Highlights
-
•
Modern IGRT has given new insight regarding organ motion in radiotherapy.
-
•
Rectal volume variation may increase the risk of biochemical and local failure.
-
•
Rectal volume decreased significantly during eight weeks of radiotherapy.
-
•
The percentage of irradiated rectal volume did not change statistically significant.
-
•
Our study shows that IGRT ensures a close to stable dose to the rectum.
1. Introduction
The introduction of modern image-guided radiotherapy (IGRT) has given new insight regarding organ motion in radiotherapy (RT), both in general and in treatment for prostate cancer (PC) [1]. IGRT with daily Cone-beam computed tomography (CBCT) is now considered as part of standard external beam radiotherapy (EBRT) in an increasingly number of cancer patients. Interfraction displacement of the prostate gland during RT is often observed in response to the variations in rectum and bladder filling, and can range from 0 to 20 mm [1], [2], [3], [4], [5], [6], [7]. Accordingly, rectal volume variation (RVV) during RT may increase the risk of biochemical and local failure [8], [9], [10], [11], [12], [13]. Moreover, the rectal volume (RV) receiving ≥ 60 Gy is associated with increased risk of grade ≥ 2 late rectal toxicity or rectal bleeding and can be a limiting factor for dose escalation [14], [15], [16], [17], [18], [19].
Some small sample-size studies have reported variable rectal dose distribution due to RVV during RT [2], [20], [21]. In order to minimize RVV, some authors advocate rectum emptying using an enema eventually combined with laxatives and dietary measures at the time of the initial planning computed tomography (CT) and during the treatment period, especially if daily IGRT is not applied [22], [23]. Consequently, studies on radiation dose distribution and variations in Organs at Risk during the total treatment period are essential to gain knowledge about the accuracy of dose delivery to the tumor and the surrounding normal tissue.
The aim of this study was to answer the following research question: Are rectal volumes reduced or increased, and are rectal doses consequently reduced or increased during eight weeks of radical 3D conformal CBCT-IGRT in patients treated for PC?
2. Material and methods
2.1. Patient selection and RT treatment
Between October 2012 and June 2013, 30 consecutively treated patients with high risk or intermediate risk PC (according to D’Amico’s risk stratification [24]) at two Norwegian hospitals (St. Olavs Hospital and Ålesund Hospital) were included in this study. All patients received 70 Gy to a planning target volume (PTV2) which included a clinical target volume (CTV2) consisting of the prostate and basal 10 mm (intermediate risk PC) or 20 mm (high risk PC) of the seminal vesicles with an additional 7 mm margin in all directions. In addition, the patients received an 8 Gy boost to a CTV1 consisting of the prostate with a 3 mm margin to a PTV1. Elective lymph node irradiation was not performed. CT-based, 3D-conformal treatment planning was mandatory, as were multi- leaf collimators (MLC). A four-field box technique with necessary supplemental field segments was applied with 15 megavoltage photon beams from 0 to 70 Gy. For the 8 Gy boost, a 5 field (1 anterior, 2 oblique anterior and 2 lateral) technique was applied. All patients had 4 prostatic fiducial gold markers implanted prior to the RT and the isocenter was placed in the fiducial gold marker located closest to the base of the prostate. The target volume doses were within 95–107% of the prescribed dose. The rectal dose constraint was defined as 60 Gy to no more than half of the rectal circumference. If necessary, posterior blocking of the rectum with MLC was accepted even if this resulted in reduced dose to the PTV. Patients were treated in supine position without rigid immobilization. After alignment by skin markers, 3D kilovoltage imaging with CBCT (XVI, Elekta AB©, Stockholm, Sweden), of prostate with fiducial markers were performed and all localization errors corrected prior to each fraction (treatment 1–39). One hour before the initial planning CT-scan (CT1) patients were asked to empty the bladder and drink two glasses of water. Emptying of the rectum was not mandatory before the dose planning CT or the subsequent RT fractions.
