peripheral nervous system. The definition of PNP was based on different methodologies, in all but 1 case relying on electrophysiological criteria in combination with neurological symptoms and/or validated clinical scales. The majority of studies found an association between PNP and l‐dopa doses, as well as with selected biochemical alterations, namely reduced vitB12 plasma levels and increased Hcy and MMA plasma levels. Less data were reported on the association between low vitB6 plasma levels and PNP. A multivitamin supplementation was empirically tried both in patients treated with oral l‐dopa and in those treated with LCIG, showing positive yet preliminary results. No studies tested the efficacy of B‐group vitamin supplementation versus placebo for the prevention of l‐dopa‐associated PNP.
Role of Aging: Prevalence of PNP in PD Versus Age‐Matched Controls
Although 4.9% of the population between the ages of 65 and 69 might present with clinical or subclinical signs of PNP, with a prevalence that reaches 6.4% between 70 and 74 years old,41, 42 aging seems to only partially explain the increased risk of PNP observed in PD patients treated with l‐dopa. Rajabally and colleagues20 found a 4.7 higher prevalence of PNP in PD versus age‐matched controls, and Ceravolo and colleagues18 estimated that age accounts for a 1.08‐fold higher risk (i.e., 8% of annual risk) of PNP in PD, whereas a long history of l‐dopa exposure might increase the risk of PNP by 2.38‐ to 3.08‐folds.
l‐dopa Iatrogenic Effect: Biochemical Considerations
Data from our systematic review confirm the association between increased Hcy plasma levels and PNP. Hcy may directly cause neurotoxicity through an increased vulnerability to mitochondrial toxins, induction of inflammatory reactions, glutamatergic excitotoxicity, and impairment of DNA repairing mechanisms.4, 5, 43, 44 Multiple evidence supports the notion that Hcy has a neurotoxic effect not only in PD,45 but also in diabetes,46 and in the general population of elderly subjects.47 A possible neuroprotective effect of COMT inhibitors has been postulated in human subjects48 and supported by animal studies.49 Cossu and colleagues11 recently found that PD patients treated with COMT inhibitors had a lower incidence of PNP, lower plasma levels of Hcy, and higher vitB12 plasma levels.
VitB12 and vitB6 are two other major players in the iatrogenic effect of l‐dopa metabolism, mostly investigated in studies from PD patients treated with LCIG. It has been suggested that a continuous delivery of the viscous enteral gel might hamper the functionality of the intestinal mucosa, eventually resulting in malabsorption of vitamins that are critical for the peripheral nervous system, such as vitB12 and vitB6.50 Deficiency of these two critical cofactors may cause neuronal damage through multiple pathogenic mechanisms, including elevation of Hcy plasma levels, reduction of succinyl‐CoA availability, and alteration of vitB12‐dependent RNA methylation, which may result in a reduction of carbohydrate and fat metabolism, and in the impairment of axonal protein production.5, 51, 52
MMA levels were found to be increased in almost all patients with PD‐associated PNP, but its pathogenic role in the complex cascade of events associated with l‐dopa‐associated PNP remains unclear.2 Some researchers suggested that MMA might represent an early marker of cobalamin “functional” insufficiency, in particular considering that up to 50% of patients with clinically significant cobalamin deficiency have vitB12 plasma levels still comprised within the normal range.2, 53, 54
l‐dopa‐Independent Peripheral Nervous System Degeneration in PD: Neuropathological Considerations
There is evidence that PNP might result from a direct involvement of the peripheral nervous system in PD.4 Skin biopsies showed an increased prevalence of pathological alterations in patients with PD compared to controls, as well as a reduction in density of myelinated fibers, Meissner corpuscles, and intraepidermal nerve fibers, regardless of l‐dopa exposure. In particular, all neuropathological studies demonstrated signs of small fiber pathology also in l‐dopa‐naïve PD patients (i.e., reduced IENFD), whereas only two studies25, 40 found a correlation between small fibers neuropathy and l‐dopa doses. Aggregates of ubiquitin, 14–3‐3 protein, and peripheral alpha‐synuclein were also documented in peripheral axons,55 as well as in the skin,26 even in the prodromal phases of PD.7, 24, 56, 57 In a cohort of over 5,000 l‐dopa‐naïve PD patients and 20,000 nonparkinsonian controls, Conradt and colleagues58 found that PD is associated with a 2.4‐fold higher prevalence of PNP. Moreover, the prevalence of EMG‐confirmed PNP, mostly asymptomatic, ranged from 4.8% to 24.0%, in the revised studies that investigated also l‐dopa‐naïve PD patients.15, 17, 18, 19 Finally, a recent study from our group9 found significant differences in the clinical phenotype of PD patients with and without PNP, suggesting that peripheral nervous system involvement represents a clinical endophenotype of a more‐aggressive form of PD, characterized by greater cognitive impairment, nonmotor symptoms burden, and daily living functional disability.
