Highlights
-
•
Bactrim failure adverse effects.
-
•
Alternative echinocandin target.
-
•
Combination echinocandin therapy.
Abstract
Trimethoprim-sulfamethoxazole (TMP-SMX, co-trimoxazole, or bactrim) has been the standard first-line treatment against Pneumocystis jirovecii pneumonia (PCP) for decades. However, adverse effects and cases of treatment failure have led to a search for alternative agents. We present a case of a 50 year old immune compromised female whose course of PCP did not improve until Caspofungin was added to TMP-SMX.
Introduction
Initially classified as a protozoa, Pneumocystis was later identified as a member of ascomycetous fungi based on ribosomal RNA sequencing in the 1980′s [1].
About eighty percent of children are exposed to this ubiquitous organism by two to three years of age but its virulence is low, often causing no more than colonization. Even in the immunocompromised host pneumocystis pneumonia often presents as a mild, insidious and nonspecific illness but can lead to respiratory failure and death [2].
Trimethoprim-sulfamethoxazole is effective for both prophylaxis and treatment of PCP [3,4]. It is not, however, without adverse effects and hypersensitivity reactions are particularly common in patients with HIV compared to those without HIV- up to two thirds of individuals versus one in twenty, respectively. Other adverse reactions include rash, cytopenia, nausea, vomiting, and metabolic derangements such as hyperkalemia, hyponatremia and acidosis. Intolerance leads to discontinuation in up to half of patients with HIV [5]. In addition, there is concern for developing antibiotic resistance. The sulfamethoxazole component, an analog of para-aminobenzoic acid, competitively binds to dihydropteroate synthetase (DHPS), blocking an intermediate step in the production of tetrahydrofolate needed for DNA synthesis. Mutations in this enzyme and not dihydrofolate reductase (DHFR), the site of action for the trimethoprim component of co-trimoxazole, is associated with prior use of co-trimoxazole and dapsone [6,7]. Whether or not this translates to poor clinical outcomes is not clear. A systematic review published in 2004 and other studies since then have either not shown or not concurred on an association between DHPS mutations and treatment failure [[8], [9], [10], [11], [12]]. Regardless, the adverse effects of co-trimoxazole along with a limited number of effective alternatives highlight the need for further drug investigation. Current alternative options include clindamycin plus primaquine, pentamidine, atovaquone, and trimethoprim plus dapsone. Only the first two options are recommended for cases of severe PCP, however, and the toxicity of intravenous pentamidine makes it a less desirable choice. [2] A meta-analysis of salvage therapy for PCP concluded that clindamycin plus primaquine is the preferred alternative therapy to TMP-SMX due to its high efficacy of 88–92% [13].
Echinocandins, caspofungin in particular, have also shown some promise against Pneumocystis. Unlike most fungi, Pneumocystis lacks ergosterol which is normally an integral component of the fungal cell membrane and thus, azole and polyene therapies are ineffective. The organism does, however, produce and depend upon beta-D Glucan in its cell wall which can be targeted by echinocandins. Prospective randomized trials are lacking but several case reports have shown success with caspofungin in treating severe refractory PCP either as monotherapy or combined with traditional agents [[14], [15], [16], [17]].
Case report
A 50-year-old female on prednisone of 60 mg daily for Sjogren’s syndrome was diagnosed with Diffuse Large B-Cell Lymphoma in May 2016. In addition, she had Mikulicz syndrome, Mixed Connective Tissue disease, Antiphospholipid syndrome and factor V Leiden deficiency. She was planned for initiation of R−CHOP but presented with a new cough, dyspnea, fevers and bilateral ground glass opacities on chest CT (Fig. 1). Broad spectrum antibiotics were administered for pneumonia and after three days she transferred hospitals for chemotherapy. Bactrim at treatment dose was promptly begun after transfer for possible PCP and Prednisone was increased to 100 mg daily for chemotherapy. She had been taking a prophylactic dose of Bactrim for only two days prior to presentation. By day seven she was sent to the intensive care monitoring for worsening hypoxia and underwent a bronchoscopy which confirmed pneumocystis organisms on cytopathology. BAL cultures including for acid fast organisms, blood cultures, common respiratory viral PCR testing and HIV screening were all negative. Despite two weeks of appropriate therapy with clinical improvement permitting Prednisone to be tapered to 40 mg daily, she had recurrence of fevers, worsening hypoxemia and progression of bilateral patchy opacities on chest radiograph (Fig. 2). Due to poor clinical response without an alternative diagnosis, she underwent a second bronchoscopy which demonstrated organizing pneumonia on pathology associated with Pneumocystis carinii infection. Intravenous caspofungin was then added to her regimen, 70 mg followed by 50 mg every 24 h. Slowly she began to improve on both bactrim and caspofungin and was eventually transferred to medical wards after one week of combination therapy. She received a total of two weeks of caspofungin and six weeks of intravenous TMP-SMX. Her bilateral opacities improved (Fig. 3) and she was discharged home on secondary oral prophylaxis and a steroid taper.