The present study was a side study to a previous published phase III randomized controlled trial (a Randomized, two centre trial on daily cone-beam IGRT vs standard weekly orthogonal IGRT in Curative radiotherapy for prostate cancer, the RIC-study) which included 260 PC patients [25].
2.2. Calculation of volume and doses on CBCT
In each patient, eight CBCT-scans (slice thickness 2 mm) obtained at fraction number 1, 6, 11, 16, 21, 26, 31 and 36 (CBCT1-8) were transferred to the Oncentra© (Elekta AB, Stockholm, Sweden) treatment planning system. The RV was manually outlined on the planning CT (CT1) and CBCT1-8, resulting in 9 rectum contours for each patient (270 in total for all 30 patients). One Clinical Oncologist (HT) outlined the RVs including the outer wall from the recto-sigmoid transition to the caudal part of the anus on all CBCTs, and the RVs (cm3) were calculated automatically. The eight rectum contours obtained from each patient were imported and merged with CT1 using the prostatic fiducial gold markers as reference. Recalculation of rectal dose was done for each CBCT1-8 using CT1 and set up beams. Dose-volume histograms (DVHs) for RVs receiving 50, 60, 65, and 70 Gy (V50Gy, V60Gy, V65Gy and V70Gy) were software estimated, both in cm3 and in percentage of irradiated RV.
2.3. Statistics
Descriptive statistics for volumes are reported as mean and standard deviation (SD), and illustrated with box plots displaying the mean, quartiles, and minimum and maximum values. A time trend in RV was analyzed using a two-level mixed model with volume as dependent variable, patient as random effect and week number (0–8) as continuous covariate, with a possible deviating volume at initial planning CT-scan (time 0). A time trend in percent irradiated volume was analyzed using a three-level mixed model with irradiated percent as dependent variable, patient as random effect, time point as random effect nested within patient, dose as a four-level categorical covariate, week number (0–8) as continuous covariate, and interaction between dose and week number, with a possible deviating volume at initial planning CT-scan (time 0). Normality of residuals was checked by visual inspection of Q-Q plots. The residuals for RV were slightly skewed. Hence, alternative analyses with log transformed RV as dependent variable were carried out. Ninety-five percent confidence intervals (CI) are reported where relevant.
Analyses were carried out in SPSS® ver. 22.
3. Results
Baseline characteristics are described in Table 1. Mean age was 71 years and 60% of the patients had high-risk PC.
Table 1.
Baseline characteristics. SD: Standard deviation, CTCAE: Common toxicity criteria for adverse effects version 4.0.
