Abstract
The role of anatomic segmentectomy as an acceptable, lung parenchymal sparing alternative to pulmonary lobectomy for the small peripheral stage I lung cancer is under great scrutiny today. This is not a new consideration, particularly for the patient with impaired cardiopulmonary reserve where preservation of lung function may be a critical issue in deciding on surgical resection for local/regional control of their cancer. In this review, we discuss the oncologic issues along with past and present evidence supporting “anatomic” lung preservational surgery in the management of lung cancer.
Keywords: Lung cancer, surgery, anatomic resection, sublobar resection, segmentectomy
Introduction
“Is segmentectomy the future?” We believe that the answer to this question in a selective “YES!” That is if you are referring to “intentionally” anatomic segmentectomy for the surgical resection approach to the small (<2 cm. diameter), non-small cell lung cancers (NSCLC) located in the periphery of the lung parenchyma and anatomically located within the boundaries of a chosen segment of the lung (1-9). The possibility of obtaining adequate resection margins must also be calculated into the clinical formulation for “intentionally” choosing to perform anatomic segmentectomy over lobectomy for such NSCLCs. Anatomic segmentectomy also appears to be a preferred approach to the indeterminant peripheral pulmonary nodule suspicious for malignancy (10). If these radiographic, anatomic criteria can be met, “sublobar resection” by either extended wedge resection, or preferably anatomic segmentectomy may have an important primary management role for the clinical stage Ia NSCLC. Of course, the sublobar resection must be accompanied with an adequate interlobar, hilar and mediastinal lymph node sampling/dissection to establish this “quality sublobar resection” (11). Short of meeting these clinical parameters, sublobar resection must be considered as a surgical approach directed “only” to the physiologically impaired patient with a small peripheral NSCLC (12-17).
What leads us to consider sublobar resection/anatomic segmentectomy for early stage NSCLC?
For as long as physicians have contemplated surgical resection of pulmonary pathology, limited resection has been considered. The goal of sublobar pulmonary resection is to be the preservation of lung parenchyma and pulmonary function and reduce postoperative morbidity without affecting the primary therapeutic goals of surgical extirpation of the pathologic condition.
The first pulmonary resections, performed over half a millennium ago, were only of portions of lung hernias that resulted from battlefield chest injuries (18). Subsequent lung resections were also “piece meal” resections of necrotic lung tissue and intentional sublobar resection of areas of limited tuberculous disease (19). As improvements in surgical approaches to lung resection emerged, such as; use of intercostal access vs. thoracic chest wall resection; single stage lung resections enabled by postoperative chest drainage (20); and the eventual acceptance of endotracheal intubation with general anesthesia as a preferred approach to local/regional analgesia with patient sedation, renewed interest in sublobar resection occurred for limited pulmonary pathologic conditions (19).
The use of anatomic segmentectomy was first noted in the resection of focal bronchiectasis of the lung reported by Belsey and Churchill, and described in detail by Overholt (21,22). The pulmonary segment has long been defined as the true anatomic unit of the lung (23), and the use of this resection for lesions contained within the segmental boundaries appeared to be justified.
However, the use of sublobar resection was not generally accepted as adequate treatment of carcinoma of the lung. An important barrier to the acceptance of sublobar resection, and even lobectomy, for the treatment of primary bronchogenic carcinoma was the oncologic surgical concepts that William Halsted championed for the treatment of breast cancer and other cancers (24). Halsted believed that the only appropriate operation was the removal of the entire organ affected by the cancer, any proximate tissues contiguous with the tumor (i.e., pectoral muscles and chest wall if necessary), and also all lymph node drainage basins proximate to the organ. These concepts defined Halsted’s “radical mastectomy” approach to breast cancer. This oncologic surgical concept was also held by most thoracic surgeons who first approached resection of lung cancer—total pneumonectomy was the only appropriate resection (25-31). Although lobectomy began to be utilized more commonly as an approach for the management of primary lung cancer by the middle of the 20th century (32-34), this lesser resection was not appreciated as a reasonable alternative to pneumonectomy until the seminal work of Shimkin et al., which described an equivalent survival between lobectomy and pneumonectomy for limited lung cancers managed at the Overholt and Ochsner Clinics (35) (Figure 1).
Figure 1.
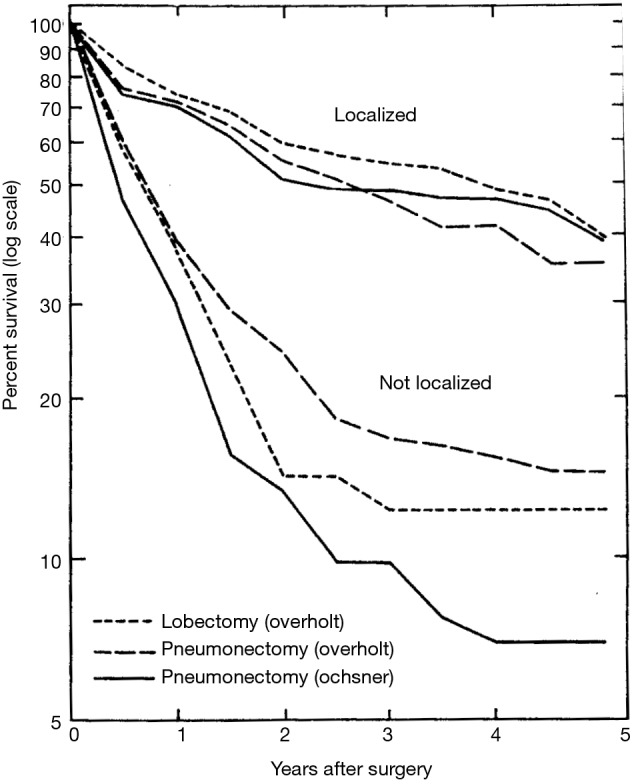
The results of the landmark study by Shimkin which compared the survival outcomes of patients undergoing either lobectomy or pneumonectomy for localized disease and not localized disease operated upon at the Ochsner and Overholt Clinics. Lobectomy found to be equivalent to pneumonectomy for localized disease. Reproduced from ref. (35).
