Abstract
Brain white matter fiber bundles in patients with mild cognitive impairment (MCI) and Alzheimer's disease (AD) have abnormalities not usually seen in unaffected subjects. Ideal algorithm of the localization-specific properties in white matter integrity might reveal the changes of tissue properties varying along each tract, while previous studies only detected the mean DTI parameters of each fiber. The aim of this study was to investigate whether these abnormalities of nerve fiber tracts are localized to specific regions of the tracts or spread throughout and to analyze which of the examined fiber tracts are involved in the early stages of Alzheimer's disease. In this study, we utilized VBA, TBSS as well as AFQ together to comprehensively investigate the white matter fiber impairment on 25 CE patients, 29 MCI patients and 34 normal control (NC) subjects. Two tract profiles, fractional anisotropy (FA) and mean diffusivity (MD), were extracted to evaluate the white matter integrity at 100 locations along each of 20 fiber tracts and then we validated the results with 27 CE patients, 21 MCI patients and 22 NC from the ADNI cohort. Also, we compare the AFQ with VBA and TBSS in our cohort. In comparison with NC, AD patients showed widespread FA reduction in 25% (5 /20) and MD increase in 65%(13/20) of the examined fiber tracts. The MCI patients showed a regional FA reduction in 5% (1/20) of the examined fiber tracts (right cingulum cingulate) and MD increase in 5%(1/20) of the examined fiber tracts (left arcuate fasciculus). Among these changed tracts, only the right cingulum cingulate showed widespread disruption of myelin or/and fiber axons in MCI and aggravated deterioration in AD, findings supported by FA/MD changes both by the mean and FA changes by point wise methods and TBSS. And the AFQ findings from ADNI cohort showed some similarity with our cohort, especially in the pointwise comparison of MD profiles between AD vs NC. Furthermore, the pattern of white matter abnormalities was different across neuronal fiber tracts; for example, the MCI and AD patients showed similar FA reduction in the middle part of the right cingulum cingulate, and the anterior part were not damaged. However, the left arcuate fasciculus showed MD elevation located at the temporal part of the fibers in the MCI patients and expanding to the temporal and middle part of the fibers in AD patients. So, the AFQ may be an alternative complementary method of VBA and TBSS, and may provide new insights into white matter degeneration in MCI and its association with AD.
Keywords: Alzheimer's disease, Mild cognitive impairment, Automated fiber quantification, Diffusion tensor imaging, Pointwise comparison
Highlights
-
•
AD and MCI patients have white matter disruption compared with NC.
-
•
The abnormalities of the fiber tracts are limited to specific regions of tracts in MCI and spread throughout in AD.
-
•
The AFQ may be an alternative complementary method of VBA and TBSS.
1. Introduction
Alzheimer's disease (AD) and its prodromal stage of mild cognitive impairment (MCI) are typical neurodegenerative disorders and the most common forms of dementia in older adults (Ferri et al., 2006). A growing number of studies suggest that white matter microstructure differs in patients with AD and MCI compared to healthy controls; therefore, diffusion tensor imaging (DTI) has received increasing attention because it can detect microstructural integrity of white matter fiber bundles(Amlien and Fjell, 2014). Fractional anisotropy (FA) and mean diffusivity (MD) are two frequently used quantitative measures of DTI that detect the anisotropy and overall displacement of water molecule diffusion (Le Bihan, 2003). Although most researchers suggest that white matter abnormalities exist in AD and MCI, whether the patterns of white matter abnormalities are different across neuronal fiber tracts remains unknown.
Several approaches for DTI analyses, such as region of interest (ROI) analysis, voxel-based analysis (VBA) and tract-based spatial statistics (TBSS) have been applied in AD and MCI studies. For example, Alves reported decreased FA and increased MD in selected brain regions or certain segments of fibers in patients with AD and MCI(Alves et al., 2015). However, this ROI based DTI analysis is hypothesis-dependent, and most of the brain area remains unexamined, making localization difficult. Several other previous studies used VBA and indicated a significant FA reduction in the fornix, the splenium, and several other regions (Medina et al., 2006; Nir et al., 2013; Struyfs et al., 2015), whereas VBA is not sufficiently precise at the individual level because of the variants in the shape of long-range fiber tracts among subjects (Wassermann et al., 2011; Yeatman et al., 2011). To improve the specificity of the detection of white matter lesions, tract-based voxel grouping analysis, such as TBSS, has been employed to reveal deterioration in the limbic fibers, the fronto-occipital fasciculi, the inferior longitudinal fasciculi and other (Damoiseaux et al., 2009; Honea et al., 2009; Stricker et al., 2009; Zarei et al., 2009; Acosta-Cabronero et al., 2010; Smith et al., 2010; Liu et al., 2011; Bosch et al., 2012). But, there was only modest agreement between TBSS-based tract definitions and the actual location of a tract in an individual's brain (Tsang and Wandell, 2010), and voxel-based technique cannot provide the localization-specific properties in white matter integrity along each tract.
In summary, these white matter analysis techniques have merits and demerits respectively, and an ideal algorithm of the localization-specific properties in white matter integrity along each tract may be needed to enrich the white matter studies. In vivo, tissue properties may vary along each tract for the following reasons: different populations of axons enter and exit the tract, and disease can strike at local positions within the tract. Therefore, the diffusion measures along each fiber tract (tract profiles) of whole brain need to be further investigated (Hattori et al., 2011). Automated Fiber Quantification (AFQ) is a new algorithm that automatically identifies white matter tracts in human brains and takes measurements at anatomically equivalent locations along their trajectories (Beaulieu et al., 2012). In this study we performed AFQ to extract FA and MD at 100 locations along 20 fiber tracts to evaluate tract profiles of the white matter integrity across AD and MCI compared with normal controls (NC). These 20 tracts include association tracts, projection tracts, commissural tracts between cortices and fibers in the limbic system. Therefore, in the current study we utilized VBA, TBSS as well as AFQ together to comprehensively investigate the white matter fiber impairment in the MCI and AD patients. We hypothesize that the abnormalities of the fiber tracts are limited to specific regions of tracts in MCI and spread throughout the tracts in AD. Furthermore, certain fibers are involved in the early stages of AD, and white matter impairment spread along fiber tracts in different patterns across all the examined fibers. We hope that as a novel algorithm, the AFQ may be an alternative complementary method of VBA and TBSS, and may provide new insights into white matter degeneration in MCI and its association with AD.
2. Materials and methods
2.1. Subjects
Eighty-eight participants were recruited from the Department of Neurology of the Affiliated Drum Tower Hospital at Nanjing University Medical School from July 2013 to December 2015. This study was approved by the ethics committee of the Nanjing Drum Tower Hospital, Nanjing, China. Written informed consent was obtained from each participant.
Each subject underwent a whole brain MRI scan, a general medical examination, and a neuropsychological assessment with the Mini–Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and clinical dementia rating scale (CDR) score, all performed by neurologists blinded to the results of MR imaging results.
AD was diagnosed according to the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria for probable AD (Rabe-Jablonska and Bienkiewicz, 1994). Twenty-five patients met these criteria. Twenty-nine consecutive patients met the criteria of Petersen et al. (Petersen, 2004) for MCI. Thirty-four subjects (NCs) were identified as individuals who (a) had no cognitive complaints, (b) had a normal level of clinical rating scales, and (c) had no evidence of any abnormality determined by conventional MRI.
The exclusion criteria were set as follows: 1) concurrent illnesses (such as diabetes mellitus, addictions or psychiatric diseases other than AD) or treatments interfering with cognitive function; 2) a score higher than 4 on the Hachinski Ischemic Scale; 3) the presence of structural abnormalities that could cause cognitive impairment, or the presence of leukoaraiosis higher than Fazekas' grade I, which were identified by conventional MRI; 4) <8 years of education; or 5) not right-handed.
At the same time, to validate the findings, we employed 70 patients (27 CE patients, 21 MCI patients and 22 NC) from ADNI with age and gender matched with our cohort.
Part of data used in the preparation of this article was obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer's disease (AD).
2.2. Magnetic resonance imaging acquisition
All magnetic resonance imaging scans were performed using a Philips 3.0 T scanner (Achieva 3.0 T TX, Philips Medical Systems, the Netherlands) equipped with an 8-channel phase array head coil. A 3-dimensional, high-resolution sagittal T1 weighted scan with turbo fast echo acquisition was performed with repetition time (TR)/echo time (TE)/inversion time (TI) at 9.8/4.6/900 ms, with a flip angle of 8° and 1.0 mm isotropic resolution for anatomical reference. Diffusion weighted images were collected using a spin-echo echo-planar imaging (EPI) sequence (TR 9154 ms, TE 55 ms, matrix size 112 × 112, FOV 224 × 224 mm, slice thickness 2.5 mm, voxel size 2 × 2 × 2.5 mm3) with both 32-directional diffusion encoding (b = 1000 s/mm2 for each direction) and no diffusion encoding (b = 0 s/mm2). All scans were conducted by an experienced neurologist with the aim of excluding gross brain abnormalities. The total acquisition time was 13 min and 10s.
2.3. Magnetic resonance image pre-processing
The pre-processing of DTI, voxel-based analysis (VBA) and tract-based spatial statistics (TBSS) were carried out by PANDA(Cui et al., 2013) by its default pipeline setting. The pre-processing of DTI, automated fiber quantification (AFQ) was performed using FSL 5.0 software (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, University of Oxford, Oxford, England; http://www.fmrib.ox.ac.uk/fsl/)(Jenkinson et al., 2012). First, brain extraction was performed to remove non-brain structures, using a brain extraction tool (BET tool of the FSL software). The raw images were corrected for head movements and eddy current by using an affine image registration (FLIRT tool of the FSL software) and eddy current program (command of the FSL software). The diffusion tensor was reconstructed by fitting a diffusion tensor model to each image voxel by using the dtifit program (command of the FSL software), producing fractional anisotropy (FA) and mean diffusivity (MD) images. Subsequently, the diffusion tensor was calculated for each subject.
2.4. Voxel-based analysis (VBA) & tract-based spatial statistics (TBSS)
First, register individual FA images of native space to the FA template in the MNI space and then apply the resultant warping transformations to write the images of the diffusion metrics into MNI space. Then smooth is needed to alleviate the registration error with an 6 mm full width at Gaussian kernel. Further, resample the volume into a voxel size of 2 × 2 × 2 mm3.
The TBSS were carried out as following: To create the mean FA skeleton which represents the centers of all tracts common to the group, the mean FA image was thinned with FA threshold value as 0.25. Then project each subject's FA data onto the mean FA skeleton, a procedure that minimizes misalignment more prevalent in standard registration procedures.
