Abstract
In this study, we investigated whether simvastatin (SVT), a statin commonly prescribed to decrease cholesterol levels, might have a therapeutic effect in OA. Primary rabbit chondrocytes were pre-treated with SVT (50 μM), then treated with sodium nitroprusside (SNP; 1 mM), a donor of nitric oxide (NO) known as a pro-inflammatory mediator, and analyzed for the expression levels of type II collagen, SOX-9, aggrecan, matrix metalloproteinases (MMPs) 1, and 13. SNP increased NO generation in a dose-dependent manner, causing a loss of type II collagen and aggrecan indicative of chondrocyte dedifferentiation, which was inhibited by SVT. SVT also reversed the increase in MMP-1 and -13 and inhibited NO production and NO synthase expression induced by SNP in articular chondrocytes. Given that MMP-1 and -13 knockdown by siRNA increased the level of type II collagen in SNP-treated cells, our results show that SVT prevented NO-induced chondrocyte damage and dedifferentiation through downregulation of MMP expression. This study showed that SVT could attenuate the degradation of articular cartilage components, which is characteristic for OA, through inhibition of MMPs in NO-treated chondrocytes, suggesting that SVT may be a novel candidate therapeutic agent for the prevention and/or treatment of OA.
Impact statement
Dedifferentiation of chondrocytes is the main character of cartilage degradation. Therefore the understanding of chondrocytes dedifferentiation is essential for arthritis therapy. However, the molecular mechanism of cartilage destroy is mostly unknown. In this work we show that simvastatin (SVT) inhibits dedifferentiation by nitric oxide by blocking the expression of matrix metalloproteinases 1 and 13. These effects of SVT on dedifferentiation suggest that SVT may be used as a drug for the cure of arthritis.
Keywords: Simvastatin, sodium nitroprusside, chondrocyte, differentiation, matrix metalloproteinase, osteoarthritis
Introduction
Osteoarthritis (OA) is described by gradual dysfunction of cartilage and inflammation of the synovium, which lead to exposure and sclerosis of the subchondral bone plate, ultimately causing pain and joint stiffness.1,2 OA is primarily triggered by joint injury, but can also be promoted by defects in limb development and/or genetic factors. Articular chondrocytes are the cells that secrete the extracellular matrix (ECM) and maintain its metabolic balance in normal cartilage3; however, a mechanical insult may induce them to release proinflammatory cytokines and express increased amounts of matrix metalloproteinases (MMPs),4 leading to inflammation, ECM degradation, and OA development.5
MMPs comprise a large family of matrix-destroying factors that play a crucial role in modulating OA.6 MMPs are induced by various factors, including pro-inflammatory cytokines in the synovium7 and are considered important determinants of OA development. In common conditions, MMPs are synthesized at low levels, providing remodeling of healthy connective tissue critical for various physiological processes such as organ development, angiogenesis, and tissue repair,8,9 whereas in pathogenic conditions, the production of MMPs increases dramatically, leading to excessive destruction of connective tissue. Consequently, high expression and secretion of MMPs are associated with different pathological conditions such as tumor invasion/metastasis, atherosclerosis, and arthritis.10–12
Among MMPs, MMP-1 and -13 which decrease type II collagen, a main constituent of the cartilage, are known to be elevated in joint disorders; thus, they play an important role in OA, in which collagen destruction is the critical pathogenic factor.13 Increasing evidence indicates that some MMP inhibitors can alleviate cartilage degradation in experimental OA,14,15 suggesting that the blockage of MMP activity can be considered as a treatment strategy for OA patients.