| Age (years) (SD) | 71.0 (5.3) |
| Aalesund Hospital | 9 |
| St. Olavs hospital | 21 |
| PSA mean (nmol/l) (SD) | 17.7 (13.2) |
| Clinical stage | |
| T1 | 6 |
| T2 | 10 |
| T3 | 14 |
| Gleason score | |
| 6 | 3 |
| 7 | 16 |
| 8 | 6 |
| 9 | 5 |
| High risk | 18 |
| Intermediate risk | 12 |
| CTCAE grade at inclusion | |
| 0 | 23 |
| 1 | 5 |
| 2 | 2 |
| CTCAE grade at end of RT | |
| 0 | 6 |
| 1 | 21 |
| 2 | 3 |
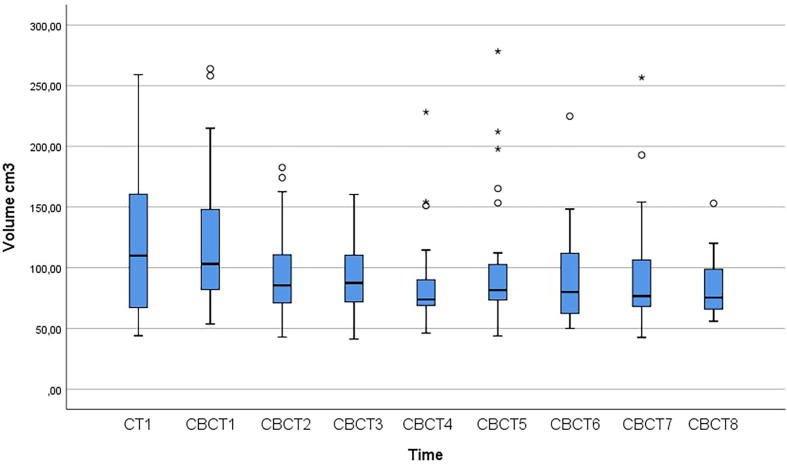
The mean RV in CT 1 was 114.6 (43.9–259.1) cm3 whereas the mean RV in all 240 CBCT scans was 94.3 (41.9–278.3) cm3. (Table 2, Fig. 1). In the two patients with the largest (202.1 cm3) and smallest (20.3 cm3) RVV, the volumes ranged from 61.8 to 263.9 cm3 and 67.4 to 87.7 cm3, respectively (Fig. 2). The individual RVV over the treatment course (including CT1) was considerable with an estimated mean of 95.6 cm3. Six out of 30 patients had a RVV of >150 cm3. Fig. 3 shows the RVs as outlined on CT1 and CBCT1-8 in one randomly selected patient with a RVV of 171.5 cm3, ranging from 87.6 cm3 (CBCT2) to 259.1 cm3 (CBCT1).
Table 2.
Mean and standard deviation (SD) for rectal volumes for the n = 30 patients at initial planning CT-scan, and then weekly during treatment.
| Initial planning CT-scan | CBCT 1 | CBCT 2 | CBCT 3 | CBCT 4 | CBCT 5 | CBCT 6 | CBCT 7 | CBCT 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Mean (cm3) | 114.6 | 119.2 | 94.9 | 91.6 | 85.1 | 99.1 | 90.2 | 91.3 | 82.8 |
| SD | 55.3 | 56.6 | 38.2 | 27.5 | 37.2 | 53.0 | 37.7 | 44.8 | 22.5 |
Fig. 1.
Boxplot of rectal volumes at initial planning CT-scan (0) and during treatment (week 1 to 8). The horizontal line represents the median, and the box covers the inter-quartile range. The ends of the whiskers show the range of observations less than 1.5 inter-quartile range from the box, and circles and asterisks show the more distant observations, more than 1.5 box-lengths and more than 3 box-lengths away from the box, respectively.
Fig. 2.
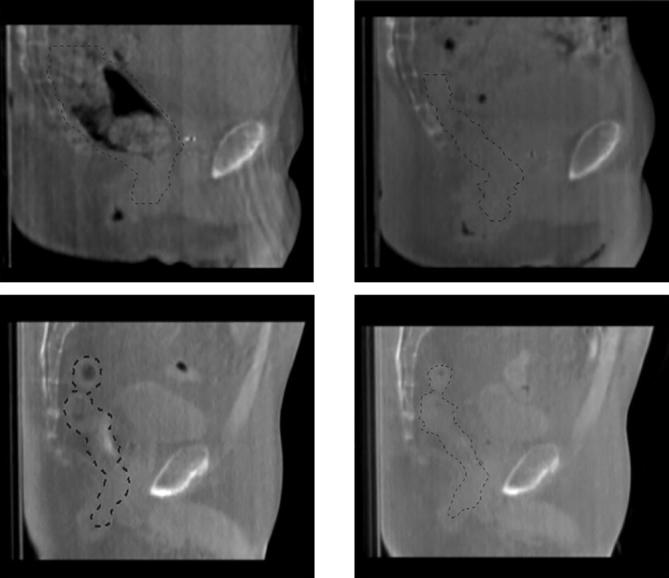
Images for the patients with the largest (above line) and smallest (below line) range on CBCT 1–8 (range 202.1 cm3 and 20.3 cm3, respectively). Green line representing outlined rectal volume. Left images represent CBCT 1 (volumes 263.9 cm3 and 87.7 cm3, respectively), right images represent CBCT 6 (61.8 cm3) and CBCT 3 (67.4 cm3), respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
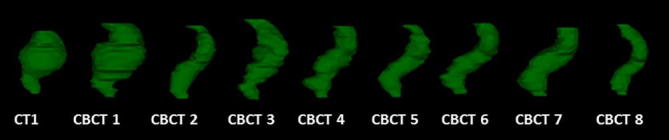
Example of rectal volume variation during radiotherapy for prostate cancer for one patient. Maximal volume on CT 1 = 259.1 cm3, minimal volume on CBCT 2 = 87.6 cm3. Range 171.5 cm3.
