ABSTRACT
Objective
To examine the effect of levodopa medication on speech dysfluency in Parkinson's disease.
Methods
Fifty‐one individuals with Parkinson's disease (IWPD) read aloud during off‐ and on‐ medication states. Total speech dysfluencies were calculated from transcriptions of recorded speech samples.
Results
Severity of speech dysfluency was not significantly related to the severity of motor symptoms, duration of disease, levodopa equivalent dosage, or age. When the IWPD were divided into two groups based on dysfluency severity, there was a significant group‐by‐medication state interaction. There was a significant correlation between the medication‐related change in speech dysfluency and the off‐medication severity of speech dysfluency measure (r = −0.46).
Conclusions
The results of this study indicate that levodopa medication can have a significant effect on speech dysfluency. The beneficial levodopa effect appears to be related to the severity of the off‐medication speech dysfluency. Results did not provide strong support for the excess dopamine theory of stuttering in IWPD. A dualistic model of the effects of dopamine on speech fluency in PD is proposed.
Keywords: dopamine therapy, levodopa, Parkinson's disease, speech dysfluency, stuttering
Introduction
Many individuals with Parkinson's disease (IWPD) develop speech impairments during the duration of the disease.1, 2, 3 Speech dysfluency, also referred to as stuttering, is one speech symptom that is observed in some IWPD and has been inconsistently reported in the literature. It should be noted that although the speech dysfluencies associated with stuttering in PD are very similar to those seen in development stuttering, the two disorders are distinct in regard to typical age of onset, presence of an associated neurological condition, and the effects of fluency enhancing conditions.4 Several researchers have attributed dopamine medication (i.e., levodopa) as a factor impacting dysfluent speech in PD.3, 5, 6 Some studies have explored the excess dopamine theory of stuttering in relation to the dysfluent pattern of speech found in IWPD.3, 7, 8, 9 This theory contends that the excess accumulation of dopamine in the brain, related to the intake of levodopa medication, results in the increased speech dysfluency. Previous preliminary studies involving the short‐term effects of levodopa in relatively small groups of IWPD have provided inconsistent findings of whether levodopa indeed has an adverse effect on speech fluency in Parkinson's disease (PD).3, 7, 8, 9 Studies involving a larger number of IWPD at various stages of PD are required. Furthermore, few studies have specifically examined the relationship between speech dysfluency and dopamine in relation to patient characteristics such as disease duration and motor severity.3 It should be noted that some studies10, 11 have examined the effects of levodopa and disease duration on the stability of a syllable repetition task, but this type of rhythmic pseudo‐speech procedure is not a typical or well‐accepted method for evaluating speech dysfluency or stuttering. Most evaluations of dysfluency involve real‐speech tasks, such as reading aloud or conversational speech.
The purpose of this study was to examine the effect of levodopa on speech dysfluency during a reading task using an on‐ versus off‐levodopa procedure. The study also examined the relationships between speech dysfluency and factors such as age, duration of disease and medication, levodopa dosage, and severity of motor symptoms.
Methods
Participants
Participants included 51 IWPD and 13 age‐matched healthy controls (HC). This study was approved by the Health Science Research Ethics Board (HSREB #107253) of Western University. Informed consent was obtained from all participants. IWPD participants were included based on the following criteria: (1) having a diagnosis of idiopathic PD for at least two years; (2) being between the ages of 45 to 85; (3) taking stable doses of anti‐Parkinson medication, including any levodopa preparation (stable doses indicate that no adjustments to medications have been made within the last six months); and (4) having the ability to give informed, written consent. IWPD participants were excluded based on the following criteria: (1) history of any surgical intervention for treating PD (i.e., deep brain stimulation, Duodopa pump); (2) extreme physical disability that impairs mobility assessment; (3) history or current diagnosis of a psychiatric condition requiring hospitalization; (4) pregnant, planning on becoming pregnant, or breastfeeding; or (5) deemed unable to understand or speak sufficient English.