It has been shown that nitric oxide (NO) can inhibit the production of ECM components by prompting the dedifferentiation of chondrocytes.16 NO is a small hydrophobic signaling molecule which can function both inside the cells where it is synthesized from arginine through the activity of NO synthases (NOSs) and in the extracellular space where it can act on the adjacent cells after penetration through the cell membrane. Stimulation of the inducible NOS isoform (iNOS or NOS2) by proinflammatory cytokines and bacterial products can upregulate NO production in various cell types, including chondrocytes, fibroblasts, and dendritic cells, resulting in generation of large NO amounts.16,17 Consistent with these data, an inhibitor of iNOS was shown to protect chondrocytes and cartilage against destruction in an in vivo experimental model.18 Furthermore, NO was reported to promote chondrocyte apoptosis, inhibit proteoglycan synthesis, and enhance MMP activity, which correlated with the increase in cartilage matrix degradation.18–20 Overall, these findings suggest an association between inflammation, NO production, and MMP activation in OA.
Simvastatin (SVT), commonly prescribed to reduce the risk of cardiovascular disease by lowering cholesterol levels, has also been shown to have a negative effect on inflammation, and several findings investigated its potential in the treatment of OA.19 Therefore, we tested the impacts of SVT on NO-induced dedifferentiation and MMP-1 and -13 expression as well as NO production in rabbit articular chondrocytes.
Materials and methods
Treatment
Chondrocytes were added with SVT (Sigma-Aldrich, Saint Louis, MO, USA). Dimethyl sulfoxide (DMSO) was used as the vehicle.20 Sodium nitroprusside (SNP; Sigma) was used for the dedifferentiation of chondrocytes. siRNA for MMP-1 and -13 was purchased from Bioneer (Daejeon, Korea).
Culture of chondrocytes
Rabbits (2 weeks old) and Wistar rats (3 weeks old) were purchased from Koatech (Pyeoungtaek, Republic of Korea), as described previously.21 Cells (2 × 104 cells/dish) were incubated in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% (v/v) Fetal Bovine Serum (FBS) and supplemented with penicillin (50 units/mL) and streptomycin (50 µg/mL). Next, we observed the morphology of chondrocytes under a microscope. Our materials and methods were accepted by the Ethics Committee of the Kongju National University.
Western blot analysis
Proteins were collated from chondrocytes, and the detection of protein was performed as described previously.22 Protein was separated SDS-PAGE and following electrophoresis, the proteins were shifted to a NC membrane. The following antibodies were used: collagen type II monoclonal antibody (Santa Cruz Biotechnology, CA, USA); MMP-1 polyclonal antibody (calbiochem, Hessen, Darmstadt, Germany); MMP-13 polyclonal antibody (Santa Cruz Biotechnology); SOX-9 polyclonal antibody (Santa Cruz Biotechnology); GAPDH (Santa Cruz Biotechnology); anti-rabbit IgG (Sigma-Aldrich, St. Louis, MO, USA); anti-goat IgG (Chemicon International, Billerica, MA, USA); and anti-mouse IgG (Enzo Life Sciences International, Farmingdale, NY, USA). An ECL reagent (Dogen, Seoul, Republic of Korea) was used to determine the blot bands using the LAS4000 system (Fuji Film, Tokyo, Japan).
Reverse Transcription polymerase chain reaction (RT-PCR)
The total RNA of chondrocytes was extracted by TRIzol reagent (Life Technologies, Gaithersburg, MD, USA) and reverse transcribed to cDNA synthesis were performed with RT premix kit (Genotech, Korea). The following primers and conditions were employed as described previously.23,24 DNA was separated on a 1% agarose gel and stained with Ecodye™ solution (BioFact, Daejeon, Republic of Korea).
Alcian blue staining
Cells were incubated with 3.5% paraformaldehyde for 20 min. Cells were stained as described previously.25 Cells were analyzed by spectrophotometer (Molecular Devices, USA) at 595 nm.
Immunofluorescence staining
Cells were seeded on sterilized coverslips in a 35 mm dish. After the cells adhered, the cells were fixed as described previously.25 Samples were incubated with type II collagen antibody (Santa Cruz), MMP-1 antibody (calbiochem, Hessen, Darmstadt, Germany), and MMP-13 antibody (Santa Cruz Biotechnology). On the next day, the cells were then incubated with peroxidase-conjugated secondary antibodies at room temperature for 1 h and then counterstained with 4′, 6-diamidino-2-phenylindole (DAPI; Invitrogen, Burlington, ON, Canada) for 10 min at RT. Immunofluorescence images were captured using a BX51 fluorescence microscope (Olympus, Tokyo, Japan).