2.5. Automated fiber quantification (AFQ)
3D-T1 weighted images were used for registration and segmentation by FSL. The pre-processed DTI images and 3DT1 weighted images were fed into the AFQ software (Yeatman et al., 2012), which is a MATLAB toolbox and is used to identify and quantify 20 white matter tracts in each subject's brain. The technique details and parameters were exactly same to those described in the original AFQ paper(Yeatman et al., 2012), except that the cingulum was divided into cingulum cingulate and cingulum hippocampus in the new versions. The identification and quantification procedure steps can be summarized as 4 steps: (1) performance of whole-brain tractography for each subject. All fibers are tracked within a white matter mask using deterministic tractography; (2) segmentation of a whole brain fiber group into fascicles using an automated ROI approach. The ROIs are defined based on a previous study, and the fiber tracts are subsequently refined by comparing each fiber within the fascicle to a probabilistic fiber tract atlas (JHU white-matter tractography atlas); (3) definition of the core of the tract and filtering out of stray fibers that deviate from the core of a fiber group by representing a fiber group as a 3D Gaussian distribution before removing the fibers from the fiber group center; (4) calculation of the diffusion measurements along the trajectory of the fiber group weighing each fiber's contribution to the measurement based on its distance from the tract core. The diffusion measurement along the fiber tract core, which is a vector of 100 values, was defined as the tract profile. In this way, the tract profiles of FA and MD at 100 points along each of the 20 tracts were evaluated for each subject to depict their global white matter status.
The 20 identified tracts included: bilateral anterior thalamic radiation (ATR), corticospinal tract (CST), cingulum cingulate (CCing), cingulum hippocampus (CHippo), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), uncinated fasciculus (UF), arcuate fasciculus (AF), splenium, and genu of the corpus callosum (CC) (Wakana et al., 2007).
2.6. Statistical analysis
Descriptive analyses were performed to describe the variables in each group. Categorical variables were compared using the Chi-squared test or Fisher's exact test. These analyses were performed using SPSS version 21 (SPSS, Chicago, IL, U.S.A). Continuous variables were compared between the three groups using one-way ANOVA, and then the false discovery rate (FDR) correction was performed in the mean FA and MD comparisons. Then pairwise comparisons among groups were conducted when necessary with the least significance difference (LSD) test.
For the VBA, the ANOVA was used to compare the FA and MD values in a voxel-.
based manner with SPM and the p level was <0.05. For the TBSS, a nonparametric statistical thresholding with permutation-based inference tool (FSL's “randomize”) was used to compare the FA and MD among three group and the p level set at p < .05, corrected for multiple comparisons using threshold-free cluster enhancement.
For the pointwise comparisons, FA and MD at 100 locations along the 20 fiber tracts were fed into a permutation-based statistical analysis with 1000 permutations. For each permutation, all the subjects were redivided into the three groups randomly, while keeping the sample sizes for each group, then the ANOVA and the post hoc test statistics were recalculated for each location of each fiber. After that, for each fiber, the maximum length of the continuous significant locations (for each location, “significant” means this location have a larger t or F statistic that corresponds to a p < .05) for each test was recorded, thus allowing the construction of the permutation distribution of the maximum length of significant locations; those locations with length within the top 5% of such distribution were considered significant at the 0.05 level (thus, familywise error rate corrected) (Nichols and Holmes, 2002).
For the comparison among the VBA, TBSS and AFQ, we calculated the percentage of significant voxels/points over the total number of evaluated voxels/points in VBA, TBSS and AFQ. Significant white matter voxels were calculated in the comparisons among groups in VBA, and tract-based voxels are calculated in TBSS. In the AFQ, we calculated the number of points with significant difference in the pointwise comparisons of the tract profiles across the 20 fiber tracts.
3. Results
3.1. Demographics and clinical information from our cohort and ADNI cohort
The full demographic and clinical characteristics of the subjects from our cohort and the ADNI cohort are provided in Table 1. In our cohort, for the 25 patients with probable AD (12 of whom were women), the mean age was 73.48 ± 11.83 years, and for the 29 patients with MCI (13 of whom were women), the mean age was 74.07 ± 10.76 years. The 34 normal control subjects (11 of whom were women) had a mean age of 69.03 ± 11.53 years. In the ADNI cohort, for the 27 patients with AD, the diagnosis of which was based on biomarkers, the mean age was 76.00 ± 9.78 years, and for the 21 patients with MCI, the mean age was 72.52 ± 8.71 years. The 22 normal control subjects had a mean age of 73.27 ± 6.05 years. The AD, MCI and NC groups did not differ in age, sex and education both in our cohort and the validation cohort. As expected, the pathological alteration among groups led to significant differences in the scores for the MMSE and MOCA among the patients with AD, MCI, and the NCs. These two cohorts did not differ in age and gender, but showed differences in education.
Table 1.
Comparison of demographic variables between the groups from our cohort and the ADNI cohort.
| Primary cohort |
Validation cohort |
t-Test/χ2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AD |
MCI |
NC |
ANOVA |
AD |
MCI |
NC |
ANOVA |
AD |
MCI |
NC |
|
| (n = 25) | (n = 29) | (n = 34) | p | (n = 27) | (n = 21) | (n = 22) | p | p | p | p | |
| Age(year) | 73.48 ± 11.83 | 74.07 ± 10.76 | 69.03 ± 11.53 | 0.163 | 76.00 ± 9.78 | 72.52 ± 8.71 | 73.27 ± 6.05 | 0.321 | 0.405 | 0.591 | 0.118 |
| Sex, n(%) | |||||||||||
| Female | 12(48%) | 13(44.8%) | 11(32.4%) | 0.420 | 15(55.5%) | 10(47.6%) | 6(27.3%) | 0.256 | 0.782 | 0.536 | 0.461 |
| Male | 13(52%) | 16(55.2%) | 23(67.6%) | 12(44.5%) | 11(52.4%) | 16(72.7%) | |||||
| Education (year) | 11.64 ± 2.72 | 12.83 ± 2.62 | 12.91 ± 2.24 | 0.121 | 14.96 ± 2.79 | 15.00 ± 2.75 | 15.41 ± 2.59 | 0.828 | <0.001* | 0.007 | 0.001 |
| MMSE score | 16.44 ± 5.98 | 25.55 ± 2.25 | 29.06 ± 1.20 | <0.001* | 22.07 ± 2.63 | 27.24 ± 1.55 | 29.50 ± 0.60 | <0.001* | <0.001* | 0.005 | 0.117 |
| MoCA score | 11.48 ± 4.14 | 21.72 ± 2.56 | 27.12 ± 1.97 | <0.001* | 14.85 ± 4.10 | 22.62 ± 2.16 | 28.23 ± 1.31 | <0.001* | 0.002 | 0.218 | 0.047 |
Note: Data are presented as means ± standard deviations. Primary cohort means our cohort; Validation cohort means the ADNI cohort; AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; MMSE, Mini–Mental State Examination (MMSE); MoCA, Montreal Cognitive Assessment; t-test/χ2 means the statistics in the demographic variables comparisons between primary cohort and the ADNI cohort; * denotes a significant difference with a p value <.05.
3.2. Mean diffusion measures of FA and MD among groups
AFQ is a new algorithm that can automatically identify white matter tracts in human brains and take measurements at anatomically equivalent locations along their trajectories. But there were some fibers failed tracked using AFQ, especially in the bilateral cingulum hippocampus tracts in this study, this may cause by the threshold setting in the fiber tracking, because the cingulum hippocampus tracts are adjacent to the gray matter. In Table 2, Table 3, we listed the successfully traced number of subjects in each fiber (labeled n1: n2: n3) with FDR correction.
Table 2.
Comparison of mean FA among the groups from our cohort.
| n1:n2:n3 | AD |
MCI |
NC |
ANOVA |
Post-hoc groupwise (LSD) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N = 25) | (N = 29) | (N = 34) | F | Df | p | AD vs NC | MCI vs NC | AD vs MCI | ||
| ATR_L | 24:29:34 | 0.454 ± 0.044 | 0.453 ± 0.038 | 0.453 ± 0.027 | 0.006 | 86 | 0.994 | 0.924 | 0.997 | 0.924 |
| ATR_R | 25:29:34 | 0.444 ± 0.042 | 0.463 ± 0.03 | 0.460 ± 0.028 | 2.449 | 87 | 0.092 | 0.070 | 0.769 | 0.044 |
| CST_L | 25:29:34 | 0.622 ± 0.044 | 0.620 ± 0.043 | 0.625 ± 0.025 | 0.152 | 87 | 0.859 | 0.750 | 0.588 | 0.846 |
| CST_R | 25:29:34 | 0.615 ± 0.042 | 0.622 ± 0.032 | 0.622 ± 0.031 | 0.345 | 87 | 0.710 | 0.465 | 0.981 | 0.467 |
| CCing_L | 23:28:33 | 0.446 ± 0.067 | 0.461 ± 0.067 | 0.487 ± 0.066 | 2.812 | 83 | 0.066 | 0.024 | 0.127 | 0.423 |
| CCing_R | 20:27:33 | 0.413 ± 0.044 | 0.434 ± 0.049 | 0.466 ± 0.036 | 10.209 | 79 | <0.001⁎⁎ | <0.001⁎ | 0.005⁎ | 0.107 |
| CHippo_L | 9:15:22 | 0.354 ± 0.059 | 0.380 ± 0.058 | 0.389 ± 0.052 | 1.257 | 45 | 0.295 | 0.120 | 0.618 | 0.282 |
| CHippo_R | 12:19:30 | 0.370 ± 0.039 | 0.393 ± 0.052 | 0.394 ± 0.070 | 0.712 | 60 | 0.495 | 0.257 | 0.952 | 0.316 |
| CC Splenium | 21:27:33 | 0.589 ± 0.056 | 0.601 ± 0.043 | 0.613 ± 0.051 | 1.535 | 80 | 0.222 | 0.086 | 0.368 | 0.391 |
| CC Genu | 24:27:34 | 0.503 ± 0.040 | 0.509 ± 0.037 | 0.519 ± 0.039 | 1.173 | 84 | 0.315 | 0.142 | 0.331 | 0.610 |
| IFOF_L | 22:26:34 | 0.438 ± 0.045 | 0.463 ± 0.030 | 0.473 ± 0.034 | 6.582 | 81 | 0.002⁎⁎ | 0.001⁎ | 0.308 | 0.016⁎ |
| IFOF_R | 23:27:31 | 0.433 ± 0.046 | 0.454 ± 0.040 | 0.467 ± 0.030 | 5.180 | 80 | 0.008⁎ | 0.002 | 0.203 | 0.058 |
| ILF_L | 23:29:33 | 0.415 ± 0.030 | 0.436 ± 0.034 | 0.436 ± 0.037 | 3.111 | 84 | 0.050⁎ | 0.028 | 0.989 | 0.032 |
| ILF_R | 24:28:34 | 0.401 ± 0.042 | 0.426 ± 0.037 | 0.415 ± 0.043 | 2.506 | 85 | 0.088 | 0.183 | 0.304 | 0.028 |
| SLF_L | 23:29:34 | 0.427 ± 0.048 | 0.427 ± 0.035 | 0.428 ± 0.051 | 0.015 | 85 | 0.985 | 0.887 | 0.879 | 0.999 |
| SLF_R | 25:29:34 | 0.452 ± 0.050 | 0.459 ± 0.048 | 0.458 ± 0.048 | 0.167 | 87 | 0.846 | 0.623 | 0.957 | 0.600 |
| UF_L | 25:29:34 | 0.406 ± 0.033 | 0.419 ± 0.038 | 0.433 ± 0.034 | 4.384 | 87 | 0.015⁎ | 0.004 | 0.107 | 0.189 |
| UF_R | 24:29:34 | 0.392 ± 0.031 | 0.398 ± 0.035 | 0.416 ± 0.030 | 4.550 | 86 | 0.013⁎ | 0.006 | 0.028 | 0.518 |
| AF_L | 24:28:34 | 0.516 ± 0.055 | 0.522 ± 0.038 | 0.532 ± 0.034 | 1.108 | 85 | 0.335 | 0.148 | 0.377 | 0.560 |
| AF_R | 24:25:33 | 0.492 ± 0.041 | 0.498 ± 0.041 | 0.489 ± 0.039 | 0.345 | 81 | 0.709 | 0.815 | 0.414 | 0.590 |
Note: Data are presented as means ± standard deviations. AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; ATR, anterior thalamic; CST, corticospinal tract; CCing, cingulum cingulate; CHippo, cingulum hippocampus; CC, corpus callosum; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; UF, uncinated fasciculus. AF, arcuate fasciculus. L, left; R, right. Least significant difference test was used in multiple comparisons. n1: n2: n3 means the successfully traced number of AD, MCI, and NC subjects in each fiber.