IWPD participants had the following characteristics: age ranged from 47 to 82 (M = 66.35, SD = 7.32); disease duration from two to 20 years (M = 9.52, SD = 4.33); duration of levodopa therapy one to 17 years (M = 7.76, SD = 4.11) and, daily levodopa equivalent dose ranged from 300 to 2200 mg (M = 1030.7, SD = 453.7). Average PD motor score off medication, obtained on the Unified Parkinson Disease Rating Scale (UPDRS; Part III) was 30.16 (SD = 8.59) with a range of 14 to 51 (total possible = 108). Average UPDRS score on medication was 16.77 (SD = 7.24), with a range of three to 32.
Procedures
IWPDs were evaluated off and on levodopa medication. The off‐state evaluation was completed in the morning, at least 12 hours after the last dose of levodopa. Following the off‐state evaluation, the IWPDs took a controlled dose of 300 mg of levodopa (three pills containing 100/25 of levodopa/carbidopa). One hour after taking the levodopa medication, the on‐state evaluation was performed. The on‐state and off‐state evaluations included an audio‐recorded reading of a standard passage (Rainbow passage) and a motor examination involving Part III of the Unified Parkinson's Disease Rating Scale (UPDRS). The audio‐recording equipment included an M‐Audio Microtrack‐2 audio recorder (16 bits; 44.1 kHz) and a DPA 4060 headset microphone placed 6 cm from the mouth. Speech samples were transcribed by two speech‐language pathology graduate students with advanced training in fluency measurement. Perceptual dysfluencies were tabulated using two dysfluency categories: within‐word and between‐word dysfluencies. This protocol was selected based on the PD dysfluency protocol conducted by Goberman and Blomgren.6 Within‐word dysfluency was hypothesized to be more atypical and therefore characteristic of motorically‐based dysfluency, compared to between‐word dysfluency which is perceived as more typical and common in normal speech. Within‐word dysfluencies included monosyllabic whole word repetitions (i.e., “like‐like a prism”), sound/syllable repetitions (i.e., “di‐division of white), audible prolongations (i.e., “Rrrr‐rainbow”), and inaudible prolongations (i.e., “beautiful c‐‐‐olours”). Between‐word dysfluencies included phrase repetitions (i.e., “The rainbow …the rainbow is…”), polysyllabic whole word repetitions (i.e., “apparently‐ apparently beyond…”), interjections (i.e., “into uh‐ many beautiful…”), and revisions (i.e., “Rainba‐ rainbow.”). For statistical analyses, within‐word and total dysfluencies (sum of within‐word and between‐word dysfluencies) were used. Reliability of dysfluency measurements was found to be significant and acceptable. This included intra‐judge reliability for within‐word dysfluencies (r = 0.99; P < 0.05) and total dysfluencies (r = .99; P < 0.05). Inter‐judge reliability included within‐word dysfluencies (r = .94; P < 0.05) and total dysfluencies (r = .97; P < 0.05).
Results
The mean percent total dysfluency scores for the PD and control groups were examined with a series of Bonferroni corrected t‐tests. The total dysfluency scores for the PD‐off‐state (M = 3.34; SD = 3.30) and PD‐on‐state (M = 2.91; SD = 3.24) were both significantly higher (P < 0.05) than those of the HCs (M = 1.09; SD = 1.04). Similarly, the mean within‐word scores for the PD‐off‐state (M = 1.16; SD = 2.11) and PD‐on‐state (M = 1.18; SD = 2.17) were both significantly higher (P < .001) than those of the HCs (M = 0.09; SD = .30).
The comparison of the PD participants in the on‐state versus off state conditions did not reach statistical significance for the total or within‐word dysfluency scores. However, when the individual data was examined more closely, there appeared to be a relationship between the total dysfluency severity score and the response to levodopa. This relationship can be seen in Fig. 1 which shows a scatterplot and regression line that relates to the significant negative correlation between the dysfluency measures off medication and medication‐related change (r = ‐0.46, P = .001). This relationship suggests that as the severity of dysfluency increases there is a greater medication‐related decrease (improvement) in dysfluency.