Acute, Subacute, and Chronic PNP: A Different Pathogenesis?
Except for one study,15 the majority of cases of PNP associated with oral l‐dopa showed a chronic onset, with predominant sensory axonal impairment. On the contrary, 35.7% of PNP cases associated with LCIG showed an acute or subacute onset, which might suggest immune medicated inflammatory mechanisms, possibly triggered by the PEG‐J tube or by the gel formulation.39 There are factors suggesting that neurodegenerative, toxic, or carential factors rather than inflammatory mechanisms might be involved in the pathogenesis of acute PNP in PD patients treated with LCIG,5 including a neurophysiological pattern predominantly characterized by axonal rather than demyelinating features,59 and the frequent association with vitB12 deficiency and increased Hcy plasma levels. It has been estimated that one third of vitB12 deficiency–induced PNP60 presents with a subacute onset and a nonspecific albumin‐cytological dissociation at the cerebrospinal fluid analysis,5 characteristics that have both been described in LCIG‐associated PNP.
Although the description of inflammatory infiltrates in the nerve biopsies of PD patients with LCIG‐associated PNP does not permit to completely exclude the hypothesis of an inflammatory mechanism underlying these unusual types of PNP, an increased production of proinflammatory cytokines has also been described in patients with vitB12 deficiency.61 Finally, acute PNPs have been reported in patients undergoing bariatric surgery,62 suggesting the possibility that any interactions with the gastric and enteric mucosa can trigger a cascade of events eventually resulting in an acute or subacute PNP.
The Role of Genetic Factors
The possibility exists that genetic factors might play a role in the susceptibility to l‐dopa‐related PNP. In particular, quantitative or functional deficiency of 5,10‐methylenetetrahydrofolate reductase (MTHFR)63, 64, 65, 66 could lead to an elevation of Hcy levels through the decreased availability of 5‐methyltetrahydrofolate and consequent impairment of Hcy re‐methylation in methionine. A “low‐activity” catechol‐O‐methyltransferase (COMT) genotype (i.e., methionine homozygosis in the single‐nucleotide polymorphisms A158G rs4680) has also been described10 and associated with a greater risk of l‐dopa‐related PNP. Carriers of this specific genetic variant might present with a particular susceptibility to the S‐adenosylhomocysteine (SAH)‐mediated inhibition, with a consequent peripheral cytotoxic effect.