Significant difference with a p value <.05 with ANOVA or Post-hoc LSD multiple comparisons.
Significant difference with a p value <.05(FDR corrected).
Table 3.
Comparison of mean MD among the groups from our cohort.
| n1:n2:n3 | AD |
MCI |
NC |
ANOVA |
Post-hoc groupwise (LSD) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N = 25) | (N = 29) | (N = 34) | F | Df | p | AD vs NC | MCI vs NC | AD vs MCI | ||
| ATR_L | 24:29:34 | 0.802 ± 0.092 | 0.771 ± 0.051 | 0.742 ± 0.04 | 6.661 | 86 | 0.002⁎⁎⁎⁎ | <0.001⁎ | 0.066 | 0.074 |
| ATR_R | 25:29:34 | 0.778 ± 0.094 | 0.770 ± 0.076 | 0.748 ± 0.061 | 1.210 | 87 | 0.303 | 0.146 | 0.270 | 0.699 |
| CST_L | 25:29:34 | 0.774 ± 0.051 | 0.755 ± 0.030 | 0.743 ± 0.031 | 4.845 | 87 | 0.010⁎⁎ | 0.003⁎ | 0.223 | 0.066 |
| CST_R | 25:29:34 | 0.759 ± 0.055 | 0.742 ± 0.031 | 0.730 ± 0.028 | 3.892 | 87 | 0.024⁎⁎ | 0.007⁎ | 0.233 | 0.118 |
| CCing_L | 23:28:33 | 0.800 ± 0.055 | 0.790 ± 0.038 | 0.766 ± 0.040 | 4.472 | 83 | 0.014⁎⁎ | 0.006⁎ | 0.039⁎ | 0.419 |
| CCing_R | 20:27:33 | 0.773 ± 0.045 | 0.766 ± 0.044 | 0.741 ± 0.034 | 4.769 | 79 | 0.011⁎⁎ | 0.007⁎ | 0.021⁎ | 0.545 |
| CHippo_L | 9:15:22 | 0.815 ± 0.049 | 0.786 ± 0.073 | 0.751 ± 0.044 | 4.581 | 45 | 0.016⁎⁎ | 0.006⁎ | 0.067 | 0.235 |
| CHippo_R | 12:19:30 | 0.795 ± 0.034 | 0.744 ± 0.054 | 0.744 ± 0.060 | 4.274 | 60 | 0.019⁎⁎ | 0.008⁎ | 0.990 | 0.013⁎ |
| CC Splenium | 21:27:33 | 0.883 ± 0.085 | 0.849 ± 0.07 | 0.853 ± 0.068 | 1.499 | 80 | 0.230 | 0.148 | 0.815 | 0.111 |
| CC Genu | 24:27:34 | 0.845 ± 0.054 | 0.833 ± 0.057 | 0.824 ± 0.046 | 1.154 | 84 | 0.320 | 0.133 | 0.494 | 0.419 |
| IFOF_L | 22:26:34 | 0.864 ± 0.059 | 0.818 ± 0.035 | 0.798 ± 0.035 | 16.124 | 81 | <0.001⁎⁎ | <0.001⁎ | 0.072 | <0.001⁎ |
| IFOF_R | 23:27:31 | 0.840 ± 0.078 | 0.800 ± 0.046 | 0.778 ± 0.033 | 9.028 | 80 | <0.001⁎⁎ | <0.001⁎ | 0.118 | 0.010⁎ |
| ILF_L | 23:29:34 | 0.859 ± 0.081 | 0.824 ± 0.047 | 0.802 ± 0.039 | 7.240 | 85 | 0.001⁎⁎ | <0.001⁎ | 0.131 | 0.024⁎ |
| ILF_R | 24:28:34 | 0.811 ± 0.056 | 0.791 ± 0.057 | 0.776 ± 0.037 | 3.495 | 85 | 0.035⁎⁎ | 0.010⁎ | 0.253 | 0.143 |
| SLF_L | 23:29:34 | 0.762 ± 0.065 | 0.747 ± 0.033 | 0.722 ± 0.029 | 6.493 | 87 | 0.002⁎⁎ | 0.001⁎ | 0.022⁎ | 0.226 |
| SLF_R | 25:29:34 | 0.768 ± 0.052 | 0.763 ± 0.055 | 0.747 ± 0.027 | 1.667 | 87 | 0.195 | 0.091 | 0.187 | 0.678 |
| UF_L | 25:29:34 | 0.854 ± 0.043 | 0.823 ± 0.039 | 0.808 ± 0.048 | 8.084 | 87 | 0.001⁎⁎ | <0.001⁎ | 0.177 | 0.011⁎ |
| UF_R | 24:29:34 | 0.843 ± 0.083 | 0.800 ± 0.042 | 0.770 ± 0.040 | 12.133 | 86 | <0.001⁎⁎ | <0.001⁎ | 0.038⁎ | 0.006⁎ |
| AF_L | 24:28:34 | 0.769 ± 0.055 | 0.751 ± 0.029 | 0.733 ± 0.029 | 6.777 | 85 | 0.002⁎⁎ | <0.001⁎ | 0.069 | 0.071 |
| AF_R | 24:25:33 | 0.759 ± 0.066 | 0.753 ± 0.035 | 0.737 ± 0.024 | 2.130 | 81 | 0.126 | 0.056 | 0.146 | 0.649 |
Note: Unless otherwise specified, data reflect the MD values × 10−3 mm2 s−1 presented as means ± standard deviations. AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; ATR, anterior thalamic; CST, corticospinal tract; CCing, cingulum cingulate; CHippo, cingulum hippocampus; CC, corpus callosum; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; UF, uncinated fasciculus; AF, arcuate fasciculus. L, left; R, right. Least significant difference test was used in multiple comparisons. n1: n2: n3 means the successfully traced number of AD, MCI, and NC subjects in each fiber.
Significant difference with a p value <.05 with Post-hoc LSD multiple comparisons.
Significant difference with a p value <.05(FDR corrected).
A group comparison of the mean diffusion measures of each tract between the NC, MCI, and AD groups using a p < .05 threshold showed a significant difference. At the global level, a summary of the mean FA and MD of each identified fiber tract from the normal controls and patients with AD and MCI is shown in Table 2, Table 3.
-
1.
AD patients vs. NC subjects
Compared with the normal controls, two fiber tracts showed a significantly decreased FA in the AD subjects: right CCing and left IFOF. In a group comparison of the mean MD, the AD group showed extensive increased MD relative to the NC group in most of the 20 fiber tracts in addition to the corpus callosum, right ATR, SLF, and AF.
-
2.
MCI patients vs. NC subjects
The FA reduction and MD elevation in MCI was not as obvious as in AD subjects when compared with NC. The FA revealed significant reduction in the right CCing. The MD revealed significant elevation in 4 fiber tracts: bilateral CCing, left SLF and right UF.
-
3.
AD patients vs. MCI patients
Compared with MCI subjects, the left IFOF showed a significantly decreased FA in AD. The MD values in AD were significantly higher than those in MCI in the following 6 fiber tracts: right CHippo, bilateral IFOF, left ILF, and bilateral UF.
3.3. VBA
-
1.
AD patients vs. MCI patients
The AD subjects showed no significant difference in FA and MD after GRF correction compared to MCI (Figs. 1A and 2A).
-
2.
MCI patients vs. NC subjects
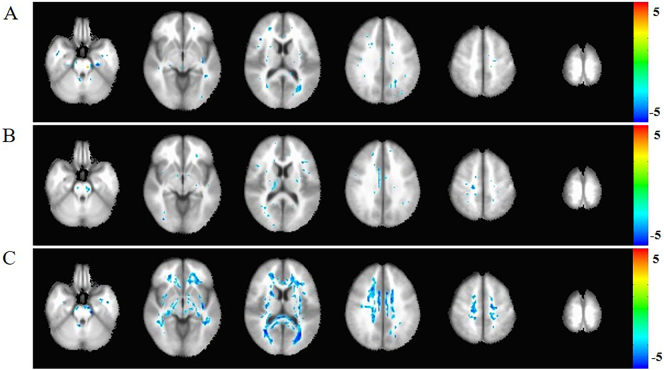
Fig. 1.
The Voxel-based analysis (VBA) of FA among AD, MCI, and NC groups.
A: comparison between AD and MCI. B: comparison between MCI and NC. C:comparison between AD and N. The blue cluster in white matter region represent the reduction FA in AD compared to NC with Gaussian Random Field (GRF) correction(p < .05). The FA difference in A and B was showed without correction and there were no differences after GRF correction (p < .05). The statistic was displayed with z-values.
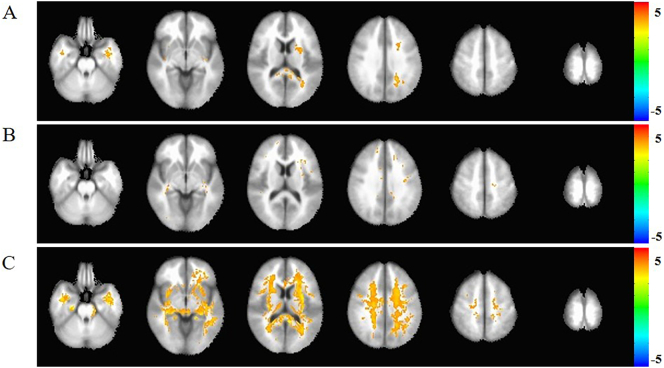
Fig. 2.