Figure 1.
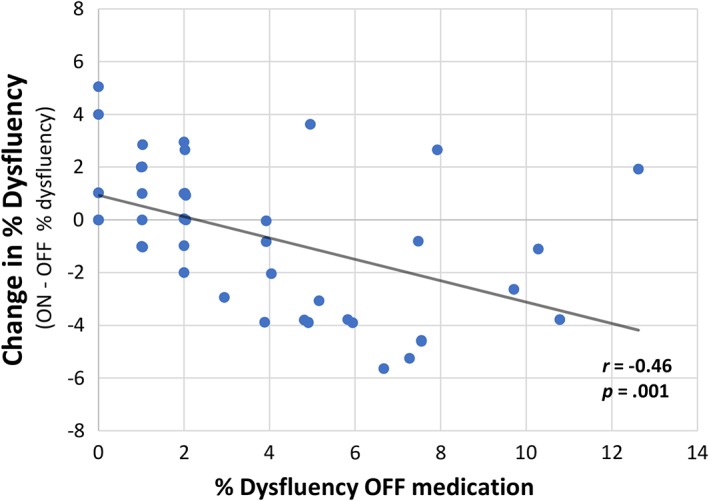
Levodopa‐related change in percent total dysfluency.
To further investigate the effects of levodopa in this group of IWPD, the PD group was split into a “fluent” and “dysfluent” group based on their off state total dysfluency scores relative to the control dysfluency scores. A group of 25 fluent PDs had total dysfluency scores (M = 0.77; SD = 0.78) that fell within one standard deviation of the control dysfluency scores (< 2.2%). A second group of 26 dysfluent PDs had dysfluency scores (M = 5.81: SD = 2.86) that were greater than 2.2%. A comparison of the medication‐related change in the dysfluency scores between the fluent and dysfluent PD groups indicated a significant group by medication‐state interaction (P < .001) that is shown in Fig. 2. This interaction is reflected by the dysfluent PD group showing a significant decrease in dysfluency (improvement) with medication (M = ‐1.83; SD = 2.7; P = .002), whereas the fluent PD group displayed a significant increase in dysfluency following levodopa intake (M = 1.04; SD = 2.38; P = 0.03).
Figure 2.
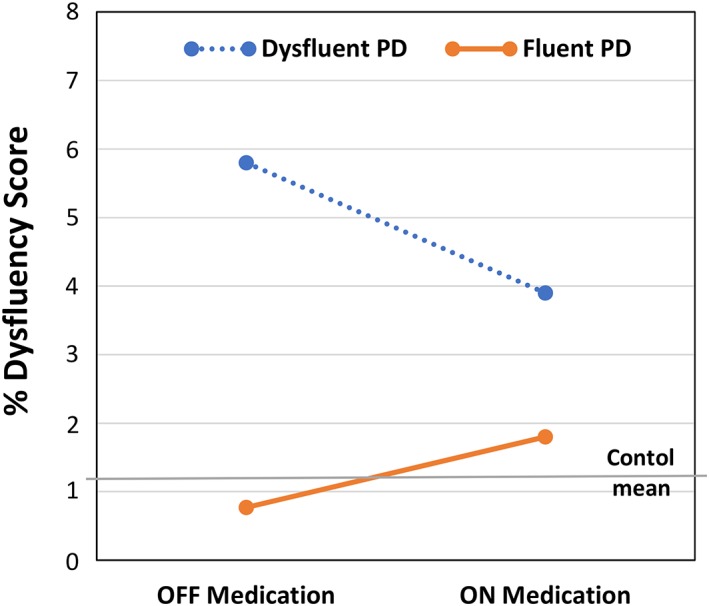
Average percent total dysfluency for the dysfluent and fluent PD groups in the OFF and ON levodopa medication states. Average value for the controls is shown by the horizontal gray line.