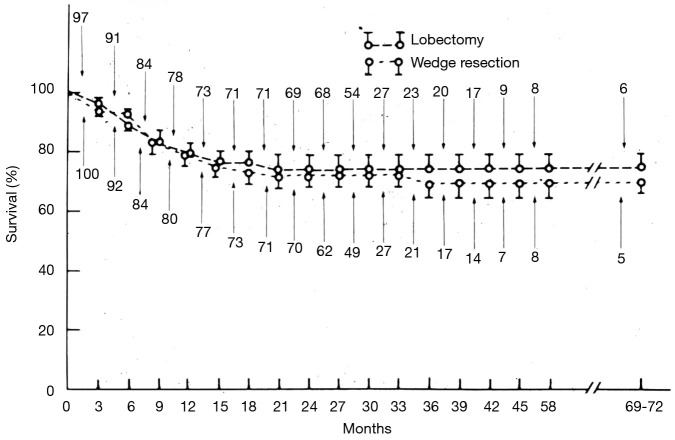
Acceptance of the use of sublobar resection for the management of resectable lung cancers by thoracic surgeons has been met with even greater challenges. We must credit Errett et al. with providing the impetus to consider sublobar resection for peripheral stage I lung cancers (12). In 1985, Errett reported his survival comparison of 100 stage I NSCLC patients with compromised cardiopulmonary reserve who were managed with sublobar resection to the survival of 97 good risk NSCLC patients undergoing lobectomy from 1965 to 1982. He found no difference in survival between these groups (Figure 2).
Figure 2.
Life-table analysis for cumulative survival rates in patients who underwent either lobectomies or wedge resections for stage I non-small cell bronchogenic carcinomas. Deaths include operative mortality. Numbers indicate patients at risk at the beginning of each interval. Reproduced from ref. (12).
It appears that this investigation was encouraged by the contemporary findings of equivalent survival of breast cancer patients undergoing either segmental breast resection with radiotherapy to that of radical mastectomy (36).
Errett’s report eventually inspired the NCI’s Lung Cancer Study Group to conduct the important randomized study of sublobar resection compared to lobectomy for the management of stage I (less 3.0 cm diameter) NSCLC (13). The results of Lung Cancer Study Group’s publication revealed a statistically equivalent cancer survival which did not change on subsequent analysis, however, a three-fold increase in local recurrence was seen among wedge resection and a two-fold plus (2.4) increase among segmental resection patients. Two thirds of the sublobar resections were performed by “segmentectomy”, although the “segmentectomy” site and extent (i.e., single segment vs. multiple segments removed) was not delineated. This study was flawed primarily from the small sample size ultimately available for analysis. Although 771 patients were registered for trial, only 247 patients (32%) were randomized to sublobar resection or lobectomy due to a variety of disqualifying circumstances (i.e., not a T1 tumor, benign disease, lesion technically inappropriate for sublobar resection, or not NSCLC by frozen section). Interestingly, although the intended resection was to have “a 2 cm margin of resection”, a lack of adherence to this marginal distance was not noted as a disqualifying factor for inclusion in the randomization process. Could the non-reported marginal distance have affected local recurrence rates? Additionally, even as the tumors resected were segregated by tumor volumes equivalent to diameters of 1, 2, and <3 cm in diameter, no objective evidence is provided regarding differential local recurrences or survival based on tumor size between sublobar and lobectomy patients.
A contemporaneous retrospective analysis of wedge resection vs. lobectomy for stage I NSCLC by Landreneau et al., also noted equivalent survival but an increased local recurrence among sublobar resection patients. The general thought developed that sublobar resection should be considered a “compromise resection” approach for the physiologically impaired NSCLC patient, and that lobectomy remained the standard of surgical care for the peripheral early stage NSCLC (14).
Another driving force leading to consideration of sublobar resection of the small peripheral lung lesion/cancer was the increasing use of computed tomographic (CT) radiographic scanning/surveillance of patients at high risk for developing lung cancer (i.e., significant smoking history, middle aged, and chronic obstructive pulmonary disease). The chest CT surveillance effort began in Japan a decade or more before being considered in North America and Europe. These surveillance efforts identified increasing numbers of small, peripheral lung nodules/cancers amenable to sublobar resection. Growing interest emerged in Japan for the use of a sublobar resection approach for small peripheral lung cancers as a result of these CT surveillance efforts.
