ABSTRACT
Introduction
Huntington's chorea (HC) is commonly managed with neuroleptic medications, though there is little evidence to support their use. This study aimed to perform a real‐world comparison of the efficacy of risperidone and olanzapine to tetrabenazine (TBZ) for HC.
Methods
The Enroll‐HD database was used to perform a propensity score‐matched comparison of risperidone and olanzapine to TBZ, regarding their efficacy in controlling chorea. Participants with motor manifest Huntington's disease (HD) were grouped according to their use of risperidone, olanzapine, or TBZ. For the three groups, independent propensity score matching was performed on participants’ baseline total functional score (TFC), baseline total motor score (TMS), disease burden score, CAG repeat length, baseline age, region, sex, and body mass index. Independent samples t test was used to calculate the differences between the groups in the annual rate of change of the TMS from the baseline to the second available visit.
Results
The risperidone (n = 72) and olanzapine groups (n = 77) had annualized increases (worsening) in the TMS of only 1.47 points and 3.20 points, respectively, compared to 5.70 points in the two matched TBZ groups (n = 72) (P = 0.019) and (n = 77) (P = 0.143), respectively.
Conclusions
In the absence of prospective data, this analysis of the Enroll‐HD database found that the neuroleptics risperidone and olanzapine seemed to at least be comparable to TBZ at controlling HC. These results demonstrate that neuroleptics may have comparable efficacy to TBZ for the treatment of HC. Further prospective studies are needed to confirm these findings.
Keywords: chorea, Enroll‐HD, Huntington's disease, olanzapine, risperidone, tetrabenazine
Introduction
Huntington's disease (HD) is an autosomal dominant, neurodegenerative disorder that causes psychiatric problems, progressive cognitive decline, and loss of motor control. Chorea can be a prominent manifestation and warrants treatment when bothersome. Having demonstrated their efficacy in large, double‐blind, placebo‐controlled clinical trials, tetrabenazine (TBZ), and more recently, deutetrabenazine, are the only FDA‐approved medications for Huntington's chorea (HC).1, 2, 3 These drugs have not been studied for the treatment of other HD manifestations and are contraindicated in patients with unmanaged depression and suicidality.4
For these reasons, antipsychotic drugs (APDs) are often prescribed off‐label for HC. They are also used for concomitant psychiatric problems, sleep dysfunction, and low weight. According to a 2011 survey of HD experts, APDs were favored for treating HC in patients with psychiatric symptoms;5 however, even in the absence of such comorbidities, European providers preferred APDs to TBZ, and there was a near tie between APDs and TBZ amongst North American and Australian experts. The most favored APDs were risperidone and olanzapine.
The popularity of APDs for HC is founded on experience‐based expert opinion.6, 7 Their anti‐choreic efficacy is supported by a few small studies and case series,8, 9 but they have never been evaluated in large, randomized, placebo‐controlled trials. Unfortunately, due to their low profitability for pharmaceutical companies, APDs are unlikely to be studied in any clinical trials. To bridge the gap between expert opinion and evidence‐based medicine, we leveraged the Enroll‐HD database to determine how APDs (risperidone and olanzapine) compared to TBZ in the management of HC.
Methods
Enroll‐HD is a multi‐center, longitudinal, observational study with over 15,000 active participants from more than 150 international sites. At the time of this analysis (April 10, 2018), data were available for 8,714 participants in the third periodic dataset of the Enroll‐HD database.10 In addition to manifest HD patients, the database includes those with premotor HD, genotype‐negative individuals, and family control patients without HD. All research participants were clinically evaluated at annual visits, during which they provided updated information, including their current and past medication use. A risk‐based monitoring approach was used to check the data for quality and accuracy. All sites are required to obtain and maintain local ethics committee approvals.
Inclusion in our analysis required participants be at least 18 years of age and have a minimum CAG repeat length of 36. Participants must have had at least two qualifying Enroll‐HD visits with a documented total motor score (TMS), and a diagnostic confidence level (DCL) of four at their baseline and subsequent Enroll‐HD visits (Fig. 1). The DCL is a diagnostically indicative component of the United Huntington's Disease Rating Scale (UHDRS); a value of four is assigned only when the clinical rater is more than 99% certain that the patient has motor‐manifest HD.11
Figure 1.