No significant correlations were found between the severity of dysfluency and the following patient characteristics: age (r = 0.12), duration of PD (r = 0.13), duration of levodopa use (r = 0.19), UPDRS score off (r = 0.14), UPDRS score on (r = 0.08), or levodopa equivalent dosage (r = 0.28). Similarly, no significant correlations were found between these patient characteristics and the levodopa‐related change in speech dysfluency. When the correlations between these selected patient characteristics and the severity of dysfluency scores were examined separately in the fluent and dysfluent groups, no significant correlations were found (Table 1). Table 1 presents the average values and the results of the t‐test comparisons related to the selected patient characteristics for the fluent and dysfluent groups. The only significant comparison relates to the LED. The dysfluent group had a significantly (P = .001) higher LED (M = 1251; SD = 453) than the LED of the fluent group (M = 811; SD = 351).
Table 1.
Pearson's correlations (r‐values) between off‐state total dysfluency scores and selected patient characteristics for the fluent IWPDs and dysfluent IWPDs (*P < 0.05 in parentheses). Also shown are the fluent IWPDs versus dysfluent IWPDs independent t‐test comparisons for the patient characteristics (*P < 0.05)
| Fluent IWPDs (N = 25) mean (SD) | Correlation with off‐ dysfluency r‐value (P‐value) | Dysfluent IWPDs (N = 26) mean (SD) | Correlation with off‐ dysfluency r‐value (P‐value) | Fluent vs dysfluent t‐test P‐value | |
|---|---|---|---|---|---|
| Age (years) | 65.6 (8.1) | ‐10 (.64) | 66.1 (7.0) | .29 (.16) | .81 |
| Duration PD | 8.9 (3.9) | ‐.03 (.89) | 9.5 (4.5) | .14 (.49) | .63 |
| Duration levodopa | 6.9 (3.6) | .07 (.73) | 8.1 (4.1) | .11 (.58) | .30 |
| LED | 811 (351) | ‐.16 (.43) | 1251 (453) | ‐.22 (.30) | .001* |
| UPDRS off | 28.1 (9.1) | ‐.11 (.61) | 31.8 (7.7) | ‐.05 (.82) | .13 |
| UPDRS on | 14.9 (7.2) | ‐.09 (.67) | 17.8 (6.6) | ‐.14 (.49) | .15 |
| UPDRS diff | 13.1 (5.9) | ‐.05 (.79) | 13.9 (4.4) | .14 (.52) | .59 |
Discussion
Levodopa medications are commonly prescribed in attempts to reduce parkinsonian motor symptoms such as rigidity, rest tremor, akinesia, and bradykinesia.7, 12 Recent literature has explored the excess dopamine theory of stuttering, which proposed that the accumulation of dopamine in the form of levodopa causes a dysfluent pattern of speech in IWPD. However, this theory has not been clearly supported in the literature. The purpose of this study was, therefore, to gain a better understanding of the effect of levodopa on speech dysfluency in IWPD.
The results of this study suggest a complex dopamine medication effect on speech dysfluency in IWPD. The most notable finding was the significant association between the severity of dysfluency in the off state and the levodopa‐related change in dysfluency. The more dysfluent the patient's speech when in the off‐medication state, the more likely that the dysfluency will improve in the on‐medication state. These results for the dysfluent IWPDs would appear to support a dopamine reduction hypothesis that proposes that reductions in the level of dopamine have a causal role in the development of stuttering in PD. This dopamine reduction hypothesis may be consistent with the results from a previous study that provided evidence for a re‐emergence of stuttering in PD.13 Also, the reduction of levodopa medication dosage following DBS surgery may play a role in the development of post‐DBS stuttering in some IWPD.14 Perhaps a trial increase in levodopa dosage should be re‐examined in cases of post‐DBS stuttering.15 Finally, low dopamine levels may have a causal role in the dysfluencies that are observed in progressive supranuclear palsy16, 17 and the stuttering that has been observed in manganese‐induced ephedrone (methcathinone) parkinsonism.18
In contrast to the dysfluent IWPDs, the opposite appeared to be the case for the fluent IWPDs, where they were more likely to experience a worsening of speech dysfluency in the on‐medication state. Thus, the results for the fluent IWPD would appear to support the excess dopamine hypothesis that proposes that increased levels of dopamine lead to the development of stuttering in PD.
