Abstract
The control of the immune response during the development of some diseases is crucial for the maintenance or restoration of homeostasis. Several mechanisms can initiate inflammation, one of which is the activation of toll-like receptors (TLRs), necessary to initiate the immune response to eliminate an infection. However, inappropriate activation can compromise immunological homeostasis, leading to pathologies such as autoimmune diseases, chronic inflammation, and even cancer. Regulatory mechanisms that intervene in the initiation or modulation of inflammation include microRNAs (miRNAs), which have emerged as key post-transcriptional regulators of proteins involved in distinct cellular processes, such as regulation of the immune response. The focus of this review is on the diverse roles of miRNAs in the regulation of TLR-signaling pathways by targeting multiple molecules, including TLRs, the signaling proteins and cytokines induced by TLRs. It will also address the relationships of these molecules with some diseases that involve inflammation such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), cancer, as well as bacterial or viral infections.
Keywords: microRNA, inflammatory diseases, toll-like receptor, TLR signaling
Introduction
In the past several decades, extensive studies have focused on how the host’s immune response discriminates between microorganisms. One of the most extensively studied recognition mechanisms is through the toll-like receptor (TLR) family. The activation of the TLR signaling pathways is necessary to initiate the immune response to eliminate an infection; however, inappropriate activation, such as a persistent infection (i.e., bacteria, virus, or other microorganisms), can compromise immunological homeostasis, leading to pathologies such as autoimmune diseases, chronic inflammation, tumor development and even cancer. TLRs identify microbe-associated molecular patterns (MAMPs), which in turn triggers an intracellular signaling cascade involving adaptor proteins and the activation of transcription factors that prompt the production of cytokines [1]. Once signaling has been initiated, transcription factors and message translation can be halted through post-transcriptional regulation of key proteins along the signaling cascade. This negative regulation can be achieved by the destabilization of encoding messenger RNA (mRNA) or by hampering the translation. One of the post-transcriptional regulatory mechanisms is through microRNAs (miRNAs), which are small non-coding RNAs, approximately 23 nucleotides that bind the seed region (2-7 nucleotides from the 5’ end) to the 3’ untranslated region (UTR) of the mRNA from target proteins [2]. The sequences of miRNAs are conserved between species; however, they are not specific for a single protein. The importance of miRNAs as a regulatory mechanism for protein expression has been the focus of several research studies in the past decade. Several of them imply diverse aspects of the immune system, particularly the inflammatory process. This review will discuss the role of some miRNAs in the regulation of TLRs and related signaling proteins, cytokines and their important roles in maintaining homeostasis, and the implications of this regulation in several diseases linked to the inflammatory response.
Regulation of toll-like receptor expression by miRNAs
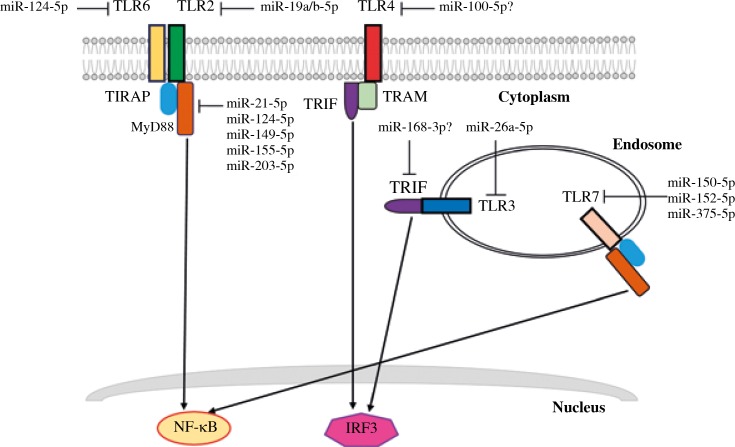
TLRs have a very well-known fundamental function in the recognition of microbial components, resulting in the increased production of inflammatory cytokines such as interleukin (IL)-6, interferon β (IFN-β), tumor necrosis factor α (TNF-α), among others. However, abnormal localization of foreign or self-molecules, or anomalous molecular complexes, can also trigger inflammation through TLR recognition [3]. Figure 1 shows miRNAs that regulate TLR expression in inflammatory diseases. For instance, high expression levels of TLR2 and TLR4 have been reported in patients with rheumatoid arthritis (RA) [4]. In fibroblast-like synoviocytes (FLS), the key effector cells for RA, the elevated expression of TLR2 can be regulated by miR-19a/b-5p, and the overexpression of these miRNAs caused a decrease in TLR2 expression and thus a reduction in the production of IL-6 in FLS [5]. Similarly, overexpression of TLR3, both mRNA and protein, promotes the development and maintenance of pristane-induced RA in rats, while TLR3 inhibition modulates the severity of the disease [6]. For RA, miR-26a-5p has been shown to downregulate the expression of TLR3 in rat macrophages, since its overexpression leads to a severe dose-dependent decrease in TLR3 mRNA expression. Remarkably, miR-26a-5p administration in arthritic rats slowed the development of RA by suppressing TLR3 [7].
