Abstract
Background
Pleural fluid pH and glucose levels are both recommended in the workup of pleural effusions. Whether their levels correlate and predict each other or contribute independent knowledge is unclear. We aimed to investigate the pH/glucose relationship, assess their concordance and ascertain whether performing both tests provides additional information to performing either test alone.
Methods
The pH and glucose measurements from 2,971 pleural fluid samples, from three centers in Spain, UK and Australia, were categorized into Cancer (n=1,045), Infection (n=544), Tuberculosis (n=249) and Others (n=1,133) groups. The relationship between pH and glucose values and their concordance at clinically relevant cutoffs (pH 7.2 and glucose 3.3 mmol/L) were assessed.
Results
The mean pH of the cohort was 7.38 (SD 0.22) and median glucose 5.99 (range, 0.00–29.36) mmol/L. A regression model of the relationship between glucose (log-transformed) and pH with a restricted cubic spline showed linear (P<0.01) and nonlinear effects (P<0.01). The relationship was strong with a narrow confidence interval but the prediction interval was wide. Most (91.9%) samples were concordant using pH and glucose levels at cutoffs of 7.20 and 3.30 mmol/L respectively. Using pH alone, without glucose, captured 95.0% of the infection-related effusions with either pH or glucose below cutoff and glucose alone identified 91.7%.
Conclusions
Pleural fluid pH and glucose have a strong non-linear relationship but, in most situations, the level of one cannot accurately predict the other. Concordance rates were high and either test is sufficient in the majority of cases.
Keywords: Clinical decision making, empyema, glucose, hydrogen-ion concentration, pleural effusion
Introduction
Pleural effusion can complicate a wide variety of conditions and has over 60 differential diagnoses (1). Detailed analysis of pleural fluid is essential to establishing its underlying cause. Pleural fluid pH and glucose are included in commonly used clinical guidelines for the work-up of pleural effusions (2,3). A correlation between pH and glucose has been noted in small studies (4,5). Whether both tests are needed is unknown. No studies have assessed if they provide duplicate information or contribute independent knowledge and, if a relationship is present, whether it is dependent on the underlying diagnosis.
Pleural fluid pH is determined by the acids generated from glucose metabolism of cells within the pleural space, as well as the efflux of those acids and the influx of glucose across the pleural membranes. Diffusion across the pleura can be affected in acute inflammatory states (e.g., infection), chronic fibrotic conditions (e.g., rheumatoid pleurisy) and tumour infiltration in pleural malignancies. Low pH is most commonly used clinically to guide the need for chest tube drainage of parapneumonic effusions (6,7). Some studies have also shown that low pH and glucose predict pleurodesis failure and poorer prognosis in malignant pleural effusions (8,9).
Varying cutoff levels for both pH and glucose have been used throughout the literature. Most commonly a pH of 7.20 is used to guide drainage in pleural infection (6) and a pH of between 7.20 and 7.30 is considered prognostic in pleural malignancy (8,10,11). Both the British Thoracic Society and the American College of Chest Physicians’ guidelines for pleural infection recommend pleural fluid drainage if the pH is <7.20 (2,3). The equivalent glucose level considered low is <3.30 mmol/L (60 mg/dL) (2,3,8,12).
pH testing is subject to variation in collection methods and analysis, to which it is vulnerable (13). Some centers do not have access to blood gas analysers to measure pH and instead rely on litmus paper or pH meters, which have been proven inaccurate (14,15). Collection and analysis methods do not have the same effect on glucose, though it is possible that serum hyperglycemia could affect pleural fluid glucose levels.
Aiming to assess if both tests need to be performed, we employed a large multi-center cohort to: (I) ascertain whether the levels of pleural fluid pH and glucose correlate; (II) assess whether the level of one test can accurately predict the other; and (III) assess if the tests are concordant at clinically used cutoff values.
Methods
Clinical centers and identification of cases
Pleural effusion pH and glucose data, measured as part of clinical care, were extracted and collated from prospectively maintained databases at three pleural centers—North Bristol Lung Centre (NBLC), Bristol, United Kingdom; Sir Charles Gairdner Hospital (SCGH), Perth, Western Australia; and Arnau de Vilanova University Hospital, Lleida, Spain. All three databases contained data on consecutive patients who presented with a new pleural effusion and underwent thoracentesis from 2008 to 2012 in Bristol, 2009 to 2013 in Perth and 1992 to 2013 in Spain. Only pleural fluid samples sent for both pH and glucose measurement at the time of thoracentesis were included. Samples sent for only one of the tests and those without a definite diagnosis were excluded.