When applying a linear mixed regression model, the mean volume reduction was estimated as 110.1 – 3.55 * t cm3, where t is week number (set to 0 at the initial planning). That is, the mean volume was reduced by an estimated 3.55 cm3 (CI 1.90 to 5.21), p < 0.001 per week. Adding an extra term for the initial planning (time 0) did not change the estimates notably. The distribution was slightly skewed at each time point, and the mean volumes and variance tended to be higher at the time of dose planning and in the first week of RT (Fig. 1). Secondary analysis with log transformed volumes gave symmetric distributions with equal variances, and gave essentially the same reduction in volume over time as for untransformed volumes.
The mean proportion of RVs irradiated to 50 Gy (V50Gy) on the planning CT-scan and CBCT1-8 was 34.1%. The corresponding figures for V60, V65, and V70Gy were 26.9, 22.3, and 15.6%, respectively.
Applying a linear mixed regression model, an increase of 0.18% (CI -0.182 to 0.550, p = 0.30) per week was estimated for V70Gy, corresponding to an absolute increase of 1.47% over 8 weeks. The absolute increase over 8 weeks for volumes irradiated to 50, 60, and 65 Gy (1.14, 1.12, and 1.20%, respectively) was not statistically significant (p = 0.42, 0.43, and 0.39, respectively). Adding an extra term for the initial planning (time 0) gave essentially the same results.
Table 3 shows the distribution of percentage of RVs receiving 50, 60, 65, and 70 Gy (V50Gy, V60Gy, V65Gy and V70Gy).
Table 3.
Mean percentage of irradiated rectal volumes for V50Gy, V60Gy, V65Gy and V70Gy, range (%) in parenthesis.
| CT 1 | CBCT 1 | CBCT 2 | CBCT 3 | CBCT 4 | CBCT 5 | CBCT 6 | CBCT 7 | CBCT 8 | |
|---|---|---|---|---|---|---|---|---|---|
| V50 Gy | 33.0% (50.4–21.6) |
35.7% (49.8–17.9) |
33.8% (57.5–12.1) |
33.5% (54.4–18.7) |
32.7% (54.8–15.5) |
33.5% (56.8–21.6) |
34.6% (51.5–22.2) |
33.9% (50.6–21.6) |
36.1% (59.9–18.3) |
| V60 Gy | 25.6% (42–16.3) |
28.7% (39.8–12.7) |
26.5% (47.3–8.6) |
26.3% (44.6–13) |
25.7% (46.1–10.3) |
26.5% (48.1–17.1) |
27.3% (43.7–15.8) |
26.6% (42.4–15.4) |
28.8% (49.5–14.3) |
| V65 Gy | 20.9% (36.3–12.8) |
24.1% (34.1–9.9) |
21.8% (41.3–6.5) |
21.9% (39.3–10.0) |
21.2% (41.1–7.6) |
22.1% (43.5–13.5) |
22.8% (38.8–11.5) |
22.0% (38.1–11.5) |
24.2% (43.7–11.3) |
| V70 Gy | 14.0% (24.4–8.0) |
16.8% (25.4–6.4) |
15.1% (29.2–4.2) |
15.2% (28.3–5.9) |
14.9% (31.9–4.2) |
15.7% (33.0–3.3) |
16.2% (28.5–7.5) |
15.1% (29.1–7.1) |
17.4% (31.6–7.9) |
4. Discussion
The principal finding in this study was that although the RV decreased significantly in this cohort of PC patients, the RVV did not influence on the percentage of planned irradiated RV receiving 50, 60, 65 and 70 Gy, which were close to constant during the eight weeks of RT. Most of the RV reduction occurred early in the treatment period and mainly between the initial planning CT and CBCT2. Moreover, the inter-individual RVV was considerable and ranged from 20.3 cm3 to 202.1 cm3 during the treatment period.