Eventually similar interests evolved in the West. Henschke and the I-ELCAP consortium of North America and Europe explored the utility of CT screening among patients with increased risk of lung cancer based upon smoking history, age and diagnosis of chronic obstructive pulmonary disease. Among those patients identified with malignant early lung cancers undergoing resection, an 80% overall survival was noted and greater than 90% survival seen among patients identified with stage I lung cancers (37). The results of this effort led to the North American, “National Lung Screening Trial (NLST)” which identified a 20% reduction in lung cancer deaths among those patients who undergo low-dose helical CT surveillance scanning compared with the observation arm of the study undergoing standard chest X-ray alone (38). The role of possible sublobar resection as a “primary surgical approach” alternative to lobectomy for the peripheral small lung cancer began to gain traction in North America and world-wide after these studies were reported (37,38).
Can anatomic segmental resection be equivalent to lobectomy for stage I NSCLC?
The Lung Cancer Study Group strongly recommended against the use of sublobar resection, by wedge resection or anatomic segmentectomy, for the stage I NSCLC patient with adequate cardiopulmonary function (13,39). Due to the overall weaknesses of such studies a number of thoracic surgeons continued to be intrigued with the earlier seminal work of Jensik et al. (1) and Read et al. (2) which supported the use of a “quality” sublobar resection for appropriately selected patients with stage I NSCLC.
As noted above, the results of the international low dose CT surveillance efforts directed for high risk patients for lung cancer, which have identifying small, peripheral lung cancers, has also inspired increased investigation of sublobar resection. Okada’s investigation of the outcomes for stage I NSCLC managed by lobectomy compared to sublobar resection (segmentectomy and wedge resection) based on the size of the lung lesion has provided important insight into the possible best approach to the small peripheral lung cancer (5) (Figure 3).
Figure 3.
Data summary of Okada experience. Although the message of this provocative study is important regarding the potential utility of segmentectomy for the small stage I NSCLC, the patient distribution compared to lobectomy for sublobar resections is comparatively small across all tumor sizes. Reproduced from (5). NSCLC, non-small cell lung cancer.
Important differences in the numbers of patients managed by sublobar resection for lesions greater than 2.0 cm in diameter in this retrospective study by Okada et al., lead to concerns in making definitive recommendations for sublobar vs. lobectomy for larger lung cancers. Certainly, other para-phenomenal circumstances may have been at play in deciding upon sublobar resection among these larger tumors (i.e., comorbidities, etc.).
Nevertheless, the data reported by Okada et al. for smaller (<2.0 cm diameter) lung cancers treated by lobectomy or sublobar resection was compelling and probably statistically relevant. Notably, in this work, Okada reported a similar survival opportunity with segmentectomy and lobectomy among patients with tumors between 2.0 and 3.0 cm in diameter. Although the disparity between patients treated by lobectomy, segmentectomy, or wedge resection for lesions less than 2.0 cm in diameter are important, the clinical outcome results between surgical treatment approaches were provocative (5) (Figures 4,5).
Figure 4.
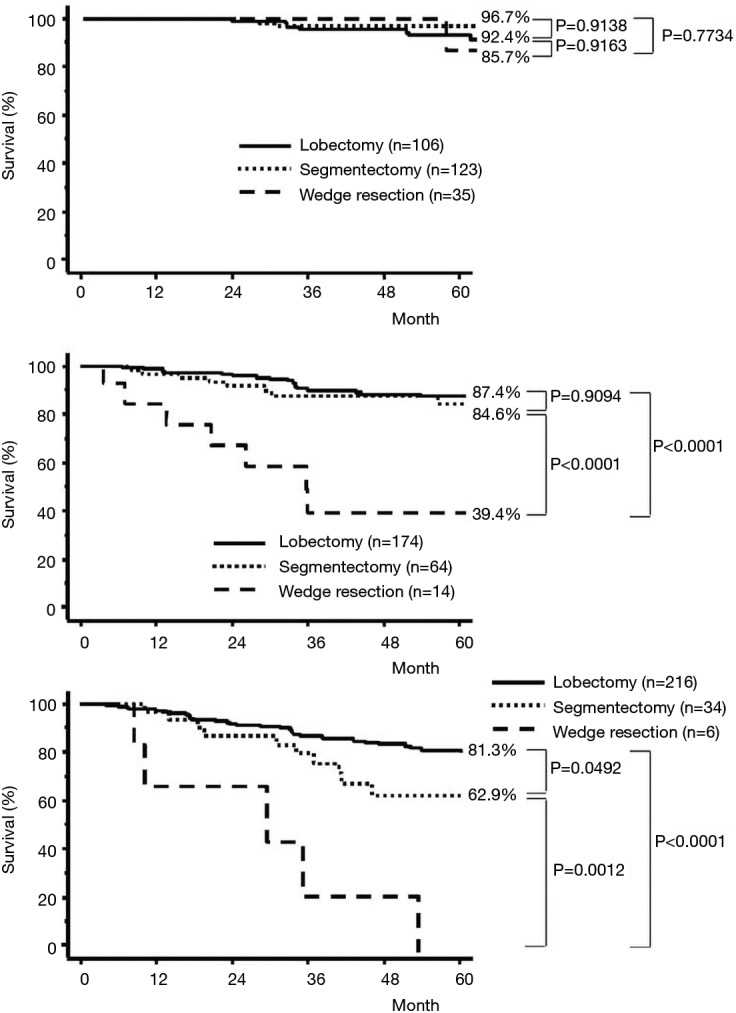
Cancer-specific survival curves for patients with complete resection for pathologic stage I non-small cell lung cancer of 20 mm or less (A), 21 to 30 mm (B), and greater than 30 mm in diameter according to operative procedure. Reproduced from (5).
Figure 5.