All patients gave written informed consent to be included in the respective databases and for their pleural fluid results to be used for research. The Institutional Review Board at each center approved the data collection [approval numbers: 08/H0102/11 (NBLC); 2009-104 and 2004-147 (Sir Charles Gairdner and Osbourne Park Health Care Group) and CEIC-1872 (Spain)].
Pleural fluid handling
At all participating centers, pleural fluid was collected into an uncoated syringe, transferred immediately to a heparinized blood gas syringe for pH analysis and processed within a maximum of two hours. The pH was measured using a GEM Premier 4000 POCT analyser, Radiometer ABL 800 Flex Analyser and GEM Premier 3000 in Bristol, Perth and Lleida respectively.
In the NBLC, a BD vacutainer containing fluoride/oxalate was used for glucose analysis, whereas in the other two centers uncoated bottles were used in accordance with local practice. Pleural fluid glucose was measured using a Roche Hitachi Cobas 6000 (C501 module) or Cobas 8000 (C701 module) in Bristol, Abbott Architech C16000 in Perth and Roche/Hitachi models 717, 917, or Modular DP Roche Diagnostics in Lleida. All machines were calibrated following manufacturers’ instructions.
Diagnostic groups
The diagnosis of the effusion was determined by interrogation of the hospital integrated clinical system, clinic letters, radiology images and medical notes by two respiratory clinicians in the UK cohort, independent to patient care, and by at least one respiratory clinician in the Australian and Spanish cohorts.
Each pleural effusion was categorized into one of four groups—“Cancer”, “Infection”, “Tuberculosis (TB)” and “Others”.
Pleural effusions were classified into the Cancer group if the patient had:
Histocytological confirmation of pleural malignancy;
Radiological evidence of malignant pleural disease on computed tomography in the context of a known malignancy;
Radiological evidence of disseminated malignancy and an effusion with no other cause identified.
Pleural effusions were classified into the Infection group if they were:
Empyema (pus and/or bacterial culture positive); or
Complicated parapneumonic effusion (CPE), defined as effusions related to pneumonia requiring definitive intervention (e.g., intercostal catheter insertion);
Simple parapneumonic effusions (SPEs) defined as other exudative effusions not included in the above categories in a patient with clinical/radiological evidence of pneumonia.
Pleural effusions were classified into the TB group if they had:
Positive Ziehl-Neelsen stains or cultures of pleural fluid or biopsy specimens for M. tuberculosis;
Presence of necrotizing granulomas on pleural biopsy;
Lymphocytic effusions after the exclusion of other causes, which improved with empirical anti-tuberculous therapy.
Cases that did not fulfil the criteria for the above groups were classified under others and included congestive cardiac failure and other transudates, benign reactive effusions and effusions secondary to connective tissue diseases.
Data management and statistics
Patient demographics, diagnoses and pleural fluid pH and glucose values were collected. Only samples with paired glucose and pH values were considered. Results reported as “less than” a value were given that value for analysis. Glucose values reported in mg/dL were converted to mmol/L (multiplied by 0.0555). All data were anonymized at source. Hereafter, pH and glucose pertain to pleural pH and glucose levels unless otherwise specified.
Data are presented as means [± standard deviation (SD)] or median (range), where appropriate. To assess the relationship between pH and glucose, smooth curves from restricted cubic spline models were plotted, which provide a flexible fitting of curves for continuous predictors in regression models. For analyses, glucose was log-transformed but the results are presented on the original scale. The concordance between pH and glucose was assessed at standard cutoff values used in clinical practice (i.e., pH of 7.20 and glucose of 3.30 mmol/L). Statistical analyses were conducted in the R environment for statistical computing (16).
Results
A total of 2,971 pleural effusion samples were included in the analysis (NBLC n=287, SCGH n=212 and Lleida n=2,472). The mean age was 65.6±18.38 years, with 60.3% of samples from male patients. The samples were categorized as Cancer in 35.2% of cases, Infection 18.3%, TB 8.4% and Others 38.1% (Table 1).
