Abstract
Background
Esophagectomy is the mainstay treatment for early stage and locoregionally advanced esophageal cancer. Anastomotic leaks following esophagectomy are associated with numerous detrimental sequelae. The management of anastomotic leaks has evolved over time. The present study is a single-institution experience of esophageal leak management over an 11-year period, in order to identify when these can be managed nonoperatively.
Methods
All patients undergoing esophagectomy with gastric reconstruction at our institution between 2004 and 2014 were identified. Preoperative patient characteristics and perioperative factors were reviewed. Failure of initial leak treatment was defined as need for escalation of therapy. Length of stay (LOS) and postoperative mortality were the primary outcomes. Follow-up was obtained through institutional medical records and the Social Security Death Index.
Results
Sixty-one of 692 (8.8%) patients developed an anastomotic leak. Forty-six patients (75.4%) first underwent observation, which was successful in 35 patients. Predictors of successful observation included higher preoperative albumin (P=0.02), leak diagnosed by esophagram (P=0.004), and contained leaks (P=0.01). Successful observation was associated with shorter LOS (P=0.001). Predictors of mortality included lower preoperative serum albumin (P=0.01) and induction therapy (P=0.03). Thirty and 90-day mortality among patients who developed an anastomotic leak were 9.8% and 16.7%, respectively.
Conclusions
Over half of anastomotic leaks were managed successfully with observation alone and did not require additional interventions. We have identified factors that may predict successful therapy with observation in these patients. Further research is warranted to determine more timely interventions for patients likely to fail conservative management.
Keywords: Esophagectomy, esophageal cancer, anastomotic leak
Introduction
Esophagectomy is considered the mainstay of treatment for the management of early stage and locoregionally advanced esophageal cancer. However, esophagectomy carries a significant risk of morbidity and mortality since it is commonly performed on patients who have poor nutritional status, multiple medical comorbidities, or other factors predisposing them to postoperative complications (1-6). Anastomotic leaks following esophagectomy are a somewhat common complication, with reported incidence ranging between 10–25% for cervical anastomoses and 3–25% for intrathoracic anastomoses (2). Intrathoracic anastomoses have a lower incidence of leak and stricture than cervical anastomoses, but they tend to carry higher morbidity and usually require more extensive treatment (3). Anastomotic leaks are associated with prolonged hospital stay, stricture formation, postoperative dysphagia, and overall morbidity and mortality (1,2). Reported mortality rates among patients who develop anastomotic leaks ranges between 30–60%, and approximately 40% of postoperative mortality following esophagectomy is directly related to anastomotic leaks (1-3).
The most effective treatment for anastomotic leaks remains controversial and there is no defined regimen for their management to date (3,4). Treatment options include either close observation or surgical reintervention. Surgical approaches for repair include thoracoscopy or thoracotomy, and surgical strategies range from drainage via T-tube placement for less severe leaks to complete gastrointestinal diversion for extensive disruption (5,6). Endoscopic stenting has also been employed, with multiple reports on its success (5).
The present study seeks to further characterize the different strategies for management of anastomotic leaks, with particular focus on non-operative management by reviewing all of the cases performed over an 11-year period at our institution.
Methods
All patients undergoing esophagectomy with gastric reconstruction at our institution between January 1, 2004 and December 31, 2014 were identified retrospectively using a prospectively compiled institutional database. The requirement for individual consent for this study was waived by the institutional review board at our institution. Patients who developed an anastomotic leak following surgery were identified. Anastomotic leak was defined as a disruption of the esophagogastric anastomosis, as identified by radiographic contrast examination, endoscopic evaluation, or clinical observations noted by healthcare providers in the patients’ medical files. Anastomotic leaks were further categorized into three grades, as previously described by Low et al. (7). Type 1 anastomotic leaks were defined as a localized defect that was treated using medical therapy or by observation alone; type 2 leaks were defined as a localized defect requiring intervention but not surgical therapy; and type 3 leaks were defined as a localized defect requiring surgical intervention.
