Abstract
Lupus nephritis is a common disease manifestation of SLE, in which immune complex deposition and macrophage activation are important contributors to disease pathogenesis. Bruton’s tyrosine kinase (BTK) plays an important role in both B cell and FcgammaR mediated myeloid cell activation. In the current study, we examined the efficacy of BI-BTK-1, a recently described irreversible BTK inhibitor, in the classical NZB×NZW F1 (NZB/W) and MRL/lpr spontaneous mouse models of SLE. NZB/W mice were randomly assigned to a treatment (0.3 mg/kg, 1 mg/kg, 3 mg/kg and 10 mg/kg) or control group and began treatment at 22 weeks of age. The experimental setup was similar in MRL/lpr mice, but with a single treated (10 mg/kg, beginning at 8–9 weeks of age) and control group. A separate experiment was performed in the MRL/lpr strain to assess the ability of BI-BTK-1 to reverse established kidney disease. Early treatment with BI-BTK-1 significantly protected NZB/W and MRL/lpr mice from the development of proteinuria, correlating with significant renal histological protection, decreased anti-DNA titers, and increased survival in both strains. BI-BTK-1 treated mice displayed a significant decrease in nephritis-associated inflammatory mediators (e.g. LCN2 and IL-6) in the kidney, combined with a significant inhibition of immune cell infiltration and accumulation. Importantly, BI-BTK-1 treatment resulted in the reversal of established kidney disease. BTK inhibition significantly reduced total B cell numbers and all B cell subsets (immature, transitional, follicular, marginal zone, and class switched) in the spleen of NZB/W mice. Overall, the significant efficacy of BIBTK-1 in ameliorating multiple pathological endpoints associated with kidney disease in two distinct murine models of spontaneous lupus nephritis provides a strong rationale for BTK inhibition as a promising treatment approach for lupus nephritis.
Keywords: Lupus nephritis, Bruton’s tyrosine kinase, Systemic lupus erythematosus, MRL/lpr, NZB/W
1. Introduction
Systemic lupus erythematosus (SLE) is a multifaceted autoimmune disease with the potential to affect multiple organ systems [1]. Kidney involvement, known as lupus nephritis (LN), is a common complication that adds considerable morbidity and mortality in affected patients [2]. LN pathogenesis is associated with immune complex (IC) deposition and complement activation within the kidney, infiltration of immune cells, and increased expression of inflammatory cytokines [3]. Current treatment options for LN are less than satisfactory, as the immunosuppressive cytotoxic therapies now in use are associated with serious adverse effects and about 10–30% of LN patients still progress to end stage renal disease [4].
Both B cells and macrophages are instrumental in the pathogenesis of LN. B cells produce autoantibodies associated with SLE, which can form ICs and deposit within target tissues [1,5]. IC activation of Fcgamma receptors on local and infiltrating Fc receptor bearing cells, including monocytes and macrophages, leads to the release of inflammatory cytokines and subsequent renal damage [3,6]. In human LN, the degree of macrophage infiltration correlates with disease severity[7]. Therefore, a treatment that targets both B cell activation and IC mediated inflammatory cytokine release can provide a significant therapeutic advantage over current treatment options for SLE/LN patients.
Bruton’s Tyrosine Kinase (BTK) is a member of the Tec family of non-receptor tyrosine kinases that is essential for intracellular signaling in B cells and cells of the myeloid lineage [8]. The role of BTK in BCR signaling is exemplified by the impaired B cell development and function observed in human X-linked agammaglobulinemia [9] and X-linked immunodeficiency mice that harbor specific BTK mutations [10]. BTK is also required for Fcgamma receptor signaling which mediates immune complex activation of myeloid cell types such as monocytes and macrophages[11]. Inhibition of BTK would consequently target two major cellular contributors to disease pathogenesis in LN.
We have previously described the use of a novel BTK inhibitor, BI-BTK-1, in an inducible kidney disease model known as nephrotoxic serum nephritis (NTN) [12]. However, these results were generated in a short term inducible model in non-autoimmune mice. To truly test the efficacy of BI-BTK-1 and its translational promise in lupus, it would be critical to assess the drug in a spontaneous model of LN.
For this study, we used two spontaneous models of SLE, the NZB × NZW F1 (NZB/W) mouse and the MRL/lpr mouse, to assess the potential of BI-BTK-1 to treat LN. Both strains are classic and commonly used models for LN [13]. Furthermore, we also assessed the effect of BIBTK-1 on the survival of lupus mice, and studied whether BTK inhibition could reverse already established disease.
2. Material and methods
2.1. Mice and treatment
2.1.1. NZB/W
NZB/W and NZW female mice at 6 weeks of age were purchased from Jackson Laboratories (Bar Harbor, ME). Beginning at 22 weeks of age, mice were dosed with BI-BTK-1 (~0.3 mg/kg, 1 mg/kg, 3 mg/kg, or 10 mg/kg) in chow formulation or control non-medicated chow (Research Diets, New Brunswick, NJ). BI-BTK-1 was described in detail in a previous publication [12]. A separate group of mice treated with cyclophosphamide (50 mg/kg intraperitoneally once weekly) was also assessed and compared to a PBS injected control group. In addition, untreated NZW mice and NZB/W mice euthanized at 12 weeks of age were included as additional non-diseased control groups. At the age of 52 weeks or upon development of overt proteinuria (>2000 mg/dl on two separate days within the same week), mice were sacrificed and various tissues were collected for analysis. All animal studies were performed under protocols approved by the Boehringer Ingelheim Institutional Animal Care and Use Committee.
2.1.1.1. Assessment of kidney disease in NZB/W mice
To assess proteinuria in NZB/W mice, twenty-four-hour urine samples were collected weekly and analyzed by the Pierce Coomassie Plus (Bradford) Assay Kit (Thermo Fisher Scientific, Waltham, MA). Creatinine was measured concurrently with protein via the Creatinine Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI). Blood urea nitrogen (BUN) was measured with the Urea Nitrogen Test Kit (STANBIO Laboratory, Boerne, TX).
2.1.2. MRL/lpr
Female MRL/lpr mice, age four to five weeks, were purchased from Jackson Laboratories (Bar Harbor, ME) and aged in the Albert Einstein College of Medicine animal facility (Bronx, NY). Treatment with medicated chow containing BI-BTK-1 (daily dosage of ~10 mg/kg) or a comparable control chow (CC)(Research Diets) began when the mice were 8–9 weeks of age and continued until sacrifice, at which time urine, serum, and tissues were collected for analysis.
For the survival study, three to four-week-old female MRL/lpr mice were purchased from Jackson and aged to eight to nine weeks at the Albert Einstein College of Medicine. Mice were then randomly divided into two groups, half receiving treatment with BI-BTK-1 at 10 mg/kg, and the other half receiving comparable control chow. Mice were monitored for proteinuria weekly and were considered terminal when they reached the humane endpoint of ≥2000 mg/dl for more than two consecutive days.
Finally, MRL/lpr mice were also used for a therapeutic study in which three to four-week-old female MRL/lpr mice were purchased from Jackson and aged at the Albert Einstein College of Medicine. Mice were checked weekly for proteinuria starting at 12 weeks of age. Once individual mice developed significant proteinuria (>300 mg/dl at least two consecutive measurements, or >2000 mg/dl once), they were randomly matched in a pairwise fashion, with one mouse beginning treatment with BI-BTK-1 and the other beginning control chow.
All animal studies were approved by the Albert Einstein College of Medicine Institutional Animal Care Committee.
2.1.2.1. Assessment of kidney disease in MRL/lpr mice
Proteinuria was assessed semi-quantitatively once a week via Uristix (Siemens Healthcare Diagnostic, Tarrytown, NY). Proteinuria levels were confirmed in terminal urine via an albumin ELISA (Mouse Albumin ELISA Quantitation Set from Bethyl Laboratories (Montgomery, TX)), which was normalized to urinary creatinine levels (Quantichrom Creatinine Assay kit (BioAssay Systems, Hayward, CA)). Blood urea nitrogen (BUN) and serum creatinine levels were measured in terminal serum using the DIUR 500 kit (BioAssay Systems).
2.2. Renal histopathology
Mice were perfused with ice cold PBS at the time of sacrifice. Kidneys were harvested, fixed, and paraffin embedded. The embedded tissues were sectioned and stained with hematoxylin and eosin (H&E) and periodic acid Schiff (PAS). NZB/W tissues were processed at the Molecular Pathology Shared Resource of the Columbia University Herbert Irving Comprehensive Cancer Center, and MRL/lpr tissues were processed at the Albert Einstein College of Medicine Histology and Comparative Pathology Core. Renal pathology of both strains was scored by a blinded, experienced nephropathologist according to a previously published method [12]. In short, wire loop deposits, glomerular deposits, endocapillary proliferation, glomerular crescent formation (glomerular indices), interstitial inflammation, and tubular casts and dilatation (tubulointerstitial indices) were each given a score of 0–4, with 0 being no disease and 4 being severe disease. In addition, kidney sections were co-stained for IgG and C3 deposition by immunofluorescence, and the deposition was quantified in the kidney cortex using ImageJ.