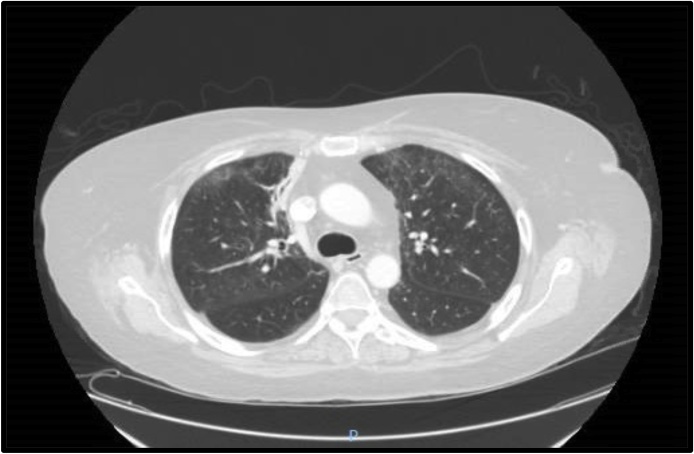
Fig. 1.
CT chest on admission showing scatter bilateral groundglass opacities.
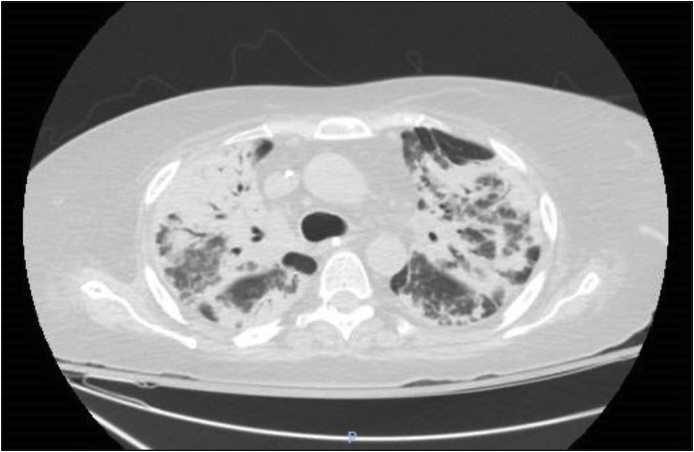
Fig. 2.
CT chest after two weeks of Bactrim therapy showing progression of bilateral consolidations with airbronchograms compatible with extensive multifocal pneumonia.
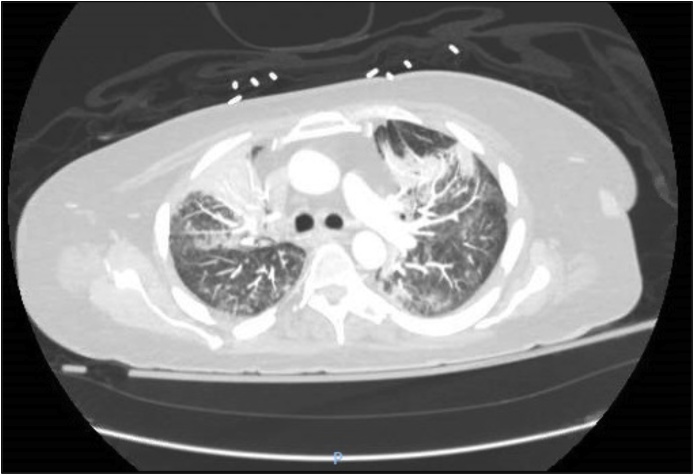
Fig. 3.
CT chest after 6 weeks of PCP treatment showing decreased bilateral groundglass opacities and dense consolidations.
Discussion
Our patient had at least a few risk factors for developing pneumocystis pneumonia including use of high-dose corticosteroids, hematologic malignancy and inflammatory rheumatologic conditions. In addition, she also had risk factors for poor outcome: low hemoglobin, elevated LDH levels despite treatment, poor oxygenation and low albumin. Although the mortality rate among HIV patients with PCP has decreased significantly with the advent of antiretroviral therapy, patients such as ours who are not infected with HIV continue to face a 30–50% mortality rate [2]. The need for new, efficacious, and safe agents against PCP is made apparent in this patient population.