Clinical Management of l‐dopa‐Induced PNP
A strong level of evidence supports a role, at least partial, for vitamins of the group B and related cofactors in the pathogenesis of l‐dopa‐related PNP. Monitoring the plasma levels of vitB6, vitB12, MMA, and Hcy is of utmost importance in patients starting and continuing LCIG therapy, as well as in patients with high oral l‐dopa intake or at higher risk of PNP (e.g., elderly, diabetic, known vitamin deficiency, and previous gastroenteric surgery). Some researchers suggested a periodic clinical, electrophysiological, and biochemical assessment in patients undergoing LCIG. A basic screening should include regular monitoring of vitB12 and folate since the beginning of the treatment, plus a determination of Hcy and MMA in cases of borderline vitamin levels. Vitamin supplementation should be started in all patients showing biochemical alterations or symptoms of PNP.6, 33 Other empirical approaches include a prophylactic supplementation with high doses of vitB12 and folate since the beginning of LCIG.4 Rispoli and colleagues27 found a very low incidence of subclinical PNP in 30 patients undergoing supramaximal supplementation with vitB12, folic acid, vitB6, and vitB2 since the early onset of LCIG treatment. Although extensive vitB6 supplementation is scarcely recommended because of the interference with decarboxylase inhibitor and the potential neurotoxic effect of pyridoxine megadoses,67 preliminary evidence suggests that COMT inhibitors might be beneficial to prevent the development of PNP in patients receiving l‐dopa treatment.11
The management of acute/subacute cases of PNP is more controversial. In these researchers’ opinion, LCIG should be discontinued in patients developing acute PNP. A clinical, electrophysiological, and biochemical extensive workup is recommended to exclude alternative causes of PNP and identify possible specific causative factors such as vitamin deficiencies. The choice of whether to restart LCIG should be taken only after vitamin supplementation and careful analysis of the risk/benefit profile associated with LCIG. Still, one third of the cases reported in the literature opted for continuing LCIG.3, 15, 29, 34, 36, 37
Conclusions
l‐dopa‐induced PNP might result from a complex interlink between peripheral nervous system neurodegenerative processes, genetic predisposition, and pathogenic mechanisms associated with the l‐dopa metabolic pathway, which may result in Hcy accumulation and vitamin deficiency. Prospective studies are required to estimate the incidence of PNP in patients receiving different dopamine‐replacement therapies, stratify risk factors for developing PNP, and clarify the protective role of vitamin supplementations.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
Dr. Romagnolo: 1A, 1B, 1C, 2A, 2B, 3A.
Dr. Merola: 1B, 2C, 3A.
Dr. Artusi: 1C, 2C, 3B.
Dr. Rizzone: 1C, 2C, 3B.
Dr. Zibetti: 1C, 2C, 3B.
Dr. Lopiano: 1A, 1B, 2A, 2C, 3B.
All the co‐authors listed above gave their final approval of this manuscript version, accepted responsibility for its content, and agreed to the order of author names.
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The authors confirm that the approval of an institutional review board and the patient consent were not required for this work.
Funding Sources and Conflicts of Interest: This study was supported by the NIH (KL2 TR001426). The authors report no conflict of interest.
Financial Disclosures for previous 12 months: Dr. Romagnolo has received grant support and speaker honoraria from AbbVie; speaker honoraria from Chiesi Farmaceutici; and travel grants from Medtronic, Lusofarmaco, and UCB Pharma. Dr. Merola is supported by NIH (KL2 TR001426) and has received speaker honoraria from CSL Behring, AbbVie, and Cynapsus Therapeutics. He has received grant support from Lundbeck. Dr. Artusi has received travel grants from Zambon. Dr. Rizzone has received honoraria for lecturing and travel grants from Medtronic and Zambon. Dr. Zibetti has received speaker's honoraria from Medtronic, Chiesi Farmaceutici, UCB Pharma, and AbbVie. Dr. Lopiano has received honoraria for lecturing and travel grants from Medtronic, UCB Pharma, and AbbVie.