2.3. Total IgG ELISA
Plates were coated with goat anti-mouse IgG (1 μg/ml in PBS (Southern Biotech, Birmingham, Alabama)) overnight at 4°C. The next day plates were washed and blocked with 2% BSA in PBS at 37°C for one hour. Serially diluted samples and standards (purified mouse IgG (Southern Biotech)) were added to the plate and placed at 37°C for two hours, and then washed. The plates were then incubated with an alkaline phosphatase conjugated goat anti-mouse IgG secondary antibody (Southern Biotech) for one hour at 37°C. After washing, the substrate solution was added, and the absorbance read at 405 nm.
2.4. Anti-double stranded (ds)DNA ELISA
2.4.1. NZB/W
Serum anti-dsDNA IgG was assessed using the mouse anti-dsDNA IgG-specific ELISA Kit (Alpha Diagnostics International, San Antonio, TX).
2.4.2. MRL/lpr
Plates were coated with 0.1 mg/ml salmon sperm DNA (ThermoFisher Scientific, Waltham, MA) in PBS overnight in a warm room, and then rinsed once the next day with water. Plates were then blocked with 1% BSA in PBS for 1 hour at 37°C, and samples added at a dilution of 1:1800. Samples were incubated for 2 hours at 37°C and then washed from the plate. Secondary antibody was then added (goat anti-mouse IgG AP, Southern Biotech), incubated for 1 hour at 37°C, and then washed off before the substrate solution was added. After developing, the plate was read at 405 nm. Sample ODs were normalized to the ODs of serum from healthy mice.
2.5. Immunofluorescent staining
Kidney sections were deparaffinized, rehydrated, and boiled in antigen retrieval buffer (citrate buffer pH 6 or Tris-EDTA buffer pH 9) for 10 minutes. Slides were then washed in PBS, and blocked with 20% normal horse serum and 0.1% triton in PBS for 1 hour at room temperature. After blocking, slides were incubated with the primary antibody cocktails. The first stain contained goat anti-C3 (1:100), rabbit anti-IBA-1 (1:250), and donkey anti-mouse IgG (1:500); whereas the second stain contained rat anti-B220 (1:100) and rabbit anti-CD4 (1:100). Finally, slides were also stained with anti-Ly6G (1:100). All primary antibody cocktails were incubated on the slides overnight at room temperature. The next day slides were washed, and then incubated with the appropriate secondary antibodies for 1 hour at room temperature (Table 1). Slides were washed after this incubation, stained with DAPI, and mounted. Staining intensity was quantified using ImageJ.
Table 1.
Antibodies used for fluorescent staining of kidney sections
| Primary antibody | Dilution | Secondary antibody | Dilution | |
|---|---|---|---|---|
| Stain 1 | goat anti-mouse C3 | 1:100 | donkey anti-goat AF594 | 1:100 |
| rabbit anti-mouse IBA-1 | 1:250 | donkey anti-rabbit AF488 | 1:250 | |
| donkey anti-mouse IgG | 1:500 | donkey anti-mouse IgG | 1:500 | |
| Stain 2 | rat anti-mouse B220 | 1:100 | donkey anti-rat AF594 | 1:100 |
| rabbit anti-mouse CD4 | 1:100 | donkey anti-rabbit AF488 | 1:100 | |
| Stain 3 | rat anti-mouse Ly6G | 1:100 | donkey anti-rat AF594 | 1:100 |
2.6. RT-PCR
At the time of sacrifice, kidney tissue was stored in RNA Later at 4°C for 24 hours and then transferred to −80°C until analysis. Frozen tissues were homogenized in Trizol using a Retsch MM300 Tissue Lyser to collect RNA. Chloroform was then added and the aqueous phase was collected and processed using the Agencourt RNAdvanced tissue kit that was modified for automation on a Biomek FXp from Beckman. RNA was quantified on a NanoDrop 8000 instrument and RNA quality was assessed based on RNA integrity numbers (RIN) using the Agilent 2200 Tape Station. Reverse transcription was achieved using the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems). The resultant cDNA was used in a ViiA 7 Real-Time PCR system (Applied Biosystems) using mouse specific probes from Applied Biosystems. Relative expression was then calculated with GAPDH used as the standardized housekeeping gene.
2.7. Flow Cytometry
2.7.1. NZB/W
At the time of sacrifice, spleens and femurs were collected. Spleens were processed into single cell suspensions and femurs were flushed for collection of bone marrow. Samples were incubated in Fc block (anti-CD16/CD32) for 15 minutes on ice, and incubated with one of the following staining cocktails, or appropriate controls, for 30 minutes: Cocktail 1 (B-cell, spleen and bone marrow): CD45, CD19, CD23, CD21, IgD, IgM, CD69; Cocktail 2 (T-cell, spleen): CD45, CD4, CD44, CD62L, CD8, CD3, CD11b, CD69; Cocktail 3 (CD220, spleen): CD45, CD3, CD220; Cocktail 4 (Myeloid cell, spleen): CD45, CD11b, CD11c, Gr-1, F4/80, CD115, B220; Cocktail 5 (Plasma cell, spleen and bone marrow): CD45, B220, CD11b, CD138, IgD, CD5, IgM, CD4. All antibodies were purchased from BD Biosciences (Franklin Lakes, NJ) or BioLegend (San Diego, CA). Cells were fixed in 2% paraformaldehyde and run on BD Facs Canto (BD Biosciences). The data was analyzed using FlowJo (BD Biosciences).
2.7.2. MRL/lpr
At the time of sacrifice, kidneys and spleens were harvested and made into single cell suspensions. The samples were incubated in Fc block (anti-CD16/CD32) for 15 minutes on ice, and then incubated with the following staining cocktails for another 30 minutes on ice in the dark: Cocktail 1 (macrophages): CD45, CD11b, CD11C, F480, Ly6C; Cocktail 2 (T cells): CD45, CD3, CD11b, CD4. All antibodies were purchased from BD Biosciences. Cells were then washed 3 times and run on the LSRII, and the data was analyzed using FlowJo.
2.8. Statistics
Statistics were performed in GraphPad Prism. For analysis of the NZB/W mice, each group was assessed for normality of distribution and subjected to an outlier’s test (Grubbs’ outlier test). To compare CC and the BI-BTK-1 treated groups (0.3 mg/kg − 10 mg/kg) a one-way Anova with multiple comparisons using either Tukey or Dunn’s (for nonparametric data) was used. For comparison of CTX vs PBS, a student’s T-test was used to compare normally distributed groups, and Mann-Whitney was used for nonparametric data. In the MRL/lpr data, both groups were assessed for normality and for outliers (Grubbs’ outlier test). A student’s T-test was used to compare normally distributed groups, and Mann-Whitney was used for nonparametric data. For all figures, *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001. When not all mice were tested in a given analysis, a random subgroup was selected.
3. Results
3.1. BI-BTK-1 inhibits kidney disease in NZB/W and MRL/lpr mice
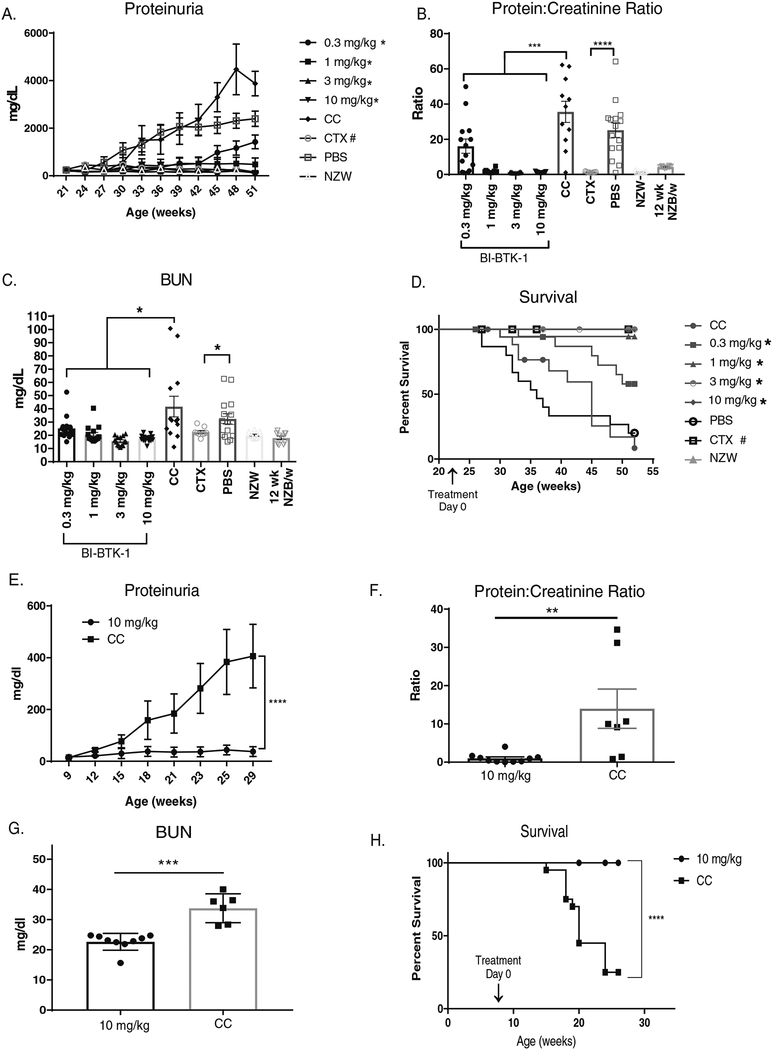
NZB/W mice were treated with various doses of BI-BTK-1 starting at 22 weeks of age, and showed a dose responsive inhibition of the development of proteinuria over the course of the experiment (Figure 1A). All four doses (0.3, 1, 3, and 10 mg/kg) of BI-BTK-1 provided significant renal protection compared to control treated mice (Figure 1B). The two highest doses of BI-BTK-1 completely prevented development of any significant levels of proteinuria, and were comparatively as effective as CTX (Figure 1A and B). BI-BTK-1 treated mice similarly showed lower serum levels of blood urea nitrogen (BUN) (Figure 1C) and serum creatinine (data not shown) compared to control treated mice. Additionally, all four doses of BI-BTK-1 significantly improved survival relative to control treated mice (Figure 1D).
