Abstract
Background
In the midst of the nationwide opioid epidemic, our institution began an effort to improve the education of opioid prescribers and disseminate procedure-specific guidelines for the number of opioid pills to prescribe post-operatively for total joint arthroplasty. The number of opioid pills suggested for total hip or knee replacement was 70 tablets.
Questions/Purposes
We sought to evaluate the impact of the new institutional guideline on opioid prescribing practices, hypothesizing that it would lead to a decrease in the number of pills prescribed but an increase in patient call volume after discharge.
Methods
After the new guidelines were implemented in February 2018, we retrospectively reviewed all opioid prescriptions written for patients on the joint-replacement service from March 2016 to March 2018. In addition, we tabulated post-operative telephone calls made to the nurse practitioner service before and after guideline implementation. The majority of calls to the nurse practitioner service are for opioid renewals.
Results
We included 9514 patients in the analysis. Prior to guideline implementation, the mean number of pills prescribed after primary total joint arthroplasty was 91 ± 26.6 pills and after it was 65 ± 16.3 pills. The monthly number of unique patient telephone interactions was statistically significantly lower after the implementation of the new guidelines.
Conclusion
An institutional guideline for opioid prescribing after total joint arthroplasty significantly reduced the number of pills prescribed to patients without causing a significant increase in the number of phone calls to the service.
Electronic supplementary material
The online version of this article (10.1007/s11420-018-9632-6) contains supplementary material, which is available to authorized users.
Keywords: opioid, arthroplasty, total joint replacement, prescriptions, pain control
Introduction
The USA is in the midst of an opioid epidemic, with increasing prevalence of opioid use disorder [9, 12] and deaths from overdose [9, 17]. Orthopedic surgeons are the third highest prescribers of opioids among medical specialties [22], and orthopedic thought leaders have recently called for orthopedic surgeons to take a leadership role in fighting the opioid epidemic [18]. Prior studies have shown that taking opioids for as little as 6 days puts patients at risk of long-term opioid use [19]. Although the development of new chronic opioid use in opioid-naïve joint replacement patients is not common [8], high post-operative opioid use increases patients’ risk for thromboembolism, longer hospitalization, infection, and respiratory, gastrointestinal, and urinary complications [3]. However, orthopedic surgeons do not currently have clear guidelines on the best practices for opioid prescribing after joint replacement surgery.
Given the lack of evidence-based recommendations for post-operative opioid prescribing after total joint replacement and other orthopedic procedures, our institution began a hospital-wide effort to educate opioid prescribers and disseminate procedure-specific guidelines for the number of opioid pills to prescribe. Guidelines were introduced in the Adult Reconstruction and Joint Replacement (ARJR) service to instruct prescribers on the total number of pills that should be prescribed at discharge after routine total hip and total knee replacement.
The purpose of this study was to evaluate the impact of the new institutional guideline on opioid prescribing after total joint arthroplasty. We hypothesized that this new guideline would lead to a decrease in opioid pills prescribed but an increase in patient call volume after discharge.
Methods
In 2016, a multidisciplinary team of anesthesiologists, orthopedic surgeons, and other hospital personnel at our institution held a series of meetings to build a hospital-wide consensus on best practices for opioid prescribing. In November 2016, all prescribers underwent a 1-hour mandatory opioid education program consisting of a discussion on the scope of the opioid epidemic, the involvement of orthopedic surgeons, and the use of the state prescription drug monitoring program and new opioid prescribing laws. In addition, each orthopedic service at our institution was tasked with creating opioid prescribing guidelines for common procedures based on expert opinion, the current literature, and consensus-based methodology [7]. Initially, the ARJR service set a maximum prescribing guideline of 90 tablets of standard-dose opioids for total hip arthroplasty and 120 tablets for total knee arthroplasty. Subsequently, after several multidisciplinary meetings and further literature review, in February 2018, the ARJR service revised its guidelines to a limit of 70 tablets for both total hip and total knee arthroplasty.
To assess the impact of these new guidelines, we retrospectively reviewed all opioid prescriptions written for patients on the ARJR service from March 2016 to March 2018. Only patients who underwent primary total hip or total knee arthroplasty and received a prescription for opioid pain medication were included. Patients undergoing all other procedures, including revision arthroplasty, partial knee replacement, and arthroscopic hip and knee procedures were excluded from analysis. Of note, patients discharged to a rehabilitation or nursing facility do not receive prescriptions from our institution and thus were excluded from analysis. Furthermore, patients who received higher than the standard dosages prescribed at our institution (Table 1) or a type of opioid not commonly prescribed were excluded from analysis (higher dosages are generally prescribed only by the pain management service under extenuating circumstances and not by the usual ARJR service prescribers).