Fig. 1.
TLR signaling regulation by miRNAs in inflammatory diseases
Moreover, overreaction of the germinal centers in secondary lymphoid tissues and activation of the TLR4 pathway in follicular dendritic cells (FDC) cause an increase in the production of IL-6, which has been found in large amounts in the synovial fluid and serum of patients with RA. Inhibition of miR-100-5p in an FDC-like cell line caused an increase in gene expression of TLR4 and IL6; however, its overexpression had no effect on those genes. Hence it is plausible that miR-100-5p indirectly regulates TLR4 signaling [8], which is important for controlling the reactivity of the germinal centers.
On the other hand, reduced expression of TLR7 has been associated with a poor response to interferons (IFNs). It has also been hypothesized that there may be a defect in innate immunity in patients with severe asthma due to a deficiency in the synthesis of TLR7, since alveolar macrophages from these patients have both decreased TLR7 mRNA and protein. Rupani et al. [9] showed a TLR7 deficiency and elevated expression of miR-150-5p, miR-152-5p and miR-375-5p in alveolar macrophages from patients with severe asthma. Moreover, blockade of those miRNAs restored TLR7 expression and increased the IFN response to rhinovirus in alveolar macrophages [9].
These findings show the anti-inflammatory functions of some miRNAs, such as miR-19a/b-5p, miR-26a-5p and miR-100-5p; therefore these miRNAs could be key factors for the control of excessive inflammation through the regulation of TLR expression and could aid in the treatment of diseases that are triggered by increased circulating IL-6, such as autoimmune diseases. It also shows that there are miRNAs that negatively regulate the expression of TLR, and that blockade of these miRNAs restores the IFNs response, alleviating viral infections.
Regulation of adaptor molecules by miRNAs
Recognition of MAMPs activates the TLR pathway, which in turn recruits a group of adapter molecules and transcription factors. The myeloid differentiation primary response 88 protein (MyD88) and the complex toll/interleukin 1 receptor (TIR) domain-containing adapter inducing IFN-β (TRIF) have been reported as the main adapter molecules associated with TLR signaling. The MyD88-dependent pathway is common to all TLRs with the exception of TLR3, and TRIF-dependent pathway that is peculiar to the TLR3 and TLR4 signaling pathways [3]. MyD88 and TRIF pathways lead to the expression of numerous cytokines, such as TNF-α, IL-1β, IL-6, IL-10, and IFN-γ, through transcriptional factors (NF-κB, transcription factor nuclear factor kappa B; and IRF3, IFN-regulatory factor 3) [10].
MyD88 is a critical downstream adaptor protein for most TLRs, and its inhibition causes abrogation of NF-κB activation. MiR-155-5p was one of the first described miRNAs that regulate TLR signaling by targeting MyD88. Hence, it plays an important role in the regulation of the innate and acquired immune response. Several reports have focused on the regulation of inflammation by miR-155-5p, and it will not be discussed further [11-13]. In addition, the direct regulation of MyD88 by miRNAs has been investigated by overexpression of miR-203-5p or miR-149-5p in mouse macrophages stimulated with LPS or Mycobacterium bovis, which results in a significant decrease of MyD88 protein. Consequently, indirect regulation of NF-κB occurs, as well as subsequent inhibition of IL-6 and TNF-α transcripts [14, 15]. Another miRNA involved in the Mycobacterium bovis response is miR-124-5p, which represses the expression of MyD88 protein in alveolar epithelial cells without affecting the mRNA levels, and this regulation has been directly observed in the protein expression levels of TLR6, TRAF6 and TNF-α [16]. However, miR-124-5p has a signal interchange with MyD88, since its silencing directly affects its transcription in alveolar macrophages [16]. These data suggest that the mechanisms of miR-124-5p, miR-149-5p and miR-203-5p action on MyD88 are to suppress or pause protein translation rather than to degrade the message. Consequently, these miRNAs might play a key role in the immune response of macrophages in the course of tuberculosis, as pro-inflammatory cytokine production is essential for the recruitment of inflammatory cells to the site of infections and the formation and maintenance of granulomas. Nevertheless, it is imperative to avoid the excessive production of pro-inflammatory cytokines to prevent excessive tissue damage or the reactivation of a latent infection.