Figure 1. BI-BTK-1 prevents renal disease in lupus mouse models.
NZB/W mice were treated with varying doses of BI-BTK-1 starting at 22 weeks of age. (A) BI-BTK-1 significantly inhibited proteinuria over the course of the experiment in a dose dependent manner compared to mice fed with control chow (CC) (* significant relative to control chow, # significant relative to PBS), and (B) significantly reduced protein: creatinine ratios measured at sacrifice. BUN levels at sacrifice were attenuated with BI-BTK-1 treatment (C). BI-BTK-1 improved survival, which was defined as the mice not reaching the humane endpoint of proteinuria of >2000 mg/dl on two separate days within the same week (D) (* significant relative to control chow, # significant relative to PBS). (BI-BTK-1 0.3 mg/kg, n=14; 1 mg/kg, n=15; 3 mg/kg, n=14; and 10 mg/kg, n=15; CC, n=13; 50 mg/kg cyclophosphamide IP, n=9; PBS IP, n=15; NZW control, n=10; 12 week old NZB/W control, n=10). MRL/lpr mice were also treated with BI-BTK-1 at 10 mg/kg starting at 8–9 weeks of age. Compared to control treated mice, BI-BTK-1 treatment significantly reduced proteinuria (E) (BI-BTK-1, n=12; control, n=12), protein:creatinine ratios (F), and BUN levels (G) (BI-BTK-1, n=9; control, n=7). BI-BTK-1 improved the survival of MRL/lpr mice, as defined by not reaching proteinuria levels of >2000 mg/dl on two separate days within the same week (BI-BTK-1, n=15; control, n=15). (*p<0.05, ** p<0.01, *** p<0.001, **** p< 0.0001, #p<0.05).
To verify that the protective effect of BTK inhibition is not limited to a single genetic background, or histological pattern of nephritis, we tested BI-BTK-1 in the MRL/lpr strain as well. Although both NZB/W and MRL/lpr mice are considered excellent LN models, these strains display differences in the disease course, renal gene expression, and kidney histopathology [13]. Treatment with 10 mg/kg BI-BTK-1 starting at 8–9 weeks of age also protected MRL/lpr mice from proteinuria over time, with control chow fed mice developing significantly higher levels of proteinuria than BI-BTK-1 treated mice (Figure 1E). These results were confirmed in terminal urine by measuring protein:creatinine ratios, which were significantly higher in control chow fed mice (Figure 1F). BUN levels were measured in terminal serum and found to be significantly lower in BI-BTK-1 treated mice (Figure 1G). Additionally, kidneys from treated mice weighed significantly less than control mice, consistent with less edema (data not shown). Finally, treatment with BI-BTK-1 significantly improved the survival of MRL/lpr mice. At 28 weeks of age, 100% of the treated mice were still alive, whereas only about 20% of the control mice survived (p<0.0001) (Figure 1H).
3.2. BTK inhibition prevents cellular infiltration of immune cells into the kidney
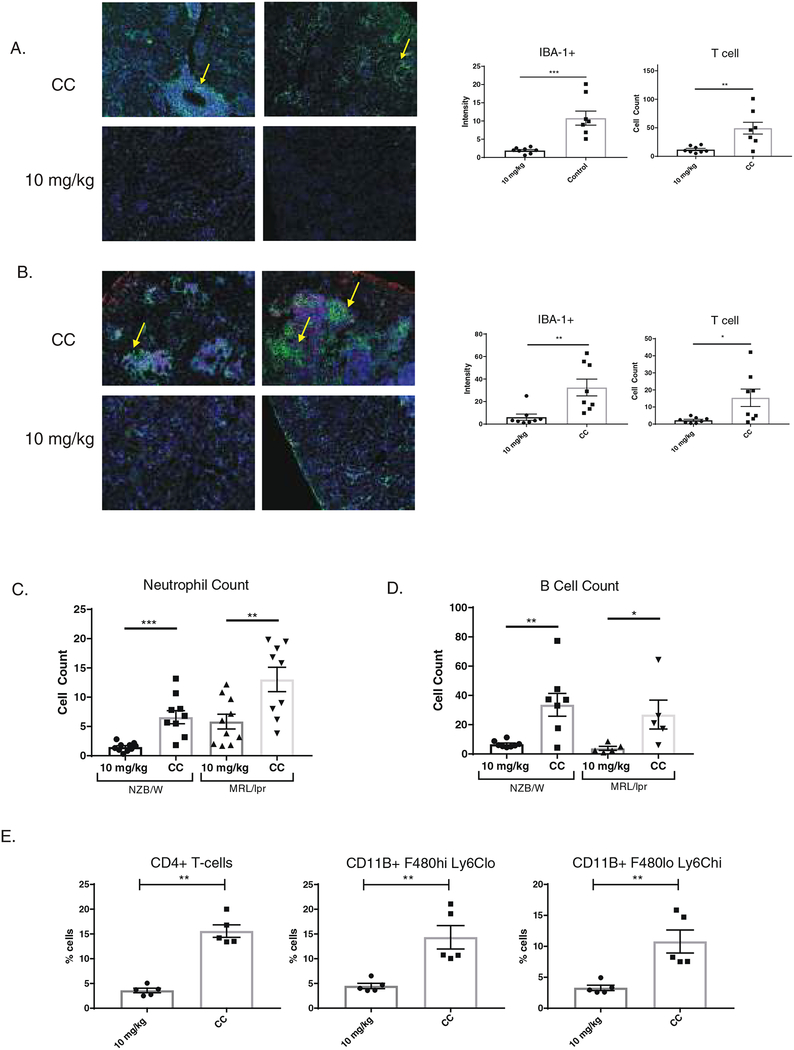
Control treated NZB/W and MRL/lpr mice had significant kidney infiltration of IBA-1+ macrophages and CD4+ T cells, as shown by immunofluorescent staining (Figure 2A and B, respectively). Periglomerular and intraglomerular accumulations of macrophages were seen in both strains (arrows). This accumulation of macrophages and T cells was ameliorated with BIBTK-1 treatment. Neutrophils were stained via Ly6G, and BI-BTK-1 decreased their accumulation in the kidney of both MRL/lpr and NZB/W mice (Figure 2C). There was also a significant reduction in B220+ B cells (Figure 2D).
Figure 2. BI-BTK-1 improves kidney pathology in lupus-prone mice.
Treatment with BI-BTK-1 improved renal histology in NZB/W and MRL/lpr mice (A-C), as evidenced by normalized glomerular and tubular histology (B, C: 100× pictures, H&E staining in left panels; 400× pictures, PAS staining in right panels). In the NZB/W graphs, * represents significance relative to control chow, whereas # represents significance relative to PBS control group (NZB/W: BI-BTK-1 0.3 mg/kg, n=14; 1 mg/kg, n=15; 3 mg/kg, n=14; and 10 mg/kg, n=15; CC, n=13; 50 mg/kg cyclophosphamide IP, n=9; PBS IP, n=15; NZW control, n=10; 12 week old NZB/W control, n=10) (MRL/lpr: CC, n=9; BI-BTK-1 10 mg/kg, n=12). Kidneys were also assessed for IgG and C3 deposition, with a significant reduction in both observed in the NZB/W and MRL/lpr mice (D). (NZB/W: BI-BTK-1 10 mg/kg, n=8; CC, n=7; MRL/lpr: BI-BTK-1 10 mg/kg, n=12; CC, n=9).
Additionally, flow cytometry was performed on the kidneys of MRL/lpr mice at the time of sacrifice. As seen in Figure 3E, we confirmed the reduction in infiltrating CD4+ T cells, as well as macrophages. Phenotype analysis revealed a decrease in the accumulation of inflammatory macrophages (CD11b+, F4/80 lo, Ly6Chi) and in the number of tissue resident macrophages (CD11b+, F4/80hi, Ly6Clo) (Figure 2E).