Table 1.
Standard types and doses of opioids prescribed after total knee or hip replacement
| Oxycodone 5 mg | |
| Oxycodone/acetaminophen 5 mg/325 mg | |
| Hydrocodone/acetaminophen 5 mg/325 mg | |
| Tramadol 50 mg | |
| Acetaminophen/codeine 300 mg/30 mg | |
| Hydromorphone 2 mg |
mg milligrams
In addition, the ARJR service maintains a telephone service staffed by nurse practitioners that patients may call with questions and concerns after total joint replacement. The service is used by 18 of the 26 surgeons on the ARJR service. While patients call the nurse practitioners about a variety of post-operative issues, a majority of phone calls received are for opioid renewals. Therefore, we also evaluated the number of calls made to this service and recorded in the electronic medical record before and after implementation of the new opioid prescribing guidelines. Telephone data was available for August 2017 to April 2018, which includes 6 months prior to implementation of the new guidelines and 3 months after implementation.
Statistics
Statistical analysis was performed to evaluate the changes in opioid prescribing before and after the most recent guidelines were established. Descriptive statistics were performed to show the opioid prescribing patterns of the ARJR service. Student’s t test was used to determine the difference in the number of pills prescribed and in the number of phone calls made to the nurse practitioner service before and after the new guidelines were disseminated. A p value of 0.050 was considered to be statistically significant.
Results
A total of 13,594 patients underwent surgery on the ARJR service and were given a prescription pain medication at our institution from March 2016 to March 2018. We excluded 3271 patients because a non-arthroplasty, partial arthroplasty, or revision arthroplasty procedure was performed; 222 patients because they underwent bilateral arthroplasty; and 587 patients because they received a non-standard dosage (e.g., oxycodone 10 mg) or type (e.g., fentanyl) of opioid. Non-standard prescriptions accounted for 6.1% of all those written before and 3.2% of all those written after the guideline implementation. After exclusion criteria were applied, 9514 patients were included in the analysis.
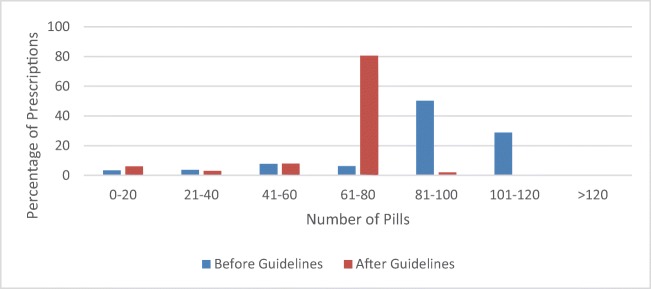
From March 2016 to March 2018, 115 prescribers wrote opioid prescriptions for patients on the ARJR service. Prior to the implementation of the new guidelines, the mean number of pills prescribed after primary total joint arthroplasty was 91 ± 26.6 pills. After implementation of the new guidelines, the mean number of pills prescribed was 65 ± 16.3 pills (p < 0.0001) (Fig. 1).
Fig. 1.
Number of pills prescribed before and after guideline implementation.
From August 2017 through January 2018, the nurse practitioners of the ARJR service had telephone interactions with 7700 patients, with an average monthly volume of 1283.3 patients. From February 2018 through April 2018, the nurse practitioners had telephone interactions with 3199 unique patients, for a monthly average of 1066.3 patients. The monthly number of unique patient telephone interactions was significantly lower after the implementation of the new guidelines (p = 0.033).
Discussion
Opioid education is lacking for both patients and prescribers. A study of online opioid information aimed at patients showed a lack of accurate information for patients online about the dangers and effects of opioids [16]. Prior studies have also shown that prescribers have many unanswered questions about the best practices in opioid prescribing [4]. Our study demonstrated that a service-wide implementation of opioid prescribing guidelines for total joint arthroplasty significantly reduced the number of pills prescribed to patients post-operatively without causing a significant increase in the number of phone calls to the service.
This study has several limitations. First, we gathered data on the number of pills prescribed post-operatively but not on the number of pills that the patients actually used or discarded. This is the subject of future work. Additionally, we were unable to track the number of refill prescriptions written in this cohort, which could have changed after the implementation of the guidelines. Furthermore, in tabulating calls to the nurse practitioner service, we were unable to determine which calls were for opioid refills and which were for other matters. While anecdotally, we know the majority of calls concern opioid refills, we were unable to determine the change in requests for refills before and after guideline implementation. Also, the number of patient phone calls included both incoming and outgoing calls from the service, and we were unable to determine if either incoming or outgoing calls decreased after the new guidelines. We accounted for patient-practitioner phone call interactions that went through the service line and not for those made directly to surgeons’ offices. Finally, only 18 of the 26 surgeons use the nurse practitioner service, while opioid prescribing data from patients of all 26 surgeons is included in the analysis, which may bias the results.