Table 1. Demographics of the overall cohort.
| Variables | N (%) | pH, mean ± SD | Glucose, median (range) (mmol/L) |
|---|---|---|---|
| Gender: male | 1,791 (60.3) | ||
| Age, mean ± SD (years) | 65.6±18.38 | ||
| Diagnosis | |||
| Overall | 2,971 (100.0) | 7.38±0.22 | 5.99 (0.00–29.36) |
| Cancer | 1,045 (35.2) | 7.39±0.14 | 5.90 (0.00–25.81) |
| Lung | 367 (35.1) | ||
| Breast | 163 (15.6) | ||
| Mesothelioma | 122 (11.7) | ||
| Gynae | 85 (8.1) | ||
| Haematological | 72 (6.9) | ||
| Gastrointestinal | 55 (5.3) | ||
| Urological | 39 (3.7) | ||
| Head and neck | 17 (1.6) | ||
| Pancreas | 17 (1.6) | ||
| Other | 23 (2.2) | ||
| Unknown primary | 85 (8.1) | ||
| Infection | 544 (18.3) | 7.15±0.35 | 4.03 (0.00–23.59) |
| Uncomplicated | 191 (35.1) | ||
| Complicated | 221 (40.6) | ||
| Empyema | 132 (24.3) | ||
| Tuberculosis | 249 (8.4) | 7.35±0.13 | 4.33 (0.06–24.53) |
| Others | 1,133 (38.1) | 7.49±0.12 | 6.94 (0.06–29.36) |
| Transudates | 846 (74.7) | ||
| Heart failure | 604 (53.3) | ||
| Pericardial disease | 91 (8.0) | ||
| Hepatic hydrothorax | 85 (7.5) | ||
| Renal disease | 6 (0.5) | ||
| Miscellaneous | 60 (5.3) | ||
| Exudates | 287 (25.3) | ||
| Benign pleuritis | 98 (8.6) | ||
| Abdominal aetiology | 97 (8.6) | ||
| Pulmonary embolism | 46 (4.1) | ||
| Connective tissue disease | 25 (2.2) | ||
| Benign asbestos disease | 19 (1.7) | ||
| Chylous effusion | 2 (0.2) |
The mean pH was 7.38±0.22 and the median glucose level 5.99 (range, 0.00–29.36) mmol/L for the overall cohort. The pH and glucose were lowest in the Infection group and highest in Others: 7.15±0.35 vs. 7.49±0.12 (P<0.01), and 4.03 (range, 0.00–23.59) vs. 6.94 (range, 0.06–29.36) mmol/L (P<0.01) respectively (Table 1). The overall pH and glucose results from NLBC, SCGH and Lleida were similar: pH 7.31±0.19, 7.35±0.23 and 7.39±0.23; glucose 5.80 (range, 0.00–15.30), 4.90 (range, 0.50–19.60) and 6.11 (range, 0.00–29.36) mmol/L respectively.
Relationship between glucose and pH
Overall
Regression modeling of the relationship between glucose (log-transformed) and pH with a restricted cubic spline showed linear (P<0.01) and nonlinear effects (P<0.01). The estimated overall trend (after transforming glucose back to the original scale) showed an increasing glucose with increasing pH values up to a pH of approximately 7.50, with a narrow confidence interval. Beyond this, a decreasing trend based on a small number of cases with a wider confidence interval was observed (Figure 1). The prediction interval was wide throughout the relationship.
Figure 1.
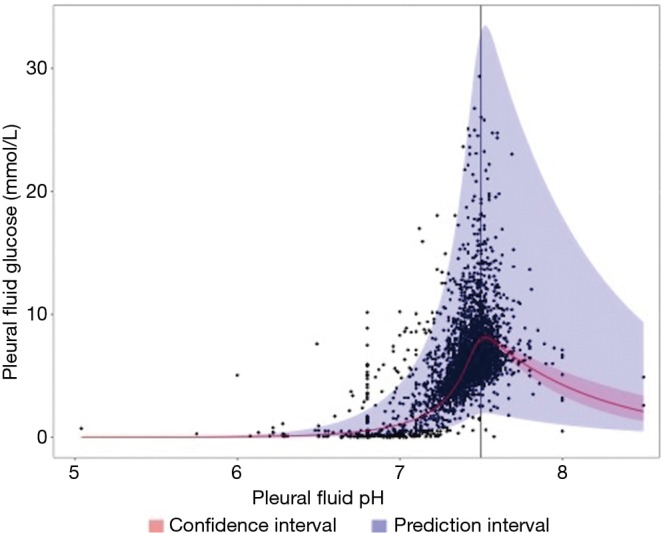
Relationship between pH and glucose modelled using a restricted cubic spline. The fitted regression line is displayed in red. The confidence interval is narrow up to a pH of 7.50. The prediction interval is broad throughout indicating that we cannot accurately estimate glucose from pH and vice versa.