The primary outcomes for this study were hospital length of stay (LOS) and 30- and 90-day mortality. In addition, preoperative risk factors and medical comorbidities, intraoperative variables, and postoperative outcomes including failure of initial leak treatment were analyzed. Preoperative risk factors analyzed included age, gender, race, smoking history, and body mass index (BMI). Preoperative comorbidities assessed for included coronary artery disease (CAD), hypertension, congestive heart failure (CHF), diabetes mellitus (DM), and neoadjuvant chemoradiation. Intraoperative variables collected included the type of esophagectomy—Ivor Lewis, three-incision, or trans-hiatal. Postoperative variables included clinical indicators, diagnostic studies [contrast esophagram, esophagogastroduodenoscopy (EGD), and/or computed tomography (CT)], and leak management strategy (observation, stenting, surgery). Observation included diet changes (restricting oral intake) as well as antibiotic use, but no other intervention. Failure of initial therapy was defined as the need for additional interventions for leak management (any interventions in patients who were initially observed, or surgery in patients who were initially stented). Subsequent interventions following failed initial therapy were also recorded. Follow-up and long-term mortality data were obtained through medical records and the Social Security Death Index, as permitted by our institutional review board.
Postoperative management of patients following esophagectomy is standardized at our institution. Feeding jejunostomy use has varied over time and was mostly performed selectively. A drain was placed at the site of anastomosis during surgery. Contrast esophagram was routinely performed on postoperative day 5–7. Patients who were unable to undergo contrast esophagram, such as those with prolonged intubation, received an endoscopic examination instead.
Statistical analysis was performed using SPSS 23 (IBM Corp., Released 2015. IBM SPSS Statistics for Windows, Version 22.0, Armonk, NY, USA) Unpaired student’s t-test was used for continuous variables, Fisher’s exact test was used for dichotomous data, and a Chi-squared test was used for categorical variables. A two-tailed P value of less than 0.05 was considered significant. Ethics approval was obtained prior to the study. This was obtained from Indiana Universities institutional review board. The approved protocol number is 1508620119.
Results
Between January 1, 2004 and December 31, 2014, 692 patients underwent esophagectomy with gastric reconstruction at our institution. A total of 61 patients [8.8%—cervical leaks (30/177) and thoracic leaks (31/515)] developed an anastomotic leak postoperatively. The preoperative characteristics of this cohort of patients are listed in Table 1. Preoperative symptomatology was available for 22 patients, all of whom presented with dysphagia. Preoperative interventions included jejunostomy tube placement in five patients, gastrostomy tube placement in two patients, and total parenteral nutrition in two patients. No patients underwent preoperative stenting.
Table 1. Preoperative characteristics of patients with post-operative anastomotic leaks.
| Variable | Total |
|---|---|
| Median age, years | 61.5 |
| Gender, No (%) | |
| Male | 52 (85.2) |
| Female | 9 (14.8) |
| Race, No (%) | |
| White | 60 (98.4) |
| African American | 1 (1.6) |
| Average BMI (kg/m2) | 27.7 |
| Smoking History, No (%) | |
| Current | 15 (24.6) |
| Former | 30 (49.2) |
| Never | 16 (26.2) |
| Coronary Disease, No (%) | 14 (22.5) |
| CHF, No (%) | 3 (4.9) |
| Hypertension, No (%) | 39 (63.9) |
| Pre-op albumin (g/dL), median | 3.7 (3.5–4.7) |
| Hyperlipidemia, No (%) | 11 (18.0) |
| Atrial fibrillation, No (%) | 10 (16.4) |
| DM, No (%) | 17 (27.9) |
| COPD, No (%) | 6 (9.8) |
| Malignancy, No (% of malignancies) | |
| Adenocarcinoma | 41 (67.2) |
| Squamous | 8 (13.1) |
| Induction therapy, No (%) | 34 (55.7) |
| Tumor location, No (% of malignancies) | |
| Proximal | 2 (3.2) |
| Mid | 7 (11.4) |
| Distal | 40 (65.6) |
BMI, body mass index; CHF, congestive heart failure; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease.