The physiological role of TRIF (also known as TICAM-1, Toll-interleukin-1 receptor domain-containing adaptor molecule-1) was revealed through its deletion in mice. In response to TLR3 and TLR4 ligands, TRIF-deficient mice showed both impaired activation of IRF3 and decreased expression of IFN-inducible genes [3]. There is less information about the direct regulation of TRIF expression by microRNAs. However, in experiments with dendritic cells (DCs) treated with FvmiR168 obtained from strawberries (Fragaria vesca) and further stimulated with poly (I : C) or LPS, a decrease of TRIF, IRF3 and IFN-β mRNA was observed after 4 h of exposure, although a direct interaction between TRIF and FvmiR168 was not tested [17]. Figure 1 shows some of the reported miRNAs that regulate the adaptor molecules MyD88 and TRIF implicated in inflammatory diseases.
Toll-like receptor-induced signaling cascade proteins and their regulation by miRNAs
The MyD88-dependent pathway relies on the activation of a family of kinase proteins termed interleukin-1 receptor (IL-1R)-associated kinase (IRAK), composed of four proteins termed IRAK1, IRAK2, IRAK3 (or IRAKM) and IRAK4 [3]. IRAK1 also interacts with TNF receptor-associated factor 6 (TRAF6), and together they play important roles in signal transduction mediated by TLRs and IL-1Rs. IRAK4 and IRAKM, in conjunction with TRAF6, are key proteins in the TLR signaling pathway. However, several other important proteins with kinase activity or adapter function are involved in signaling that leads to activation of the transcription factor NF-κB.
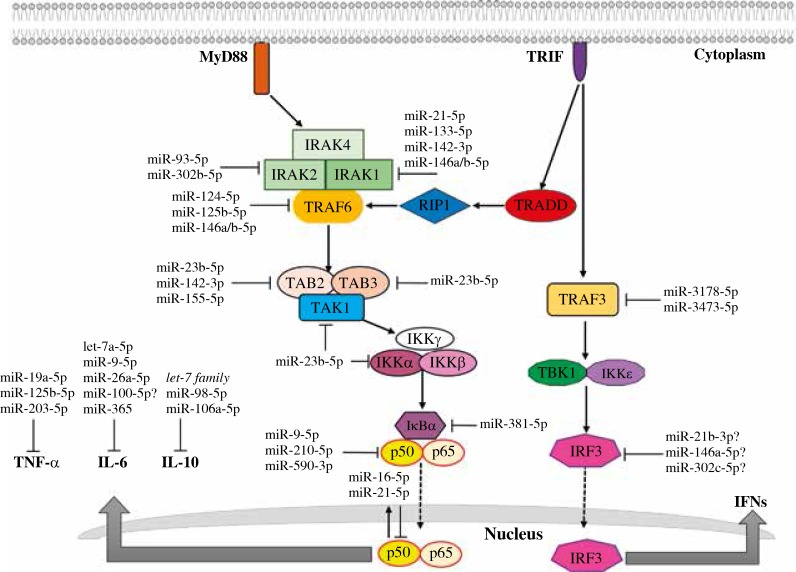
The IRAK family proteins are independently regulated by different miRNAs. Figure 2 shows some of the reported miRNAs that regulate proteins involved in the signaling cascade implicated in inflammatory diseases. For instance, miR-21-5p targets several cellular processes, which suggests a multifaceted role. In hepatitis C virus-infected PBMCs, miR-21-5p regulates IRAK1 and MyD88 expression [18]. The microRNAs miR-146a-5p and miR-155-5p are the most extensively implicated in the regulation of TLR downstream signaling [13, 19]; in human DCs, miR-146a-5p and miR-146b-5p regulate apoptosis and cytokine production by targeting TRAF6 and IRAK1 [20, 21]. Other miRNAs have been reported to regulate TLR downstream signaling, such as miR-133-5p and miR-142-3p, which target IRAK1 [22, 23]. Mycobacterium bovis-infected mouse macrophages show high levels of IRAK1; however, miR-142-3p overexpression results in the downregulation of IRAK1 and the consequent downregulation of NF-κB, TNF-α and IL-6 [23]. This finding suggests that miR-142-3p contributes to the control of the exacerbated inflammatory response to Mycobacterium bovis and could be related to the induction of tolerance.
Fig. 2.
NF-κB and IRF3 signaling pathway regulation by miRNAs implicated in inflammatory diseases
Several studies have indicated the importance of IRAK4 in TLR4 signaling. Patients who carry IRAK4 mutations are extremely susceptible to bacterial infections. Infection with Pseudomonas aeruginosa, an important etiological agent in nosocomial infections, strongly induces miR-302b-5p expression in mouse alveolar macrophages, which targets IRAK4, affecting NF-κB activation and the associated inflammatory response [24]; thus, miR-302b-5p is a negative regulator of the innate immune response. Conversely, miR-93-5p exhibits reduced expression in a rat model of endotoxin-induced uveitis, as well as in LPS-treated RAW 264.7 cells, and its overexpression greatly reduced IL-1β, IL-6, and TNF-α. Further assays demonstrated that IRAK4 is the target of miR-93-5p, which thereby hampers NF-κB signaling, resulting in low production of the pro-inflammatory cytokines [25].