The Voxel-based analysis (VBA) of MD among AD, MCI, and NC groups.
A: comparison between AD and MCI. B: comparison between MCI and NC. C:comparison between AD and NC. The yellow cluster in white matter region represent the elevation MD in AD compared to NC with Gaussian Random Field (GRF) correction (p < .05). The MD difference in A and B was showed without correction and there were no differences after GRF correction (p < .05). The statistic was displayed with z-values.
The MCI subjects showed no significant difference in FA and MD after GRF correction compared to NC (Figs. 1B and 2B).
-
3.
AD patients vs. NC subjects
Compared with the normal controls, the FA reduction and MD elevation in AD involved almost all the white matter region after Gaussian Random Field (GRF) correction (Figs. 1C and 2C).
3.4. TBSS
-
1.
AD patients vs. MCI patients
The MD values in AD were significantly higher than those in MCI in the following fiber tracts: bilateral SLF, ILF, IFOF, ATR, UF, CC, CHippo and left CST. There were no fibers showed FA decreased in AD, compared to MCI (Fig. 3A).
-
2.
MCI patients vs. NC subjects
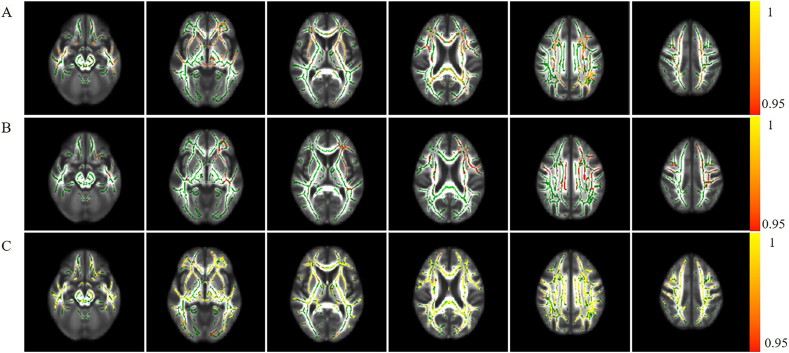
Fig. 3.
The Tract-based spatial statistics (TBSS) of MD among AD, MCI, and NC groups.
A: comparison between AD and MCI. B: comparison between MCI and NC. C:comparison between AD and NC. The FA skeleton is displayed in green, which represent the center of the white matter tracts. Red-yellow color bar indicates significant MD elevate (p < .05 corrected for multiple comparisons using threshold-free cluster enhancement, the statistic value beside the color bar is 1-p). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The MD elevation in MCI was not as obvious as in AD subjects when compared with NC. The MD revealed significant elevation in the following fiber tracts: bilateral SLF, ILF, IFOF, Ccing, CST, CC, left UF. There were no fibers showed FA decreased in MCI (Fig. 3B).
-
3.
AD patients vs. NC subjects
Compared with the normal controls, AD showed widespread MD reduction among the white matter tract threshold-free cluster enhancement (TFCE) correction. There were no significant fibers showed FA decreased in AD after TFCE correction (Fig. 3C).
3.5. Tract profiles of FA and MD among the group from our cohort
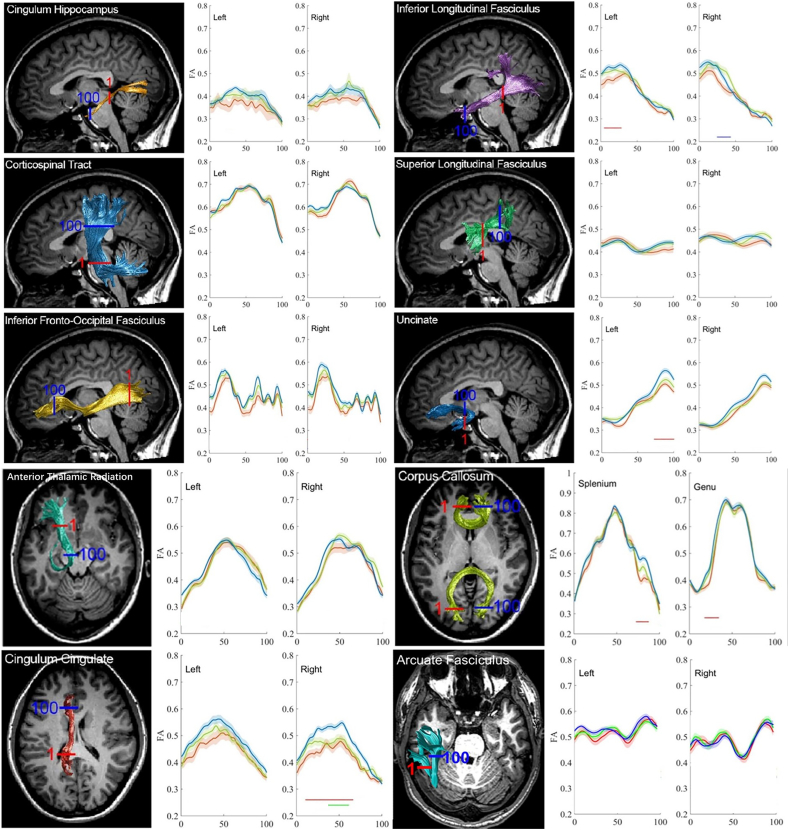
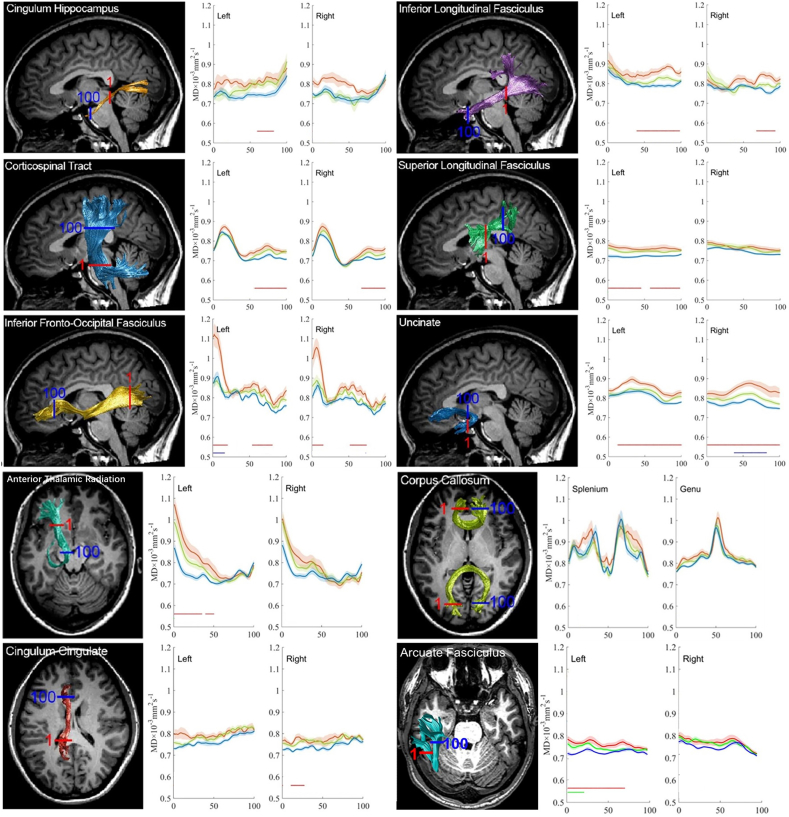
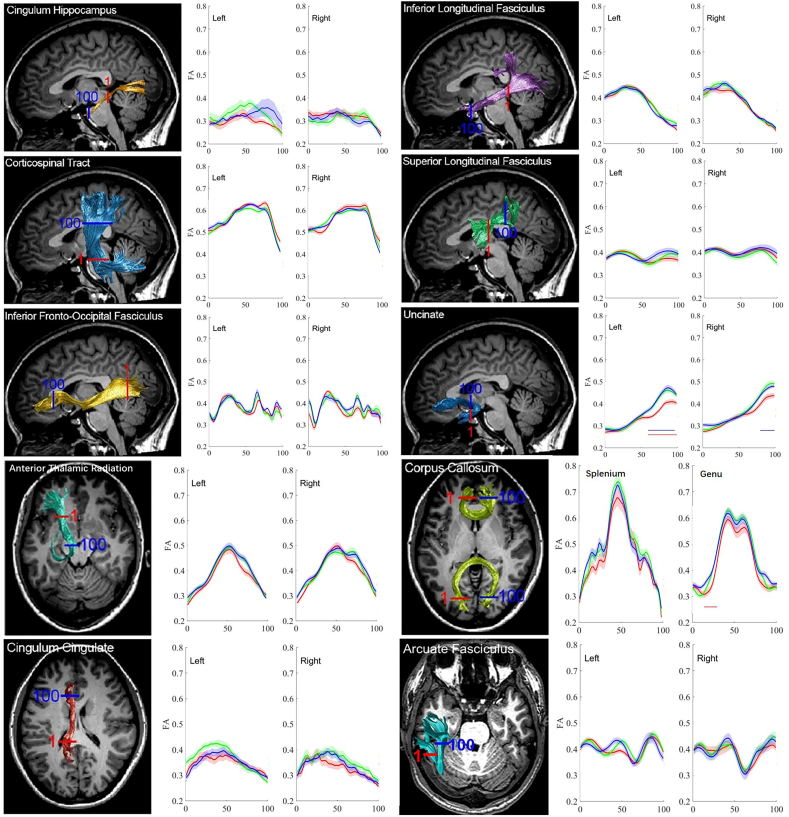
In general, group comparisons of tract profiles between the NC, MCI, and AD groups using a p < .05 threshold(FWE-corrected)identified many points of significant difference. The position of the 20 fiber tracts and tract profiles of 100 locations along the fiber tracts and the results of a pointwise comparison of FA and MD profiles among AD, MCI, and NC groups are illustrated in Fig. 4, Fig. 5.
Fig. 4.
The Pointwise Comparison of FA Profiles among AD, MCI, and NC groups from our cohort.
The plots of the FA profiles of 20 tracts from AD patients (red), MCI patients (green) and NC subjects (blue) as means (SD) (solid lines for means and shaded areas for SDs). The color bars under the FA profiles indicate the regions of significant difference between AD and NC (red), MCI and NC (green), and between MCI and AD (blue). The X-axis represents the location between the beginning and termination waypoint regions of interest. Abbreviations in context: ATR, anterior thalamic; CST, corticospinal tract; CCing, cingulum cingulate; CHippo, cingulum hippocampus; CC, corpus callosum; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; UF, uncinated fasciculus. AF, arcuate fasciculus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
The Pointwise Comparison of MD Profiles among AD, MCI, and NC groups from our cohort.