Thirty-two patients (52.5%) underwent an Ivor Lewis esophagectomy, 24 (39.3%) underwent a three-incision esophagectomy, and five patients (8.2%) underwent a transhiatal esophagectomy. Seventeen patients (27.9%) underwent a minimally invasive procedure. The majority of patients (52/61, 85.2%) had an operation for esophageal cancer. From the remaining patients, one underwent esophagectomy for gastro-esophageal junction perforation, one for gastrointestinal stromal tumor, two for achalasia, three for benign stricture, one for bronchoesophageal fistula, and one for gastric outlet obstruction.
The median date of diagnosis of anastomotic leak was on postoperative day seven with a range of 2 to 20 days. Initial anastomotic leak detection methods included contrast esophagram in 42 patients, EGD in 10 patients, chest CT in three patients, and clinical indicators in six patients. Of note, two of the chest CT scans were performed with PO contrast. Esophagram was negative in three of the patients who were subsequently diagnosed by EGD, one patient subsequently diagnosed by CT, and three patients subsequently diagnosed by clinical indicators. Patients who were diagnosed with an anastomotic leak by EGD or clinical indicators were diagnosed later in the postoperative period than those diagnosed by contrast esophagram (median postoperative day of diagnosis 11 vs. 7, P=0.03). Twenty-eight of the 42 patients diagnosed with a leak by contrast esophagram were characterized as contained and 14 as uncontained (communicating with the drain).
Initial management strategies based upon diagnostic methods are shown in Table 2. We did not identify any differences in our management based on location of anastomosis. Overall, 46 patients (75.4%) were treated with observation only. Eleven of those (23.9%) failed observation and required re-intervention. Six of these patients underwent stent placement and five were managed surgically, two having received a stent prior to revisional surgery. Predictors of success for observation alone included higher preoperative albumin (median level in successful vs. failed observation, 3.8 vs. 3.6 g/dL, P=0.02), leak diagnosed by esophagram (successful vs. failed observation 93.1% vs. 63.1%, P=0.004), and identification of a contained leak on esophagram (successful vs. failed observation 68.6% vs. 36.4%, P=0.01). Patients managed successfully with observation alone had a shorter median LOS and median intensive care unit (ICU) stay than those treated with other management strategies (15 vs. 27 days and 3 vs. 9 days, both P=0.001). Of interest, LOS and ICU stay were similar in patients who were initially observed and then needed a stent compared to those stented successfully as an initial therapy (median values: 20 vs. 21 days and 4.5 vs. 5.5 days, P=NS). Similarly, those requiring surgery after being initially observed had similar LOS and ICU stay compared to those operated on immediately (median values 36 vs. 37.5 days and 19.5 vs. 17 days, P= NS). Four patients treated conservatively died. None of these patients had required further intervention, although one of them died shortly after the diagnosis due to aspiration pneumonia. The other 3 deaths were unrelated to the leak.
Table 2. Initial management based on diagnostic method. All numbers are absolute values.
| Modality | Diagnosis | Observation | Stent | Surgery |
|---|---|---|---|---|
| Esophagram | 42 | 39 | 2 | 1 |
| Endoscopy | 10 | 4 | 5 | 1 |
| Clinical | 6 | 3 | 3 | 0 |
| CT scan | 3 | 0 | 1 | 2 |
CT, computed tomography.
Correlation between initial treatment strategy and ultimate leak severity by type 1–3 is shown in Figure 1. Esophageal stents were placed in a total of 19 patients. Eight of the 19 patients had anastomoses at the cervical location and 11 had intra-thoracic anastomoses. Five of the 19 patients failed stent therapy and required surgery (two of whom were “salvage” stents after failed observation.
Figure 1.