TRAF6 is another important protein in the NF-κB signaling cascade, and is a target for miRNAs. The microRNA miR-124-5p also targets TRAF6, together with TLR6, MyD88, and TNF-α in epithelial cells and alveolar macrophages in response to mycobacterial infection [16]. The miRNA miR-125b-5p is downregulated after LPS-treatment in mouse macrophages; its overexpression led to decrease in pro-inflammatory cytokines by targeting TRAF6 [26]. Interestingly, miR-125b-5p has a dual inhibitory effect in the immune response, since it not only represses the transcription of pro-inflammatory cytokines by inhibiting TRAF6 but also has the ability to transcriptionally regulate TNF-α, by binding to TNF-α mRNA, inhibiting its production [12].
In addition, tissue from patients with autoimmune diseases such as SLE and RA exhibit reduced expression of miR-23b-5p and consequently higher expression of TAB2, TAB3 and IKK-α, and increased production of TNF-α, IL-1β and IL-17 [27]. On the other hand, the increased expression of miR-381-5p in LPS-stimulated human lung epithelial carcinoma cells reduces the expression of the inhibitor IκBα, which allows the release of NF-κB and the subsequent production of pro-inflammatory cytokines [28].
The TRIF-dependent pathway culminates in the activation of both IRF3 and NF-κB [3]. TRIF recruits TRAF6, TRADD and TRAF3. TRAF6 in turn initiates the signaling cascade described above. On the other hand, TRADD recruits RIP1 and activates TAK1 (transforming growth factor beta-activated kinase 1), which in turns activates NF-κB for the production of inflammatory cytokines. Conversely, TRAF3 activates the kinases TBK1 and IKKε, which phosphorylate and activate IRF3, inducing the production of type I interferons (mainly IFN-α and β), with anti-viral activity [3]. Figure 2 shows some of the reported miRNAs that regulate proteins involved in the signaling cascade implicated in inflammatory diseases.
Patients with Helicobacter pylori-positive gastric cancer had downregulation of miR-3178-5p and elevated levels of TRAF3 and in IL-6 and IL1β; further experiments found TRAF3 as the target for miR-3178-5p. Treatment with miR-3178-5p mimic impeded the proliferation of gastric cancer cells via inhibition of TRAF3 and the concomitant production of inflammatory cytokines [29]. Other microorganisms also use miRNAs to target TRAF3 and induce an inflammatory response, for example, Burkholderia pseudomallei, the causative agent of melioidosis, induces a strong inflammatory response mediated by TNF-α, IL-10, IL-1β, IL-8, IL-6 and IFN-γ that has been associated with mortality among patients. Fang et al. (2016) demonstrated that B. pseudomallei-infected mouse macrophages had elevated levels of miR-3473-5p and its target was TRAF3. Inhibition of miR-3473-5p resulted in reduced levels of TNF-α; however, the in vivo administration of miR-3473 did not reduce the death rate of infected mice [30].
Regulation of transcription factors by miRNAs
The inflammatory response is a complex mechanism that requires controlled programs of gene induction regulators. The signaling pathways involved in the inflammatory response are regulated by several transcription factors such as the NF-κB and IRF family. NF-κB encompasses a family of five protein members, NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB, and c-Rel, which function as homodimers or heterodimers. IκB sequesters the dimer in the cytoplasm until its release [3]. The NF-κB protein subunits can also be regulated by miRNAs before their translation into protein since the dimer-forming subunits are individually regulated. Many cancer studies have focused on the regulation of NF-κB because its increase is related to the inhibition of apoptosis, increased proliferation and metastasis. Several miRNAs have been implicated in this process; for example, in murine macrophages miR-210-5p regulates the activation of NF-κB in the TLR4 signaling pathway. MiR-210-5p controls the production of pro-inflammatory cytokines induced by LPS stimulation and the greater the stimulus, the greater the increase in the expression of miR-210-5p, which occurs through inhibition of the NF-κB1 mRNA subunit [31]. Other miRNAs involved in the regulation of NF-κB1 in different pathologies have been identified, such as miR-9-5p in cancer [32, 33] and miR-590-3p in myocarditis [34]. It is important to note that there is a cross-talk mechanism between NF-κB and several miRNAs; for instance, the transcription of miR-16-5p and miR-21-5p depends on the activation of NF-κB [35]; similarly, transcription of let-7a-3-5p, let-7b-5p and miR-15b-5p depends on activation of the p50 homodimer [36, 37]. Figure 2 shows some of the reported miRNAs implicated in inflammatory diseases that regulate the transcription factors NF-κB and IRF3.