The plots of the MD profiles of 20 tracts from AD patients (red), MCI patients (green) and NC subjects (blue) as means (SD) (solid lines for means and shaded areas for SDs). The color bars under MD profiles indicate the regions of significant difference between AD and NC (red), MCI and NC (green) and between MCI and AD (blue). The X-axis represents the location between the beginning and termination waypoint regions of interest. Abbreviations in context: ATR, anterior thalamic; CST, corticospinal tract; CCing, cingulum cingulate; CHippo, cingulum hippocampus; CC, corpus callosum; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; UF, uncinated fasciculus. AF, arcuate fasciculus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In comparison with the NC subjects, the AD patients showed widespread FA reduction in 25% (5 /20) of examined fiber tracts and MD increase in 65%(13/20) of examined fiber tracts. Relative to the NC subjects, the MCI patients only showed regional FA reduction in 5% (1/20) of examined fiber tracts and an MD increase in 5%(1/20) of examined fiber tracts, primarily in the right CCing and the left AF. Compared with the MCI patients, the AD patients showed FA reduction in 5% (1/20) of examined fiber tracts and an MD increase in 10%(2/20) examined fiber tracts.
-
1.
AD patients vs. NC subjects
Generally, the tract profiles in the AD group showed a significant difference compared with the NC group.
The FA decrease did not reach a significant level in mean diffusion measures in the bilateral ATR, bilateral CST, bilateral SLF, bilateral ILF, bilateral AF, left CCing, bilateral CHippo, splenium and genu of CC, whereas significant FA reduction at certain points was found in group comparison of FA profiles.
In a pointwise comparison of FA profiles, the AD group showed widespread FA reduction relative to the NC group. Changed locations of fibers were demonstrated as follows: (1) the posterior portion of the left ILF; (2) the frontier portion of the left UF; (3) the middle and posterior component of the right CCing; (4) the left part of the splenium of the CC; (5) the right part of genu of the CC.
The mean diffusion measures failed to show significant elevation in the following fiber tracts: right ATR, right SLF, right AF, splenium, and genu of CC, although they displayed points with significantly increased MD in group comparisons of the MD profiles.
In a pointwise comparison of MD profiles, the AD group showed extensive MD elevation relative to the NC group. Changed locations of fibers were as the follows: (1) the anterior and intermediate component of the left ATR and the left ILF; (2) the posterior and intermediate component of the bilateral IFOF; (3) the anterior part of the right ILF; (4) the posterior portion of the right CCing and the left CHippo; (5) the superior portion of the right CST; (5) the superior and intermediate component of the left CST; (6) the temporal lobe potion and intermediate component of the left AF; (7) the anterior and posterior component of the left CST; (9) the whole right UF; (10) the frontier and intermediate component of the left UF.
-
2.
MCI patients vs. NC subjects
Compared with the NC subjects, the following locations revealed regional decreased FA in the MCI patients: the intermediate component of the right CCing. However, in group comparison of mean diffusion measures, none of the fibers showed reduced FA reached the level of significant after FDR correction.
The MD revealed significant elevations in the temporal lobe portion of left AF. However, in group comparison of mean diffusion measures, none of the fibers showed increased MD reached the level of significant after FDR correction.
-
3.
AD patients vs. MCI patients
The AD versus MCI contrast demonstrated significant FA reduction in the AD patients in the intermediate component of right ILF. However, in the group comparison of mean diffusion measures, none of the fibers showed reduced FA reached the level of significant after FDR correction.
The MD values were significantly higher than those of the MCI patients in the following locations: the posterior portion of the left IFOF and the intermediate component of the right UF. The mean diffusion measures failed to show significant MD elevation after FDR correction.
-
4.
Number of points with significant differences in tract profiles
To evaluate the percentage of points with white matter lesions for each fiber tract, we calculated the number of points with significant difference in the pointwise comparisons of the tract profiles across the 20 fiber tracts (as described in Table 4, Table 5).
Table 4.
Number of points with significant difference in pointwise comparison of tract profiles across 20 fiber tracts after permutation based FWE correction from our cohort.
| FA |
MD |
|||||
|---|---|---|---|---|---|---|
| AD vs NC | MCI vs NC | AD vs MCI | AD vs NC | MCI vs NC | AD vs MCI | |
| ATR_L | 0 | 0 | 0 | 35 | 0 | 0 |
| ATR_R | 0 | 0 | 0 | 0 | 0 | 0 |
| CST_L | 0 | 0 | 0 | 44 | 0 | 0 |
| CST_R | 0 | 0 | 0 | 33 | 0 | 0 |
| Ccing_L | 0 | 0 | 0 | 0 | 0 | 0 |
| Ccing_R | 56 | 31 | 0 | 17 | 0 | 0 |
| Chippo_L | 0 | 0 | 0 | 23 | 0 | 0 |
| Chippo_R | 0 | 0 | 0 | 0 | 0 | 0 |
| CC Splenium | 15 | 0 | 0 | 0 | 0 | 0 |
| CC Genu | 17 | 0 | 0 | 0 | 0 | 0 |
| IFOF_L | 0 | 0 | 0 | 36 | 0 | 16 |
| IFOF_R | 0 | 0 | 0 | 38 | 0 | 0 |
| ILF_L | 24 | 0 | 0 | 59 | 0 | 0 |
| ILF_R | 0 | 0 | 19 | 26 | 0 | 0 |
| SLF_L | 0 | 0 | 0 | 86 | 0 | 0 |
| SLF_R | 0 | 0 | 0 | 0 | 0 | 0 |
| UF_L | 28 | 0 | 0 | 88 | 0 | 0 |
| UF_R | 0 | 0 | 0 | 100 | 0 | 45 |
| AF_L | 0 | 0 | 0 | 72 | 21 | 0 |
| AF_R | 0 | 0 | 0 | 0 | 0 | 0 |
Note: AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; ATR, anterior thalamic radiation; CST, corticospinal tract; CCing, cingulum cingulate; CHippo, cingulum hippocampus; CC, corpus callosum; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; UF, uncinated fasciculus; AF, arcuate fasciculus. L, left; R, right. FWE corrected with p value <.05.
Table 5.
Summary of change in mean FA, mean MD, location of points with significant difference in pointwise comparison of tract profiles across 20 fiber tracts among the groups from our cohort.
| Changes in mean FA |
Location of changes in FA |
Changes in mean MD |
Location of changes in MD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD vs NC | MCI vs NC | AD vs MCI | AD vs NC | MCI vs NC | AD vs MCI | AD vs NC | MCI vs NC | AD vs MCI | AD vs NC | MCI vs NC | AD vs MCI | |
| ATR_L | − | − | − | − | − | − | + | − | − | A/M | − | − |
| ATR_R | − | − | − | − | − | − | − | − | − | − | − | − |
| CST_L | − | − | − | − | − | − | + | − | − | S/M | − | − |
| CST_R | − | − | − | − | − | − | + | − | − | S | − | − |
| CCing_L | − | − | − | − | − | − | + | − | − | − | − | − |
| CCing_R | + | − | − | P/M | M | − | + | − | − | P | − | − |
| CHippo_L | − | − | − | − | − | − | + | − | − | P | − | − |
| CHippo_R | − | − | − | − | − | − | + | − | − | − | − | − |
| CC Splenium | − | − | − | L | − | − | − | − | − | − | − | − |
| CC Genu | − | − | − | R | − | − | − | − | − | − | − | − |
| IFOF_L | + | − | − | − | − | − | + | − | − | P/M | − | P |
| IFOF_R | + | − | − | − | − | − | + | − | − | P/M | − | − |
| ILF_L | − | − | − | P | − | + | − | − | A/M | − | − | |
| ILF_R | − | − | − | − | − | M | + | − | − | A | − | − |
| SLF_L | − | − | − | − | − | − | + | − | − | A/P | − | − |
| SLF_R | − | − | − | − | − | − | − | − | − | − | − | − |
| UF_L | + | − | − | F | − | + | − | − | M/F | − | − | |
| UF_R | + | − | − | − | − | − | + | − | − | W | − | M |
| AF_L | − | − | − | − | − | − | + | − | − | T/M | T | − |
| AF_R | − | − | − | − | − | − | − | − | − | − | − | − |
Note: AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; ATR, anterior thalamic; CST, corticospinal tract; CCing, cingulum cingulate; CHippo, cingulum hippocampus; CC, corpus callosum; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; UF, uncinated fasciculus; AF, arcuate fasciculus. L, left; R, right. +, difference between the groups; −, no difference between the groups; A, difference in anterior part of the fiber; P, difference in posterior part of the fiber; S, difference in superior part of the fiber; M, difference in middle part of the fiber; T, difference in the temporal lobe part of the fiber; W, difference in the entire fiber. FDR correction was used in comparison in mean FA with a p value<.05. FWE correction was used in point wised location changes with a p value<.05.
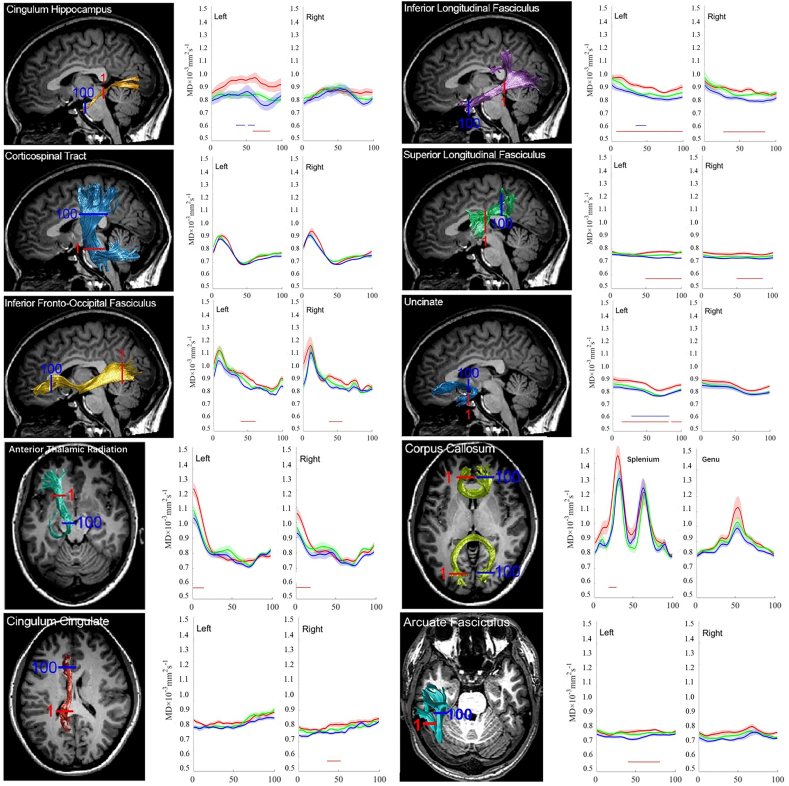
Tract Profiles of FA and MD among the groups from ADNI cohort.