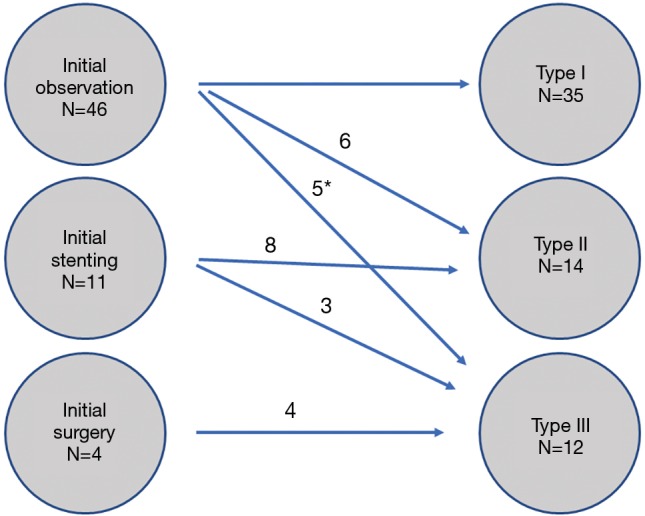
Correlation between initial treatment and ultimate leak type. *, stent then surgery.
Overall 12 patients developed a type 3 leak—eventually required surgical intervention; four patients underwent surgery initially, three straights after failed observation and 5 after failed stenting. Four of these patients underwent takedown of the conduit with esophageal diversion: two dues to an uncontrolled leak and two dues to the development of tracheoesophageal fistula. Of the remaining patients managed surgically, one patient required neck wound drainage and six underwent a decortication.
Outcomes between the leak groups by type are summarized in Table 3. Median hospital LOS was 19 days and overall 30- and 90-day mortality was 9.8% and 17.3%, respectively, among patients who developed an anastomotic leak. The only predictors of mortality following anastomotic leak included lower preoperative serum albumin (P=0.01) and induction chemoradiation therapy (P=0.03).
Table 3. Outcome by leak type.
| Outcome | Type I | Type II | Type III | P value |
|---|---|---|---|---|
| 30-day mortality (%) | 8.8 | 0 | 28.5 | 0.07 |
| 90-day mortality (%) | 8.8 | 15.7 | 42.8 | 0.18 |
| Median LOS (days) | 15 | 21.5 | 40 | 0.0001 |
| Median ICU stay (days) | 3 | 5 | 20.5 | 0.0001 |
LOS, length of stay; ICU, intensive care unit.
Discussion
Anastomotic leaks are one of the most significant complications after esophagectomy. Several risk factors contribute in the development of anastomotic leaks and can generally be classified into four categories: systemic disease, esophageal structure, operative technical factors, and post-operative factors (2). Pre-operative malnutrition, diabetes, and advanced age are all known pre-operative risk factors. Kassis et al. showed that obesity, heart failure, coronary disease, vascular disease, renal disease, and tobacco use were associated with post-operative anastomotic leak (8). Absence of serosa and extraperitoneal anastomoses all comprise aspects of esophageal structure that contribute to anastomotic leaks (2). Site of anastomosis, surgery type, anastomotic tension, type of anastomosis, and experience of the surgeon define the operative-technical category. Finally, gastric distension, reoperation, and prolonged mechanical ventilation may all contribute to anastomotic leak in the post-operative period (2,9).
The diagnosis of an anastomotic leak may be made via physical examination, laboratory studies or radiographic imaging (2). Clinical signs vary widely but the most common include fever, tachycardia, increased drainage from chest tube, or recurrent coughing with swallowing (10). At our institution, we generally evaluate the esophagus for leak on the 7th post-operative day by means of a contrast esophagram in patients without a suspected leak clinically. It has been suggested that patients only be evaluated with an esophagram when there is a clinical suspicion for leak due to risk for aspiration (11). However, we believe that there is additional information to be obtained by a routine esophagram, including evaluation of conduit emptying and risk for aspiration, particularly in cervical anastomoses.