On the other hand, IRF3 is a transcription factor crucial in the TRIF-dependent pathway. IRF3 is a 53-kDa protein located in the cytoplasm in its inactive unphosphorylated form. Following phosphorylation, IRF3 dimerizes and translocates to the nucleus, where DNA binding and transcriptional activation of target genes occur [3]. IRF3 itself has an important anti-inflammatory function, since its overexpression in IRF3-knockout mice alleviates systemic and hepatic inflammation [38]; also, overexpression of IRF3 switches the microglia cells and astrocytes (central nervous system-resident immune cells) from the pro-inflammatory to the anti-inflammatory phenotype [39]. Furthermore, IRF3 plays a crucial role in development of Th17 responses in experimental autoimmune encephalomyelitis (an animal model of multiple sclerosis), since IRF3-deficient mice developed less severe disease [40]. Additionally, LPS-treated human monocytic cells had increased expression of IRF3, IFN-β and miR-146a-5p; however, when LPS-stimulated cells were treated with IVIg (intravenous immunoglobulin, commonly used to treat sepsis and inflammatory disorders), they had significantly reduced expression and protein levels of IRF3 and IFN-β, and increased levels of miR-146a, suggesting that miR-146a-5p degraded the IRF3 transcript [41]. Also, miR-302c-5p downregulates IRF3 expression in the human lung epithelial cell line A549 infected with influenza A virus, which in turn decreased the IFN-β production, impeding viral clearance [42]. Therefore, the use of microRNAs targeting IRF3 may alleviate inflammatory conditions. There is insufficient information about the microRNAs involved in the regulation of IRF3 mRNA. However, through bioinformatics tools, possible regulation of IRF3 by miR-21b-3p binding has been predicted in Atlantic salmon [43].
Regulation of cytokines by miRNAs
MAMPs’ recognition by TLRs triggers signaling pathways ending with the activation of transcription factors for cytokine production. Very precise regulation of this signaling occurs from start to finish; hence, the mRNA of cytokines can also be post-transcriptionally regulated. Cytokines act as soluble mediators of the innate and adaptive immune system. Nonetheless, a response to pathogens or debris from damaged host cells could cause elevated and uncontrolled cytokine production, and also inadequately resolved chronic inflammation such as chronic gastritis, inflammatory bowel diseases (IBD), prostatitis or endometriosis, which in turn may increase the risk of cancer. Some of the miRNAS implicated in the regulation of the cytokines described herein are shown in Figure 2.
Tumor necrosis factor α
In addition to the pathological implications related to the overproduction of TNF-α mentioned above, this cytokine has been considered the main effector associated with septic shock syndrome. In chronic inflammatory diseases, such as psoriasis, it has been observed that miR-203-5p and TNF-α are abundant in cells in psoriatic lesions. However, in primary keratinocytes and a keratinocyte cell line, the overexpression of miR-203-5p inhibits TNF-α transcript and protein production. The increase in TNF-α and miR-203-5p in psoriatic lesions skin is contradictory [44], since miR-203 also directly targets SOCS3 (suppressor of cytokine signaling 3) and SOCS6 [45], negative regulators of cytokine signaling. Hence, miR-203 acts as both an anti- and a pro-inflammatory regulator. Due to the key role that TNF-α plays in the pathogenesis of IBD, miR-19a-5p may also regulate the production of TNF-α in cells from patients with ulcerative colitis and colitis experimentally induced in mice; this finding is of great importance since many therapies for this condition include anti-TNF-α treatments [46].
Interleukin 6
Interleukin 6 is a cytokine involved in the acute phase response to infection and injury but, in addition to its role in the immune system, it plays a crucial role in hematopoiesis, neuronal and liver regeneration, embryonic development and fertility. Increased levels of circulating IL-6 have been implicated in several autoimmune diseases such as systemic lupus erythematosus (SLE) and RA, and the control of IL-6 production is a therapeutic target for these diseases. In addition to RA, IL-6 dysregulation contributes to the onset of other diseases such as inflammatory bowel disease (IBD), osteoporosis, multiple sclerosis, multiple myeloma, Hodgkin’s lymphoma, epithelial cancer and other several cancers. There is evidence that the let-7 family is involved in the regulation of tumorigenesis since low expression of the let-7 family has been found in several cancers [47, 48]. The overexpression of let-7g in a mouse model of lung cancer reduces tumorigenesis [49], while let-7a inhibits IL-6 transcript in the epithelial tissue of breast and prostate cancers [50]. However, it was found that miR-365-5p is a more potent regulator, since its overexpression in cervical cancer cells induced greater inhibition of IL-6 production than let-7a [51]. IL-6 can also be suppressed by miR-9-5p in cervical cancer cells, as inhibiting this cytokine also inhibits the activation pathways involved in progression of cervical adenocarcinoma [52]. Another miRNA related to the evolution of IL-6 and cancer is miR-26a-5p, as it is implicated in apoptosis induction and its low expression causes metastasis and recurrence of hepatocellular carcinoma (HCC); transfection of HCC cells with miR-26a-5p resulted in reduced proliferation, migration and invasion [53].