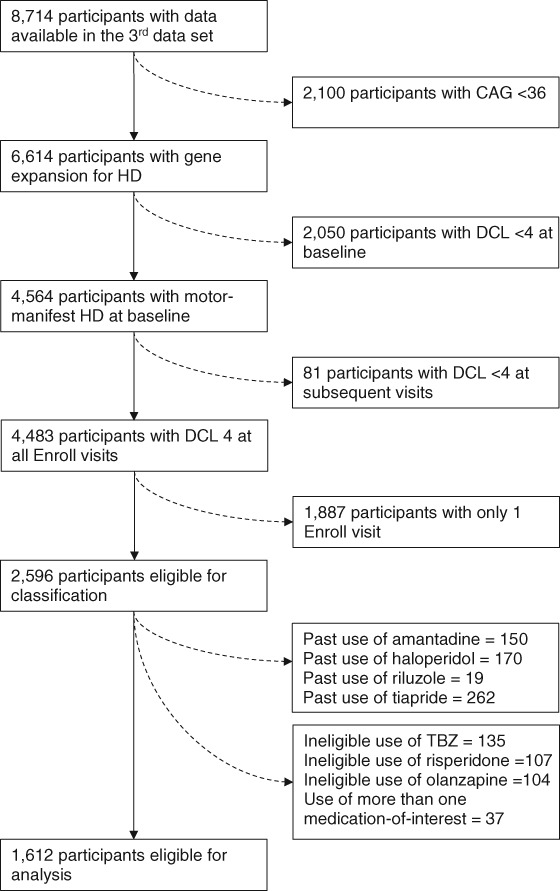
Description of exclusions from Enroll‐HD database.
Abbreviations: CAG, cytosine‐adenine‐guanine repeats; DCL, diagnostic confidence level; HD, Huntington disease; TBZ, tetrabenazine.
Participants who met the eligibility requirements also had to be taking risperidone, olanzapine, or TBZ. Participants were considered to be taking one of these medications if they started treatment before or within 14 days of their baseline Enroll‐HD visit. The medication‐of‐interest had to have been continued without interruption until the participant's second Enroll‐HD visit. Participants were excluded from the analysis if they had taken more than one of the drugs, even if the combination was not used concomitantly. Participants were also excluded if, precluding study participation, they had a current or prior history of being on haloperidol, tiapride, amantadine, or riluzole, as these are sometimes used off‐label to treat HC.12 These exclusions were done to ensure that anti‐choreic effects of other medications did not confound the results. To compare the anti‐choreic efficacy of these medications, risperidone (n = 104), olanzapine (n = 158), and TBZ (n = 137) users were divided into separate groups.
Two propensity score‐matching procedures matched TBZ users to users of (1) risperidone and (2) olanzapine, in both cases based on the calculated likelihood that the participant would be using TBZ. A propensity score is calculated when analyzing observational data to account for potential confounders that may affect a participant's likelihood of receiving a particular intervention.13 These matching procedures focused on ensuring that risperidone users and olanzapine users were adequately matched to TBZ users regarding factors that may affect the progression of HD. These factors included baseline measures of age, sex, body mass index (BMI), region, TMS, total functional capacity (TFC) score, CAG repeat length, and CAG‐Age Product (CAP) score. The CAP score was used as a marker of overall disease burden and was calculated as follows: age0 * (CAG ‐ 30)/6.27.14 A maximum difference in propensity scores of 0.01 (1%) was allowed for the matching procedures.
The primary outcome measure was the TMS's annual rate of change between the baseline and second available visits. Secondary outcome measures included the changes in the TMS (absolute, percent and annualized percent) and the rates of change between the baseline and the second available visits of weight, BMI, TFC score, and the total chorea score (defined as the sum of the maximal chorea scores for facial, buccal‐oral‐lingual, truncal, and each extremity from the UHDRS motor subscale).11 The primary and secondary outcome measures were evaluated using independent sample t tests that separately compared TBZ users to users of risperidone and olanzapine. Finally, in the TETRA‐HD study,1 which led to the FDA's approval of TBZ, the drug had a 3.3 units reduction (improvement) in the TMS over the placebo arm. Therefore, the odds of this occurrence between groups were used as an exploratory outcome measure.
A sub‐analysis was performed to partially account for the different lengths of time that participants were taking the medications and for the notion that the medications’ anti‐choreatic effects may change over time. This analysis included only participants who started therapy on the day of, or no more than 14 days after, an Enroll‐HD study visit. Excluding those who initiated the medication before a study visit ensured that baseline assessments were of participants when they were not on any anti‐choreic medication. Similar to the initial analyses, the medication‐of‐interest had to have been continued for the remainder of their time in the Enroll‐HD study without any gaps in treatment. Independent samples t tests were again performed to compare the same primary and secondary outcomes between (1) TBZ and risperidone and (2) TBZ and olanzapine.