In addition to contrast esophagram, there are multiple modalities that may be utilized for diagnosis. A CT scan with radiographic contrast is routinely used and has proven to be highly sensitive (1,12,13). Guo et al. have advocated for CT with oral contrast, suggesting that it allows for observation of leak, characterization of its magnitude, leak location, and extent of abscess if present (1). Three patients in our study were diagnosed with an anastomotic leak using CT scan. Oral contrast was used in 2 of those patients. Endoscopy may also be used to diagnose an anastomotic leak, and may be the most definitive test. Page and colleagues suggest it is the best way to determine if there is ischemia or necrosis and allows for selection of the best treatment option (14). In our experience, endoscopy is typically utilized in evaluation of leak extent and appropriateness of endoscopic intervention, and also as the initial mode of diagnosis in patients who may be unable to undergo an esophagram, such as mechanically ventilated patients. This was the case in 2 out of 10 patients initially diagnosed with EGD in our study. Two patients developed hematemesis and required immediate EGD, while two others developed sepsis ultimately leading to EGD.
The clinical spectrum varies widely for anastomotic leaks, which creates difficulties when creating algorithms for treatment (15). In our study, anastomotic leak was more common when the anastomosis was at the cervical location. This is similar to reports by Kassis and colleagues where they showed that the leak rates for cervical and intra-thoracic anastomosis were 12.3% and 9.3%, respectively (8). In general, if a cervical leak is confined to the neck and is not associated with systemic sepsis, treatment with antibiotics, exploration of the wound and daily bedside dressing changes will suffice (2). If the leak is more substantial, drainage along with nasogastric decompression and nutritional support may be necessary. If a leak is uncontained or cannot be controlled by drainage alone, then one may need to advance to decortication, or resection with diversion (2). The management of intrathoracic anastomotic leaks is less straightforward and should be individualized to each patient. Asymptomatic patients and a minority of patients with symptoms can often be treated conservatively when the leaks are contained (2,10,16,17). Surgical intervention is warranted if the leak is uncontained and for those who fail conservative management (4). While it is thought by some that intrathoracic leaks are more ominous than cervical ones, our findings suggested that outcomes were similar regardless of leak location.
In our study, 75% of patients were initially treated with conservative management. Eleven of these patients failed observation, eight receiving stents (2 of whom required subsequent surgery) and 3 proceeding to surgery. Overall, the success rate for observation was 59% (36/61). Our analysis indicated several factors that may be useful indicators of successful observation. Adequate nutritional status, as judged by preoperative albumin, and a less extensive leak, as diagnosed by an esophagram (and not by clinical picture or endoscopic findings), especially one demonstrating a contained leak were such factors. The significance of preoperative nutrition in overall complications is previously documented, so it is perhaps not surprising that if also affects success of a conservative strategy in anastomotic leak management. In terms of the contained nature of the leak, Crestanello et al. showed that 1 out of 4 patients with symptomatic non-contained leak who were managed non-operatively died. They suggest that patients with non-contained leaks with clinical signs of sepsis should be resuscitated and re-explored in the operating room. They also recommend that the pleural cavity and mediastinum be debrided and drained (4,18). Although intuitive to an extent, it is important to recognize that not all leaks require aggressive interventions. It is also notable that those patients who failed observation had similar LOS and ICU stay to other patients with the same type of leak (meaning observation was not detrimental to the outcomes). Along these lines, no deaths occurred on patients who failed observation. We are not suggesting that all leaks can be treated with observation, but rather that many can, and the outcomes are not worse—even if observation fails.
More recently, endoluminal stenting has been popularized and has demonstrated effectiveness. Schaheen et al. performed a review and showed that endoscopic placement of stents was successful in managing anastomotic leaks in 72% of patients. Mortality in patients receiving stents was approximately 15% compared to 3.3% to 11% for surgery (3). We have previously reported that stenting is technically feasible in all leaks and successful in 83% of patients with esophageal leaks, fistulas or perforations of various etiologies, including following esophagectomy. In total, 28% of patients required stent revisions in our study (19). In the current study, 19 patients underwent stent placement at any point of their management. Stenting alone as an intervention was only successful in 14 of those patients. The 30-day mortality rate was 0% for patients who underwent stenting, but 90-day mortality was 15.7%. It is interesting to note that enthusiasm about stenting is not shared by all; a recent report from a high-volume center in the UK showed excellent results without the use of stents, with surgical therapy for all patients who failed conservative management, although the median LOS was 41.5 days (20). We found a similar increase in LOS after stenting. While it is possible that stents may allow earlier resumption of diet and thus earlier discharge, we were not able to show any decrease in the LOS in patients receiving stents compared to patients who were observed.