Interleukin 10
Interleukin 10 is an anti-inflammatory cytokine and plays a crucial role in the prevention of inflammatory and autoimmune disorders. Mice deficient in IL-10 develop IBD and show an exacerbated response to bacteria challenges; thus, it is suggested that IL-10 may play a protector role and may be used as a therapy for IBD. Bioinformatics analysis followed by experimental validation revealed that miR-106a-5p is a post-transcriptional regulator of IL-10 in several cell lines [54]. In general, members of the let-7 family have been shown to participate in the regulation of IL-10. In T cells, this microRNA family responds to infection by human immunodeficiency virus (HIV). IL-10 levels in plasma of HIV-infected patients are increased, which contributes to an abnormal response of cytotoxic T cells to infection; meanwhile, let-7 family levels are repressed by the infection [55]. In LPS-stimulated macrophages, miR-98-5p expression decreases, which contributes to the production of IL-10. Similarly, overexpression of miR-98 in LPS-stimulated macrophages promotes the generation of pro-inflammatory cytokines such as IL-6 and TNF-α. Therefore, miR-98-5p may play a role in inflammatory diseases since it might be involved in IL-10 deficiency and exaggerated pro-inflammatory responses in the intestine [56]. Given that cytokines share pathways and transcription factors, the role of miRNAs in the post-transcriptional regulation of cytokines further clarifies the fine mechanism that manages the immune response to maintain control of homeostasis.
Conclusions
The importance of miRNAs as a regulatory mechanism for protein expression has been the focus of several research studies in the past decade, several of which imply diverse aspects of the immune system. On the other hand, the TLR signaling cascade has been implicated in several inflammatory disorders. Hence, it is plausible that miRNAs play a regulatory function as well. In fact, the microRNA screening or microtranscriptome profile has been applied to explore the mechanism of immune system disorders. It is noteworthy that a single disease could harbor overexpression of several genes and discrepant miRNA values.
While many proteins participate in the TLR signaling cascade, few are known targets of miRNA. In this review, we have presented key aspects of cellular signaling regulation by miRNAs, emphasizing those inflammatory disorders in which their dysregulation has been described.
It may be possible to regulate the inflammatory processes associated with these diseases through the manipulation of key miRNAs. However, given that miRNAs can regulate several proteins, further studies are needed to elucidate whether they could really be used therapeutically. In the near future, the knowledge of how to modify the microtranscriptome could be useful for designing new therapies that target miRNAs, which in turn could restore or maintain immune system homeostasis altered by an excessive inflammatory response. Caution should be taken, as their application would not only affect the gene of interest but also other genes not involved in the immune response.
Acknowledgments
This work was financed by CONACyT grant 257462 to VMH. MAP is the recipient of a CONACyT PhD fellowship.
Footnotes
The authors declare no conflict of interest.
References
- 1.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 4.Huang QQ, Pope RM. The role of toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–364. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philippe L, Alsaleh G, Suffert G, et al. TLR2 expression is regulated by microRNA miR-19 in rheumatoid fibroblast-like synoviocytes. J Immunol. 2012;188:454–461. doi: 10.4049/jimmunol.1102348. [DOI] [PubMed] [Google Scholar]
- 6.Meng L, Zhu W, Jiang C, et al. Toll-like receptor 3 upregulation in macrophages participates in the initiation and maintenance of pristane-induced arthritis in rats. Arthritis Res Ther. 2010;12:R103. doi: 10.1186/ar3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang C, Zhu W, Xu J, et al. MicroRNA-26a negatively regulates toll-like receptor 3 expression of rat macrophages and ameliorates pristane induced arthritis in rats. Arthritis Res Ther. 2014;16:R9. doi: 10.1186/ar4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aungier SR, Ohmori H, Clinton M, Mabbott NA. MicroRN-100-5p indirectly modulates the expression of Il6, Ptgs1/2 and Tlr4 mRNA in the mouse follicular dendritic cell-like cell line, FL-Y. Immunology. 2015;144:34–44. doi: 10.1111/imm.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rupani H, Martinez-Nunez RT, Dennison P, et al. Toll-like receptor 7 is reduced in severe asthma and linked to an altered microRNA profile. Am J Respir Crit Care Med. 2016;194:26–37. doi: 10.1164/rccm.201502-0280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 11.Tang B, Xiao B, Liu Z, et al. Identification of MyD88 as a novel target of miR-155, involved in negative regulation of Helicobacter pylori-induced inflammation. FEBS Lett. 2010;584:1481–1486. doi: 10.1016/j.febslet.2010.02.063. [DOI] [PubMed] [Google Scholar]