All statistical analyses utilized IBM SPSS Statistics Version 25 software. Fisher's exact test and independent student t tests were used to compare baseline characteristics between the risperidone, olanzapine, and tetrabenazine groups. A P value of less than 0.05 was considered significant.
Results
Risperidone versus TBZ
Table 1 shows the baseline demographics for participants taking either TBZ or risperidone. The only significant difference between the two groups is that the risperidone users had a higher baseline depression score than the TBZ users (3.09 vs 1.39, respectively, P = 0.003). The groups’ similarities indicate that the propensity score matching procedure was successful. In the sub‐analysis of participants who started therapy on the day of, or in the days following, a study visit, the one baseline difference between the groups was that risperidone users (n = 11) had significantly worse TFC scores compared to the TBZ users (n = 18), (8.6 vs 10.8, respectively, P = 0.044).
Table 1.
Baseline demographics between tetrabenazine and risperidone groups
| Tetrabenazine | Risperidone | P‐value | |
|---|---|---|---|
| N | 72 | 72 | |
| % Female | 63.6 | 50.0 | 53.1 |
| CAG, mean ± S.D. | 44.26 ± 3.24 | 43.79 ± 3.18 | 0.379 |
| Age0 (yrs), mean ± S.D. | 53.77 ± 11.55 | 54.01 ± 11.85 | 0.904 |
| AMO (yrs), mean ± S.D. | 44.42 ± 10.63 | 46.72 ± 10.55 | 0.199 |
| CAP0, mean ± S.D. | 117.60 ± 14.82 | 114.47 ± 17.40 | 0.247 |
| TMS0, mean ± S.D. | 40.81 ± 17.70 | 39.56 ± 17.89 | 0.674 |
| TFC0, mean ± S.D. | 7.10 ± 3.20 | 7.24 ± 3.14 | 0.793 |
| Weight0 (kg), mean ± S.D. | 73.03 ± 15.80 | 77.50 ± 17.33 | 0.108 |
| BMI0 (kg/m2), mean ± S.D. | 25.57 ± 4.86 | 26.45 ± 4.79 | 0.272 |
| Baseline depression score, mean ± S.D. | 1.39 ± 2.53 | 3.09 ± 3.94 | 0.003 |
| Baseline suicidality score, mean ± S.D. | 0.07 ± 0.35 | 0.24 ± 0.75 | 0.082 |
| Baseline aggression score, mean ± S.D. | 0.83 ± 1.99 | 1.01 ± 2.16 | 0.603 |
| Baseline irritability score, mean ± S.D. | 1.99 ± 2.85 | 2.73 ± 3.70 | 0.178 |
| Time between first and second visit (yrs), mean ± S.D. | 1.09 ± 0.22 | 1.07 ± 0.25 | 0.641 |
| Daily dose (mg), mean ± S.D.1 | 56.53 ± 41.17 | 1.76 ± 1.60 | N/A |
| Prior time on therapy (yrs), mean ± S.D. | 1.80 ± 2.05 | 2.39 ± 2.49 | 0.125 |
| Medication indicated for chorea, %1 | 100 | 55.6 | N/A |
Abbreviations: AMO, Age of motor onset; BMI0, Baseline body mass index; CAG, Cytosine‐Adenine‐Guanine; CAP0, Baseline disease burden score calculated (age0 x [CAG‐30]/6.27); TFC0, Baseline Total Functional Capacity score; TMS0, Baseline total motor score.
Statistical significance not calculated
The annual rate of change in the TMS, the study's primary outcome measure, was significantly slower (better) in the risperidone group compared to the TBZ group (1.47 vs 5.70 points per year, P = 0.019). Similarly, slower absolute increases in the TMS and smaller percent changes in the TMS were seen with the use of risperidone over TBZ (Table 2). Risperidone users were also significantly more likely to experience at least a 3.3 point decrease (improvement) in their TMS from visits one to two, compared to TBZ users (33.3% vs 16.7%, P = 0.023).
Table 2.