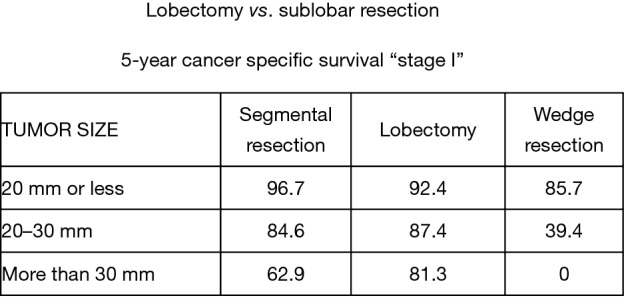
Lobectomy vs. sublobar resection—5-year cancer specific survival ‘stage I’. Reproduced from (5).
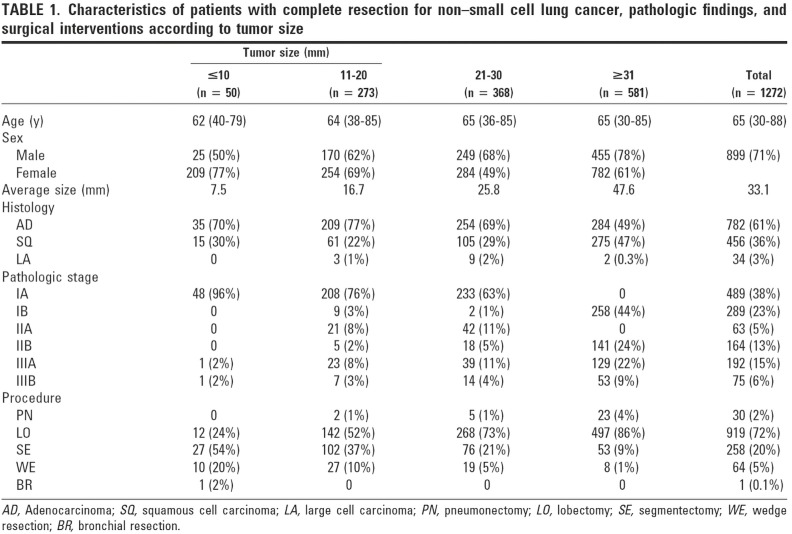
The influence of tumor size on outcome as influenced by the method of resection used has also been emphasized in the recent report by Carr et al. (40). Carr and associates evaluated the relative efficacy of anatomic segmentectomy compared to lobectomy performed among 429 patients with pathologic stage Ia–Ib NSCLC at their institution over a 7-year period [2002–2009] (40). Stage Ia patients had similar survival between surgical approaches as noted in Figures 6,7.
Figure 6.
Clinical peripheral stage IA NSCLC potentially amenable to anatomic segmental resection for cure. NSCLC, non-small cell lung cancer.
Figure 7.
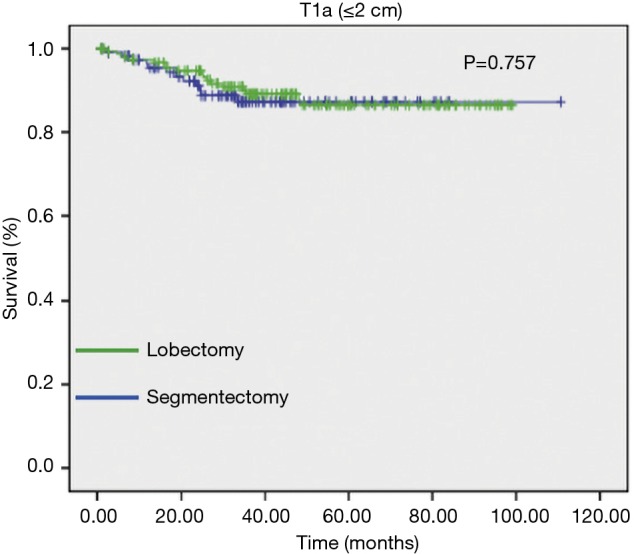
Survival between anatomic segmentectomy vs. lobectomy for stage IA NSCLC (segmentectomy: 121; lobectomy: 163). Reproduced from (40). NSCLC, non-small cell lung cancer.
Earlier, Schuchert et al. reported the University of Pittsburgh’s general experience with sublobar resection (specifically anatomic segmentectomy) for stage I NSCLC. In this work, this single institutional experience also analyzed the relative survival and the potential risk for “local recurrence” following segmentectomy (6). This review reported equivalent survivals between lobectomy and segmentectomy resections among patients with stage I NSCLC. Schuchert reaffirmed the work of Bando et al. (41), that the risk for local recurrence was uncommon after sublobar resection of lesions less than 2 cm diameter, particularly when adequate margins of resection were obtained.
Figure 8 from Schuchert’s work illustrates this point, where they confirmed that an increased local recurrence rate was noted among segmentectomy patients with margin of resection was less than the diameter of the cancer being resected (6).
Figure 8.
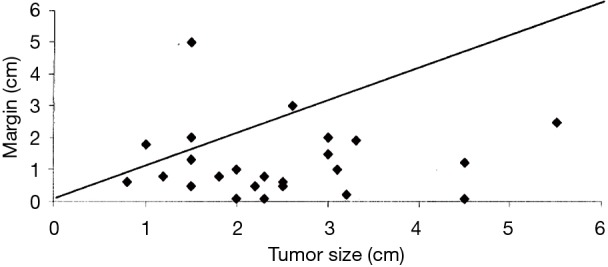
Analysis of recurrences (diamonds) based on tumor size and surgical margin. The diagonal line represents a surgical margin/tumor size ratio of 1 over the range of tumors encountered. Note that the significant majority of recurrences (23/27=85%) are seen when the margin/tumor ratio is less than 1. Reproduced from (6).