One major limitation in previous studies on RVV in prostatic RT have been few (24 or less) included patients [20], [21], [26], [27]. Our study included 30 consecutive PC patients from the experimental arm of a randomized controlled trial [25]. It is possible that rarely occurring extreme variations in RV may have been missed also in our study due to a limited sample size of 30 patients. On the other hand, a total of 270 CT-scans were included, and we believe our results to be representative of RVVs amongst patients receiving RT for PC.
Several authors have found a decrease in RV during EBRT for PC patients. In accordance with our findings, the main reduction in RV occurs early in the treatment period [21], [26], [27]. Zellars et al. reported a significant decrease in RV in 18 of 24 patients when comparing the planning CT-scan with a single CT-scan 4–5 weeks after prostatic irradiation [27]. Antolak et al. compared the planning CT-scan with three CT-scans obtained during the 8 weeks treatment period and also found a significant decrease in RV in 17 patients receiving prostatic irradiation [26]. Sripadam et al. found a significant decrease in rectal cross-sectional area (CSA) in CBCT-scans obtained immediately after the daily treatment in 13 of 15 PC patients receiving RT (50 Gy in 16 fractions) [21]. Other authors have reported significant variations, but no systematic changes [2], [20].
A reduced CTV-PTV safety margin has the potential to lower side effects. Nevertheless, it is important not to reduce the margins excessively. Previous studies have indicated that RVV during RT may increase the risk of geographical miss, especially in patients with a distended rectum on the planning CT-scan [8], [9], [10], [11]. Heemsbergen et al. analysed 549 patients included in the Dutch prostate cancer dose-escalation trial (78 Gy vs. 68 Gy), and found a significantly reduced freedom from clinical failure in patients with anorectal volumes ≥90 cm3 on the planning CT-scan [10]. Engels et al. analysed freedom from biochemical failure (FFBF) in 238 patients given conformal RT to a total dose 70–78 Gy [9] and found that an average rectal CSA of ≥16 cm2 was associated with worse FFBF. In another study reported by Engels et al., 50 patients treated with IGRT and daily positioning using fiducial gold markers were analysed [11]. This study demonstrated a reduced 5-year FFBF in patients with a larger rectal distention on the planning CT-scan compared to those with limited rectal distention (75% vs 89%). Other authors claim that the adverse effects of rectal distention on local control can be compensated by the use of modern IGRT [12], [28]. Park et al. measured CSA on the planning CT-scans in 962 PC patients receiving adaptive RT with a median prescribed dose of 75.6 Gy [28]. The authors found that initial rectal distention was not significantly associated with reduced 5-years biochemical cancer control or grade ≥ 2 genitourinary and gastrointestinal toxicity, and concluded that adaptive IGRT reduces the risk of geographical miss. Silverman et al. examined 172 PC patients receiving conformal RT to a total dose of 74 Gy at a median of 72 months follow up [12]. The rectal diameter was measured at the midpoint of the PTV on the planning CT-scan. A large (>4.5 cm) rectal diameter on the planning CT-scan was not associated with increased risk of PSA-relapse. The PTV margins applied in Park and Silvermans studies were, however, larger than the studies reported by Engels et al. [9].