Risperidone compared to TBZ
| Results of Propensity Score Matched Analysis | ||||
|---|---|---|---|---|
| Direction of favorable Δ | TBZ Group | Risperidone Group | P‐value | |
| N | 72 | 72 | ||
| Primary Outcome Measure | ||||
| Annual rate of Δ of TMS | − | 5.70 ± 10.92 | 1.47 ± 10.49 | 0.019 |
| Secondary Outcome Measures | ||||
| Absolute Δ of TMS | − | 6.29 ± 11.95 | 1.29 ± 10.57 | 0.009 |
| Percent Δ of TMS | − | 20.86 ± 40.86 | 8.26 ± 33.94 | 0.046 |
| Annualized percent Δ of TMS | − | 19.05 ± 38.25 | 8.97 ± 35.34 | 0.103 |
| Annual rate of Δ of total chorea score | − | −0.02 ± 5.09 | −0.20 ± 4.73 | 0.826 |
| Annual rate of Δ of TFC | + | −0.70 ± 1.89 | −0.47 ± 1.80 | 0.454 |
| Annual rate of Δ of weight (kg) | + | −1.08 ± 7.03 | −1.12 ± 6.55 | 0.972 |
| Annual rate of Δ of BMI (kg/m2) | + | −0.34 ± 2.38 | −0.34 ± 2.40 | 0.998 |
| Exploratory Outcome Measures | ||||
| % with annual rate of Δ of TMS ≤ −3.3 | + | 16.7% | 33.3% | .023 |
| Results of Sub‐Analysis | ||||
|---|---|---|---|---|
| Direction of favorable Δ | TBZ Group | Risperidone Group | p‐value | |
| N | 18 | 11 | ||
| Primary Outcome Measure | ||||
| Annual rate of Δ of TMS | − | 3.58 ± 8.28 | 0.40 ± 10.56 | 0.374 |
| Secondary Outcome Measures | ||||
| Absolute Δ of TMS | − | 3.56 ± 9.60 | 0.36 ± 8.62 | 0.375 |
| Percent Δ of TMS | − | 16.89 ± 30.38 | −4.58 ± 26.12 | 0.063 |
| Annualized percent Δ of TMS | − | 17.58 ± 29.96 | −5.23 ± 26.89 | 0.048 |
| Annual rate of Δ of total chorea score | − | −0.76 ± 0.3.98 | −0.34 ± 4.07 | 0.788 |
| Annual rate of Δ of TFC | + | −0.33 ± 1.82 | 0.37 ± 1.15 | 0.263 |
| Annual rate of Δ of weight (kg) | + | 0.72 ± 4.54 | 5.45 ± 14.45 | 0.204 |
| Annual rate of Δ of BMI (kg/m2) | + | 0.31 ± 1.63 | 1.89 ± 4.91 | 0.215 |
| Exploratory Outcome Measures | ||||
| % with annual rate of Δ of TMS ≤ −3.3 | + | 11.1% | 45.5% | .049 |
Abbreviations: BMI, baseline body mass index; TBZ, tetrabenazine; TFC, total functional capacity score; TMS, total motor score.
All results that are not percentages represent mean ± standard deviation.
In the sub‐analysis, participants taking risperidone (n = 11) had a 5.23 annual percent decrease (improvement) in the TMS, compared to a 17.58 annual percent increase (worsening in the participants taking TBZ (n = 18, P = 0.048). Risperidone users also had a higher likelihood of experiencing at least a 3.3 point drop (improvement) in the TMS from visits one to two, compared to the TBZ users (P = 0.049; Table 2).
Olanzapine versus TBZ
Table 3 shows the baseline demographics of participants taking either TBZ or olanzapine. Compared to TBZ users, those on olanzapine had significantly higher suicidality and irritability scores. The two groups were otherwise similar, again indicating that the propensity score matching procedure was successful. However, the groups in the sub‐analysis had differences (results not shown). Specifically, compared to the TBZ users (n = 18), the olanzapine users (n = 27) had significantly higher (worse) motor scores (31.3 vs 42.6, P = 0.008), lower TFC scores (10.8 vs 8.0, P = 0.001), and higher disease burden scores (120.4 vs 106.2, P = 0.020). These differences indicate that of the sub‐analysis participants, those taking olanzapine seemed to have more advanced disease than those on TBZ.
Table 3.