Figure 3. BI-BTK-1 ameliorates immune cell accumulation in the kidney.
BI-BTK-1 (10 mg/kg) reduced the accumulation of macrophages (IBA-1+ cells, green) and T cells (CD4+, red) in the kidneys compared to control treated NZB/W mice. Fluorescence intensity was measured using ImageJ as a means of quantifying and comparing cellular infiltrations (A). Similar reductions in macrophages and T cells were seen in the kidneys of MRL/lpr mice (B). There was also a significant reduction of both kidney infiltrating neutrophils and B cells in both strains (C and D). Flow cytometry of the kidneys of MRL/lpr mice confirmed significant reduction in CD4+ T cells, tissue resident macrophages (CD11b+ F480hi Ly6Clo), and inflammatory macrophages (CD11b+ F480lo Ly6Chi) (E). (NZB/W staining: BI-BTK-1 treated 10 mg/kg, n=8; CC, n=7) (MRL/lpr staining: BI-BTK-1 treated 10 mg/kg, n=8; CC, n=8) (MRL/lpr flow and B cell stain: BI-BTK-1 treated, n=5; CC, n=5).
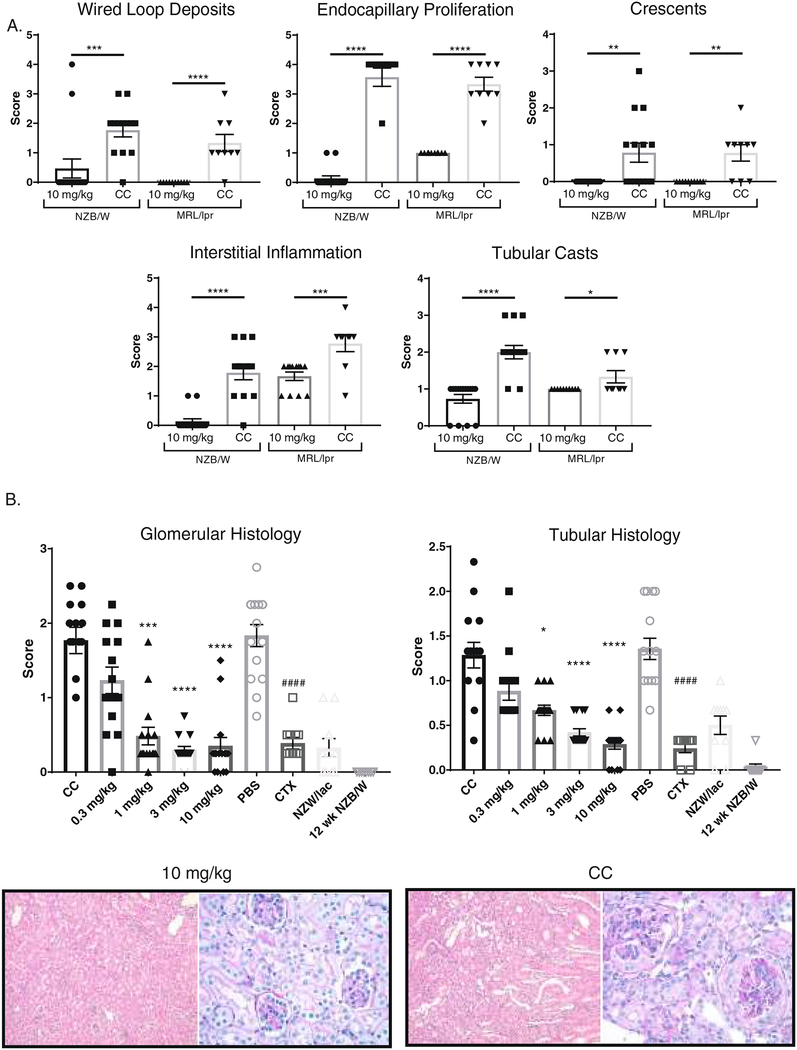
3.3. BI-BTK-1 treatment improves renal histopathology
BI-BTK-1 significantly impeded the development of wire loop deposits, endocapillary proliferation, crescent formation, interstitial inflammation, and tubular casts in both NZB/W and MRL/lpr mice (Figure 3A). Overall, a significant dose response was seen in the NZB/W mice, with protection observed in both the glomerular and tubular compartments (Figure 3B). MRL/lpr mice treated with BI-BTK-1 showed similarly improved renal histopathology compared to control treated mice, as shown in Figure 3C.
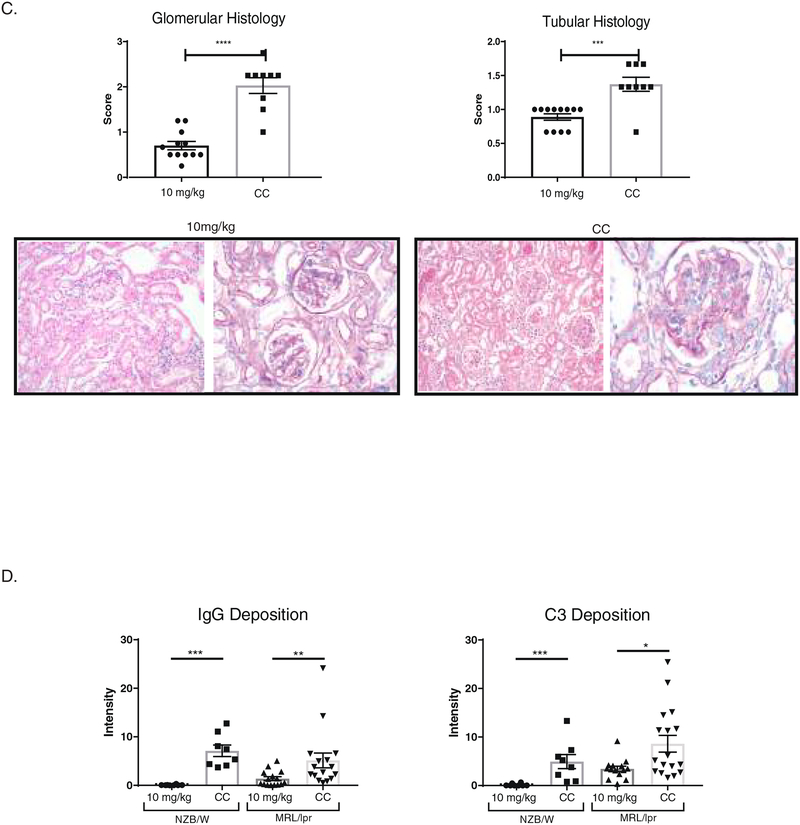
3.4. Renal immune deposits are decreased with BI-BTK-1 treatment
To further assess the effect of BI-BTK-1 on the pathogenesis of LN, kidney sections were stained for IgG and C3 deposition. We found that treated NZB/W and MRL/lpr mice had significant decreases in both IgG and C3 deposition in the kidney (Figure 3D).