Supporting information
Table S1. Reviewed articles
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Toth C, Brown MS, Furtado S, Suchowersky O, Zochodne D. Neuropathy as a potential complication of levodopa use in Parkinson's disease. Mov Disord 2008; 23:1850–9. [DOI] [PubMed] [Google Scholar]
- 2. Toth C, Breithaupt K, Ge S et al Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol 2010; 68:28–36. [DOI] [PubMed] [Google Scholar]
- 3. Loens S, Chorbadzhieva E, Kleimann A, Dressler D, Schrader C. Effects of levodopa/carbidopa intestinal gel versus oral levodopa/carbidopa on B vitamin levels and neuropathy. Brain Behav 2017; 7:e00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cossu G, Melis M. The peripheral nerve involvement in Parkinson disease: a multifaceted phenomenon. Parkinsonism Relat Disord 2016; 25:17–20. [DOI] [PubMed] [Google Scholar]
- 5. Uncini A, Eleopra R, Onofrj M. Polyneuropathy associated with duodenal infusion of levodopa in Parkinson's disease: features, pathogenesis and management. J Neurol Neurosurg Psychiatry 2015; 86:490–5. [DOI] [PubMed] [Google Scholar]
- 6. Muller T, van Laar T, Cornblath DR et al Peripheral neuropathy in Parkinson's disease: levodopa exposure and implications for duodenal delivery. Parkinsonism Relat Disord 2013; 19:501–7. [DOI] [PubMed] [Google Scholar]
- 7. Nolano M, Provitera V, Lanzillo B, Santoro L. Neuropathy in idiopathic Parkinson disease: an iatrogenic problem? Ann Neurol 2011; 69:427–8. [DOI] [PubMed] [Google Scholar]
- 8. Park JS, Park D, Ko PW, Kang K, Lee HW. Serum methylmalonic acid correlates with neuropathic pain in idiopathic Parkinson's disease. Neurol Sci 2017; 38:1799–804. [DOI] [PubMed] [Google Scholar]
- 9. Merola A, Rosso M, Romagnolo A et al Peripheral neuropathy as marker of severe Parkinson's disease phenotype. Mov Disord 2017; 32:1256–8. [DOI] [PubMed] [Google Scholar]
- 10. Andreasson M, Brodin L, Laffita‐Mesa JM, Svenningsson P. Correlations between methionine cycle metabolism, COMT genotype, and polyneuropathy in L‐dopa treated Parkinson's disease: a preliminary cross‐sectional study. J Parkinsons Dis 2017; 7:619–28. [DOI] [PubMed] [Google Scholar]
- 11. Cossu G, Ceravolo R, Zibetti M et al Levodopa and neuropathy risk in patients with Parkinson disease: effect of COMT inhibition. Parkinsonism Relat Disord 2016; 27:81–4. [DOI] [PubMed] [Google Scholar]
- 12. de Araujo DF, de Melo Neto AP, Oliveira IS et al Small (autonomic) and large fiber neuropathy in Parkinson disease and parkinsonism. BMC Neurol 2016; 16:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szadejko K, Dziewiatowski K, Szabat K et al Polyneuropathy in levodopa‐treated Parkinson's patients. J Neurol Sci 2016; 371:36–41. [DOI] [PubMed] [Google Scholar]
- 14. Grambalova Z, Kaiserova M, Vastik M et al Peripheral neuropathy in Parkinson's disease. Neuro Endocrinol Lett 2015; 36:363–7. [PubMed] [Google Scholar]
- 15. Mancini F, Comi C, Oggioni GD et al Prevalence and features of peripheral neuropathy in Parkinson's disease patients under different therapeutic regimens. Parkinsonism Relat Disord 2014; 20:27–31. [DOI] [PubMed] [Google Scholar]
- 16. Jugel C, Ehlen F, Taskin B, Marzinzik F, Muller T, Klostermann F. Neuropathy in Parkinson's disease patients with intestinal levodopa infusion versus oral drugs. PLoS One 2013; 8:e66639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shahrizaila N, Mahamad UA, Yap AC, Choo YM, Marras C, Lim SY. Is chronic levodopa therapy associated with distal symmetric polyneuropathy in Parkinson's disease? Parkinsonism Relat Disord 2013; 19:391–3. [DOI] [PubMed] [Google Scholar]