- 12.Tili E, Michaille JJ, Cimino A, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 13.Doxaki C, Kampranis SC, Eliopoulos AG, et al. Coordinated regulation of miR-155 and miR-146a Genes during induction of endotoxin tolerance in macrophages. J Immunol. 2015;195:5750–5761. doi: 10.4049/jimmunol.1500615. [DOI] [PubMed] [Google Scholar]
- 14.Wei J, Huang X, Zhang Z, et al. MyD88 as a target of microRNA-203 in regulation of lipopolysaccharide or Bacille Calmette-Guerin induced inflammatory response of macrophage RAW264.7 cells. Mol Immunol. 2013;55:303–309. doi: 10.1016/j.molimm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Xu G, Zhang Z, Xing Y, et al. MicroRNA-149 negatively regulates TLR-triggered inflammatory response in macrophages by targeting MyD88. J Cell Biochem. 2014;115:919–927. doi: 10.1002/jcb.24734. [DOI] [PubMed] [Google Scholar]
- 16.Ma C, Li Y, Li M, et al. MicroRNA-124 negatively regulates TLR signaling in alveolar macrophages in response to mycobacterial infection. Mol Immunol. 2014;62:150–158. doi: 10.1016/j.molimm.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Cavalieri D, Rizzetto L, Tocci N, et al. Plant microRNAs as novel immunomodulatory agents. Sci Rep. 2016;6:25761. doi: 10.1038/srep25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Chen J, Wang H, et al. HCV-induced miR-21 contributes to evasion of host immune system by targeting MyD88 and IRAK1. PLoS Pathog. 2013;9:e1003248. doi: 10.1371/journal.ppat.1003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulte LN, Westermann AJ, Vogel J. Differential activation and functional specialization of miR-146 and miR-155 in innate immune sensing. Nucleic Acids Res. 2013;41:542–553. doi: 10.1093/nar/gks1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park H, Huang X, Lu C, et al. MicroRNA-146a and microRNA-146b regulate human dendritic cell apoptosis and cytokine production by targeting TRAF6 and IRAK1 proteins. J Biol Chem. 2015;290:2831–2841. doi: 10.1074/jbc.M114.591420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taganov KD, Boldin MP, Chang KJ, Baltimore D, et al. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu M, Zhang PJ, Li CH, et al. miRNA-133 augments coelomocyte phagocytosis in bacteria-challenged Apostichopus japonicus via targeting the TLR component of IRAK-1 in vitro and in vivo. Sci Rep. 2015;5:12608. doi: 10.1038/srep12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu G, Zhang Z, Wei J, et al. MicroR-142-3p down-regulates IRAK-1 in response to Mycobacterium bovis BCG infection in macrophages. Tuberculosis (Edinb) 2013;93:606–611. doi: 10.1016/j.tube.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, Li X, Ye Y, et al. MicroRNA-302b augments host defense to bacteria by regulating inflammatory responses via feedback to TLR/IRAK4 circuits. Nat Commun. 2014;5:3619. doi: 10.1038/ncomms4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Jin H, Yang X, et al. MicroRNA-93 inhibits inflammatory cytokine production in LPS-stimulated murine macrophages by targeting IRAK4. FEBS Lett. 2014;588:1692–1698. doi: 10.1016/j.febslet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Ma H, Wang X, Ha T, et al. MicroRNA-125b prevents cardiac dysfunction in polymicrobial sepsis by targeting TRAF6-mediated nuclear factor kappaB activation and p53-mediated apoptotic signaling. J Infect Dis. 2016;214:1773–1783. doi: 10.1093/infdis/jiw449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu S, Pan W, Song X, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 28.Xu Z, Dong D, Chen X, et al. MicroRNA-381 negatively regulates TLR4 signaling in A549 cells in response to LPS stimulation. Biomed Res Int. 2015;2015:849475. doi: 10.1155/2015/849475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou M, Wang F, Jiang A, et al. MicroRNA-3178 ameliorates inflammation and gastric carcinogenesis promoted by Helicobacter pylori new toxin, Tip-α, by targeting TRAF3. Helicobacter. 2017;22:e12348–n/a. doi: 10.1111/hel.12348. [DOI] [PubMed] [Google Scholar]
- 30.Fang Y, Chen H, Hu Y, et al. Burkholderia pseudomallei-derived miR-3473 enhances NF-kappaB via targeting TRAF3 and is associated with different inflammatory responses compared to Burkholderia thailandensis in murine macrophages. BMC Microbiol. 2016;16:283. doi: 10.1186/s12866-016-0901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi J, Qiao Y, Wang P, et al. MicroRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-kappaB1 in murine macrophages. FEBS Lett. 2012;586:1201–1207. doi: 10.1016/j.febslet.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Kumar SM, Lu H, et al. MicroRNA-9 up-regulates E-cadherin through inhibition of NF-kappaB1-Snail1 pathway in melanoma. J Pathol. 2012;226:61–72. doi: 10.1002/path.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan HY, Guo LM, Liu T, et al. Regulation of the transcription factor NF-kappaB1 by microRNA-9 in human gastric adenocarcinoma. Mol Cancer. 2010;9:16. doi: 10.1186/1476-4598-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao S, Yang G, Liu PN, et al. miR-590-3p Is a Novel MicroRNA in Myocarditis by Targeting Nuclear Factor Kappa-B in vivo. Cardiology. 2015;132:182–188. doi: 10.1159/000433596. [DOI] [PubMed] [Google Scholar]