3.5. BI-BTK-1 treatment modulates immune cell populations in the spleen
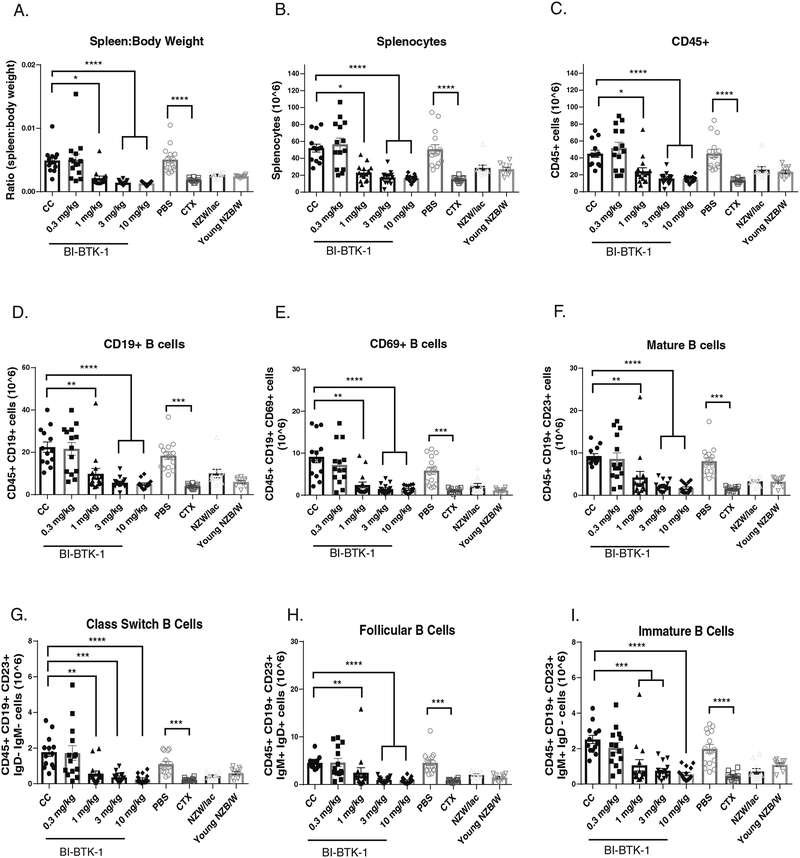
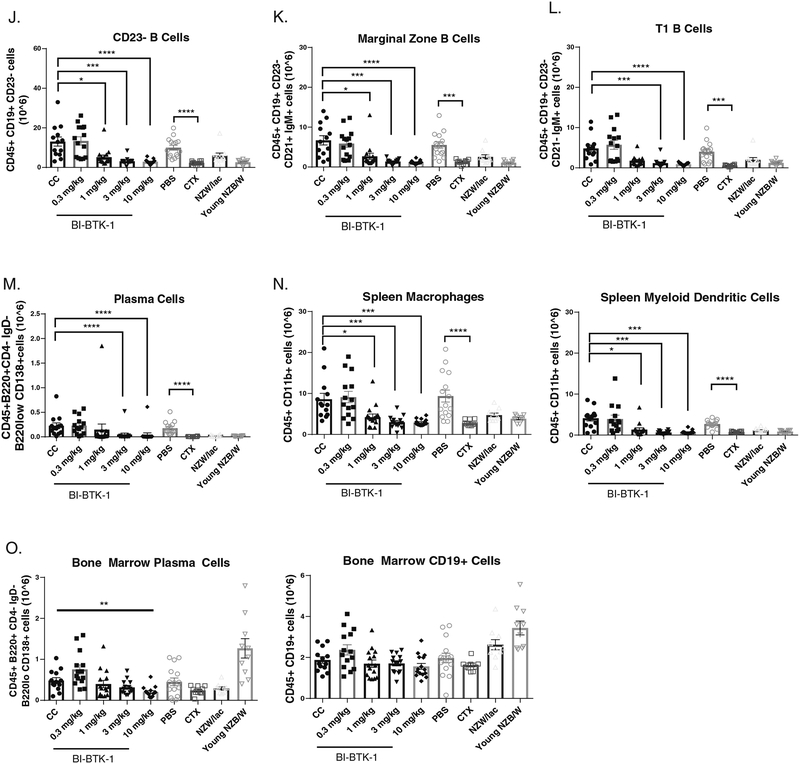
Both NZB/W (Figure 4A) and MRL/lpr (data not shown) mice exhibit marked splenomegaly due to accumulation of proliferating immune cells in the spleen. To assess the effect of BTK inhibition on B cell populations in the spleen, we performed flow cytometry on the spleens of NZB/W mice in the different treatment groups. At higher doses, BI-BTK-1 inhibited expansion of multiple B cell subtypes (Figure 4). Spleen to body weight ratios were significantly less in the three highest doses of BI-BTK-1 compared to control mice (Figure 4A), correlating with cellularity of the spleen (Figure 4B) and a significant reduction in CD45+ cells (Figure 4C). BTK inhibition was associated with a significant reduction of CD19+ B cells (Figure 4D), including CD69+ B cells, mature B cells, class switched B cells, follicular B cells, immature B cells, transitional B cells, marginal zone B cells, T1 B cells, and plasma cells in the spleen (Figure 4E–M). There was also a significant reduction in splenic myeloid cells, including macrophages and myeloid dendritic cells (Figure 4N). Flow cytometry was also performed on bone marrow cells. While there was no effect on overall CD19+ B cell numbers, at 10 mg/kg of BI-BTK-1 there was a significant decrease in CD138+ plasma cells (Figure 4O).
Figure 4. BI-BTK-1 affects multiple B cell subtypes in the spleens of NZB/W mice.
BI-BTK-1 treatment reduced spleen size (A) and total cell numbers within the spleen (B), including total CD45+ cells (C) and, specifically, CD19+ B cells (D). We assessed different B cell subtypes, and found that BI-BTK-1 treatment reduced all tested subsets in a dose responsive manner (E-M). Splenic macrophages and myeloid dendritic cells were also reduced (N). While BI-BTK-1 did not reduce overall B cell numbers in the bone marrow, plasma cell numbers were significantly reduced at the highest dose of 10 mg/kg of BI-BTK-1 compared to control mice (O). (NZB/W mice: CC, n=13; BI-BTK-1: 1 mg/kg, n=15; 3 mg/kg, n=14; 10 mg/kg, n=15; PBS, n=15; CTX, n=10; NZW, n=10; 12 week old NZB/W control, n=10).
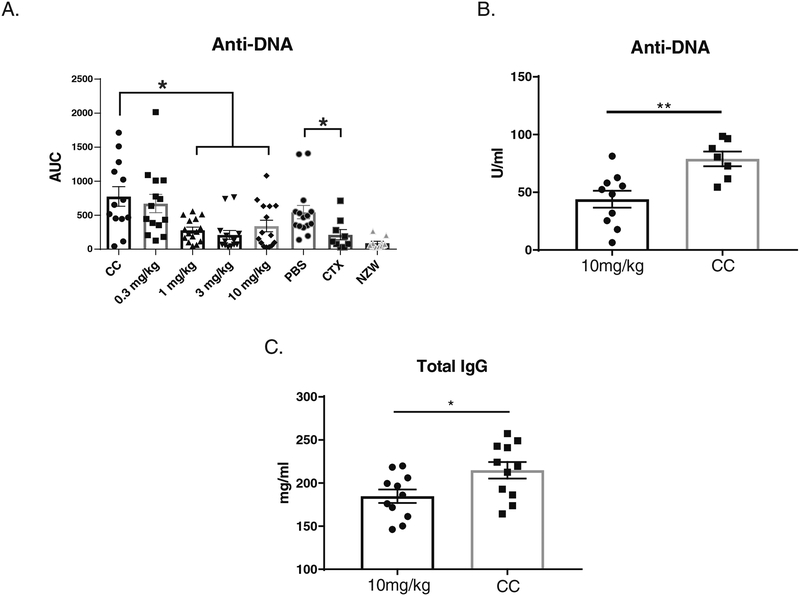
3.6. Auto-antibodies in BI-BTK-1 mice
Consistent with the profound effect of BI-BTK-1 on B cell populations, we found decreased serum IgG anti-dsDNA antibody titers in treated NZB/W mice (Figure 5A, p<0.05). BIBTK-1 treatment similarly decreased IgG anti-dsDNA antibody levels in the MRL/lpr strain (Figure 5B). Circulating total IgG levels were additionally significantly reduced in MRL/lpr mice (Figure 5C).
Figure 5. BI-BTK-1 treatment decreases circulating antibody levels.
NZB/W mice had significantly lower levels serum of anti-DNA antibodies when treated with three different doses of BI-BTK-1 (A). A similar effect was seen in MRL/lpr mice (B), which also demonstrated lower levels of circulating total IgG (C). (NZB/W mice: CC, n=13; BI-BTK-1 0.3 mg/kg, n=14; 1 mg/kg, n=15; 3 mg/kg, n=14; 10 mg/kg, n=15; PBS, n=15; CTX, n=9; NZW, n=10) (MRL/lpr: BI-BTK-1 treated, n=11; control, n=11) (*p<0.05).
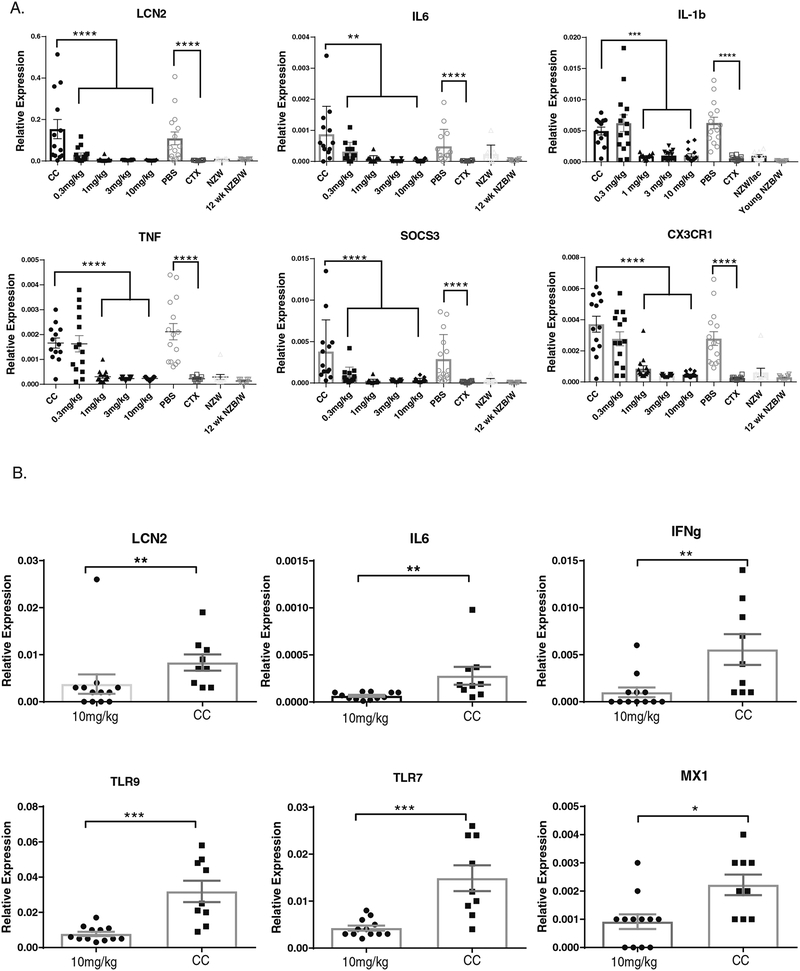
3.7. Inflammatory mediators are decreased in the kidneys of BI-BTK-1 treated mice
We assessed levels of inflammatory mediators in the kidneys by RT-PCR. In NZB/W mice, BI-BTK-1 treatment reduced expression of LCN2 (lipocalin-2), IL-6, IL-1b, TNF, SOCS3 and CX3CR1, among others (Figure 6A), consistent with an effect on macrophages and a notable reduction in the inflammatory mediators associated with LN. Similar findings were seen in MRL/lpr mice (Figure 6B), with a significant reduction in kidney expression of LCN2, IL-6, IFNg, TLR9, TLR7, and MX1.