- 18. Ceravolo R, Cossu G, Bandettini di Poggio M et al Neuropathy and levodopa in Parkinson's disease: evidence from a multicenter study. Mov Disord 2013; 28:1391–7. [DOI] [PubMed] [Google Scholar]
- 19. Rajabally YA, Martey J. Levodopa, vitamins, ageing and the neuropathy of Parkinson's disease. J Neurol 2013; 260:2844–8. [DOI] [PubMed] [Google Scholar]
- 20. Rajabally YA, Martey J. Neuropathy in Parkinson disease: prevalence and determinants. Neurology 2011; 77:1947–50. [DOI] [PubMed] [Google Scholar]
- 21. Kimber T, Blumbergs P, Thompson P. Severe ataxic polyneuropathy associated with chronic levodopa use in Parkinson's disease. Parkinsonism Relat Disord 2013; 19:847–9. [DOI] [PubMed] [Google Scholar]
- 22. Gondim Fde A, de Oliveira GR, Peixoto AA, Jr., Horta WG. A case series of peripheral neuropathy in patients with Parkinson's disease. Ann Neurol 2010;68:973–975. [DOI] [PubMed] [Google Scholar]
- 23. Bulling MT, Wing LM, Burns RJ. Controlled release levodopa/carbidopa (Sinemet CR4) in Parkinson's disease—an open evaluation of efficacy and safety. Aust N Z J Med 1991; 21:397–400. [DOI] [PubMed] [Google Scholar]
- 24. Nolano M, Provitera V, Manganelli F et al Loss of cutaneous large and small fibers in naive and l‐dopa‐treated PD patients. Neurology 2017; 89:776–84. [DOI] [PubMed] [Google Scholar]
- 25. Kass‐Iliyya L, Javed S, Gosal D et al Small fiber neuropathy in Parkinson's disease: a clinical, pathological and corneal confocal microscopy study. Parkinsonism Relat Disord 2015; 21:1454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doppler K, Ebert S, Uceyler N et al Cutaneous neuropathy in Parkinson's disease: a window into brain pathology. Acta Neuropathol 2014; 128:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rispoli V, Simioni V, Capone JG et al Peripheral neuropathy in 30 duodopa patients with vitamins B supplementation. Acta Neurol Scand 2017; 136:660–7. [DOI] [PubMed] [Google Scholar]
- 28. Merola A, Romagnolo A, Zibetti M, Bernardini A, Cocito D, Lopiano L. Peripheral neuropathy associated with levodopa‐carbidopa intestinal infusion: a long‐term prospective assessment. Eur J Neurol 2016; 23:501–9. [DOI] [PubMed] [Google Scholar]
- 29. Chang FC, Kwan V, van der Poorten D et al Intraduodenal levodopa‐carbidopa intestinal gel infusion improves both motor performance and quality of life in advanced Parkinson's disease. J Clin Neurosci 2016; 25:41–5. [DOI] [PubMed] [Google Scholar]
- 30. Lehnerer SM, Fietzek UM, Messner M, Ceballos‐Baumann AO. Subacute peripheral neuropathy under duodopa therapy without cobalamin deficiency and despite supplementation. J Neural Transm (Vienna) 2014; 121:1269–72. [DOI] [PubMed] [Google Scholar]
- 31. Caceres‐Redondo MT, Carrillo F, Lama MJ et al Long‐term levodopa/carbidopa intestinal gel in advanced Parkinson's disease. J Neurol 2014; 261:561–9. [DOI] [PubMed] [Google Scholar]
- 32. Galazky I, Schoof J, Stallforth S, Kupsch A, Heinze HJ, Kluge C. Guillain‐Barre/CIDP‐like neuropathy in two parkinsonian patients following intestinal levodopa/carbidopa treatment. Parkinsonism Relat Disord 2014; 20:125–7. [DOI] [PubMed] [Google Scholar]