More recently, Altorki et al. reported the survival differences between stage I NSCLC patients identified by the LCAP study group, which was previously mentioned in this review (37,42). These investigators identified 347 patients with stage I NSCLC (<3.0 cm in diameter solid lesions) from the LCAP database who underwent lobectomy (n=294) or sublobar resection (n=53). Similar actuarial survivals were noted between lobectomy (85%) and sublobar resection (86%) patients (P=0.86) (42).
The general interest in identifying the appropriate role of sublobar resection for definitive management of small peripheral lung cancers led to the Japanese and North American studies comparing sublobar resection to lobectomy for stage Ia NSCLC (43,44). These large clinical studies are presently closed to accrual to patient participation and under analysis. The fact that continued scrutiny of the local recurrence and survival outcomes continue is interesting. Certainly, if important differences in local recurrence were noted, at this juncture something may have been reported. Overall survival and cancer related survival differences between the sublobar and lobectomy patients of these important investigations are certainly approaching a point where reporting is appropriate.
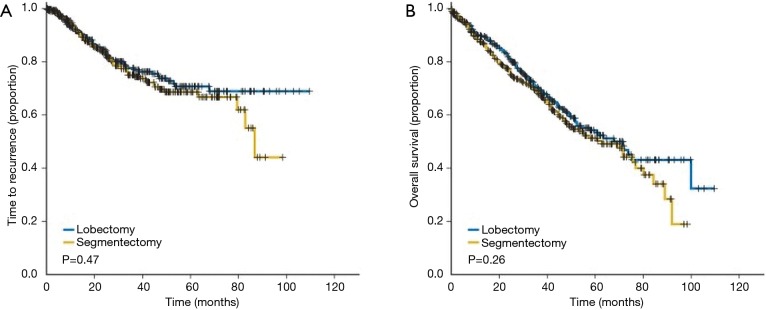
We must stand in alert until results of these randomized trials of sublobar resection trials reach maturity. However, during the course waiting for these findings, we can reflect on the results of anatomic segmentectomy compared to lobectomy for stage I NSCLC was recently reported by the group at the University of Pittsburgh. These investigators used a propensity matched design to compare 312 matched stage I NSCLC lung cancer patients who had underwent anatomic segmentectomy vs. lobectomy (45). No difference in survival or local recurrence was identified between the anatomic segmentectomy and lobectomy patients (Figure 9).
Figure 9.
The time to recurrence (A) and overall Survival (B) between stage I NSCLC patients undergoing anatomic segmentectomy vs. lobectomy. Reproduced from (45). NSCLC, non-small cell lung cancer.
Preservation of pulmonary functionality following surgery for resectable lung cancer is an important concern. Objective evidence of preservation of pulmonary functionality between sublobar resection and lobectomy is relatively limited in the literature. Indeed, this was an important failure of the Lung Cancer Study Group trial noted earlier in this review (13). Keenan et al. did note a significant change in spirometric and diffusion capacity between patients undergoing lobectomy compared to segmentectomy during a common time line in their clinical experience (46). These postoperative functional differences have been subsequently reported by others (47-49). These pulmonary functional differences primarily seen among patients undergoing segmentectomies involving 2 or less anatomic segments (47,49).
On the other hand, today we also have local intense radiotherapy, “stereotactic body radiotherapy—SBRT”, aka, “Stereotactic Ablative Body Radiotherapy—SABR” as a non-surgical alternative to the management of the peripheral stage I NSCLC (50). Although not the focus of this review, there is ongoing consideration for the primary use of this local radiation therapy as an alternative to surgical resection for the peripheral, small lung cancer, particularly among patients who are marginal candidates for surgical resection by lobectomy. This push toward nonsurgical approaches to the peripheral small lung cancer makes it even more important that we understand the utility of “well done” wedge resection and anatomic segmentectomy for stage I NSCLC.
What is an acceptable sublobar resection/segmentectomy and how should it be performed?
The definition of an “anatomic segmentectomy” has been debated. Should middle lobectomy be considered an equivalent resection to a left sided lingulectomy? What should we consider a left upper lobe tri-segmentectomy? Should we consider it an equivalent to a right upper lobectomy—sparing the lingula as we do the middle lobe on the right—or should it be considered true segmental resection? What do we consider a four segment complete lower lobe basilar resection—sparing the superior resection of the lower lobe? Is this major resection really a segmentectomy? Functionally, such basilar resections seem to deserve a separate consideration (47,49). These anatomic distinctions appear to have both clinical oncologic and functional importance.
Regardless, whenever sublobar resection for primary NSCLC is considered, the primary goal is to obtain clear surgical margins. Ideally the margin of resection should be equal to the diameter of the resected lesion (6,43-45,51-53). A representative regional lymph node sampling of the interlobar, hilar, and mediastinal lymph nodes should also accompany the resection to minimize understaging of the cancer. This “clinical to pathologic stage shift” is an important concern when attempting to determine the true efficacy of any resective/ablative therapy for “presumed” early stage lung cancer. Ginsburg and Rubenstein identified a 25% stage shift in their randomized trial (13). Other investigators have also raised concern for this phenomenon, reporting stage shift in up to 30% of lung cancer patients undergoing resection (6,13,45,54-56).