Nonetheless, this study has several strengths. It describes the opioid prescribing patterns of a large cohort of patients undergoing total joint arthroplasty. Over 100 prescribers are involved in the ARJR service, and we demonstrated the effectiveness of clinical opioid-prescribing guidelines even among a large group of prescribers.
Previous studies have shown mixed compliance with national opioid-prescribing guidelines on a national scale, though recent evidence has shown improved compliance through changes made to default settings in electronic medical record systems [2, 4, 11]. The present study has shown close adherence to new guidelines set for post-operative prescribing after total joint arthroplasty, leading to a significant decrease in the number of pills prescribed. Although we are unable to determine the factors leading to compliance, we hypothesize that specific targeted guidelines for these procedures, as well as prior opioid-education efforts, helped increase prescriber adherence. A study by Donaldson et al. also showed a decrease in the number of opioid pills prescribed after targeted prescriber education in an emergency setting [5]. Furthermore, multiple studies in the hand surgery literature have shown a decrease in opioid pills prescribed after procedure-specific guidelines were implemented [6, 20].
The American Academy of Orthopaedic Surgeons (AAOS) has called for increased prescriber education on opioid safety and management strategies [1]. In the present study, the widespread education for attending physicians, residents, nurse practitioners, and physician assistants allowed all prescribers to gain a thorough understanding of the opioid epidemic and to align their goals in prescribing opioids. Also, recent changes in state legislation have made it more difficult for prescribers to write large opioid prescriptions, further contributing to the decline in opioids prescribed in this study. Based on the results of this study, further education and guidelines will likely be useful for decreasing the amount of opioids prescribed and ultimately decreasing the amount consumed after orthopedic surgery.
Curbing opioid use after total joint replacement is an important goal for all orthopedic surgeons. Goesling et al. found that of opioid-naïve patients undergoing total knee replacement and total hip replacement, 8.2% and 4.3%, respectively, were still using opioids 6 months later [8]. In a review of online patient resources about opioid use after total knee replacement, Schairer et al. showed that less than half of websites discussed the risk of opioid dependency and only 12.5% gave any information on tapering opioids post-operatively [16]. Further patient education is needed to help decrease excessive opioid use after joint replacement.
Over-prescribing opioids is detrimental to both the patient and the people around them. The majority of people misusing or diverting opioid pain medications obtain the pills through a friend or relative who received them through an authentic medical prescription [14]. Kumar et al. showed in a prospective study of outpatients undergoing shoulder surgery that patients had a mean of 20.1 unused opioid pills at 90 days after surgery, and most patients did not receive instructions on how to dispose of unused pills [13]. Several other studies have noted similar findings of excess unused pills after outpatient hand surgery [12, 15, 21]. Further work is needed to define the optimal number of pills to provide to patients after orthopedic procedures and to educate patients on safe opioid storage and disposal.
There is a wide range in opioid prescribing practices after total joint arthroplasty. Hernandez et al. studied post-operative opioid use and prescriptions after total knee replacement and found that a mean of 74 pills were prescribed at discharge, though the amount ranged from 20 to 300 pills [10]. The authors found that the number of requests for refills did not differ between patients who received large and small prescriptions. They concluded that larger prescriptions should be avoided because they did not decrease refill requests and they increased risk for opioid divergence and misuse [10]. Similarly, we did not find an increase in telephone calls to the nurse practitioner service after the new opioid-prescribing guideline was issued. We therefore advocate guidelines to help prescribers decrease the number of opioid pills prescribed after total joint replacement. Further research is needed to provide evidence-based guidelines on the optimal number of pills.
Overall, an institutional guideline for opioid prescribing after total joint arthroplasty significantly reduced the number of pills prescribed to patients post-operatively without causing a significant increase in the number of phone calls to the service. Future studies are needed to evaluate the number of unused pills and effects on patient pain and satisfaction after total joint replacement surgery.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)
Conflict of Interest
Cynthia A. Kahlenberg, MD, Jeffrey G. Stepan, MD, Ajay Premkumar, MD, MPH, and Francis D. Lovecchio, MD, declare that they have no conflicts of interest. Michael B. Cross, MD, reports receiving grants or personal fees from or owning stock in Acelity, Exactech, Inc., Intellijoint, Link Orthopaedics, Smith & Nephew, Zimmer, Flexion Therapeutics, Imagen, Insight Medical, and Parvizi Surgical Innovation, as well as serving on the editorial or governing boards of Bone and Joint Journal 360, Journal of Orthopaedics and Traumatology, and Techniques in Orthopaedics, all outside the submitted work.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent
Informed consent was waived from all patients for being included in this study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Level IV, retrospective review
References
- 1.American Academy of Orthopaedic Surgeons. Information statement 1045. Opioid use, misuse, and abuse in orthopaedic practice. 2015. Available at https://www.aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1045 Opioid Use, Misuse, and Abuse in Practice.pdf.