As shown in previous studies anastomotic leaks account for a significant portion of mortality after esophagectomy (2,3). Our findings confirm these statements. We observed higher 30- and 90-day mortality in patients requiring surgical therapy, presumably reflecting the increased severity of their overall condition. LOS and ICU stay was also longer in those patients. It is unclear whether timeliness of surgical intervention contributed to that finding, and indeed patients who failed non-surgical management had similar outcomes to those treated initially with an operation—although we recognize that the urgency for each of these patients was different and direct comparisons may be misleading. Given that 4 of the patients required takedown of the conduit, typically a last resort when not necessitated by conduit necrosis, it is questionable whether earlier intervention would have indeed prevented that outcome.
The limitations of our study include its retrospective nature, which limits the ability to account for all confounding factors. Despite the development of postoperative care pathways typical of a high-volume esophageal surgery center, there remains a significant degree of individualized care based on surgeon preference at our institution. As such, this study is unable to make specific recommendations regarding suitability for observation, stenting, or surgical intervention on post-operative leaks, but rather can provide a baseline for expected outcomes for these interventions. Specific management algorithms, particularly relating to stent selection, timing of intervention, experience of gastroenterologists deploying the stents, and endoscopic suturing to minimize migration have evolved over time. Additionally, while the current grading systems rank leaks by the ultimate clinical significance, this is not known a priori. For example, a leak treated with observation upon initial presentation may indeed evolve and require additional therapy. This dynamic nature of this problem makes ideal comparisons difficult. Finally, information about long-term sequelae of leaks, specifically stricture formation, could not be fully evaluated in our database. At our institution, being a regional referral center, many of the patients receive subsequent care at local institutions and exact information on need for dilations or ultimate functional outcomes were not always available. Finally, while a promising form of endoscopic leak management has recently emerged, namely endo-sponge vacuum therapy (21). We have not had experience with this type of therapy but it is certainly possible that it may alter some of the decision making in terms of leak management in the future.
In conclusion, we have reported our experience with management of anastomotic leaks after esophagectomy in a single, high-volume esophageal center. We described the incidence of leaks, the frequency of different grades and the success rates of different management methods, focusing on patients who were treated conservatively with observation which was successful in over half of anastomotic leaks We identified risk factors for failure of observation and mortality after leak. Further study is warranted on more timely intervention on patients likely to fail conservative management.
Acknowledgements
None.
Ethical Statement: Ethics approval was obtained prior to the study. This was obtained from Indiana Universities institutional review board. The approved protocol number is 1508620119.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Guo J, Chu X, Liu Y, et al. Choice of therapeutic strategies in intrathoracic anastomotic leak following esophagectomy. World J Surg Oncol 2014;12:402. 10.1186/1477-7819-12-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turkyilmaz A, Eroglu A, Aydin Y, et al. The management of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma. Dis Esophagus 2009;22:119-26. 10.1111/j.1442-2050.2008.00866.x [DOI] [PubMed] [Google Scholar]