In general, group comparisons of tract profiles between the NC, MCI, and AD groups using a p < .05 threshold (FWE-corrected) identified many points of significant difference. The position of the 20 fiber tracts and tract profiles of 100 locations along the fiber tracts and the results of a pointwise comparison of FA and MD profiles among AD, MCI, and NC groups are illustrated in Fig. 6, Fig. 7.
Fig. 6.
The Pointwise Comparison of FA Profiles among AD, MCI, and NC groups from the ADNI cohort.
The plots of the FA profiles of 20 tracts from AD patients (red), MCI patients (green) and NC subjects (blue) as means (SD) (solid lines for means and shaded areas for SDs). The color bars under the FA profiles indicate the regions of significant difference between AD and NC (red), MCI and NC (green), and between MCI and AD (blue). The X-axis represents the location between the beginning and termination waypoint regions of interest. Abbreviations in context: ATR, anterior thalamic; CST, corticospinal tract; CCing, cingulum cingulate; CHippo, cingulum hippocampus; CC, corpus callosum; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; UF, uncinated fasciculus. AF, arcuate fasciculus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
The Pointwise Comparison of MD Profiles among AD, MCI, and NC groups from the ADNI cohort.
The plots of the MD profiles of 20 tracts from AD patients (red), MCI patients (green) and NC subjects (blue) as means (SD) (solid lines for means and shaded areas for SDs). The color bars under MD profiles indicate the regions of significant difference between AD and NC (red), MCI and NC (green) and between MCI and AD (blue). The X-axis represents the location between the beginning and termination waypoint regions of interest. Abbreviations in context: ATR, anterior thalamic; CST, corticospinal tract; CCing, cingulum cingulate; CHippo, cingulum hippocampus; CC, corpus callosum; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; UF, uncinated fasciculus. AF, arcuate fasciculus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In comparison with the NC subjects, the AD patients showed FA reduction in 10% (2 /20) of examined fiber tracts and MD increase in 65%(13/20) of examined fiber tracts. Relative to the NC subjects, the MCI patients showed no FA reduction nor MD increase of examined fiber tracts. Compared with the MCI patients, the AD patients showed FA reduction in 10% (2/20) of examined fiber tracts and an MD increase in 15%(3/20) examined fiber tracts.
-
1.
AD patients vs. NC subjects
Generally, the tract profiles in the AD group showed a significant difference compared with the NC group.
In a pointwise comparison of FA profiles, the AD group showed FA reduction relative to the NC group. Changed locations of fibers were demonstrated as follows: (1) the right part of the genu of the CC; (2) the frontier portion of the left UF.
In a pointwise comparison of MD profiles, the AD group showed extensive MD elevation relative to the NC group. Changed locations of fibers were as the follows: (1) the anterior component of the bilateral ATR and the left CHippo; (2) the posterior and intermediate component of the bilateral SLF and the right ILF; (3) the intermediate part of the bilateral IFOF and the right CCing; (4) the right portion of the splenium of the CC; (5) the frontier and intermediate component of the left AF; (6) almost all part of the left UF and ILF.
-
2.
MCI patients vs. NC subjects
Compared with the NC subjects, none of the fiber locations showed reduced FA nor increased MD reached the level of significant after FWE correction.
-
3.
AD patients vs. MCI patients
The AD versus MCI contrast demonstrated significant FA reduction in the AD patients in the frontal component of bilateral UF.
The MD values were significantly higher than those of the MCI patients in the following locations: the posterior and middle portion of the left ILF and the intermediate component of the left UF and CCing.
-
4.
Number of points with significant differences in tract profiles
To evaluate the percentage of points with white matter lesions for each fiber tract, we calculated the number of points with significant difference in the pointwise comparisons of the tract profiles across the 20 fiber tracts (as described in Table 6, Table 7).
Table 6.
Number of points with significant difference in pointwise comparison of tract profiles across 20 fiber tracts from the ADNI cohort after permutation based FWE correction.
| FA |
MD |
|||||
|---|---|---|---|---|---|---|
| AD vs NC | MCI vs NC | AD vs MCI | AD vs NC | MCI vs NC | AD vs MCI | |
| ATR_L | 0 | 0 | 0 | 14 | 0 | 0 |
| ATR_R | 0 | 0 | 0 | 18 | 0 | 0 |
| CST_L | 0 | 0 | 0 | 0 | 0 | 0 |
| CST_R | 0 | 0 | 0 | 0 | 0 | 0 |
| Ccing_L | 0 | 0 | 0 | 0 | 0 | 0 |
| Ccing_R | 0 | 0 | 0 | 17 | 0 | 0 |
| Chippo_L | 0 | 0 | 0 | 25 | 0 | 23 |
| Chippo_R | 0 | 0 | 0 | 0 | 0 | 0 |
| CC Splenium | 0 | 0 | 0 | 10 | 0 | 0 |
| CC Genu | 15 | 0 | 0 | 0 | 0 | 0 |
| IFOF_L | 0 | 0 | 0 | 21 | 0 | 0 |
| IFOF_R | 0 | 0 | 0 | 19 | 0 | 0 |
| ILF_L | 0 | 0 | 0 | 93 | 0 | 15 |
| ILF_R | 0 | 0 | 0 | 58 | 0 | 0 |
| SLF_L | 0 | 0 | 0 | 52 | 0 | 0 |
| SLF_R | 0 | 0 | 0 | 37 | 0 | 0 |
| UF_L | 40 | 0 | 37 | 83 | 0 | 55 |
| UF_R | 0 | 0 | 20 | 0 | 0 | 0 |
| AF_L | 0 | 0 | 0 | 40 | 0 | 0 |
| AF_R | 0 | 0 | 0 | 0 | 0 | 0 |
Note: AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; ATR, anterior thalamic radiation; CST, corticospinal tract; CCing, cingulum cingulate; CHippo, cingulum hippocampus; CC, corpus callosum; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; UF, uncinated fasciculus; AF, arcuate fasciculus. L, left; R, right. FWE corrected with p value <.05.
Table 7.
Summary of change in the location of points with significant difference in pointwise comparison of tract profiles across 20 fiber tracts among the groups from the ADNI cohort.
| Location of changes in FA |
Location of changes in MD |
|||||
|---|---|---|---|---|---|---|
| AD vs NC | MCI vs NC | AD vs MCI | AD vs NC | MCI vs NC | AD vs MCI | |
| ATR_L | – | – | – | A | – | – |
| ATR_R | – | – | – | A | – | – |
| CST_L | – | – | – | – | – | – |
| CST_R | – | – | – | – | – | – |
| CCing_L | – | – | – | – | – | – |
| CCing_R | – | – | – | M | – | – |
| CHippo_L | – | – | – | A | – | M |
| CHippo_R | – | – | – | – | – | – |
| CC Splenium | – | – | – | R | – | – |
| CC Genu | R | – | – | – | – | – |
| IFOF_L | – | – | – | M | – | – |
| IFOF_R | – | – | – | M | – | – |
| ILF_L | – | – | A/M/P | – | M/P | |
| ILF_R | – | – | – | M/P | – | – |
| SLF_L | – | – | – | M/P | – | – |
| SLF_R | – | – | – | M/P | – | – |
| UF_L | F | – | F | F/M/T | – | M |
| UF_R | – | – | F | – | – | – |
| AF_L | – | – | – | M/F | – | – |
| AF_R | – | – | – | – | – | – |
Note: AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; ATR, anterior thalamic; CST, corticospinal tract; CCing, cingulum cingulate; CHippo, cingulum hippocampus; CC, corpus callosum; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; UF, uncinated fasciculus; AF, arcuate fasciculus. R, right; A, difference in anterior part of the fiber; P, difference in posterior part of the fiber; M, difference in middle part of the fiber; T, difference in the temporal lobe part of the fiber; F, difference in the frontal lobe part of the fiber. FDR correction was used in comparison in mean FA with a p value<.05. FWE correction was used in point wised location changes with a p value<.05.
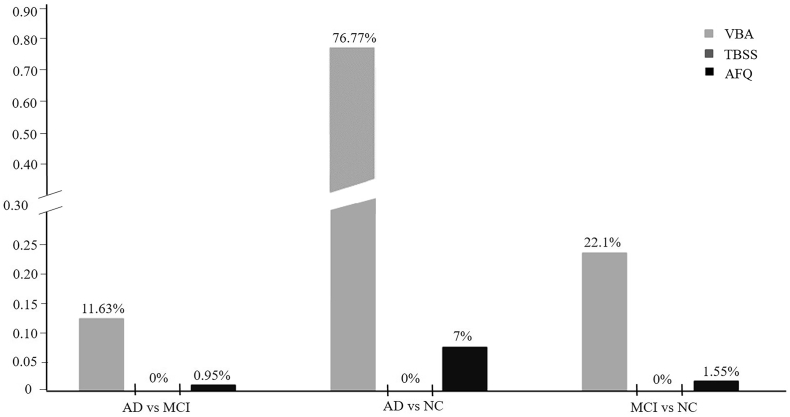
3.6. The comparison among the VBA, TBSS and AFQ
The quantification FA and MD comparisons among the VBA, TBSS and AFQ were respectively showed in Fig. 8, Fig. 9.
Fig. 8.
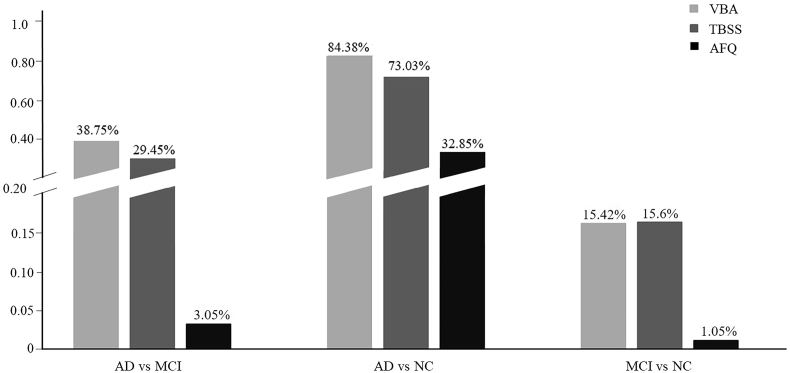
The percentage of the significant FA reduction region of VBA, TBSS and AFQ, respectively, among AD, MCI, and NC groups. 0% means there were no differences of FA in the comparison between AD vs NC, MCI vs NC and AD vs MCI with TBSS. The y-axis is the percentage of significant voxels/points over the total number of evaluated voxels/points in VBA, TBSS and AFQ.