Emptying of the rectum was not routine before the planning CT-scan or the subsequent RT fractions in our study. Several authors recommend use of laxatives and/or rectal emptying before dose planning/each fraction, especially if daily IGRT is not applied [2], [22], [23]. Chen et al. advocate that prostatic IMRT should be planned with an empty rectum in order to increase the accuracy of the dose distribution [2]. Engels et al. aimed at predicting which PC patients may benefit from daily rectal emptying by analysing 18 patients receiving RT to the prostate and iliac nodes with daily IGRT [23]. Before the planning CT-scan all patients had an enema. Two typical groups were observed; one with a limited and stable rectal diameter and one with a large RVV. The authors suggest that a CSA cut-off value estimated using data from the first 3–5 fractions may be helpful in deciding which patients would benefit from an enema. A review regarding effectiveness of rectal emptying reported by McNair et al. compared dietary interventions, oral and intravenous laxatives, enemas, or combinations [29]. No evidence in support of a superior strategy was found. Moreover, no significant difference in RV was observed in another cohort of 80 PC patients given EBRT at Aalesund hospital, of whom 40 were given laxatives regularly during the eight weeks treatment period [30]. Thus the optimal regime and effectiveness of rectal emptying is still unknown. Although the shrinkage in total RV in our study was significant, the percentage of irradiated RV remained unchanged. These findings indicate that the IGRT-technique applied in the RIC-study eliminates the risk of increased dose to the rectum caused by volume shrinkage. Based on our results, we believe rectal emptying and other more invasive and expensive methods for limiting the movement of internal organs can be omitted if fiducial markers and daily CBCT are used.
The dose constraint to the rectum in the RIC-study was 60 Gy to no more than 50% of the rectum circumference on the planning CT-scan. If exceeded, a posterior shielding of the PTV with multi leaf collimators was accepted. Accordingly, the limit of 60 Gy to 50% of the estimated RV was not exceeded in any of the included patients in this subset of 30 patients. The grade of Common toxicity criteria for adverse effects (CTCAE) in this side study corresponded well to the overall grade of CTCAE reported in the main study (Table 1). We found no grade 3 toxicity in the 30 patients compared to 1 out of 125 patient in the corresponding arm in the RIC-study.
The volume of rectum receiving ≥ 60 Gy is associated with the risk of grade ≥ 2 late rectal toxicity or rectal bleeding and may be a limiting factor for dose escalation [14], [15], [16], [17], [18], [19]. Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) recommend a number of rectal dose-volume constraints in RT for PC including V60Gy < 35% and V70Gy < 20% [19]. In our study five patients had a mean V60Gy > 35% and five a mean V70Gy > 20% despite the small margins from CTV to PTV. Since the irradiated RV remained unchanged in this study, we believe that the use of daily IGRT with sufficient PTV-margins may have the potential to counteract adverse effects of RVV and initial distention on rectal toxicity.
In conclusion, the RV decreased significantly by an estimated 3.55 cm3 per week during 8 weeks of radical, 3D conformal RT in 30 PC patients. The majority of the reduction occurred during the initial 2–3 weeks. However, the irradiated RVs during the treatment period remained unchanged. Consequently, the use of frequent IGRT with CBCT and fiducial gold markers seems to eliminate possible adverse effects of RVV ensuring an acceptable and stable radiation dose to the rectum which corresponds with the initial planned rectal dose and within acceptable dose levels.
Acknowledgements
This study was funded by the Norwegian Cancer Society.