In order to reconcile the opposite effects of levodopa on dysfluency in the fluent and dysfluent groups, it may be useful to propose a dualistic model of dopamine levels and stuttering in PD. In a dualistic model, both abnormally high levels and low levels of dopamine could cause stuttering in PD. This dualistic model would predict that there is a range of dopamine levels that allow for fluent speech but as dopamine levels go higher or lower than this fluent speech range, there will be an increased risk of the development of dysfluencies (stuttering). In an attempt to apply this model to the present results, we suggest that when the fluent PDs are in the off state, they are operating within the “normal” fluent range of dopamine levels, but they can be pushed to excessively high dopamine levels via levodopa medication that is too high for fluent speech, and they may start to show the high‐dopamine stuttering effect. On the other hand, the dysfluent PDs may be operating below the bottom of the “normal” fluent range of dopamine levels that allow for fluent speech when they are in the off state, and this could cause them to show the low dopamine stuttering effect. These dysfluent PDs would be likely to demonstrate a reduction in stuttering following levodopa medication if it was sufficient to push their dopamine levels into the “normal” fluent range of dopamine levels associated with fluent speech. The model would also predict that if these patients had their levodopa progressively increased, there would be a point where they would move into the high dopamine dysfluency range, and the beneficial effects of levodopa would be reversed.
Some additional support for this dualistic interpretation of the results may come from the finding that the dysfluent IWPDs in the present study had significantly higher LED values than the fluent IWPDs. The higher LED values for the dysfluent IWPDs would suggest that they may be at a relatively lower dopamine level than the fluent IWPDs when they are in the off state and that this reduced dopamine level may be causing a low‐dopamine stuttering effect. It should be noted that support for this proposed dualistic model of dopamine levels in stuttering can be found in a recent physiologically‐based computational model of the basal ganglia and speech production.19 In the Civier et al.,19 model they similarly propose that both abnormally high dopamine levels and abnormally low dopamine levels could cause stuttering.
While this study could not directly assess the role of dopamine in the development of speech dysfluency in PD, the results suggest that future investigations should give consideration to testing the novel hypothesis of a dualistic model of dopamine with particular emphasis on the prediction that a reduction in dopamine may lead to an increase in speech dysfluency and stuttering in some IWPDs. Future studies could also examine the effect of different doses of levodopa on the severity of speech dysfluency in IWPD. Additional, levodopa challenge studies involving other impaired speech components, such as abnormal rate of speech and imprecise oral articulation, could be used to further examine the hypothesized relationship between levodopa responsiveness and speech severity. These multi‐component studies also may reveal important causal interactions between other speech components and speech dysfluency.
A limitation of the present study is that patients were given a uniform dose of levodopa (300 mg), rather than their usual dose. While this uniform dose provided increased inter‐subject consistency in dosage, it introduced some new variability in terms of under‐ versus over‐treatment of the speech dysfluency. In general, a concern was that the patient's usual dose, while appropriate for the limb motor symptoms, may have been associated with undertreating the speech symptoms. There was also a concern that the overnight washout period may have added risk, and the patient's standard morning dose would undertreat the speech symptoms. It is possible that the IWPD in the fluent group were slightly over‐medicated by the standard 300 mg levodopa dose used in this study. As previously suggested by the daily LED values, the fluent group may have had a baseline dopamine state that was generally higher than that of the dysfluent group and therefore the fluent group may have been more susceptible to the high‐dopamine effect on dysfluency when they were given a levodopa dose that was near or above their regular levodopa dose. It is recommended that future studies include a systematic levodopa dose escalation to examine speech dysfluency responsiveness across a range of levodopa dose levels.