Baseline demographics between tetrabenazine and olanzapine groups
| Tetrabenazine | Olanzapine | P‐value | |
|---|---|---|---|
| N | 77 | 77 | |
| % Female | 50.0 | 50.0 | 1.000 |
| CAG, mean±S.D. | 43.14 ± 2.87 | 43.48 ± 2.85 | 0.466 |
| Age0 (yrs), mean±S.D. | 57.91 ± 10.57 | 56.30 ± 11.00 | 0.355 |
| AMO (yrs), mean±S.D. | 48.03 ± 10.70 | 47.47 ± 10.83 | 0.752 |
| CAP0, mean±S.D. | 117.86 ± 17.80 | 117.45 ± 17.51 | 0.884 |
| TMS0, mean±S.D. | 43.75 ± 18.02 | 42.78 ± 19.04 | 0.745 |
| TFC0, mean±S.D. | 7.04 ± 3.09 | 6.96 ± 3.10 | 0.876 |
| Weight0 (kg), mean±S.D. | 74.17 ± 16.41 | 72.63 ± 17.35 | 0.573 |
| BMI0 (kg/m2), mean±S.D. | 25.64 ± 5.90 | 25.18 ± 5.23 | 0.606 |
| Baseline depression score, mean±S.D. | 1.62 ± 2.70 | 2.61 ± 3.50 | 0.052 |
| Baseline suicidality score, mean±S.D. | 0.05 ± 0.28 | 0.43 ± 1.52 | 0.035 |
| Baseline aggression score, mean±S.D. | 0.62 ± 1.71 | 1.21 ± 2.07 | 0.058 |
| Baseline irritability score, mean±S.D. | 1.56 ± 2.63 | 2.70 ± 3.44 | 0.022 |
| Time between first and second visit (yrs), mean±S.D. | 1.09 ± 0.27 | 1.06 ± 0.23 | 0.477 |
| Daily dose (mg), mean±S.D.1 | 60.07 ± 53.84 | 6.95 ± 5.88 | N/A |
| Prior time on therapy (yrs), mean±S.D. | 2.23 ± 2.20 | 2.16 ± 2.55 | 0.840 |
| Medication indicated for chorea, %1 | 100 | 41.6 | N/A |
Abbreviations: AMO, age of motor onset; BMI0, baseline body mass index; CAG: cytosine‐adenine‐guanine; CAP0: baseline disease burden score calculated age0 x ([CAG‐30]/6.27); TFC0: baseline total functional capacity score; TMS0: baseline total motor score.
Statistical significance not calculated.
The annual rate of change in the TMS was not significantly slower in the olanzapine users compared to the TBZ users (3.20 vs 5.69 points per year, P = 0.143). Treatment effects between the two groups were comparable, except for the measure of BMI, which had an average increase of 0.75 points per year in the olanzapine group, compared to an average loss of 0.06 points per year in the TBZ group (P = 0.042; Table 4).
Table 4.
Olanzapine compared to TBZ
| Results of Propensity Score Matched Analysis | ||||
|---|---|---|---|---|
| Direction of favorable Δ | TBZ Group | Olanzapine Group | P‐value | |
| N | 77 | 77 | ||
| Primary Outcome Measure | ||||
| Annual rate of Δ of TMS | − | 5.69 ± 11.09 | 3.20 ± 9.77 | 0.143 |
| Secondary Outcome Measures | ||||
| Absolute Δ of TMS | − | 5.96 ± 12.06 | 3.43 ± 9.87 | 0.156 |
| Percent Δ of TMS | − | 20.61 ± 43.40 | 12.93 ± 31.95 | 0.213 |
| Annualized percent Δ of TMS | − | 19.08 ± 39.75 | 11.96 ± 30.58 | 0.215 |
| Annual rate of Δ of total chorea score | − | 0.30 ± 4.65 | −0.13 ± 4.47 | 0.563 |
| Annual rate of Δ of TFC | + | −0.84 ± 1.60 | −0.73 ± 1.38 | 0.637 |
| Annual rate of Δ of weight (kg) | + | −0.34 ± 4.54 | 1.52 ± 8.69 | 0.105 |
| Annual rate of Δ of BMI (kg/m2) | + | −0.06 ± 1.60 | 0.75 ± 3.01 | 0.042 |
| Exploratory Outcome Measures | ||||
| % with annual rate of Δ of TMS ≤ −3.3 | + | 20.8% | 23.4% | .698*^ |
| Results of Sub‐Analysis | ||||
|---|---|---|---|---|
| Direction of favorable Δ | TBZ Group | Olanzapine Group | p‐value | |
| N | 18 | 27 | ||
| Primary Outcome Measure | ||||
| Annual rate of Δ of TMS | − | 3.58 ± 8.28 | 0.55 ± 9.28 | 0.270 |
| Secondary Outcome Measures | ||||
| Absolute Δ of TMS | − | 3.56 ± 9.60 | 0.82 ± 8.59 | 0.323 |
| Percent Δ of TMS | − | 16.89 ± 30.38 | 2.35 ± 21.78 | 0.068 |
| Annualized percent Δ of TMS | − | 17.58 ± 29.96 | 1.23 ± 25.57 | 0.057 |
| Annual rate of Δ of total chorea score | − | −0.76 ± 0.3.98 | −1.46 ± 5.82 | 0.658 |
| Annual rate of Δ of TFC | + | −0.33 ± 1.82 | −1.03 ± 1.74 | 0.201 |
| Annual rate of Δ of weight (kg) | + | 0.72 ± 4.54 | 5.15 ± 6.86 | 0.022 |
| Annual rate of Δ of BMI (kg/m2) | + | 0.30 ± 1.63 | 1.87 ± 2.52 | 0.027 |
| Exploratory Outcome Measures | ||||
| % with annual rate of Δ of TMS ≤ −3.3 | + | 11.1% | 29.6% | .158*^ |
Abbreviations: BMI: Baseline body mass index; TBZ: tetrabenazine; TFC: Total Functional Capacity score; TMS: total motor score.