Pneumocystis produces 1-3-beta-d-glucan, an essential component of its fungal cell wall in the cystic form and thus, a potential target for echinocandin therapy. Rodent models have demonstrated the efficacy of echinocandins in enhancing survival, reducing cyst burden and preventing pathogen transmission [18,19]. Case reports, however, demonstrated inconsistent results. Hong et al reported four cases of HIV-negative patients from Seoul, South Korea who did not respond with caspofungin as salvage therapy, three of whom died from complications of their infection [20]. Kamboj et al described two patients from Memorial Sloan Kettering Cancer Center with hematologic malignancies who each died while on combination therapy - one with bactrim and micafungin, the other with caspofungin and intravenous pentamidine [21].
For our patient, however, the addition of caspofungin was the turning point in her course and others have had similar results. Tu et al reported three renal transplant patients in China who received the same combination therapy for PCP and were successfully weaned off either mechanical or noninvasive ventilation [22]. An AIDS patient in Taiwan had complete recovery from PCP with caspofungin after bactrim was discontinued due to adverse effects of rash and leukopenia [17]. Li et al described a case of caspofungin plus clindamycin successfully treating PCP in a patient with IgA nephropathy who did not tolerate bactrim due to oral ulcerations and hemorrhages [14]. The discrepancy between case reports may be due to difference in immune suppression and unclear risk factors for poor outcome including DHPS mutations. Randomized control trials will be necessary to clarify the role of echinocandins in treatment regimens for PCP.
CRediT authorship contribution statement
Robin Koshy: Conceptualization, Writing - review & editing, Visualization, Supervision. Thomas Chen: Writing - original draft, Writing - review & editing, Visualization.
Contributor Information
Robin Koshy, Email: rkoshy@northwell.edu.
Thomas Chen, Email: tchen10@northwell.edu.
References
- 1.Edman J.C., Kovacs J.A., Masur H., Santi D.V., Elwood H.J., Sogin M.L. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the Fungi. Nature. 1988;334:519–522. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- 2.Walzer P.D., Smulian A.G., Miller R.F. Pneumocystis Species. In: Mandell, Douglas, Bennett's, editors. Principles and Practice of Infectious Diseases. 8th ed. Elsevier Saunders; Philadelphia, PA: 2015. pp. 3016–3029. [Google Scholar]
- 3.Thomas C.F., Jr., Limper A.H. Pneumocystis pneumonia. N. Engl. J. Med. 2004;350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 4.Hughes W.T., Rivera G.K., Schell M.J., Thornton D., Lott L. Successful intermittent chemoprophylaxis for Pneumocystis carinii pneumonitis. N. Engl. J. Med. 1987;316:1627–1632. doi: 10.1056/NEJM198706253162604. [DOI] [PubMed] [Google Scholar]
- 5.Rex J.H., Stevens D.A. Drugs Active against Fungi, Pneumocystis, and Microsporidia. In: Mandell, Douglas, Bennett's, editors. Principles and Practice of Infectious Diseases. 8th ed. Elsevier Saunders; Philadelphia, PA: 2015. p. p. 492. [Google Scholar]
- 6.Ma L., Borio L., Masur H., Kovacs J.A. Pneumocystis carinii Dihydropteroate Synthase but Not Dihydrofolate Reductase Gene Mutations Correlate with Prior Trimethoprim-Sulfamethoxazole or Dapsone Use. J. Infect. Dis. 1999;180(6):1969–1978. doi: 10.1086/315148. [DOI] [PubMed] [Google Scholar]