Another potential limitation of the present study is the lack of control for the occurrence of dyskinesia. Previous studies have suggested both an association20 and no association5 between the levodopa on state and abnormal repetitive speech behavior in dyskinetic IWPDs. Although none of the IWPDs in the present study demonstrated obvious oral dyskinesia in the levodopa on state, this behavior was not evaluated systematically. Concerns about the potential effect of levodopa‐induced dyskinesias on dysfluency seem to be mitigated by the main finding that the dysfluent IWPDs showed a significant reduction in dysfluency during the on state. Also, the correlation between LED and dysfluency was not significant. To further evaluate the potential role of dyskinesias in speech dysfluency, it is recommended that future studies of levodopa and dysfluency consider including a more systematic quantification of oral and limb dyskinesias.
The correlational analyses revealed no significant associations between any of the major patient characteristics (i.e., PD severity, disease duration, duration of levodopa use) and levodopa‐related effects on speech dysfluency or severity of dysfluency. These findings lead us to conclude that these characteristics do not play an important role in medication effects on speech dysfluency or the severity of speech dysfluency.
Conclusions
The results of this study indicate that levodopa medication can have a significant beneficial effect on speech dysfluency in PD. This beneficial levodopa effect appears to be moderately associated with the severity of speech dysfluency.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
H.I.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
S.A.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
A.A.: 1C, 3B
M.P.: 1A, 1B, 1C, 3B
G.G.: 1C, 3B
M.J.: 1A, 1B, 3B
Disclosures
Ethical Compliance Statement: This study was approved by the Health Science Research Ethics Board (HSREB #107253) of Western University. Informed consent was obtained from all participants. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: The authors report no sources of funding and no conflicts of interest relevant to this work.
Financial Disclosures for the preceding 12 months: H.I., M.P., and G.G. were graduate students at Western University and have no financial disclosures. S.A. received salary support from Western University and received research grants from Parkinson Canada, Parkinson Society of Southwestern Ontario, and Merz Pharma. A.A. was a graduate student at Western University and received a graduate student award from Parkinson Canada. M.J. reports the following disclosures: research grant, consultancy, speaker honoraria from: Merz Pharma, Allergan, Boston Scientific, Abbvie, UCB Pharma; Research Grants from: Parkinson Society of Southwestern Ontario, Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council, Mitacs Canada, Ontario Centres of Excellence, and Western Foundation. M.J. has stock ownership in Movement Disorders Diagnostic Technologies Inc. and Manjog Enterprises Limited.
Acknowledgments
We want to acknowledge and thank the IWPD participants recruited from the Movement Disorders Centre, University Hospital, London, Ontario.
Relevant disclosures and conflicts of interest are listed at the end of this article.
Correction added on April 15, 2019, after publication: Anita Abeyesekera's last name was misspelled, and it is now spelled correctly.