Figure 6. BI-BTK-1 reduces kidney inflammatory gene expression.
RT-PCR was performed on NZB/W (A) and MRL/lpr kidneys (B). For both strains, BI-BTK-1 significantly reduces the expression of inflammatory mediators expressed within the kidney. (NZB/W : CC, n=13; BI-BTK-1 0.3 mg/kg, n=14; 1 mg/kg, n=15; 3 mg/kg, n=14; 10 mg/kg, n=15; PBS, n=15; CTX, n=9; NZW, n=10; young NZB/W, n=10) (MRL/lpr mice: CC, n=9; BI-BTK-1 10 mg/kg, n=12) (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
3.8. Disease Reversal
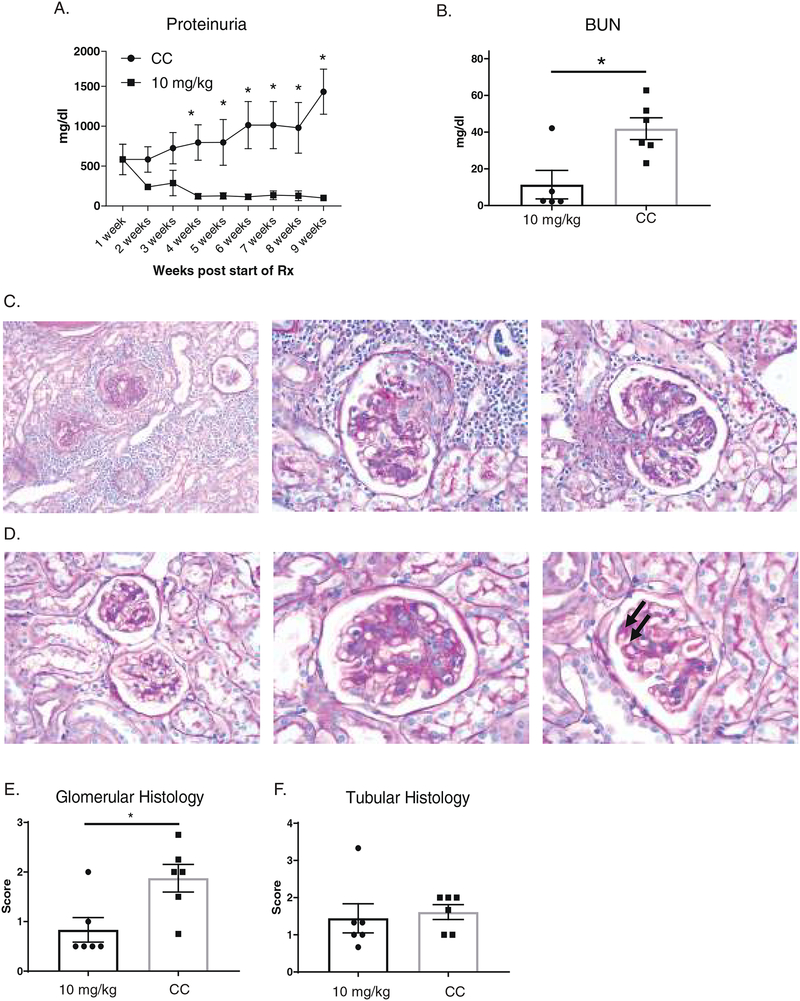
To assess the potential clinical applicability of BI-BTK-1, we tested its ability to reverse established kidney disease. MRL/lpr mice were allowed to age until the onset of significant proteinuria, at which point they were randomly started on either medicated or control chow on a rolling basis. As shown in Figure 7A, treatment was initiated when the mice averaged 500 mg/dl of proteinuria. Within 4 weeks of treatment, the BI-BTK-1 treated group had significantly reduced proteinuria levels compared to control mice treated for the same duration. This trend continued throughout the remainder of the experiment. At sacrifice, BI-BTK-1 therapy normalized BUN levels compared to control treated mice (Figure 7B). Treated mice also had significantly improved kidney histology. Control treated mice had prominent sclerotic glomeruli (Figure 7C, left panel), cellular crescents (7C, center panel), and endocapillary proliferation (7C, right panel). Treated mice, however, had healthier looking glomeruli (Figure 7D, left panel), with generally mild endocapillary proliferation (7D, center) and some immune deposits (7D, right, arrows), but with less inflammation than seen in the control treated mice. Moreover, there was a significant difference in glomerular histology (Figure 7E), although there was no difference in tubular histopathological indices (Figure 7F).
Figure 7. Delayed treatment with BI-BTK-1 reverses established renal disease in MRL/lpr mice.
Mice were allowed to reach levels of significant proteinuria (>300 mg/dl on two or more consecutive measurements, or an individual read of >2000 mg/dl) as measured by Uristix, before being randomly matched in a pairwise fashion, with one mouse beginning treatment with BI-BTK-1, and the other receiving control chow. Within 4 weeks of starting treatment with BI-BTK-1, treated mice had significantly lower proteinuria than control treatment mice (A). (4 weeks treatment, BI-BTK-1, n=10; control, n=9; 5 weeks treatment, BI-BTK-1, n=10; control=8; 6 weeks treatment, BI-BTK-1, n=9; control=8; 7 weeks treatment, BI-BTK-1, n=7; control, n=4; 8 weeks treatment, BI-BTK-1, n=6; control=3; 9 weeks treatment, BI-BTK-1, n=4; control=2). Despite delayed treatment, BI-BTK-1 reversed the high levels of BUN seen in control mice (B). CC treated mice showed sclerotic glomeruli, crescent formation, and endocapillary proliferation (C, left picture 200×, middle and right, 400×). BI-BTK-1 treated mice had more normal appearing glomeruli, with mild endocapillary proliferation and some immune deposits (arrows) (D, left picture 200×, middle and right, 400×). Representative pictures are shown. Histology was scored, showing significant differences in glomerular parameters, but not tubular histology (E, F). “Rx” denotes treatment.
4. Discussion
Lupus nephritis is a serious end organ complication of SLE. Treatment options are still inadequate and rely heavily on non-specific immunosuppression that is often associated with unfavorable side effects. In this study we showed that treatment of MRL/lpr and NZB/W mice with BI-BTK-1 significantly attenuates kidney disease relative to control treated mice. Further, we showed that treatment significantly improves the survival rate of the treated mice and, in a key and novel finding, reverses already established disease. It is important to clarify that reduced proteinuria alone does not indicate reversal of existing tissue injury; drug therapy can improve proteinuria independent of pathology. While in the delayed treatment experiment we did not sacrifice a subset of animals with proteinuria before beginning treatment to have a baseline for histology comparison, nevertheless we clearly demonstrated that even delaying the start of BIBTK-1 therapy initiation to a time point at which proteinuria was already established resulted in significantly improved glomerular histology as compared to control treated mice.
SLE is a disease largely driven by autoantibodies [14]. The systemic process that leads to LN potentially begins with mishandling of apoptotic cells, which then undergo secondary necrosis [15]. Ultimately, this results in nuclear particles activating cells through stimulation of TLR7, 8, or 9, leading to the release of type I IFNs. Type I IFNs activate the adaptive immune system, including autoreactive B cells. Autoantibodies can then deposit in the kidney, activating local cells, including macrophages, via Fc receptors. These activated cells initiate a localized inflammatory immune response which damages the kidney, leading to renal disease [16].
Increased peripheral blood expression of BTK is associated with LN in SLE patients, and it is found expressed at high levels within glomeruli of LN patients [17], further emphasizing its potential role in LN. BTK inhibition is a promising treatment option because it would interfere with several of the key pathogenic mechanisms known to be involved in LN. Specifically, BTK is important for B cell survival due to its role in downstream BCR signaling, and therefore its inhibition decreases autoantibody production and modulates B cell cytokine release and antigen presentation [8]. BTK inhibition would also interfere with TLR and Fc receptor mediated signaling, thereby limiting the damage induced by autoantibody deposition within tissues [11,18]. Thus, BTK inhibition would target both the adaptive and innate arms of the immune system involved in disease pathogenesis.