- 3.Schaheen L, Blackmon SH, Nason KS. Optimal approach to the management of intrathoracic esophageal leak following esophagectomy: a systematic review. Am J Surg 2014;208:536-43. 10.1016/j.amjsurg.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crestanello JA, Deschamps C, Cassivi SD, et al. Selective management of intrathoracic anastomotic leak after esophagectomy. J Thorac Cardiovasc Surg 2005;129:254-60. 10.1016/j.jtcvs.2004.10.024 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen NT, Rudersdorf PD, Smith BR, et al. Management of gastrointestinal leaks after minimally invasive esophagectomy: conventional treatments vs. endoscopic stenting. J Gastrointest Surg 2011;15:1952-60. 10.1007/s11605-011-1658-8 [DOI] [PubMed] [Google Scholar]
- 6.Nguyen NT, Follette DM, Roberts PF, et al. Thoracoscopic management of postoperative esophageal leak. J Thorac Cardiovasc Surg 2001;121:391-2. 10.1067/mtc.2001.110485 [DOI] [PubMed] [Google Scholar]
- 7.Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. 10.1097/SLA.0000000000001098 [DOI] [PubMed] [Google Scholar]
- 8.Kassis ES, Kosinski AS, Ross P, Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. 10.1016/j.athoracsur.2013.07.119 [DOI] [PubMed] [Google Scholar]
- 9.Cassivi SD. Leaks, strictures, and necrosis: a review of anastomotic complications following esophagectomy. Semin Thorac Cardiovasc Surg 2004;16:124-32. 10.1053/j.semtcvs.2004.03.011 [DOI] [PubMed] [Google Scholar]
- 10.Junemann-Ramirez M, Awan MY, Khan ZM, et al. Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg 2005;27:3-7. 10.1016/j.ejcts.2004.09.018 [DOI] [PubMed] [Google Scholar]
- 11.Sauvanet A, Baltar J, Le Mee J, et al. Diagnosis and conservative management of intrathoracic leakage after oesophagectomy. Br J Surg 1998;85:1446-9. 10.1046/j.1365-2168.1998.00869.x [DOI] [PubMed] [Google Scholar]
- 12.van der Schaaf M, Lagergren J, Lagergren P. Persisting symptoms after intrathoracic anastomotic leak following oesophagectomy for cancer. Br J Surg 2012;99:95-9. 10.1002/bjs.7750 [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Esquivel R, Raju GS. Endoscopic closure of acute esophageal perforations. Curr Gastroenterol Rep 2013;15:321. 10.1007/s11894-013-0321-9 [DOI] [PubMed] [Google Scholar]
- 14.Page RD, Shackcloth MJ, Russell GN, et al. Surgical treatment of anastomotic leaks after oesophagectomy. Eur J Cardiothorac Surg 2005;27:337-43. 10.1016/j.ejcts.2004.10.053 [DOI] [PubMed] [Google Scholar]
- 15.Anbari MM, Levine MS, Cohen RB, et al. Delayed leaks and fistulas after esophagogastrectomy: radiologic evaluation. AJR Am J Roentgenol 1993;160:1217-20. 10.2214/ajr.160.6.8498220 [DOI] [PubMed] [Google Scholar]
- 16.Cameron JL, Kieffer RF, Hendrix TR, et al. Selective nonoperative management of contained intrathoracic esophageal disruptions. Ann Thorac Surg 1979;27:404-8. 10.1016/S0003-4975(10)63335-8 [DOI] [PubMed] [Google Scholar]
- 17.Barkley C, Orringer MB, Iannettoni MD, et al. Challenges in reversing esophageal discontinuity operations. Ann Thorac Surg 2003;76:989-94; discussion 995. 10.1016/S0003-4975(03)00825-7 [DOI] [PubMed] [Google Scholar]
- 18.Matory YL, Burt M. Esophagogastrectomy: reoperation for complications. J Surg Oncol 1993;54:29-33. 10.1002/jso.2930540109 [DOI] [PubMed] [Google Scholar]
- 19.El H II, Imperiale TF, Rex DK, et al. Treatment of esophageal leaks, fistulae, and perforations with temporary stents: evaluation of efficacy, adverse events, and factors associated with successful outcomes. Gastrointest Endosc 2014;79:589-98. 10.1016/j.gie.2013.08.039 [DOI] [PubMed] [Google Scholar]
- 20.Dent B, Griffin SM, Jones R, et al. Management and outcomes of anastomotic leaks after oesophagectomy. Br J Surg 2016;103:1033-8. 10.1002/bjs.10175 [DOI] [PubMed] [Google Scholar]
- 21.Brangewitz M, Voigtländer T, Helfritz FA, et al. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 2013;45:433-8. 10.1055/s-0032-1326435 [DOI] [PubMed] [Google Scholar]