Fig. 9.
The percentage of the significant MD elevation region of VBA, TBSS and AFQ respectively, among AD, MCI, and NC groups. The y-axis is the percentage of significant voxels/points over the total number of evaluated voxels/points in VBA, TBSS and AFQ.
We found that VBA detected the highest percentage of abnormalities either in the FA or MD analysis. The FA decrease and MD elevate percentage in AD was respectively 11.63% and 38.75% in VBA, compared to MCI. TBSS found none FA abnormalities in AD vs MCI, MCI vs NC and AD vs NC, but it showed 29.45% abnormalities in AD, compared to MCI. There were 73.03% abnormalities of MD in AD when compared to NC, and 15.6% in MCI compared to NC. The AFQ detected changes in all the comparisons of FA and MD, mostly in the AD vs NC. When compared to NC, AD showed 7% points among 100 points of 20 fibers respectively FA reduction, and 32.85% in MD elevate. There were 0.95% points showed FA reduction in AD, compared to MCI, and 3.05% points showed MD elevate. The MCI subjects showed 1.55% FA reduction and 1.05% MD elevate when compared to normal control.
3.7. The comparison of the AFQ findings between ADNI cohort and our cohort
In a pointwise comparison of FA profiles, the AD group from ADNI cohort showed FA reduction in the genu of the CC and the left UF, which is included in our findings. The AD versus MCI contrast demonstrated significant FA reduction in the AD patients in the bilateral UF, but it showed no similar results in our cohort.
In a pointwise comparison of MD profiles, the AD group from ADNI cohort showed significantly similar fibers changes in MD elevation except the right UF and the splenium of the CC compared to NC. The right portion of the splenium of the CC showed MD elevation in AD patients from ADNI cohort, but there is nothing abnormal in our cohort. Also, we find the MD elevation in whole right UF, while the fiber showed normal in AD from ADNI cohort. (as described in Table 8).
Table 8.
Summary of change in the location of points with significant difference in pointwise comparison of tract profiles across 20 fiber tracts among the groups between our cohort and the ADNI cohort.
| Location of changes in FA |
Location of changes in MD |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD vs NC |
MCI vs NC |
AD vs MCI |
AD vs NC |
MCI vs NC |
AD vs MCI |
|||||||
| Ours | ADNI | Ours | ADNI | Ours | ADNI | Ours | ADNI | Ours | ADNI | Ours | ADNI | |
| ATR_L | – | – | – | – | – | – | A/M | A | – | – | – | – |
| ATR_R | – | – | – | – | – | – | – | A | – | – | – | – |
| CST_L | – | – | – | – | – | – | S/M | – | – | – | – | – |
| CST_R | – | – | – | – | – | – | S | – | – | – | – | – |
| CCing_L | – | – | – | – | – | – | – | – | – | – | – | – |
| CCing_R | P/M | – | M | – | – | – | P | M | – | – | – | – |
| CHippo_L | – | – | – | – | – | – | P | A | – | – | – | M |
| CHippo_R | – | – | – | – | – | – | – | – | – | – | – | – |
| CC Splenium | L | – | – | – | – | – | – | R | – | – | – | – |
| CC Genu | R | R | – | – | – | – | – | – | – | – | – | – |
| IFOF_L | – | – | – | – | – | – | P/M | M | – | – | P | – |
| IFOF_R | – | – | – | – | – | – | P/M | M | – | – | – | – |
| ILF_L | P | – | – | – | – | A/M | A/M/P | – | – | – | M/P | |
| ILF_R | – | – | – | – | M | – | A | M/P | – | – | – | – |
| SLF_L | – | – | – | – | – | – | A/P | M/P | – | – | – | – |
| SLF_R | – | – | – | – | – | – | – | M/P | – | – | – | – |
| UF_L | F | F | – | – | – | F | M/F | F/M/T | – | – | – | M |
| UF_R | – | – | – | – | – | F | W | – | – | – | M | – |
| AF_L | – | – | – | – | – | – | T/M | M/F | T | – | – | – |
| AF_R | – | – | – | – | – | – | – | – | – | – | – | – |
Note: AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; ATR, anterior thalamic; CST, corticospinal tract; CCing, cingulum cingulate; CHippo, cingulum hippocampus; CC, corpus callosum; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; UF, uncinated fasciculus; AF, arcuate fasciculus. L, left; R, right. +, difference between the groups; −, no difference between the groups; A, difference in anterior part of the fiber; P, difference in posterior part of the fiber; S, difference in superior part of the fiber; M, difference in middle part of the fiber; T, difference in the temporal lobe part of the fiber; W, difference in the entire fiber. FDR correction was used in comparison in mean FA with a p value<.05. FWE correction was used in point wised location changes with a p value<.05.
4. Discussion
As a relatively new algorithm, AFQ can automatically identify white matter tracts in human brains and anatomically measure at equivalent locations along these fibers. In this study, we performed this method to extract FA and MD at 100 locations along 20 fiber tracts to evaluate tract profiles of the white matter integrity across AD and MCI. In comparison with NC, AD patients showed widespread FA reduction in 25% (5/20) examined fiber tracts and MD increase in 65% (13/20) examined fiber tracts. While MCI subjects showed regional FA reduction in 5% (1/20) examined fiber tracts (right cingulum cingulate) and MD increase in 5% (1/20) of examined fiber tracts (left arcuate fasciculus). Then we validated the findings with ADNI cohort, which showed significantly similar changes in the pointwise comparison of MD in AD compared to NC. In the mean FA and MD comparisons, there were no FA or MD differences reach statistical differences in MCI and NC, which was also similar finding in AD and MCI. In contrast, in the VBA analysis, widespread FA reduction and MD increase were found in AD, when compared to NC. But there was not any difference in the AD and MCI or NC and MCI. In the TBSS analysis, no significant difference was found for FA among all the three groups, while widespread MD increasing was found in AD when compared to NC, which was similar to the VBA results. In summary, using the point wise method of AFQ, we have found the location specific white matter abnormalities, which could not be achieved with the mean FA and MD analyses. Therefore, AFQ is an important supplement in the white matter studies.
In our results of VBA analysis, almost all the white matter voxels showed difference of FA and MD in AD, compared to normal controls, but there is no significant difference between MCI and NC or AD and MCI. Therefore, VBA seems to be very sensitive to those larger differences, like AD vs. NC but have low spatial specificity. When the difference is milder (like MCI and NC or AD and MCI), it showed no difference either with FA or MD. This “all-or-none” result may because low spatial precisely of the fibers when using morphology based normalization. For TBSS, we also see an “all-or-none” result. MD elevation in inferior longitudinal fasciculus, superior longitudinal fasciculus and many other fibers in AD, compared to normal controls, but no significant results were found between AD and MCI or MCI and NC. None results was found for FA. As a tract-based voxel grouping analysis technique, TBSS partially overcome the problem of each voxel not totally on the same fiber after registration in VBA. But the inconsistent shape of fiber tract across participants may lead to error registration, such as the corpus callosum and so on. Jin et al. reported that Tract-specific analysis (TSA) can reveal detailed local alterations in each tract, which provide a 3D detailed profile that reflects local alterations in a particular tract associated with AD or MCI(Jin et al., 2017). Meanwhile the AFQ showed more specific locations in fibers of MD difference in AD and NC, while it is sensitive to detect some mild alteration between MCI and NC or AD and MCI, which indicate that it has advantages, and could be a complementary method to the classic ones.
Neurobiological systematical variation along each tract has been considered as the potential result of different patterns of white matter abnormalities across neuronal fiber tracts. Using AFQ, we found that most of the examined fiber tracts showed widespread white matter abnormalities in AD, while in MCI, only a few of the fiber tracts showed FA and MD alterations, whose findings were similar in the ADNI cohort. Moreover, among these changed tracts, only the right cingulum cingulate and the left arcuate fasciculus displayed obvious disruption of myelin and/or fiber axons in MCI and aggravated deterioration in AD. This finding was supported by FA/MD changes through both the mean, the TBSS and point wise method. We also found the location of white matter abnormalities to be different across 20 examined neuronal fiber tracts; for example, the MCI and AD patients showed similar FA reduction in the middle part of the right cingulum cingulate, whereas the anterior part were reserved. However, the left arcuate fasciculus showed MD elevation located at temporal part of the fibers in MCI and expanded to the temporal and middle part of the fibers in AD.
In the limbic system, the fMRI studies have suggested that hypometabolism or hypo-perfusion initially occur in the posterior cingulate cortex (PCC) (Nestor et al., 2003a; Nestor et al., 2003b). We found the middle part or the middle component of the right cingulum cingulate showed FA reduction in both MCI and AD patients with AFQ analyses. Previous studies found hypo-metabolism of the middle cingulate gyrus in AD using 18F-FDG PET (Villain et al., 2008) and showed an Aβ load in the right posterior cingulate using 11C-PIB EPT (Furst and Lal, 2011), which were partially consistent with our results. Among association fiber tracts, the uncinated fasciculus is associated with episodic memory, the formation and retrieval of memory and cognition(Ebeling and von Cramon, 1992). Previous studies have reported that FA values in the uncinated fasciculus are significantly lower and MD values are significantly higher in patients with AD than in healthy controls (Taoka et al., 2006; Yasmin et al., 2008). In this study, we noted supportive results with TBSS and AFQ, and the ADNI cohort showed same findings with AFQ. Multiple AD-risk studies have reported reduced white matter integrity in the inferior fronto-occipital fasciculus (Persson et al., 2006; Gold et al., 2010; Smith et al., 2010). One study revealed that the Aβ42/p-Tau181 ratio was positively correlated with FA in the bilateral inferior fronto-occipital fasciculus, suggesting this tract is involved in AD pathology (Molinuevo et al., 2014). An MD increase more than an FA reduction in the inferior fronto-occipital fasciculus indicates that the deterioration from the MCI to AD might be primarily caused by the myelin degradation progress. We also found that even in AD, FA reduction and MD elevate was not significant in terms of mean diffusion measures in the genu and splenium of the corpus callosum, whereas using AFQ we found the left part of splenium of corpus callosum and the right part of genu of corpus callosum showed significant FA reduction. The ADNI cohort also showed similar pointwise changes of FA reduction in the corpus callosum. A previous study showed that Aβ deposition was correlated with reduced FA in the splenium of the corpus callosum but not in the entire corpus callosum(Chao et al., 2013). Interestingly, we also found that there were no differences among AD, MCI or NC subjects in terms of the mean FA and MD, but there were some abnormal locations in the tract profiles using AFQ.