All results that are not percentages represent mean ± standard deviation.
In the sub‐analysis, there were no differences between the groups except that the use of olanzapine was associated with a relatively greater weight gain and an increased BMI (Table 4).
Discussion
There are two major classes of drugs commonly used in the management of HC. One class is the vesicular monoamine transporter 2 (VMAT2) inhibitors (including TBZ), which have been well studied and are FDA‐approved for this purpose. The other class is the APDs, which are typically used in the setting of depression and/or suicidality (when TBZ would be contraindicated) or significant behavioral problems such as irritability or anger outbursts. The anti‐choreic efficacy of APDs is presumed due to their long‐standing use by HD experts. Results from large clinical trials have not yet been reported; however, there is currently an ongoing randomized control trial (NEURO‐HD; NCT00632645) comparing the APDs olanzapine and tiapride with TBZ.15, 16
Our analysis of the Enroll‐HD database provides objective data to support the anti‐choreic benefit of APDs. Our findings show that risperidone and olanzapine may have at least similar efficacy to TBZ for the treatment of HC. When compared to participants on TBZ, those on risperidone had a slower rate of worsening in their TMS. They were also more likely to experience a TMS reduction (improvement) of at least 3.3 points. In contrast, none of the variables analyzed supported TBZ being superior to either of the APDs studied.
Olanzapine was comparable to risperidone and TBZ in all measures with the exception of weight gain and BMI increase. Both of these variables were greater in olanzapine users compared to TBZ users. Given that unintended weight loss is a hallmark of HD, this difference has clinical relevance for HD management.17 Though the association is likely not causal, weight loss and low BMI are associated with worse chorea18 and motor disability,17 as well as with a faster rate of HD progression.19
Compared to those on TBZ, the group prescribed risperidone had significantly higher baseline depression scores, and the olanzapine group had significantly higher suicidality and irritability scores. These psychiatric problems are likely why they were prescribed the APDs in the first place. It is important to note that for approximately half of the participants in this study on APDs, the medication was indicated for a reason other than chorea. Possibly, the chorea may have been even better controlled if the dosing was titrated for this purpose rather than another cause (presumably a psychiatric one).
The knowledge that the APDs anti‐choric efficacy is comparable to TBZ's will allow for other determinants to play a relatively greater role when providers are considering medications for HC. The APDs are already being used to benefit the psychiatric symptoms that they are indicated for, typically mood instability with irritability and anger outbursts or psychosis. The APDs are also known to be useful in sleep dysfunction20 and are commonly used for this purpose in HD. As previously mentioned, olanzapine promotes weight gain. Of course, this may be seen as a negative side effect in some. Other differential side effects that should be considered include depression and daytime drowsiness seen with TBZ; however, TBZ‐induced depression may be far less problematic than previously believed.21
When prescribing any of these anti‐choreic drugs, it is important to be aware that they all have the risk of causing QTc prolongation, with risperidone and olanzapine (at 3.6 and 1.7 milliseconds, respectively22 being slightly better than TBZ and deutetrabenazine (averaging at 8 and 4.5 milliseconds, respectively).23, 24 Additionally, when prescribing doses of TBZ above 50 mg/day, there is also the recommendation to perform CYP2D6 genotyping to screen for poor metabolizer status.23 Finally, the prohibitively high cost of the VMAT2 inhibitors may be a limiting factor for some.