When these two concepts of adequate surgical management are honored, a “quality wedge resection/ segmentectomy” may be considered to have occurred which can provide a significant therapeutic advantage over wedge resections without these characteristics and non-surgical approaches (i.e., SBRT/SABR) (6,11,45,46,51).
As compared to non-anatomic “wedge resection”, anatomic segmentectomy commonly affords the opportunity for improved surgical margins of resection and mediastinal/hilar lymph node assessment/dissection (1,2,4-6,45).
What surgical approach should be considered for performance of anatomic segmentectomy?
The clinical information at hand leads us to believe that anatomic segmentectomy can provide equivalent outcomes to lobectomy for the small peripheral NSCLC. Since the introduction of video-assisted thoracic surgery (VATS) into our technical armamentarium, the “Ancien Regime” of thoracic surgery has been skeptical of this approach to thoracic malignancies (15,57-61).
Recent reports from the West and Asia have noted an equivalent clinical outcome for segmentectomy performed by open and VATS techniques (62). The expanded role of robotic assisted VATS approaches for pulmonary resection has also demonstrated efficacy of this technique for performance of anatomic segmentectomy (63,64). Additionally, advocates of the “Uniportal” VATS approach to lung resection have noted success in accomplishing anatomic segmentectomy for the early lung cancer (65-67).
Conclusions
As our group has stated earlier, “Anatomic segmental resection can be safely performed for the small peripheral lung cancer anatomically confined to segmental boundaries. However, confirmation of clear, generous margins of resection and the assurance of accurate intraoperative pathologic nodal staging of the lesion are important considerations that should lead us to favor lobectomy over segmentectomy when an issue. For the small peripheral lung cancer, however, anatomic segmentectomy appears to offer comparable local control and the opportunity for prolonged disease-free and overall survival that is not statistically different when compared with lobectomy.” (45).
As a competitive therapeutic approach to non-surgical ablative procedures (50,68-70), we have further objectified that, “Surgical resection of peripheral lung cancers represents the standard of care. Similar to the arguments with breast cancer surgery, the advantages of surgery are pathologic assessment of surgical margins, the establishment of pathologic regional nodal status, and, in this era of increasing enthusiasm for adjuvant systemic therapy, provision of tissue for pharmacogenomic assessment.” (45). As the opportunity for preservation of pulmonary functionality exists without compromise of surgical oncologic therapeutic intent, “anatomic segmentectomy with objective, thorough assessment of mediastinal/hilar lymph node stations”, may be the future for the management of the small peripheral lung cancer.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jensik RJ, Faber LP, Milloy FJ, et al. Segmental resection for lung cancer. A fifteen year experience. J Thorac Cardiovasc Surg 1973;66:563-72. [PubMed] [Google Scholar]
- 2.Read RC, Yoder G, Schaeffer RC. Survival after conservative resection for T1 N0 M0 non-small cell lung cancer. Ann Thorac Surg 1990;49:391-8. 10.1016/0003-4975(90)90242-X [DOI] [PubMed] [Google Scholar]
- 3.Bonfils-Roberts EA, Claggett OT. Contemporary indication for pulmonary segmental resections. J Thorac Cardiovasc Surg 1972;63:433-8. [PubMed] [Google Scholar]
- 4.Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph nodeassessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60. 10.1016/S0003-4975(00)02223-2 [DOI] [PubMed] [Google Scholar]
- 5.Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. 10.1016/j.jtcvs.2004.04.030 [DOI] [PubMed] [Google Scholar]
- 6.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926–932. 10.1016/j.athoracsur.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 7.Schuchert MJ, Pettiford BL, Luketich JD, et al. Parenchymal-sparing resections: why, when, and how. Thorac Surg Clin 2008;18:93-105. 10.1016/j.thorsurg.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 8.Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6; discussion 1667-8. [DOI] [PubMed]
- 9.Landreneau RJ, D'Amico TA, Schuchert MJ, et al. Segmentectomy and Lung Cancer: Why, When, How, and How Good? Semin Thorac Cardiovasc Surg 2017;29:119-28. 10.1053/j.semtcvs.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 10.Schuchert MJ, Abbas G, Awais O, et al. Anatomic segmentectomy for the solitary pulmonary nodule and early-stage lung cancer. Ann Thorac Surg 2012;93:1780-5; discussion 1786-7. [DOI] [PubMed]
- 11.Ajmani GS, Wang CH, Kim KW, et al. Surgical quality of wedge resection affects overall survival in patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg 2018;156:380-91.e2. 10.1016/j.jtcvs.2018.02.095 [DOI] [PubMed] [Google Scholar]
- 12.Errett LE, Wilson J, Chiu RC, et al. Wedge resection as an alternative procedure for peripheral bronchogenic carcinoma in poor-risk patients. J Thorac Cardiovasc Surg 1985;90:656-61. [PubMed] [Google Scholar]