The above abnormality of white matter bundles indicated the pathological process of AD. The pathology of neurofibrillary tangles shows a significantly correlation with cognitive impairment in AD, which develops first in the trans entorhinal cortex and then may spread to the entorhinal cortex in stages I and II, adjacent to the hippocampus, which is part of the limbic system in stages III and IV, followed by the medial temporal isocortex (“isocortical”, stages V/VI), and finally into the brain(Mufson et al., 2015). We found that in MCI, FA reduction occurs primarily in the right cingulum cingulate, bilateral anterior thalamic radiation and uncinated fasciculus, and the genu and splenium of the corpus callosum. At the AD stage, these pathological changes occur in additional fibers, including the bilateral corticospinal tract, left cingulum cingulate, and left cingulum hippocampus, which is consistent with previous reports of the Aβ progression pathway.
Although we found more abnormalities of myelin or/and fiber axons in the 20 examined fiber tracts by pointwise tract profiles among the groups than mean diffusion measures, and prove AFQ is an important supplement of VBA and TBSS, there are a few limitations in this study. First, given that the AD patients are at a late disease stage, it is possible that gray matter atrophy in these patients may have a technical influence on the tractographic results, and therefore such atrophy may contribute to the observed abnormality of the tract profile. Second, DTI measurement could be associated with the prominent white matter hyper-intense lesions found in these patients. The current data are limited in their ability to distinguish these two issues. Third, because of the threshold setting in the fiber tracking, some fibers like bilateral cingulum hippocampus tracts are adjacent to the gray matter and the FA in this part were low, this may lead to fail track some fibers. Forth, the AD diagnosis was not based on biomarkers but only on the neuropsychological scales, which was subjected by our research conditions and might cause imprecise diagnosis.
In conclusion, AFQ can automatically reconstruct white matter tracts in human brains and detect at equivalent locations along these fasciculi, which can be a complementary method for VBA and TBSS technique. We used this method in AD, MCI patients and NC subjects, and examined two diffusion parameters (FA and MD) at 100 locations along 20 white matter fiber tracts. Our results indicated that AFQ can detect changes in specific location along fibers, and the pattern of the disrupted location was different among the fibers, but in most, the disruption started from a certain region in MCI before, expanding to include entire fibers in AD, whose findings were validated by the ADNI cohort. These results may enrich the study on neurodevelopmental alterations and the progression of AD, which can provide new insights into the determination of white matter in the progression from MCI to AD with AFQ.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81720108022, 91649116, 81571040); the social development project of science and technology project in Jiangsu Province (BE2016605, BE201707); Key Medical Talents of the Jiangsu province, the “13th Five-Year” Health Promotion Project of the Jiangsu province (B.Z.2016-2020); Jiangsu Provincial Key Medical Discipline (Laboratory) (ZDXKA2016020); the Nanjing Science and Technology Development Program (YKK15065, X.Z.); the Project of the Sixth Peak of Talented People (WSN-138, BZ). National Key R&D Program of China (2016YFC0100100). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Part of the data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
References
- Acosta-Cabronero J., Williams G.B., Pengas G., Nestor P.J. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease. Brain. 2010;133(2):529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- Alves G.S., Oertel Knochel V., Knochel C., Carvalho A.F., Pantel J., Engelhardt E. Integrating retrogenesis theory to Alzheimer's disease pathology: insight from DTI-TBSS investigation of the white matter microstructural integrity. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/291658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlien I.K., Fjell A.M. Diffusion tensor imaging of white matter degeneration in Alzheimer's disease and mild cognitive impairment. Neuroscience. 2014;276:206–215. doi: 10.1016/j.neuroscience.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Beaulieu C., Yeatman J.D., Dougherty R.F., Myall N.J., Wandell B.A., Feldman H.M. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B., Arenaza-Urquijo E.M., Rami L., Sala-Llonch R., Junqué C., Solé-Padullés C. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiol. Aging. 2012;33(1):61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Chao L.L., Decarli C., Kriger S., Truran D., Zhang Y., Laxamana J. Associations between white matter hyperintensities and beta amyloid on integrity of projection, association, and limbic fiber tracts measured with diffusion tensor MRI. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Zhong S., Xu P., He Y., Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. 2013;7:42. doi: 10.3389/fnhum.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J.S., Smith S.M., Witter M.P., Sanz-Arigita E.J., Barkhof F., Scheltens P. White matter tract integrity in aging and Alzheimer's disease. Hum. Brain Mapp. 2009;30(4):1051–1059. doi: 10.1002/hbm.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling U., von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir. 1992;115(3–4):143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- Ferri C.P., Prince M., Brayne C., Brodaty H., Fratiglioni L., Ganguli M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2006;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst A.J., Lal R.A. Amyloid-beta and glucose metabolism in Alzheimer's disease. J. Alzheimers Dis. 2011;26(Suppl. 3):105–116. doi: 10.3233/JAD-2011-0066. [DOI] [PubMed] [Google Scholar]
- Gold B.T., Powell D.K., Andersen A.H., Smith C.D. Alterations in multiple measures of white matter integrity in normal women at high risk for Alzheimer's disease. NeuroImage. 2010;52(4):1487–1494. doi: 10.1016/j.neuroimage.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Yuasa T., Aoki S., Sato R., Sawaura H., Mori T. Altered microstructure in corticospinal tract in idiopathic normal pressure hydrocephalus: comparison with Alzheimer disease and Parkinson disease with dementia. AJNR Am. J. Neuroradiol. 2011;32(9):1681–1687. doi: 10.3174/ajnr.A2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R.A., Vidoni E., Harsha A., Burns J.M. Impact of APOE on the healthy aging brain: a voxel-based MRI and DTI study. J. Alzheimers Dis. 2009;18(3):553–564. doi: 10.3233/JAD-2009-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jin Y., Huang C., Daianu M., Zhan L., Dennis E.L., Reid R.I. 3D tract-specific local and global analysis of white matter integrity in Alzheimer's disease. Hum. Brain Mapp. 2017;38(3):1191–1207. doi: 10.1002/hbm.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat. Rev. Neurosci. 2003;4(6):469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Liu Y., Spulber G., Lehtimäki K.K., Könönen M., Hallikainen I., Gröhn H. Diffusion tensor imaging and tract-based spatial statistics in Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging. 2011;32(9):1558–1571. doi: 10.1016/j.neurobiolaging.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Medina D., Urresta F., Gabrieli J.D., Moseley M., Fleischman D., Bennett D.A. White matter changes in mild cognitive impairment and AD: a diffusion tensor imaging study. Neurobiol. Aging. 2006;27(5):663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Molinuevo J.L., Ripolles P., Simo M., Llado A., Olives J., Balasa M. White matter changes in preclinical Alzheimer's disease: a magnetic resonance imaging-diffusion tensor imaging study on cognitively normal older people with positive amyloid beta protein 42 levels. Neurobiol. Aging. 2014;35(12):2671–2680. doi: 10.1016/j.neurobiolaging.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Mufson E.J., Mahady L., Waters D., Counts S.E., Perez S.E., DeKosky S.T. Hippocampal plasticity during the progression of Alzheimer's disease. Neuroscience. 2015;309:51–67. doi: 10.1016/j.neuroscience.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor P., Fryer T., Ikeda M., Hodges J. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer's disease) Eur. J. Neurosci. 2003;18(9):2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- Nestor P.J., Fryer T.D., Smielewski P., Hodges J.R. Limbic hypometabolism in Alzheimer's disease and mild cognitive impairment. Ann. Neurol. 2003;54(3):343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir T.M., Jahanshad N., Villalon-Reina J.E., Toga A.W., Jack C.R., Weiner M.W. Effectiveness of regional DTI measures in distinguishing Alzheimer's disease, MCI, and normal aging. NeuroImage. 2013;3:180–195. doi: 10.1016/j.nicl.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J., Lind J., Larsson A., Ingvar M., Cruts M., Van Broeckhoven C. Altered brain white matter integrity in healthy carriers of the APOE ε4 allele a risk for AD? Neurology. 2006;66(7):1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- Petersen R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Rabe-Jablonska J., Bienkiewicz W. Anxiety disorders in the fourth edition of the classification of mental disorders prepared by the American Psychiatric Association: diagnostic and statistical manual of mental disorders (DMS-IV — options book) Psychiatr. Pol. 1994;28(2):255–268. [PubMed] [Google Scholar]
- Smith C.D., Chebrolu H., Andersen A.H., Powell D.A., Lovell M.A., Xiong S. White matter diffusion alterations in normal women at risk of Alzheimer's disease. Neurobiol. Aging. 2010;31(7):1122–1131. doi: 10.1016/j.neurobiolaging.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker N.H., Schweinsburg B., Delano-Wood L., Wierenga C.E., Bangen K.J., Haaland K. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer's disease supports retrogenesis. Neuroimage. 2009;45(1):10–16. doi: 10.1016/j.neuroimage.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyfs H., Van Hecke W., Veraart J., Sijbers J., Slaets S., De Belder M. Diffusion Kurtosis Imaging: a possible MRI biomarker for AD diagnosis. J. Alzheimer's Dis. 2015;48(4):937–948. doi: 10.3233/JAD-150253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka T., Iwasaki S., Sakamoto M., Nakagawa H., Fukusumi A., Myochin K. Diffusion anisotropy and diffusivity of white matter tracts within the temporal stem in Alzheimer disease: evaluation of the “tract of interest” by diffusion tensor tractography. Am. J. Neuroradiol. 2006;27(5):1040–1045. [PMC free article] [PubMed] [Google Scholar]
- J. M. Tsang, B. A. Wandell (2010). "Tract Alignment Errors Decrease Detection Power in Group Analyses of Diffusion Data With TBSS", In: Society for Neuroscience.).
- Villain N., Desgranges B., Viader F., de la Sayette V., Mezenge F., Landeau B., Baron J.C., Eustache F., Chetelat G. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer's disease. J. Neurosci. 2008;28:6174–6181. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Caprihan A., Panzenboeck M.M., Fallon J.H., Perry M., Gollub R.L. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann D., Rathi Y., Bouix S., Kubicki M., Kikinis R., Shenton M. Information Processing in Medical Imaging. Springer; 2011. White matter bundle registration and population analysis based on Gaussian processes; pp. 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin H., Nakata Y., Aoki S., Abe O., Sato N., Nemoto K. Diffusion abnormalities of the uncinate fasciculus in Alzheimer's disease: diffusion tensor tract-specific analysis using a new method to measure the core of the tract. Neuroradiology. 2008;50(4):293–299. doi: 10.1007/s00234-007-0353-7. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Rykhlevskaia E., Sherbondy A.J., Deutsch G.K., Wandell B.A. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J. Cogn. Neurosci. 2011;23(11):3304–3317. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Myall N.J., Wandell B.A., Feldman H.M. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M., Damoiseaux J.S., Morgese C., Beckmann C.F., Smith S.M., Matthews P.M. Regional white matter integrity differentiates between vascular dementia and Alzheimer disease. Stroke. 2009;40(3):773–779. doi: 10.1161/STROKEAHA.108.530832. [DOI] [PubMed] [Google Scholar]