- 7.Kazanjian P., Armstrong W., Hossler P.A., Burman W., Richardson J., Lee C. Pneumocystis carinii mutations are associated with duration of sulfa or sulfone prophylaxis exposure in AIDS patients. J. Infect. Dis. 2000;182:551–557. doi: 10.1086/315719. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Martinez M.J., Moreno A., Miro J.M., Valls M.E., Rivas P.V., Lazzari E. Pneumocystis jirovecii pneumonia in Spanish HIV-infected patients in the combined antiretroviral therapy era: prevalence of dihydropteroate synthase mutations and prognostic factors of mortality. Diagn. Microbiol. Infect. Dis. 2008;62:34–43. doi: 10.1016/j.diagmicrobio.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Stein C.R., Poole C., Kazanjian P., Meshnick S.R. Sulfa use, dihydropteroate synthase mutations, and Pneumocystis jirovecii pneumonia. Emerg. Infect. Dis. 2004;10(10):1760–1765. doi: 10.3201/eid1010.040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Hal S.J., Gilgado F., Doyle T., Barratt J., Stark D., Meyer W., Harkness J. Clinical significance and phylogenetic relationship of novel Australian Pneumocystis jirovecii genotypes. J. Clin. Microbiol. 2009;47(6):1818–1823. doi: 10.1128/JCM.02102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crothers K., Beard C.B., Turner J., Groner G., Fox M., Morris A. Severity and outcome of HIV-associated Pneumocystis pneumonia containing Pneumocystis jirovecii dihydropteroate synthase gene mutations. AIDS. 2005;19(8):801–805. doi: 10.1097/01.aids.0000168974.67090.70. [DOI] [PubMed] [Google Scholar]
- 12.Valerio A., Tronconi E., Mazza F., Fantoni G., Atzori C., Tartarone F. Genotyping of Pneumocystis jiroveci pneumonia in Italian AIDS patients. Clinical outcome is influenced by dihydropteroate synthase and not by internal transcribed spacer genotype. J. Acquir. Immune Defic Syndr. 2007;45(5):521–528. doi: 10.1097/QAI.0b013e3180decbe2. [DOI] [PubMed] [Google Scholar]
- 13.Smego R.A., Jr, Nagar S., Maloba B., Popara M. A Meta-analysis of Salvage Therapy for Pneumocystis carinii Pneumonia. Arch. Intern. Med. 2001;161(12):1529–1533. doi: 10.1001/archinte.161.12.1529. [DOI] [PubMed] [Google Scholar]
- 14.Li H., Huang H., He H. Successful treatment of severe Pneumocystis pneumonia in an immunosuppressed patient using caspofungin combined with clindamycin: a case report and literature review. BMC Pulm. Med. 2016;16(1):144. doi: 10.1186/s12890-016-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annaloro C., Della Volpe A., Usardi P., Lambertenghi Deliliers G. Eur. J. Clin. Microbiol. Infect. Dis. 2006;25:52. doi: 10.1007/s10096-005-0065-z. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G., Chen M., Zhang S., Zhou H., Ji X., Cai J. Efficacy of caspofungin combined with trimethoprim/sulfamethoxazole as first-line therapy to treat non‑HIV patients with severe pneumocystis pneumonia. Exp. Ther. Med. 2018;15.2:1594–1601. doi: 10.3892/etm.2017.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee W., Hsueh P., Hsieh T., Chen F., Ou T., Jean S. Caspofungin salvage therapy in Pneumocystis jirovecii pneumonia. J. Microbiol. Immunol. Infect. 2017;50(4):547–548. doi: 10.1016/j.jmii.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Cushion M.T., Linke M.J., Ashbaugh A., Sesterhenn T., Collins M.S., Lynch K. Echinocandin Treatment of Pneumocystis Pneumonia in Rodent Models Depletes Cysts Leaving Trophic Burdens That Cannot Transmit the Infection. PLoS ONE. 2010;5(1):e8524. doi: 10.1371/journal.pone.0008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun P., Tong Z. Efficacy of caspofungin, a 1,3-β-D-glucan synthase inhibitor, on Pneumocystis carinii pneumonia in rats. Med. Mycol. 2014;52(8):798–803. doi: 10.1093/mmy/myu060. [DOI] [PubMed] [Google Scholar]
- 20.Hong H.L., Lee Y.M., Sung H., Kim S.H., Choi S.H., Kim Y.S. Is caspofungin really an effective treatment for Pneumocystis jirovecii pneumonia in immunocompromised patients without human immunodeficiency virus infection? Experiences at a single center and a literature review. Scand. J. Infect. Dis. 2013;45:484–488. doi: 10.3109/00365548.2012.760842. [DOI] [PubMed] [Google Scholar]
- 21.Kamboj M., Weinstock D., Sepkowitz K.A. Progression of Pneumocystis jiroveci Pneumonia in Patients Receiving Echinocandin Therapy. Clin. Infect. Dis. 2006;43(9):e92–e94. doi: 10.1086/508282. [DOI] [PubMed] [Google Scholar]
- 22.Tu G.W., Ju M.J., Xu M., Rong R.M., He Y.Z., Xue Z.G. Combination of caspofungin and low-dose trimethoprim/sulfamethoxazole for the treatment of severe Pneumocystis jirovecii pneumonia in renal transplant recipients. Nephrology. 2013;18:736–742. doi: 10.1111/nep.12133. [DOI] [PubMed] [Google Scholar]