NO assay
NO was detected by using Griess reagent according to manufacturer's protocols. Supernatants of cultured cells (100 μL) were harvested and mixed with same volume of Griess reagent and read at 550 nm. OD was measured at 550 nm on a microplate reader (Molecular Devices).
Immunohistochemical staining
The tissue were fixed for 4% paraformaldehyde, washed twice with PBS, and dehydrated with graded ethanol. After standard processing, the samples were embedded in paraffin. Specimens were cut in 4 μm sections. To destroy the activity of endogenous peroxidase, added 1% H2O2 for 10 min at room temperature. Cartilage sections were incubated with primary antibodies type II collagen (Mouse Anti-COL2A1/type II collagen) at 4°C for overnight and washed with PBS. After washing, the sections were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG at 37°C for 30 min. The antibody bound to the samples was determined using a DAKO kit. After washing, the images were obtained using a light microscope.
siRNA transfection
Cells were transfected with scrambled or MMPs siRNAs after changing medium with 2 mL of DMEM (Invitrogen Life Technologies) per well. For MMP-1, the sense sequence was 5′-cag guu auc cca aaa uga u-3′ and the antisense sequence was 5′-a uca uuu ugg gau aac cug-3′, used at 100 nM; For MMP-13, the sense sequence was 5′-cuc aug uuu ccc auc uac a-3′ and the antisense sequence was 5′-u gua gau ggg aaa cau gag-3′, used at 100 nM. TurboFect transfection reagent (Fisher Thermo Scientific) was used as a transporter.
Data analysis and statistics
Results were from at least three times experiments and presented as the means ± standard deviation (SD). Statistical analysis was indicated using a one-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test and all values are expressed as the means ± SD. Statistical significance with p values <0.05.
Results
SVT increases differentiation of chondrocytes
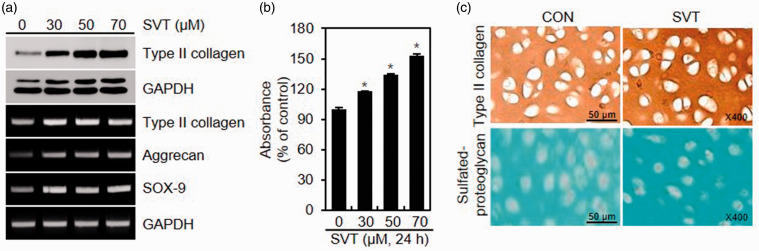
First, we examined the effects of SVT on the differentiation of chondrocytes by evaluating the expression of type II collagen and synthesis of sulfated proteoglycans. RT-PCR and western blotting analyses indicated that type II collagen in chondrocytes treated with SVT for 24 h was upregulated in both at the mRNA and protein levels (Figure 1(a)). Furthermore, the mRNA expression of SOX-9 and aggrecan, cartilage-specific proteoglycan, were also induced by SVT (Figure 1(a)). In parallel to the upregulation of type II collagen, SVT also increased the accumulation of sulfated proteoglycans (Figure 1(b)). The effect of SVT on chondrocyte differentiation was further examined by immunocytochemistry, which showed that staining for type II collagen and sulfated proteoglycans in SVT-treated cells was stronger compared to control, untreated chondrocytes (Figure 1(c)).
Figure 1.
Effect of SVT on the differentiation of chondrocytes (a, b). Cells were added with SVT for 24 h and analyzed for protein and mRNA expression by western blotting and RT-PCR (a). Sulfated proteoglycans was determined by Alcian blue staining (*p < 0.05 compared to control) (b). (c) Chondrocytes were treated with 50 μM SVT for 24 h and analyzed by Alcian blue staining and immunocytochemistry. SVT: simvastatin; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; CON: control. (A color version of this figure is available in the online journal.)