References
- 1. Ho AK, Bradshaw JL, Iansek R. For better or worse: the effect of levodopa on speech in Parkinson's disease. Movement Disord 2008; 23:575–80. [DOI] [PubMed] [Google Scholar]
- 2. Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and co‐occurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. J Speech Hear Res 1978; 43:47–57. [DOI] [PubMed] [Google Scholar]
- 3. Tykalova T, Rusz J, Cmejla R et al Effect of dopaminergic medication on speech dysfluency in Parkinson's disease: a longitudinal study. J Neural Transm 2015; 122(8):1135–42. [DOI] [PubMed] [Google Scholar]
- 4. Juste FS, Sassi FC, Costa JB, De Andrade CRF. Frequency of speech disruptions in Parkinson's disease and developmental stuttering: a comparison among speech tasks. PLoS ONE 2018; 13(6):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benke TH, Hohenstein C, Poewe W, Butterworth B. Repetitive speech phenomena in Parkinson's disease. J Neurol Neurosurg Psychiatry 2000; 69(3):319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goberman AM, Blomgren M, Metzger E. Characteristics of speech dysfluency in Parkinson disease. J Neurolinguistics 2010; 23:470–8. [Google Scholar]
- 7. Goberman AM, Blomgren M. Parkinsonian speech disfluencies: effects of levodopa‐related fluctuations. J Fluency Disord 2003; 28(1):55–70. [DOI] [PubMed] [Google Scholar]
- 8. Leder S. Adult onset of stuttering as a presenting sign in a Parkinson‐like syndrome: a case report. J Commun Disord 1996; 29(6):471–7. [DOI] [PubMed] [Google Scholar]
- 9. Louis ED, Winfield L, Fahn S, Ford B. Speech dysfluency exacerbated by levodopa in Parkinson's disease. Mov Disord 2001; 16(3):562–5. [DOI] [PubMed] [Google Scholar]
- 10. Skodda S, Flasskamp A, Schlegel U. Instability of syllable repetition as a marker of disease progression in parkinson's disease: a longitudinal study. Mov Disord 2011; 26(1):59–64. [DOI] [PubMed] [Google Scholar]
- 11. Skodda S, Flasskamp A, Schlegel U. Instability of syllable repetition in parkinson's disease‐influence of levodopa and deep brain stimulation. Mov Disord 2011; 26(4):728–30. [DOI] [PubMed] [Google Scholar]
- 12. Connolly BS, Lang AE. Pharmacological treatment of parkinson disease: a review. JAMA 2014; 311(16):1670–83. [DOI] [PubMed] [Google Scholar]
- 13. Shahed J, Jankovic J. Re‐emergence of childhood stuttering in Parkinson's disease: a hypothesis. Mov Disord 2001; 16:114–8. [DOI] [PubMed] [Google Scholar]
- 14. Picillo M, Vincos GB, Sammartino F, Lozano AM, Fasano A. Exploring risk factors for stuttering development in Parkinson disease after deep brain stimulation. Parkinsonism Relat Disord 2017; 38:85–9. [DOI] [PubMed] [Google Scholar]
- 15. Walker HC, Phillips DE, Boswell DB et al Relief of acquired stuttering associated with Parkinson's disease by unilateral left subthalamic brain stimulation. J Speech Lang Hear Res 2009; 52(6):1652–7. [DOI] [PubMed] [Google Scholar]
- 16. Kluin KJ, Foster NL, Berent S, Gilman S. Perceptual analysis of speech disorders in progressive supranuclear palsy. Neurology 1993; 43:563–6. [DOI] [PubMed] [Google Scholar]
- 17. Rusz J, Megrelishvili M, Bonnet C et al A distinct variant of mixed dysarthria reflects parkinsonism and dystonia due to ephedrone abuse. J Neural Transm (Vienna) 2014; 121(6):655–64. [DOI] [PubMed] [Google Scholar]
- 18. Rusz J, Bonnet C, Klempir J et al Speech disorders reflect differing pathophysiology in Parkinson's disease, progressive supranuclear palsy and multiple system atrophy. J Neurol 2015; 262:992–1001. [DOI] [PubMed] [Google Scholar]
- 19. Civier O, Bullock D, Max L, Guenther FH. Computational modeling of stuttering caused by impairments in a basal ganglia thalamo‐cortical circuit involved in syllable selection and initiation. Brain Lang 2013; 126(3):263–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ackermann H, Ziegler W, Oertel WH. Palilalia as a symptom of levodopa induced hyperkinesia in Parkinson's disease. J Neurol Neurosurg Psychiatry 1989; 52(6):805–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