In this study, we used two well established, spontaneous models of SLE to study the effect of BTK inhibition on LN progression. In the NZB/W mice, early treatment with BI-BTK-1 reduced various B cell subtypes within the spleen. While a previous study reported that BTK inhibition is protective in LN with associated reductions in activated (CD69+) and germinal center (GL7+) B cells, we demonstrated that the BTK inhibitor used here can affect an even broader array of B cell subtypes [19], including antibody producing plasma cells. We also saw a reduction of plasma cells in the bone marrow. Consequently, we measured a significant decrease in circulating anti-DNA antibodies in NZB/W BI-BTK-1 treated mice, in addition to decreased antibody deposition within the kidneys. BI-BTK-1 treated mice also had decreased accumulation of macrophages both within the kidneys and spleen, as well as decreased renal inflammatory cytokine expression. These multiple effects of BTK inhibition were associated with decreased proteinuria levels, as well as improved kidney function.
BI-BTK-1 treatment also decreased serum anti-DNA antibody levels in MRL/lpr mice. Additionally, treated mice had decreased total levels of circulating IgG and less IgG deposited in the kidneys. Despite not having as dramatic an effect on B cells in this model as seen in NZB/W mice, treated MRL/lpr mice still had significantly improved kidney disease. We believe this is because BTK inhibition, as mentioned above, would also modulate Fc receptor mediated macrophage activation and function. Indeed, we demonstrated a decrease of intrarenal macrophages together with a reduction in macrophage associated cytokines.
We have previously reported that macrophages are important to LN development, both in inducible and spontaneous models of nephritis [19–21]. Additionally, our previous work has shown that BI-BTK-1 can not only prevent nephritis in an inducible model of LN known as NTN, but also reverse proteinuria in established disease [12]. In the NTN model, however, any requirement for B cell autoantibody production is circumvented, as the nephrotoxic antibodies are given passively through intravenous injection. While this does not discount the possibility of B cells contributing to nephritis through other mechanisms (e.g. cytokine secretion), it is believed that the pathogenesis of NTN relies also on other cell types, particularly macrophage effector functions.
Collectively, our data shows that BI-BTK-1 can affect both B cells and macrophages, two important contributors to disease pathogenesis. By inhibiting Fc receptors and certain TLRs, BI-BTK-1 could prevent the activation of macrophages. BTK inhibition could additionally affect other cell types such as neutrophils which may also contribute to disease. BTK is also thought to be important in macrophage polarization, and has been shown to favor an M2 phenotype that could be beneficial in ameliorating disease [22]. Furthermore, BTK is relevant to the NLRP3 inflammasome pathway [11,23,24] which has been linked to LN pathogenesis [25], both in immune cells such as macrophages [11], and local resident cells such as podocytes [26–28]. Our data showed decreased expression of IL-1b with BI-BTK-1 treatment, consistent with inhibition of NLRP3 activation.
There have been several earlier studies of BTK inhibition in LN. A previous report examined the BTK inhibitor ibrutinib in MRL/lpr mice starting at 8 weeks of age. Mice were treated with various doses of PCI-32756 for 12 weeks, and while there was a significant improvement in proteinuria in the higher doses no effect on glomerular histology was found [29]. Interestingly, in our study we demonstrated that treatment with BI-BTK-1 markedly improves glomerular histopathology, and can even reverse disease, highlighting the promise of this particular compound.
In a separate study, Hutchenson et al. evaluated the effects of ibrutinib in the SLE1.3 lupus model, at a dose of 30 mg/kg. They noted attenuated nephritis, delayed production of autoantibodies, and decreased splenomegaly [30]. Mina-Osorio et al. investigated the BTK inhibitor RN486 in NZB/W F1 mice at a similar dose. Starting treatment after mild proteinuria was present reduced urine protein to normal levels. They further noted the effect of the drug on macrophage Fc receptor activation through assessing macrophage related interferon inducible genes in the spleen and kidneys [31].
There have also been studies that showed the benefit of inhibiting BTK in BXSB-7aa mice, a murine model driven by TLR7, with a reduction in autoantibodies, nephritis, and mortality; however, in the pristane-induced DBA/1 model, no effect was seen on autoantibodies or the IFN gene signature [32]. A separate study by Katewa et al. examined BTK inhibition in an alpha-IFN accelerated model in NZB/W mice, with a reduction in plasmablasts, autoantibodies, and renal disease [16]. Finally, Kim et al recently reported beneficial effects of HM71224, a selective BTK inhibitor, on renal disease and mortality when given prophylactically in NZB/W and MRL/lpr mice[19].
Our study has several important advantages over those previously conducted. To begin, BI-BTK-1 is a highly selective and potent inhibitor of BTK [12]. We report here that BI-BTK-1 ameliorated kidney disease in two spontaneous lupus models that develop more severe renal involvement than some of the models reported above, despite being used at a lower dose (10 mg/kg). We have now demonstrated its ability to prevent disease in three separate models of LN (NTN, MRL/lpr, and NZB/W), and moreover its ability to significantly improve survival. Importantly, we have also shown that BI-BTK-1 can reverse established and severe kidney disease both within the short term NTN model of inducible antibody mediated glomerulonephritis [12], and in this study in the MRL/lpr strain. This therapeutic endpoint is critical for transition of BTK inhibition into clinical use, where treatment is generally initiated after the onset of disease. We are the first to show reversal of established disease in a spontaneous lupus strain, a desired characteristic in a drug being developed for application in human disease. Finally, besides a salutary effect on renal involvement, we have recently demonstrated that BI-BTK-1 improves cutaneous lesions and cognitive dysfunction associated with murine SLE [33].
Conclusions
Our results emphasize the potential of BTK inhibition as a therapeutic option in treating LN. Moreover, we show here for the first time that BTK inhibition is effective in murine lupus not only when given preventively, but also in reversing established disease. BTK inhibition is attractive because it is more selective than current treatment options that involve broad spectrum immunosuppression. Moreover, BTK inhibitors are already being used in human trials and are relatively well tolerated, and severe side effects that require the discontinuation of therapy are uncommon. The major side effects reported thus far are upper respiratory tract infection, fatigue, and diarrhea, at a grade of 1 or 2, thus allowing relatively extended treatment periods [34]. There is now strong evidence that BTK inhibition is highly effective in multiple murine lupus models, and for different organ manifestations; it is hoped that similar effects will be seen in currently ongoing early phase studies of BTK inhibition in human SLE.
Highlights.
BI-BTK-1, a potent BTK inhibitor, attenuates kidney disease in both MRL/lpr and NZB/W lupus mouse models, and significantly increases survival;
Treatment with BI-BTK-1 decreases kidney immune cell infiltration and diminishes the local expression of inflammatory mediators;
BI-BTK-1 reverses established kidney disease in MRL/lpr mice.
Acknowledgments
We thank Shawn Anderson (Boehringer Ingelheim Pharmaceuticals) for his help with the flow cytometry experiments.
Funding
Boehringer Ingelheim (Ridgefield, CT) funded these studies. With exception of the author contributions detailed in the section below, the company did not have a role in the design of the studies, collection/analysis/interpretation of the data, or writing and submission of the manuscript.
Abbreviations
- LN
Lupus nephritis
- IC
Immune complex
- BTK
Bruton’s tyrosine kinase
- NTN
Nephrotoxic serum nephritis
- BUN
Blood urea nitrogen
- CTX
Cyclophosphamide
- H&E
Hematoxylin and Eosin
- PAS
Periodic Acid Schiff
- CC
Control chow
Footnotes
Competing interests
Elizabeth Glynn, Mark Panzenbeck, Josephine Pelletier, Janice Dimock, Elise Seccareccia, Todd Bosanac, Sara Khalil, Christian Harcken, Deborah Webb, Gerald Nabozny, Jay S. Fine, Donald Souza, Elliott Klein, and Meera Ramanujam are full time employees of Boehringer Ingelheim (Ridgefield, CT). The other authors declare that they have no conflicts of interests.