- 35.Shin VY, Jin H, Ng EK, et al. NF-kappaB targets miR-16 and miR-21 in gastric cancer: involvement of prostaglandin E receptors. Carcinogenesis. 2011;32:240–245. doi: 10.1093/carcin/bgq240. [DOI] [PubMed] [Google Scholar]
- 36.Garzon R, Pichiorri F, Palumbo T, et al. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148–4157. doi: 10.1038/sj.onc.1210186. [DOI] [PubMed] [Google Scholar]
- 37.Zhao C, Zhao Q, Zhang C, et al. miR-15b-5p resensitizes colon cancer cells to 5-fluorouracil by promoting apoptosis via the NF-κB/XIAP axis. Sci Rep. 2017;7:4194. doi: 10.1038/s41598-017-04172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang XA, Zhang R, She ZG, et al. Interferon regulatory factor 3 constrains IKKβ/NF-κB signaling to alleviate hepatic steatosis and insulin resistance. Hepatology. 2014;59:870–885. doi: 10.1002/hep.26751. [DOI] [PubMed] [Google Scholar]
- 39.Tarassishin L, Loudig O, Bauman A, et al. Interferon regulatory factor 3 inhibits astrocyte inflammatory gene expression through suppression of the proinflammatory miR-155 and miR-155*. Glia. 2011;59:1911–1922. doi: 10.1002/glia.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitzgerald DC, O’Brien K, Young A, et al. Interferon regulatory factor (IRF) 3 is critical for the development of experimental autoimmune encephalomyelitis. J Neuroinflammation. 2014;11:130. doi: 10.1186/1742-2094-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loubaki L, Chabot D, Paré I, et al. MiR-146a potentially promotes IVIg-mediated inhibition of TLR4 signaling in LPS-activated human monocytes. Immunol Lett. 2017;185:64–73. doi: 10.1016/j.imlet.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Gui S, Chen X, Zhang M, et al. Mir-302c mediates influenza A virus-induced IFNβ expression by targeting NF-κB inducing kinase. FEBS Lett. 2015;589(24 Pt B):4112–4118. doi: 10.1016/j.febslet.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Andreassen R, Woldemariam NT, Egeland IØ, et al. Identification of differentially expressed Atlantic salmon miRNAs responding to salmonid alphavirus (SAV) infection. BMC Genomics. 2017;18:349. doi: 10.1186/s12864-017-3741-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Primo MN, Bak RO, Schibler B, Mikkelsen JG. Regulation of pro-inflammatory cytokines TNFαlpha and IL24 by microRNA-203 in primary keratinocytes. Cytokine. 2012;60:741–748. doi: 10.1016/j.cyto.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 45.Moffatt CE, Lamont RJ. Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect Immun. 2011;79:2632–2637. doi: 10.1128/IAI.00082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen B, She S, Li D, et al. Role of miR-19a targeting TNF-αlpha in mediating ulcerative colitis. Scand J Gastroenterol. 2013;48:815–824. doi: 10.3109/00365521.2013.800991. [DOI] [PubMed] [Google Scholar]
- 47.Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 49.Kumar MS, Erkeland SJ, Pester RE, et al. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Z, Xiao SB, Xu P, et al. miR-365, a novel negative regulator of interleukin-6 gene expression, is cooperatively regulated by Sp1 and NF-kappaB. J Biol Chem. 2011;286:21401–21412. doi: 10.1074/jbc.M110.198630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Jia J, Zhao L, et al. Down-regulation of microRNA-9 leads to activation of IL-6/Jak/STAT3 pathway through directly targeting IL-6 in HeLa cell. Mol Carcinog. 2016;55:732–742. doi: 10.1002/mc.22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X, Liang L, Zhang XF, et al. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology. 2013;58:158–170. doi: 10.1002/hep.26305. [DOI] [PubMed] [Google Scholar]
- 54.Sharma A, Kumar M, Aich J, et al. Posttranscriptional regulation of interleukin-10 expression by hsa-miR-106a. Proc Natl Acad Sci. 2009;106:5761–5766. doi: 10.1073/pnas.0808743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swaminathan S, Suzuki K, Seddiki N, et al. Differential regulation of the Let-7 family of microRNAs in CD4+ T cells alters IL-10 expression. J Immunol. 2012;188:6238–6246. doi: 10.4049/jimmunol.1101196. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Chen Q, Song Y, et al. MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett. 2011;585:1963–1968. doi: 10.1016/j.febslet.2011.05.029. [DOI] [PubMed] [Google Scholar]