References
- 1.Langen K.M., Jones D.T. Organ motion and its management. Int J Radiat Oncol Biol Phys. 2001;50(1):265–278. doi: 10.1016/s0360-3016(01)01453-5. [DOI] [PubMed] [Google Scholar]
- 2.Chen L., Paskalev K., Xu X., Zhu J., Wang L., Price R.A. Rectal dose variation during the course of image-guided radiation therapy of prostate cancer. Radiother Oncol. 2010;95(2):198–202. doi: 10.1016/j.radonc.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Brierley J.D., Dawson L.A., Sampson E., Bayley A., Scott S., Moseley J.L. Rectal motion in patients receiving preoperative radiotherapy for carcinoma of the rectum. Int J Radiat Oncol Biol Phys. 2011;80(1):97–102. doi: 10.1016/j.ijrobp.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z., Yang Z., Wang J., Hu W. Dosimetric impact of different bladder and rectum filling during prostate cancer radiotherapy. Radiat Oncol. 2016;11:103. doi: 10.1186/s13014-016-0681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Button M.R., Staffurth J.N. Clinical application of image-guided radiotherapy in bladder and prostate cancer. Clin Oncol (R Coll Radiol) 2010;22(8):698–706. doi: 10.1016/j.clon.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Gwynne S., Webster R., Adams R., Mukherjee S., Coles B., Staffurth J. Image-guided radiotherapy for rectal cancer: a systematic review. Clin Oncol (R Coll Radiol) 2012;24(4):250–260. doi: 10.1016/j.clon.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Oates R., Gill S., Foroudi F., Joon M.L., Schneider M., Bressel M. What benefit could be derived from on-line adaptive prostate radiotherapy using rectal diameter as a predictor of motion? J Med Phys. 2015;40(1):18–23. doi: 10.4103/0971-6203.152237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Crevoisier R., Tucker S.L., Dong L., Mohan R., Cheung R., Cox J.D. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62(4):965–973. doi: 10.1016/j.ijrobp.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Engels B., Soete G., Verellen D., Storme G. Conformal arc radiotherapy for prostate cancer: increased biochemical failure in patients with distended rectum on the planning computed tomogram despite image guidance by implanted markers. Int J Radiat Oncol Biol Phys. 2009;74(2):388–391. doi: 10.1016/j.ijrobp.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Heemsbergen W.D., Hoogeman M.S., Witte M.G., Peeters S.T., Incrocci L., Lebesque J.V. Increased risk of biochemical and clinical failure for prostate patients with a large rectum at radiotherapy planning: results from the Dutch trial of 68 GY versus 78 Gy. Int J Radiat Oncol Biol Phys. 2007;67(5):1418–1424. doi: 10.1016/j.ijrobp.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Engels B., Soete G., Gevaert T., Storme G., Michielsen D., De Ridder M. Impact of planning target volume margins and rectal distention on biochemical failure in image-guided radiotherapy of prostate cancer. Radiother Oncol. 2014;111(1):106–109. doi: 10.1016/j.radonc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Silverman R., Johnson K., Perry C., Sundar S. Degree of rectal distension seen on prostate radiotherapy planning CT Scan is not a negative prognostic factor in the modern era of image-guided radiotherapy. Oncology. 2016;90(1):51–56. doi: 10.1159/000441225. [DOI] [PubMed] [Google Scholar]
- 13.Kupelian P.A., Lee C., Langen K.M., Zeidan O.A., Manon R.R., Willoughby T.R. Evaluation of image-guidance strategies in the treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(4):1151–1157. doi: 10.1016/j.ijrobp.2007.07.2371. [DOI] [PubMed] [Google Scholar]
- 14.Heemsbergen W.D., Peeters S.T., Koper P.C., Hoogeman M.S., Lebesque J.V. Acute and late gastrointestinal toxicity after radiotherapy in prostate cancer patients: consequential late damage. Int J Radiat Oncol Biol Phys. 2006;66(1):3–10. doi: 10.1016/j.ijrobp.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien P.C., Franklin C.I., Poulsen M.G., Joseph D.J., Spry N.S., Denham J.W. Acute symptoms, not rectally administered sucralfate, predict for late radiation proctitis: longer term follow-up of a phase III trial–trans-tasman radiation oncology group. Int J Radiat Oncol Biol Phys. 2002;54(2):442–449. doi: 10.1016/s0360-3016(02)02931-0. [DOI] [PubMed] [Google Scholar]