Cutoff levels and concordance
The concordance between pH and glucose, at commonly used cutoff values of pH 7.20 and glucose 3.30 mmol/L, was 91.9%. The degree of concordance within each diagnostic group was highest for the others group (98.2%) and lowest for TB (80.7%) (Figure 2, Table 2). When sub-divided into simple and CPEs and empyema, the concordance was lower in the CPEs subgroup compared to the other two subgroups combined (P=0.0242).
Figure 2.
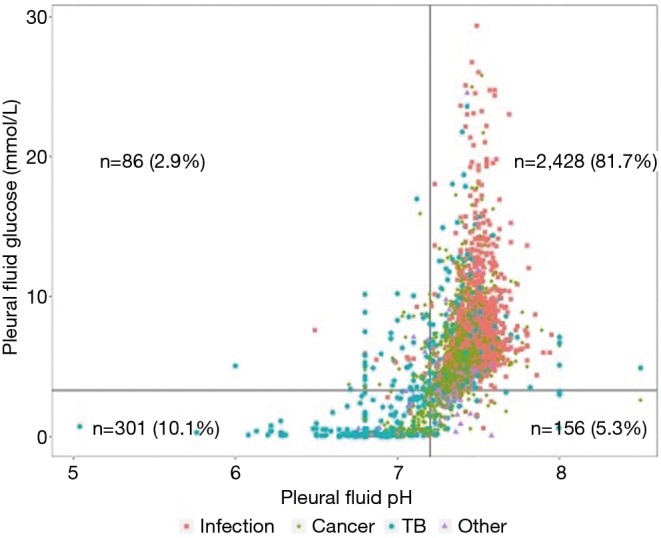
Discordant vs. concordant samples: this graph displays samples in relation to the commonly used cutoff values of pleural fluid pH 7.20 and glucose 3.30 mmol/L. The majority of samples were concordant (right upper and left lower quadrants). The most common cause of discordant samples in which the pH, but not the glucose, fell below the cutoff was infection (left upper quadrant, n=86). TB, tuberculosis.
Table 2. Concordance and discordance rates within each diagnostic group.
| Diagnosis | Concordance, n (%) | Discordance, n (%) | |
|---|---|---|---|
| Glucose normal, pH low | Glucose low, pH normal | ||
| Infection (n=544) | 472 (86.8) | 45 (8.3) | 27 (5.0) |
| Uncomplicated (n=191) | 172 (90.1) | 11 (5.8) | 8 (4.2) |
| Complicated (n=221) | 183 (82.8) | 25 (11.3) | 13 (5.9) |
| Empyema (n=132) | 117 (88.6) | 9 (6.8) | 6 (4.5) |
| TB (n=249) | 201 (80.7) | 3 (1.2) | 45 (18.1) |
| Others (n=1,133) | 1,113 (98.2) | 11 (1.0) | 9 (0.8) |
| Cancer (n=1,045) | 943 (90.2) | 27 (2.6) | 75 (7.2) |
| Overall (n=2,971) | 2,729 (91.9) | 86 (2.9) | 156 (5.3) |
Identification of complicated parapneumonic effusion
Of infection-related effusion samples with a pH or glucose below cut-off, pH was low in 90.5% vs. glucose which was low in 84.2%. Therefore, pH alone appears to identify more cases of potentially CPEs than glucose alone. In 95.0% of cases secondary to infection (n=517), the glucose result did not provide additional information to pH in the separation of biochemically complicated parapneumonic from SPEs. Conversely, if glucose alone is tested, the addition of pH testing did not change the outcome in 91.7% of infection-related cases.
Discordant results
Overall, 8.1% (n=242) of the entire cohort had discordant pH and glucose results. Discordance occurred more commonly with glucose below cutoff and pH above (n=156, 5.3% of the cohort), than vice versa (n=86). The former group were largely due to malignancy and TB (n=120, 76.9%). In the group with pH, but not glucose, below cutoff, infection accounted for >50% of cases. Of the others group, discordance was more common in cases of connective tissue disease than the other subgroups (20% vs. 1.8% overall), however the total number of these cases was small (n=25).