- 33. Santos‐Garcia D, de la Fuente‐Fernandez R, Valldeoriola F, et al. Polyneuropathy while on duodenal levodopa infusion in Parkinson's disease patients: we must be alert. J Neurol 2012;259:1668–1672. [DOI] [PubMed] [Google Scholar]
- 34. Klostermann F, Jugel C, Muller T, Marzinzik F. Malnutritional neuropathy under intestinal levodopa infusion. J Neural Transm (Vienna) 2012; 119:369–72. [DOI] [PubMed] [Google Scholar]
- 35. Meppelink AM, Nyman R, van Laar T, Drent M, Prins T, Leenders KL. Transcutaneous port for continuous duodenal levodopa/carbidopa administration in Parkinson's disease. Mov Disord 2011; 26:331–4. [DOI] [PubMed] [Google Scholar]
- 36. Urban PP, Wellach I, Faiss S et al Subacute axonal neuropathy in Parkinson's disease with cobalamin and vitamin B6 deficiency under duodopa therapy. Mov Disord 2010; 25:1748–52. [DOI] [PubMed] [Google Scholar]
- 37. Gusmaroli G, Barbagli D, Ravagnani M et al Axonal polyneuropathy during duodenal levodopa treatment in a woman with idiopatic Parkinson's disease. Mov Disord 2010; 25:S720–0. [Google Scholar]
- 38. Manca D, Cossu G, Murgia D et al Reversible encephalopathy and axonal neuropathy in Parkinson's disease during duodopa therapy. Mov Disord 2009; 24:2293–4. [DOI] [PubMed] [Google Scholar]
- 39. Antonini A, Isaias IU, Canesi M et al Duodenal levodopa infusion for advanced Parkinson's disease: 12‐month treatment outcome. Mov Disord 2007; 22:1145–9. [DOI] [PubMed] [Google Scholar]
- 40. Devigili G, Rinaldo S, Lettieri C, Eleopra R. Levodopa/carbidopa intestinal gel therapy for advanced Parkinson disease: an early toxic effect for small nerve fibers? Muscle Nerve 2016; 54:970–2. [DOI] [PubMed] [Google Scholar]
- 41. Hanewinckl R, van Oijen M, Ikram MA, van Doorn PA. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol 2016; 31:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baldereschi M, Inzitari M, Di Carlo A et al Epidemiology of distal symmetrical neuropathies in the Italian elderly. Neurology 2007; 68:1460–7. [DOI] [PubMed] [Google Scholar]
- 43. Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson's disease. J Neurochem 2002; 80:101–10. [DOI] [PubMed] [Google Scholar]
- 44. Kruman II, Kumaravel TS, Lohani A et al Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer's disease. J Neurosci 2002; 22:1752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muller T, Renger K, Kuhn W. Levodopa‐associated increase of homocysteine levels and sural axonal neurodegeneration. Arch Neurol 2004; 61:657–60. [DOI] [PubMed] [Google Scholar]
- 46. Wile DJ, Toth C. Association of metformin, elevated homocysteine, and methylmalonic acid levels and clinically worsened diabetic peripheral neuropathy. Diabetes Care 2010; 33:156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leishear K, Ferrucci L, Lauretani F et al Vitamin B12 and homocysteine levels and 6‐year change in peripheral nerve function and neurological signs. J Gerontol A Biol Sci Med Sci 2012; 67:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Valkovic P, Benetin J, Blazicek P, Valkovicova L, Gmitterova K, Kukumberg P. Reduced plasma homocysteine levels in levodopa/entacapone treated Parkinson patients. Parkinsonism Relat Disord 2005; 11:253–6. [DOI] [PubMed] [Google Scholar]
- 49. Kambur O, Mannisto PT, Pusa AM, Kaenmaki M, Kalso EA, Kontinen VK. Nitecapone reduces development and symptoms of neuropathic pain after spinal nerve ligation in rats. Eur J Pain 2011; 15:732–40. [DOI] [PubMed] [Google Scholar]