- 16.Denham J.W., O'Brien P.C., Dunstan R.H., Johansen J., See A., Hamilton C.S. Is there more than one late radiation proctitis syndrome? Radiother Oncol. 1999;51(1):43–53. doi: 10.1016/s0167-8140(99)00027-4. [DOI] [PubMed] [Google Scholar]
- 17.Jackson A., Skwarchuk M.W., Zelefsky M.J., Cowen D.M., Venkatraman E.S., Levegrun S. Late rectal bleeding after conformal radiotherapy of prostate cancer. II. Volume effects and dose-volume histograms. Int J Radiat Oncol Biol Phys. 2001;49(3):685–698. doi: 10.1016/s0360-3016(00)01414-0. [DOI] [PubMed] [Google Scholar]
- 18.Fransson P., Lund J.A., Damber J.E., Klepp O., Wiklund F., Fossa S. Quality of life in patients with locally advanced prostate cancer given endocrine treatment with or without radiotherapy: 4-year follow-up of SPCG-7/SFUO-3, an open-label, randomised, phase III trial. Lancet Oncol. 2009;10(4):370–380. doi: 10.1016/S1470-2045(09)70027-0. [DOI] [PubMed] [Google Scholar]
- 19.Michalski J.M., Gay H., Jackson A., Tucker S.L., Deasy J.O. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kupelian P.A., Langen K.M., Zeidan O.A., Meeks S.L., Willoughby T.R., Wagner T.H. Daily variations in delivered doses in patients treated with radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66(3):876–882. doi: 10.1016/j.ijrobp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Sripadam R., Stratford J., Henry A.M., Jackson A., Moore C.J., Price P. Rectal motion can reduce CTV coverage and increase rectal dose during prostate radiotherapy: a daily cone-beam CT study. Radiother Oncol. 2009;90(3):312–317. doi: 10.1016/j.radonc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Stasi M., Munoz F., Fiorino C., Pasquino M., Baiotto B., Marini P. Emptying the rectum before treatment delivery limits the variations of rectal dose – volume parameters during 3DCRT of prostate cancer. Radiother Oncol. 2006;80(3):363–370. doi: 10.1016/j.radonc.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Engels B., Tournel K., Soete G., Storme G. Assessment of rectal distention in radiotherapy of prostate cancer using daily megavoltage CT image guidance. Radiother Oncol. 2009;90(3):377–381. doi: 10.1016/j.radonc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 24.D'Amico A.V., Whittington R., Malkowicz S. BIochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 25.Tondel H., Lund J.A., Lydersen S., Wanderas A.D., Aksnessaether B., Jensen C.A. Radiotherapy for prostate cancer - Does daily image guidance with tighter margins improve patient reported outcomes compared to weekly orthogonal verified irradiation? Results from a randomized controlled trial. Radiother Oncol. 2018;126(2):229–235. doi: 10.1016/j.radonc.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Antolak J.A., Rosen I.I., Childress C.H., Zagars G.K., Pollack A. Prostate target volume variations during a course of radiotherapy. Int J Radiat Oncol Biol Phys. 1998;42(3):661–672. doi: 10.1016/s0360-3016(98)00248-x. [DOI] [PubMed] [Google Scholar]
- 27.Zellars R.C., Roberson P.L., Strawderman M., Zhang D., Sandler H.M., Ten Haken R.K. Prostate position late in the course of external beam therapy: patterns and predictors. Int J Radiat Oncol Biol Phys. 2000;47(3):655–660. doi: 10.1016/s0360-3016(00)00469-7. [DOI] [PubMed] [Google Scholar]
- 28.Park S.S., Yan D., McGrath S., Dilworth J.T., Liang J., Ye H. Adaptive image-guided radiotherapy (IGRT) eliminates the risk of biochemical failure caused by the bias of rectal distension in prostate cancer treatment planning: clinical evidence. Int J Radiat Oncol Biol Phys. 2012;83(3):947–952. doi: 10.1016/j.ijrobp.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 29.McNair H.A., Wedlake L., Lips I.M., Andreyev J., Van Vulpen M., Dearnaley D. A systematic review: effectiveness of rectal emptying preparation in prostate cancer patients. Pract Radiat Oncol. 2014;4(6):437–447. doi: 10.1016/j.prro.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Jensen C., Johansen M., Resell M., Kylling A., Skottner N., Fremsæter O. The use of laxative and the impact on rectum filling, intra-fraction motion and dose to ptv for prostate patients. Radiother Oncol. 2009;92:S175–S176. [Google Scholar]