Discussion
This is the first, large, multi-center project to examine the relationship between pleural fluid pH and glucose levels. The correlation between pH and glucose in pleural fluid has been the subject of many smaller studies, which showed similar but less definitive results (4,5,8,17,18). Ours is the first study that has adequate numbers to delineate the complex non-linear relationship between these two parameters (Figure 1). We found a large variability in the prediction interval, suggesting that the two parameters are influenced by separate factors. Although the relationship curve is tight, the large prediction interval means that the pH cannot adequately predict the glucose value and vice versa. In relation to clinical cutoffs, however, the concordance of pH and glucose was very high, suggesting that either test may be adequate in most circumstances.
Concordance varied among diagnostic groups and within diagnostic subgroups. Discordance was highest in TB effusions (n=48), more commonly due to a low glucose than a low pH (45 vs. 3 cases). This is in keeping with older literature that reported low glucose values on TB effusions, akin to cerebrospinal fluid in TB meningitis (19). On the contrary, effusions secondary to non-infective/non-malignant causes (Others, n=1,133) had a concordance rate of 98.2%. Importantly, in pleural infection, where pH and glucose have the most impact on clinical decision-making, a pH below the cutoff for chest-tube drainage was not always matched with a low glucose. Hyperglycemia may contribute to a number of these discrepancies.
In vitro and in vivo models have demonstrated significant decreases in both pleural pH and glucose in the presence of bacteria, polymorphonuclear cells and malignant cells (20,21). In vivo, the measured levels are believed to relate to the degree of metabolic activity in the effusion and pleural membrane dysfunction (20). It is hypothesized that pH decreases earlier in the presence of significant inflammation, followed by a reduction in glucose (5,20). The generation of hydrogen ions from glucose metabolism continues as long as glucose, the necessary substrate, is available, which is dependent on influx of glucose across the pleural membrane. This, in turn, is likely to be affected by the degree of pleural membrane thickening, which varies depending on the underlying aetiology. Some of our discordant results may be explained by differences in the degree of inflammation in the effusion vs. the limitation of glucose influx due to thickening of the pleura.
This study has limitations. It is a retrospective project, although the large databases and unselected nature of inclusion helped to minimize selection bias. Data on diabetes and matching serum glucose levels were not available to evaluate their effects on pleural fluid glucose concentrations, which limited our interpretation of the concordance demonstrated. Despite this, the correlation between pH and glucose was strong. To our knowledge there are no major studies or established parameters on how serum glucose level should be interpreted or used to adjust for the fluid glucose levels. Future studies should address if serum-fluid glucose ratio, their gradient, or a specific cutoff of the blood level would surpass the use of pleural fluid glucose level alone. The definition of CPE was the requirement of a chest drain, which may have been influenced by the pH value, however, this would not affect the concordance of pH and glucose in that subgroup and would not alter our conclusions overall. In our study, the data on pH and glucose would have been known to those making the diagnosis of CPEs and therefore it is outside the scope of this study to ascertain the validity of pH or glucose in the diagnosis of a CPE.
This study focuses on the scientific relationship between pH and glucose levels in pleural fluid. Often in clinical settings, other disease-specific biomarkers would be used and contribute to the diagnostic process. The relations of the levels of these markers with the pleural fluid pH and glucose concentrations may be an interesting subject for future studies. In conclusion, this largest study to date on pleural fluid pH and glucose found a non-linear relationship. Overall, these findings suggest that performing either pH or glucose will provide the same information in over 90% of cases. This may be of particular benefit in resource limited countries and raises the question of whether either test could be used alone in the first instance and the other added only if clinically indicated.
Acknowledgements
Pleural fluid samples were obtained with the support of the Xarxa Catalana de Bancs de Tumors, a tumor bank platform of RTICC and RETIC Biobancos RD09/0076/00059. Administrative support was provided by Hui Min Cheah.
YC Lee was a National Health & Medical Research Council (NHMRC) Career Development Fellow and has received research project grant funding from the NHMRC, New South Wales Dust Disease Board, Sir Charles Gairdner Research Advisory Committee, Westcare and the Cancer Council of Western Australia. DB Fitzgerald is supported by a fellowship from the Western Australia Cancer and Palliative Care Network and the European Respiratory Society.
Ethical Statement: The Institutional Review Board at each center approved the data collection [approval numbers: 08/H0102/11 (NBLC); 2009-104 and 2004-147 (Sir Charles Gairdner and Osbourne Park Health Care Group) and CEIC-1872 (Spain)].