5. Works Cited
- [1].Tsokos GC, Lo MS, Reis PC, Sullivan KE, New insights into the immunopathogenesis of systemic lupus erythematosus, Nat. Rev. Rheumatol 12 (2016) 716. doi: 10.1038/nrrheum.2016.186. [DOI] [PubMed] [Google Scholar]
- [2].Kwok S-K, Tsokos GC, New insights into the role of renal resident cells in the pathogenesis of lupus nephritis, Korean J. Intern. Med (2018). doi: 10.3904/kjim.2017.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lech M, Anders H-J, The Pathogenesis of Lupus Nephritis, J. Am. Soc. Nephrol 24 (2013) 1357–1366. doi: 10.1681/ASN.2013010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Maroz N, Segal MS, Lupus Nephritis and End-stage Kidney Disease, Am. J. Med. Sci 346 (2013) 319–323. doi: 10.1097/MAJ.0b013e31827f4ee3. [DOI] [PubMed] [Google Scholar]
- [5].Davidson A, What is damaging the kidney in lupus nephritis?, Nat. Rev. Rheumatol 12 (2016) 143–153. doi: 10.1038/nrrheum.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bergtold A, Gavhane A, D’Agati V, Madaio M, Clynes R, FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis, J. Immunol. Baltim. Md 1950. 177 (2006) 7287–7295. [DOI] [PubMed] [Google Scholar]
- [7].Hill GS, Delahousse M, Nochy D, Rémy P, Mignon F, Méry JP, Bariéty J, Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages, Kidney Int. 59 (2001) 304–316. doi: 10.1046/j.1523-1755.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- [8].Corneth OBJ, Klein Wolterink RGJ, Hendriks RW, BTK Signaling in B Cell Differentiation and Autoimmunity, Curr. Top. Microbiol. Immunol 393 (2016) 67–105. doi: 10.1007/82_2015_478. [DOI] [PubMed] [Google Scholar]
- [9].Vetrie D, Vorechovský I, Sideras P, Holland J, Davies A, Flinter F, Hammarström L, Kinnon C, Levinsky R, Bobrow M, The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases, Nature. 361 (1993) 226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- [10].Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG, Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice, Science. 261 (1993) 358–361. [DOI] [PubMed] [Google Scholar]
- [11].Weber ANR, Bittner Z, Liu X, Dang T-M, Radsak MP, Brunner C, Bruton’s Tyrosine Kinase: An Emerging Key Player in Innate Immunity, Front. Immunol 8 (2017) 1454. doi: 10.3389/fimmu.2017.01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chalmers SA, Doerner J, Bosanac T, Khalil S, Smith D, Harcken C, Dimock J, Der E, Herlitz L, Webb D, Seccareccia E, Feng D, Fine JS, Ramanujam M, Klein E, Putterman C, Therapeutic Blockade of Immune Complex-Mediated Glomerulonephritis by Highly Selective Inhibition of Bruton’s Tyrosine Kinase, Sci. Rep 6 (2016) 26164. doi: 10.1038/srep26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Perry D, Sang A, Yin Y, Zheng Y-Y, Morel L, Murine Models of Systemic Lupus Erythematosus, BioMed Res. Int (2011). doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tanaka Y, Kubo S, Iwata S, Yoshikawa M, Nakayamada S, B cell phenotypes, signaling and their roles in secretion of antibodies in systemic lupus erythematosus, Clin. Immunol (2017). doi: 10.1016/j.clim.2017.07.010. [DOI] [PubMed] [Google Scholar]
- [15].Munoz LE, van Bavel C, Franz S, Berden J, Herrmann M, van der Vlag J, Apoptosis in the pathogenesis of systemic lupus erythematosus, Lupus. 17 (2008) 371–375. doi: 10.1177/0961203308089990. [DOI] [PubMed] [Google Scholar]
- [16].Katewa A, Wang Y, Hackney JA, Huang T, Suto E, Ramamoorthi N, Austin CD, Bremer M, Chen JZ, Crawford JJ, Currie KS, Blomgren P, DeVoss J, DiPaolo JA, Hau J, Johnson A, Lesch J, DeForge LE, Lin Z, Liimatta M, Lubach JW, McVay S, Modrusan Z, Nguyen A, Poon C, Wang J, Liu L, Lee WP, Wong H, Young WB, Townsend MJ, Reif K, Btk-specific inhibition blocks pathogenic plasma cell signatures and myeloid cell-associated damage in IFNα-driven lupus nephritis, JCI Insight. 2 (n.d.). doi: 10.1172/jci.insight.90111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kong W, Deng W, Sun Y, Huang S, Zhang Z, Shi B, Chen W, Tang X, Yao G, Feng X, Sun L, Increased expression of Bruton’s tyrosine kinase in peripheral blood is associated with lupus nephritis, Clin. Rheumatol 37 (2018) 43–49. doi: 10.1007/s10067-017-3717-3. [DOI] [PubMed] [Google Scholar]
- [18].López-Herrera G, Vargas-Hernández A, González-Serrano ME, Berrón-Ruiz L, Rodríguez-Alba JC, Espinosa-Rosales F, Santos-Argumedo L, Bruton’s tyrosine kinase—an integral protein of B cell development that also has an essential role in the innate immune system, J. Leukoc. Biol 95 (2014) 243–250. doi: 10.1189/jlb.0513307. [DOI] [PubMed] [Google Scholar]
- [19].Kim Y-Y, Park KT, Jang SY, Lee KH, Byun J-Y, Suh KH, Lee Y-M, Kim YH, Hwang KW, HM71224, a selective Bruton’s tyrosine kinase inhibitor, attenuates the development of murine lupus, Arthritis Res. Ther 19 (2017). doi: 10.1186/s13075-017-14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chalmers SA, Wen J, Shum J, Doerner J, Herlitz L, Putterman C, CSF-1R inhibition attenuates renal and neuropsychiatric disease in murine lupus, Clin. Immunol. Orlando Fla. 185 (2017) 100–108. doi: 10.1016/j.clim.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chalmers SA, Chitu V, Herlitz LC, Sahu R, Stanley ER, Putterman C, Macrophage depletion ameliorates nephritis induced by pathogenic antibodies, J. Autoimmun 57 (2015) 42–52. doi: 10.1016/j.jaut.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ní Gabhann J, Hams E, Smith S, Wynne C, Byrne JC, Brennan K, Spence S, Kissenpfennig A, Johnston JA, Fallon PG, Jefferies CA, Btk regulates macrophage polarization in response to lipopolysaccharide, PloS One. 9 (2014) e85834. doi: 10.1371/journal.pone.0085834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ito M, Shichita T, Okada M, Komine R, Noguchi Y, Yoshimura A, Morita R, Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury, Nat. Commun 6 (2015) 7360. doi: 10.1038/ncomms8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Henrickson SE, Teaching an old pathway new tricks: Targeting BTK to block NLRP3, Sci. Immunol 2 (2017). doi: 10.1126/sciimmunol.aar2548. [DOI] [PubMed] [Google Scholar]
- [25].Hyperactivation of the NLRP3 Inflammasome in Myeloid Cells Leads to Severe Organ Damage in Experimental Lupus. - PubMed - NCBI, (n.d.). https://www.ncbi.nlm.nih.gov/pubmed/28039299 (accessed April 16, 2018). [DOI] [PubMed]
- [26].Carney EF, Lupus nephritis: Role of NLRP3 inflammasomes in podocyte injury, Nat. Rev. Nephrol 13 (2017) 444. doi: 10.1038/nrneph.2017.91. [DOI] [PubMed] [Google Scholar]
- [27].Fu R, Guo C, Wang S, Huang Y, Jin O, Hu H, Chen J, Xu B, Zhou M, Zhao J, Sung S-SJ, Wang H, Gaskin F, Yang N, Fu SM, Podocyte Activation of NLRP3 Inflammasomes Contributes to the Development of Proteinuria in Lupus Nephritis, Arthritis Rheumatol. Hoboken NJ. 69 (2017) 1636–1646. doi: 10.1002/art.40155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ummarino D, Lupus nephritis: NLRP3 inflammasome ignites podocyte dysfunction, Nat. Rev. Rheumatol 13 (2017) 451. doi: 10.1038/nrrheum.2017.97. [DOI] [PubMed] [Google Scholar]
- [29].Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ, The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hutcheson J, Vanarsa K, Bashmakov A, Grewal S, Sajitharan D, Chang BY, Buggy JJ, Zhou XJ, Du Y, Satterthwaite AB, Mohan C, Modulating proximal cell signaling by targeting Btk ameliorates humoral autoimmunity and end-organ disease in murine lupus, Arthritis Res. Ther 14 (2012) R243. doi: 10.1186/ar4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mina-Osorio P, LaStant J, Keirstead N, Whittard T, Ayala J, Stefanova S, Garrido R, Dimaano N, Hilton H, Giron M, Lau K-Y, Hang J, Postelnek J, Kim Y, Min S, Patel A, Woods J, Ramanujam M, DeMartino J, Narula S, Xu D, Suppression of glomerulonephritis in lupus-prone NZB × NZW mice by RN486, a selective inhibitor of Bruton’s tyrosine kinase, Arthritis Rheum. 65 (2013) 2380–2391. doi: 10.1002/art.38047. [DOI] [PubMed] [Google Scholar]
- [32].Bender AT, Pereira A, Fu K, Samy E, Wu Y, Liu-Bujalski L, Caldwell R, Chen Y-Y, Tian H, Morandi F, Head J, Koehler U, Genest M, Okitsu SL, Xu D, Grenningloh R, Btk inhibition treats TLR7/IFN driven murine lupus, Clin. Immunol 164 (2016) 65–77. doi: 10.1016/j.clim.2016.01.012. [DOI] [PubMed] [Google Scholar]
- [33].Chalmers SA, Wen J, Doerner J, Stock A, Cuda CM, Makinde HM, Perlman H, Bosanac T, Webb D, Nabozny G, Fine JS, Klein E, Ramanujam M, Putterman C, Highly selective inhibition of Bruton’s tyrosine kinase attenuates skin and brain disease in murine lupus, Arthritis Res. Ther 20 (2018). doi: 10.1186/s13075-017-1500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O’Brien S, Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia, N. Engl. J. Med 369 (2013) 32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]