There are several strengths of this study, most notably, by leveraging the Enroll‐HD database we performed a novel analysis. Clinical trials aimed at investigating the anti‐choreic efficacy of neuroleptics are needed, but presumably, have been avoided due to the lack of financial incentives for funding such studies.6, 11 Therefore, this analysis has filled a significant gap in our knowledge without a costly clinical trial. Also, this was a rigorous study in regards to controlling for potentially confounding factors; specifically, the analysis excluded participants with the prior use of relevant medications, and the use of propensity score matching to account for any differences between the groups. Another strength of this study was the discerning subgroup analysis that enabled the assessment of each participant's baseline scores, similar to a clinical trial. The sub‐analysis also excluded participants who were not taking the medication‐of‐interest long‐term to account for the notion that TBZ's anti‐choreic effect wanes over time, as prior studies have indicated.25, 26 Finally, this study demonstrates the long‐term efficacy of three medications in a real‐world setting.
Despite these strengths, our study has limitations. First, though the groups were matched for their baseline TMS, the analysis did not differentiate between HD sub‐types. It has been suggested that compared to relatively akinetic‐rigid HD patients, the more choreic ones have faster motor progression,27 and presumably this latter group is more likely to be on TBZ. Second, due to limited data availability, the analysis utilized information from only two study visits. Future analyses employing linear mixed effect regression models that monitor change over multiple time points may provide more robust results. Third, the medication information that is collected from participants is subjective data that may be inaccurate. Lastly, the analysis is based on observational data, which may compromise the validity of the results. Ideally, our findings would be confirmed in a large, prospective, randomized controlled trial.
In conclusion, this study provides objective data that seem to support experienced‐based expert opinion on the anti‐choreic efficacy of risperidone and olanzapine. To the best of our knowledge, these are also the first reported results comparing these APDs to TBZ for their effect on HC. Our results show that risperidone and olanzapine may have comparable efficacy to TBZ. Furthermore, based on the primary outcome measure, risperidone seemed to be superior compared to TBZ. These findings would ideally be confirmed by a large randomized control trial, but until that time, our results may enable providers to be better informed regarding their treatment options for the management of HD.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
J.L.S.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
J.A.K.: 1A, 1B, 1A, 1C, 3A, 3B
P.C.N.: 1A, 1B, 2C, 3A, 3B
A.K.: 1A, 1B, 2C, 3A, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The authors confirm that the presented analysis was exempt from approval by their local institutional review board. All Enroll‐HD sites were required to obtain and maintain local Ethics Committee approvals. Participants in the Enroll‐HD study must have signed informed consent forms for their data to be included in the datasets.
Funding Sources and Conflict of Interest: Jordan L. Schultz was supported by the following grant: KL2TR002536. The authors declare that there are no conflicts of interest relevant to this work.
Financial disclosures from previous 12 months: The authors report no sources of funding and no conflicts of interest.
Acknowledgments
Enroll‐HD is a longitudinal observational study for Huntington's disease families intended to accelerate progress towards therapeutics. It is sponsored by CHDI Foundation, a nonprofit biomedical research organization exclusively dedicated to developing therapeutics for HD. All sites were required to obtain and maintain local ethics committee approvals. Enroll‐HD would not be possible without the vital contribution of the research participants and their families.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Huntington Study Group . Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology 2006;66:366–372. [DOI] [PubMed] [Google Scholar]
- 2. Frank S. Tetrabenazine as anti‐chorea therapy in Huntington disease: an open‐label continuation study. Huntington Study Group/TETRA‐HD Investigators. BMC Neurol 2009;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huntington Study Group , Frank S, Testa CM, et al., Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. JAMA 2016;316:40–50. [DOI] [PubMed] [Google Scholar]