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Light RW, Lee YCG. Textbook of pleural diseases. 3rd edition. Boca Raton: CRC Press, Taylor & Francis Group, 2016. [Google Scholar]
- 2.Davies HE, Davies RJ, Davies CW, et al. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii41-53. 10.1136/thx.2010.137000 [DOI] [PubMed] [Google Scholar]
- 3.Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000;118:1158-71. 10.1378/chest.118.4.1158 [DOI] [PubMed] [Google Scholar]
- 4.Potts DE, Taryle DA, Sahn SA. The glucose-pH relationship in parapneumonic effusions. Arch Intern Med 1978;138:1378-80. 10.1001/archinte.1978.03630340048016 [DOI] [PubMed] [Google Scholar]
- 5.Good JT, Jr, Taryle DA, Maulitz RM, et al. The diagnostic value of pleural fluid pH. Chest 1980;78:55-9. 10.1378/chest.78.1.55 [DOI] [PubMed] [Google Scholar]
- 6.Light RW, Girard WM, Jenkinson SG, et al. Parapneumonic effusions. Am J Med 1980;69:507-12. 10.1016/0002-9343(80)90460-X [DOI] [PubMed] [Google Scholar]
- 7.Davies CW, Kearney SE, Gleeson FV, et al. Predictors of outcome and long-term survival in patients with pleural infection. Am J Respir Crit Care Med 1999;160:1682-7. 10.1164/ajrccm.160.5.9903002 [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Panadero F, Lopez Mejias J. Low glucose and pH levels in malignant pleural effusions. Diagnostic significance and prognostic value in respect to pleurodesis. Am Rev Respir Dis 1989;139:663-7. 10.1164/ajrccm/139.3.663 [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Panadero F, Lopez-Mejias J. Survival time of patients with pleural metastatic carcinoma predicted by glucose and pH studies. Chest 1989;95:320-4. 10.1378/chest.95.2.320 [DOI] [PubMed] [Google Scholar]
- 10.Sahn SA, Good JT., Jr Pleural fluid pH in malignant effusions. Diagnostic, prognostic, and therapeutic implications. Ann Intern Med 1988;108:345-9. 10.7326/0003-4819-108-3-345 [DOI] [PubMed] [Google Scholar]
- 11.Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest 2000;117:79-86. 10.1378/chest.117.1.79 [DOI] [PubMed] [Google Scholar]
- 12.Clarkson B. Relationship between Cell Type, Glucose Concentration, and Response to Treatment in Neoplastic Effusions. Cancer 1964;17:914-28. [DOI] [PubMed] [Google Scholar]
- 13.Rahman NM, Mishra EK, Davies HE, et al. Clinically important factors influencing the diagnostic measurement of pleural fluid pH and glucose. Am J Respir Crit Care Med 2008;178:483-90. 10.1164/rccm.200801-062OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesho EP, Roth BJ. Is pH paper an acceptable, low-cost alternative to the blood gas analyzer for determining pleural fluid pH? Chest 1997;112:1291-2. 10.1378/chest.112.5.1291 [DOI] [PubMed] [Google Scholar]
- 15.Cheng DS, Rodriguez RM, Rogers J, et al. Comparison of pleural fluid pH values obtained using blood gas machine, pH meter, and pH indicator strip. Chest 1998;114:1368-72. 10.1378/chest.114.5.1368 [DOI] [PubMed] [Google Scholar]
- 16.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. [Google Scholar]
- 17.Potts DE, Levin DC, Sahn SA. Pleural fluid pH in parapneumonic effusions. Chest 1976;70:328-31. 10.1378/chest.70.3.328 [DOI] [PubMed] [Google Scholar]
- 18.Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005;99:1183-7. 10.1016/j.rmed.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 19.Barber LM, Mazzadi L, Deakins DD, et al. Glucose level in pleural fluid as a diagnostic aid. Dis Chest 1957;31:680-7. 10.1378/chest.31.6.680 [DOI] [PubMed] [Google Scholar]
- 20.Sahn SA, Taryle DA, Good JT., Jr Experimental empyema. Time course and pathogenesis of pleural fluid acidosis and low pleural fluid glucose. Am Rev Respir Dis 1979;120:355-61. [DOI] [PubMed] [Google Scholar]
- 21.Good JT, Jr, Taryle DA, Sahn SA. The pathogenesis of low glucose, low pH malignant effusions. Am Rev Respir Dis 1985;131:737-41. [DOI] [PubMed] [Google Scholar]