- 13.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. 10.1016/0003-4975(95)00537-U [DOI] [PubMed] [Google Scholar]
- 14.Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small cell lung cancer. J Thorac Cardiovasc Surg 1997;113:691-8; discussion 698-700. 10.1016/S0022-5223(97)70226-5 [DOI] [PubMed] [Google Scholar]
- 15.Lewis RJ. The role of video-assisted thoracic surgery for carcinoma of the lung: wedge resection to lobectomy by simultaneous individual stapling. Ann Thorac Surg 1993;56:762-8. Discussion 768-9. [DOI] [PubMed]
- 16.Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005;128:237-45. 10.1378/chest.128.1.237 [DOI] [PubMed] [Google Scholar]
- 17.Tsutani Y, Tsubokawa N, Ito M, et al. Postoperative complications and prognosis after lobar resection versus sublobar resection in elderly patients with clinical Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2017;53:366-71. 10.1093/ejcts/ezx296 [DOI] [PubMed] [Google Scholar]
- 18.Paget S. Surgery of the Chest. Bristol: John Wright & Co., 1896;78,81,85,326. [Google Scholar]
- 19.Kittle CF. A history of lobectomy and segmentectomy, including sleeve resection. Chest Surg Clin N Am 2000;10:105-30. [PubMed] [Google Scholar]
- 20.Brunn H. Surgical principles underlying one-stage lobectomy. Arch Surg 1929;18:490-6. 10.1001/archsurg.1929.04420020312020 [DOI] [Google Scholar]
- 21.Churchill ED, Belsey R. Segmental pneumonectomy in bronchiectasis. Ann Surg 1939;109:481-99. 10.1097/00000658-193904000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overholt RH, Langer L. A new technique for pulmonary segmental resection, its application in the treatment of bronchiectasis. Surg Gynecol Obstet 1947;84:257. [PubMed] [Google Scholar]
- 23.Brock RC. The anatomy of the bronchial tree with special reference to surgery for lung abscesses. London: Oxford University press, 1952. [Google Scholar]
- 24.Halsted WS. I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg 1894;20:497-555. 10.1097/00000658-189407000-00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham EA, Singer JJ. Successful removal of an entire lung for carcinoma of the bronchus. JAMA 1984;25:257-60. 10.1001/jama.1933.02740430017005 [DOI] [PubMed] [Google Scholar]
- 26.MacLean LD, Martin AE. Norman Bethune and Edward Archibold: Sung and unsung heros. Ann Thorac Surg 2000;70:1746-52. 10.1016/S0003-4975(00)02043-9 [DOI] [PubMed] [Google Scholar]
- 27.Archibald E. The technique of total unilateral pneumonectomy. Ann Surg 1934;100:796-811. 10.1097/00000658-193410000-00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhoff WF., Jr The surgical technique of total pneumonectomy. Arch Surg 1936;32:218-31. 10.1001/archsurg.1936.01180200028002 [DOI] [Google Scholar]
- 29.Crafoord C. On the technique of pneumonectomy in man. A critical survey of the experimental and clinical development and a report of the author’s material and technique. Acta Chir Scand 1938;81:5-142. [Google Scholar]
- 30.Oschner A, DeBakey M. Surgical considerations of primary carcinoma of the lung – review of the literature and report of 19 cases. Surgery 1940;8:992-1023. [Google Scholar]
- 31.Ochsner A. Lobectomy or Pneumonectomy. Surg Clin N Amer 1966;46:1255-64. 10.1016/S0039-6109(16)38040-9 [DOI] [PubMed] [Google Scholar]
- 32.Churchill ED. The surgical management of carcinoma of the lung. J Thorac Surg 1933;2:254-66. [Google Scholar]
- 33.Chamberlain JM, Finnerty JJ. Modern techniques in thoracic surgery. Surg Clin North Am 1949;29:557-72. 10.1016/S0039-6109(16)32700-1 [DOI] [PubMed] [Google Scholar]
- 34.Overholt RH, Langer L. The technique of pulmonary resection. Springfield: Charles C. Thomas, 1951. [Google Scholar]
- 35.Shimkin MB, Connelly RR, Marcus SC, et al. Pneumonectomy and lobectomy in bronchogenic carcinoma. A comparison of end results of the Overholt and Ochsner Clinics. J Thorac Cardiovasc Surg 1962;44:503-19. [PubMed] [Google Scholar]
- 36.Fisher ER, Fisher B. Relationship of pathologic and some clinical discriminants to the spread of breast cancer. Int J Radiat Oncol Biol Phys 1977;2:747-50. 10.1016/0360-3016(77)90058-X [DOI] [PubMed] [Google Scholar]
- 37.International Early Lung Cancer Action Program I , Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. 10.1056/NEJMoa060476 [DOI] [PubMed] [Google Scholar]
- 38.National Lung Screening Trial Research T ; Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 1994;107:1087-93; discussion 1093-4. [PubMed] [Google Scholar]
- 40.Carr SR, Schuchert MJ, Pennathur A, et al. Impact of tumor size on outcomes after anatomic lung resection for stage 1 A non-small cell lung cancer based on the current staging system. J Thorac Cardiovasc Surg 2012;143:390-7. 10.1016/j.jtcvs.2011.10.023 [DOI] [PubMed] [Google Scholar]
- 41.Bando T, Yamigahara K, Kitayama Y, et al. A new method of segmental resection for primary lung cancer: intermediate results. Eur J Cardiothorac Surg 2002;21:894-9. 10.1016/S1010-7940(02)00122-7 [DOI] [PubMed] [Google Scholar]
- 42.Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62. 10.1016/j.jtcvs.2013.09.065 [DOI] [PubMed] [Google Scholar]
- 43.A phase III randomized trial of lobectomy versus sublobar resection for small (<2 cm) peripheral non-small cell lung cancer. CALGB 140503. Clinical Trials.gov Identifier: NCT00499330.