- 50. Weber GA, Sloan P, Davies D. Nutritionally induced peripheral neuropathies. Clin Podiatr Med Surg 1990; 7:107–28. [PubMed] [Google Scholar]
- 51. Roos D. Neurological complications in patients with impaired vitamin B12 absorption following partial gastrectomy. Acta Neurol Scand Suppl 1978; 69:1–77. [PubMed] [Google Scholar]
- 52. Walerych WS, Venkataraman S, Johnson BC. The methylation of transfer RNA by methyl cobamide. Biochem Biophys Res Commun 1966; 23:368–74. [DOI] [PubMed] [Google Scholar]
- 53. McCombe PA, McLeod JG. The peripheral neuropathy of vitamin B12 deficiency. J Neurol Sci 1984; 66:117–26. [DOI] [PubMed] [Google Scholar]
- 54. Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med 1994; 96:239–46. [DOI] [PubMed] [Google Scholar]
- 55. Vital A, Lepreux S, Vital C. Peripheral neuropathy and parkinsonism: a large clinical and pathogenic spectrum. J Peripher Nerv Syst 2014; 19:333–42. [DOI] [PubMed] [Google Scholar]
- 56. Doppler K, Jentschke HM, Schulmeyer L et al Dermal phospho‐alpha‐synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson's disease. Acta Neuropathol 2017; 133:535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Antelmi E, Donadio V, Incensi A, Plazzi G, Liguori R. Skin nerve phosphorylated alpha‐synuclein deposits in idiopathic REM sleep behavior disorder. Neurology 2017; 88:2128–31. [DOI] [PubMed] [Google Scholar]
- 58. Conradt C, Guo D, Miclea A et al Increased prevalence of polyneuropathy in Parkinson's disease patients: an observational study. J Parkinsons Dis 2018; 8:141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain‐Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol 2014; 10:469–82. [DOI] [PubMed] [Google Scholar]
- 60. Saperstein DS, Wolfe GI, Gronseth GS et al Challenges in the identification of cobalamin‐deficiency polyneuropathy. Arch Neurol 2003; 60:1296–301. [DOI] [PubMed] [Google Scholar]
- 61. Scalabrino G. The multi‐faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: lessons learned from its deficiency. Prog Neurobiol 2009; 88:203–20. [DOI] [PubMed] [Google Scholar]
- 62. Thaisetthawatkul P, Collazo‐Clavell ML, Sarr MG, Norell JE, Dyck PJ. A controlled study of peripheral neuropathy after bariatric surgery. Neurology 2004; 63:1462–70. [DOI] [PubMed] [Google Scholar]
- 63. Tsuji M, Takagi A, Sameshima K et al 5,10‐Methylenetetrahydrofolate reductase deficiency with progressive polyneuropathy in an infant. Brain Dev 2011; 33:521–4. [DOI] [PubMed] [Google Scholar]
- 64. Yuan RY, Sheu JJ, Yu JM et al Methylenetetrahydrofolate reductase polymorphisms and plasma homocysteine in levodopa‐treated and non‐treated Parkinson's disease patients. J Neurol Sci 2009; 287:64–8. [DOI] [PubMed] [Google Scholar]
- 65. Todorovic Z, Dzoljic E, Novakovic I et al Homocysteine serum levels and MTHFR C677T genotype in patients with Parkinson's disease, with and without levodopa therapy. J Neurol Sci 2006; 248:56–61. [DOI] [PubMed] [Google Scholar]
- 66. Yasui K, Kowa H, Nakaso K, Takeshima T, Nakashima K. Plasma homocysteine and MTHFR C677T genotype in levodopa‐treated patients with PD. Neurology 2000; 55:437–40. [DOI] [PubMed] [Google Scholar]
- 67. Schaumburg H, Kaplan J, Windebank A et al Sensory neuropathy from pyridoxine abuse. A new megavitamin syndrome. N Engl J Med 1983; 309:445–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Reviewed articles