- 4. Hubers AA, van Duijn E, Roos RA, et al. REGISTRY investigators of the European Huntington's Disease Network. Suicidal ideation in a European Huntington's disease population. J Affect Disord 2013;151:248–258. [DOI] [PubMed] [Google Scholar]
- 5. Burgunder J‐M, Guttman M, Perlman S, Goodman N, van Kammen DP, Goodman L. An international survey‐based algorithm for the pharmacologic treatment of chorea in Huntington's disease. PLoS Currents 2011;3:RRN1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Killoran A, Biglan KM. Current therapeutic options for Huntington's disease: good clinical practice versus evidence‐based approaches? Mov Disord 2014;29:1404–1413. [DOI] [PubMed] [Google Scholar]
- 7. Reilmann R. Pharmacological treatment of chorea in Huntington's disease–good clinical practice versus evidence‐based guideline. Mov Dis 2013;28:1030–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Killoran, A. Biglan, KM . Therapeutics in Huntington's disease. Curr Treat Options Neurol. 2012;14:2:137–149. [DOI] [PubMed] [Google Scholar]
- 9. Duff K, Beglinger LJ, O'Rourke ME, Nopoulos P, Paulson HL, Paulsen JS. Risperidone and the treatment of psychiatric, motor, and cognitive symptoms in Huntington's disease. Ann Clin Psychiatry 2008;20:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landwehrmeyer GB, Fitzer‐Attas CJ, Giuliano JD, et al. Data analytics from Enroll‐HD, a global clinical research platform for Huntington's disease. Mov Disord Clin Pract 2017;4:212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huntington Study Group . Unified Huntington's Disease Rating Scale: reliability and consistency. Mov Disord 1996;11:136–142. [DOI] [PubMed] [Google Scholar]
- 12. Coppen EM, Roos RAC. Current pharmacological approaches to reduce chorea in Huntington's disease. Drugs 2017;77:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warner JH, Sampaio C. Modeling variability in the progression of Huntington's disease: a novel modeling approach applied to structural imaging markers from Track‐HD. CPT Pharmacometrics Syst Pharmacol 2016;5:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Assistance Publique – Hopitaux de Paris . Neuroleptic and Huntington disease comparison of: olanzapine, tetrabenazine, and tiapride (NEUROHD). Available at: https://clinicaltrials.gov/ct2/show/NCT00632645. Accessed July 30, 2018.
- 16. Desamericq G, Dolbeau G, Verny C, et. al. Effectiveness of anti‐psychotics and related drugs in the Huntington French‐speaking group cohort. PLoS One. 2014;9:e85430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trejo A, Tarrats RM, Alonso ME, Boll MC, Ochoa A, Velasquez L. Assessment of the nutrition status of patients with Huntington's disease. Nutrition 2004;20:192–196. [DOI] [PubMed] [Google Scholar]
- 18. Hamilton et al. Rate and correlates of weight change in Huntington's disease. J Neurol Neurosurg Psychiatry 2004;75:209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Burg JMM, Gardiner SL, Ludolph AC, Landwehrmeyer GB, Roos RAC, Aziz NA. Body weight is a robust predictor of clinical progression in Huntington disease. Ann Neurol 2017;82:479–483. [DOI] [PubMed] [Google Scholar]
- 20. Fasano, A , Cadeddu, F , Guidubaldi, A , et al. The Long‐term Effect of Tetrabenazine in the Management of Huntington Disease. Clinical Neuropharmacology. 2008;31:313‐318. [DOI] [PubMed] [Google Scholar]
- 21. Frank, S . Tetrabenazine as anti‐chorea therapy in Huntington disease: an open‐label continuation study. BMC Neurol 2009;9:62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monti JM, Torterolo P, Pandi Perumal SR. The effects of second‐generation antipsychotic drugs on sleep variables in healthy patients and patients with schizophrenia. Sleep Med Rev 2017;33:51–57. [DOI] [PubMed] [Google Scholar]
- 23. Schultz JL, Killoran A, Nopoulos PC, Chabal CC, Moser DJ, Kamholz JA. Evaluating depression and suicidality in tetrabenazine users with Huntington disease. Neurology. 2018;9:e202–e207. [DOI] [PubMed] [Google Scholar]
- 24. Harrigan EP, Miceli JJ, Anziano R, et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol 2004;24:62–69. [DOI] [PubMed] [Google Scholar]
- 25.NDA 21‐894 Xenazine® (tetrabenazine) Risk evaluation and mitigation strategy. Food and Drug Administration. 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021894s001s002REMS.pdf. Accessed 3/2/2018
- 26. Austedo (deutetrabenazine) [package insert]. Teva Pharmaceuticals USA, Inc; North Wales, PA; 2017. [Google Scholar]
- 27. Jacobs M, Hart E, van Zwet E, et al. Progression of motor subtypes in Huntington's disease: a 6‐year follow‐up study. J Neurol 2016;263:2080–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]