- 2.Chiu AS, Jean RA, Hoag JR, Freedman-Weiss M, Healy JM, Pei KY. Association of lowering default pill counts in electronic medical record systems with postoperative opioid prescribing. JAMA Surg. Published online July 18, 2018. 10.1001/jamasurg.2018.2083. [DOI] [PMC free article] [PubMed]
- 3.Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic surgery. Pain. 2017;158(12):2422–2430. doi: 10.1097/j.pain.0000000000001047. [DOI] [PubMed] [Google Scholar]
- 4.Cushman PA, Liebschutz JM, Hodgkin JG, et al. What do providers want to know about opioid prescribing? A qualitative analysis of their questions. Subst Abus. 2017;38:222–229. doi: 10.1080/08897077.2017.1296525. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson SR, Harding AM, Taylor SE, Vally H, Greene SL. Evaluation of a targeted prescriber education intervention on emergency department discharge oxycodone prescribing. Emerg Med Australas. 2017;29:400–406. doi: 10.1111/1742-6723.12772. [DOI] [PubMed] [Google Scholar]
- 6.Dwyer CL, Soong M, Hunter A, Dashe J, Tolo E, Kasparyan NG. Prospective evaluation of an opioid reduction protocol in hand surgery. J Hand Surg Am. 2018;43(6):516–522. doi: 10.1016/j.jhsa.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Pub Health. 1984;74:979–983. doi: 10.2105/AJPH.74.9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157:1259–1265. doi: 10.1097/j.pain.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han B, Compton WM, Jones CM, Cai R. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA. 2015;314:1468–1478. doi: 10.1001/jama.2015.11859. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez NM, Parry JA, Taunton MJ. Patients at risk: large opioid prescriptions after total knee arthroplasty. J Arthroplast. 2017;32:2395–2398. doi: 10.1016/j.arth.2017.02.060. [DOI] [PubMed] [Google Scholar]
- 11.Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16:480–487. doi: 10.1111/pme.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim N, Matzon JL, Abboudi J, et al. A prospective evaluation of opioid utilization after upper-extremity surgical procedures: identifying consumption patterns and determining prescribing guidelines. J Bone Joint Surg Am. 2016;98:e89. doi: 10.2106/JBJS.15.00614. [DOI] [PubMed] [Google Scholar]
- 13.Kumar K, Gulotta LV, Dines JS, et al. Unused opioid pills after outpatient shoulder surgeries given current perioperative prescribing habits. Am J Sports Med. 2017;45:636–641. doi: 10.1177/0363546517693665. [DOI] [PubMed] [Google Scholar]
- 14.Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9–38. [PubMed] [Google Scholar]
- 15.Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37:645–650. doi: 10.1016/j.jhsa.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 16.Schairer WW, Kahlenberg CA, Sculco PK, Nwachukwu BU. What is the quality of online resources about pain control after total knee arthroplasty? J Arthroplasty. 2017;32(12):3616–3620. doi: 10.1016/j.arth.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349–358. doi: 10.15585/mmwr.mm6712a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seymour RB, Ring D, Higgins T, Hsu JR. Leading the way to solutions to the opioid epidemic: AOA critical issues. J Bone Joint Surg Am. 2017;99(21):e113. doi: 10.2106/JBJS.17.00066. [DOI] [PubMed] [Google Scholar]
- 19.Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66:265–269. doi: 10.15585/mmwr.mm6610a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanek JJ, Renslow MA, Kalliainen LK. The effect of an educational program on opioid prescription patterns in hand surgery: a quality improvement program. J Hand Surg Am. 2015;40:341–346. doi: 10.1016/j.jhsa.2014.10.054. [DOI] [PubMed] [Google Scholar]
- 21.Stepan JG, London DA, Osei DA, Boyer MI, Dardas AZ, Calfee RP. Perioperative celecoxib and postoperative opioid use in hand surgery: a prospective cohort study. J Hand Surg Am. 2018;43:346–353. doi: 10.1016/j.jhsa.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305:1299–301. doi: 10.1001/jama.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)