- 44.Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607 L). Jpn J Clin Oncol 2010;40:271-4. 10.1093/jjco/hyp156 [DOI] [PubMed] [Google Scholar]
- 45.Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. 10.1200/JCO.2013.50.8762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keenan RJ, Landreneau RJ, Maley RH, Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. 10.1016/j.athoracsur.2004.01.024 [DOI] [PubMed] [Google Scholar]
- 47.Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. 10.1016/j.athoracsur.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 48.Saito H, Nakagawa T, Ito M, et al. Pulmonary function after lobectomy versus segmentectomy in patients with stage I non-small cell lung cancer. World J Surg 2014;38:2025-31. 10.1007/s00268-014-2521-3 [DOI] [PubMed] [Google Scholar]
- 49.Macke RA, Schuchert MJ, Odell DD, et al. Parenchymal preserving anatomic resections result in less pulmonary function loss in patients with Stage I non-small cell lung cancer. J Cardiothorac Surg 2015;10:49-53. 10.1186/s13019-015-0253-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paul S, Lee PC, Mao J, et al. Long term survival with stereotactic ablative radiotherapy (SABR) versus thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. BMJ 2016;354:i3570. 10.1136/bmj.i3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hennon M, Landreneau RJ. Role of Segmentectomy in Treatment of Early-Stage Non-Small Cell Lung Cancer. Ann Surg Oncol 2018;25:59-63. 10.1245/s10434-017-5787-5 [DOI] [PubMed] [Google Scholar]
- 52.Cao J, Xu J, He Z, et al. Prognostic impact of lymphadenectomy on outcomes of sublobar resection for stage IA non-small cell lung cancer ≤2 cm. J Thorac Cardiovasc Surg 2018;156:796-805.e4. 10.1016/j.jtcvs.2018.03.122 [DOI] [PubMed] [Google Scholar]
- 53.Yendamuri S, Dhillon SS, Groman A, et al. Effect of the number of lymph nodes examined on the survival of patients with stage I non-small cell lung cancer who undergo sublobar resection. J Thorac Cardiovasc Surg 2018;156:394-402. 10.1016/j.jtcvs.2018.03.113 [DOI] [PubMed] [Google Scholar]
- 54.López-Encuentra A, Garcia-Lujan R, Rivas JJ, et al. Comparison between clinical and pathologic staging in 2,994 cases of lung cancer. Ann Thorac Surg 2005;79:974-9. 10.1016/j.athoracsur.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 55.El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400-5. 10.1245/s10434-007-9421-9 [DOI] [PubMed] [Google Scholar]
- 56.El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 415-6. 10.1016/j.athoracsur.2006.02.029 [DOI] [PubMed] [Google Scholar]
- 57.Ginsberg RJ. Thoracoscopy: a cautionary note. Ann Thorac Surg 1993;56:801-3. 10.1016/0003-4975(93)90985-Q [DOI] [PubMed] [Google Scholar]
- 58.Kirby TJ, Mack MJ, Landreneau RJ, et al. Initial experience with video-assisted thoracoscopic lobectomy. Ann Thorac Surg 1993;56:1248-52. 10.1016/0003-4975(93)90661-Z [DOI] [PubMed] [Google Scholar]
- 59.Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995;109:997-1001; Discussion 1001-2. 10.1016/S0022-5223(95)70326-8 [DOI] [PubMed] [Google Scholar]
- 60.McCormack PM, Bains MS, Begg CB, et al. Role of video-assisted thoracic surgery in the treatment of pulmonary metastases: results of a prospective trial. Ann Thorac Surg 1996;62:213-6; discussion 216-7. 10.1016/0003-4975(96)00253-6 [DOI] [PubMed] [Google Scholar]
- 61.Landreneau RJ. Commentary. Ann Thorac Surg 1996;62:216-7. [Google Scholar]
- 62.Lex JR, Naidu B. In patients with resectable nonsmall cell lung cancer, is video-assisted thoracoscopic segmentectomy an alternative to video-assisted thoracoscoic lobectomy? Interact Cardiovasc Thorac Surg 2016;23:826-31. 10.1093/icvts/ivw202 [DOI] [PubMed] [Google Scholar]
- 63.Wei B, Cerfolio R. Technique of robotic segmentectomy. J Vis Surg 2017;3:140. 10.21037/jovs.2017.08.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. 10.21037/jtd.2018.01.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434-38. 10.1016/j.athoracsur.2013.04.044 [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez-Rivas D. Uniportal thoracoscopic surgery: from medical thoracoscopy to non-intubated uniportal video-assisted major pulmonary resections. Ann Cardiothorac Surg 2016;5:85-91. 10.21037/acs.2016.03.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez-Rivas D, Sihoe ADL. Important Technical Details During Uniportal Video-Assisted Thoracoscopic Major Resections. Thorac Surg Clin 2017;27:357-72. 10.1016/j.thorsurg.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 68.Iyengar P, Westover K, Timmerman RD. Stereotactic ablative radiotherapy (SABR) for non-small cell lung cancer. Semin Respir Crit Care Med 2013;34:845-54. 10.1055/s-0033-1358554 [DOI] [PubMed] [Google Scholar]
- 69.Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. 10.1016/j.ijrobp.2015.07.2278 [DOI] [PubMed] [Google Scholar]
- 70.Baisi A, De Simone M, Raveglia F, et al. Thermal ablation in the treatment of lung cancer: present and future. Eur J Cardiothorac Surg 2013;43:683-6. 10.1093/ejcts/ezs558 [DOI] [PubMed] [Google Scholar]