SVT inhibits MMP-1/-13 expression induced by SNP-generated NO
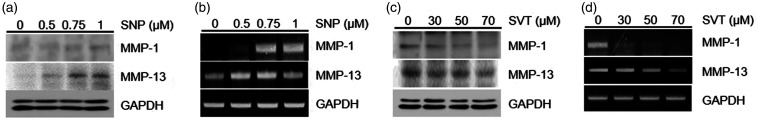
Consistent with these data, we observed a dose-dependent increase in mRNA and protein expression of MMP-1 and -13 in chondrocytes treated with different concentrations of SNP compared to control cells (Figure 2(a) and (b)). However, SVT abolished the SNP-induced upregulation of MMP expression (Figure 2(c) and (d)).
Figure 2.
Effects of SNP and SVT on MMP-1 and -13 expression in chondrocytes. (a, b) Cells were treated with the specific doses of SNP for 24 h and analyzed for MMP protein expression by western blotting (a) or mRNA expression by RT-PCR (b). (c, d) Chondrocytes were treated with the indicated concentrations of SVT for 24 h and analyzed for MMP protein expression by western blotting (c) or mRNA expression by RT-PCR (d). MMP: metalloproteinase; SNP: sodium nitroprusside; SVT: simvastatin; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
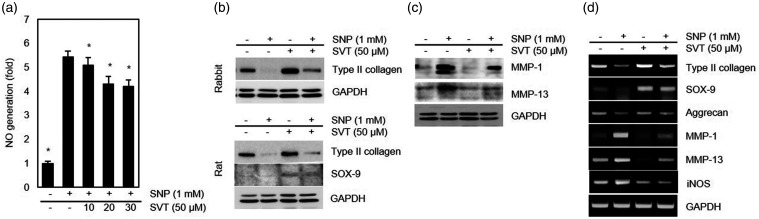
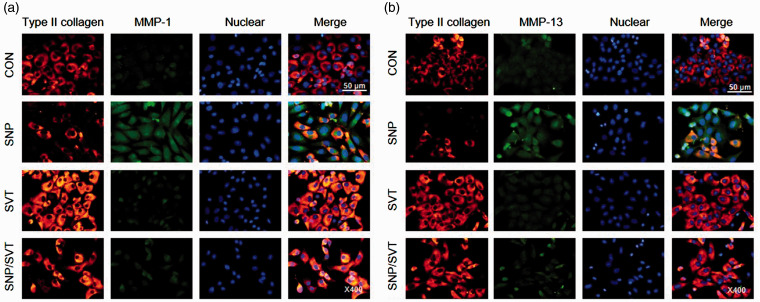
To determine whether SVT may exert protective effects on SNP-treated chondrocytes, we tested the differentiation status and NO generation in chondrocytes incubated in the presence of both SNP and SVT. As shown in Figure 3(a), the production of NO was markedly induced by SNP but the effect was significantly attenuated by SVT. Furthermore, pretreatment with SVT for 1 h prior to the addition of SNP provided recovery of SNP-induced type II collagen loss both in rabbit and rat chondrocytes (Figure 3(b)), indicating that SVT could prevent SNP-induced chondrocyte dedifferentiation. We also analyzed the expression of SOX-9, an important protein for maintaining type II collagen expression and, consequently, a stable chondrocyte phenotype. A marked induction of SOX-9 expression was observed in SVT-treated rat articular chondrocytes cultured with or without SNP (Figure 3(b)), although we could not detect SOX-9 in rabbit chondrocytes with the tested antibodies. Then, we examined whether SVT could inhibit MMP-1 and -13 expression in cells treated with SNP. Western blotting analysis showed that SVT pretreatment markedly reduced the level of both MMP-1 and -13 proteins increased in SNP-exposed chondrocytes (Figure 3(c)), suggesting that SVT may reverse SNP-caused dedifferentiation by inhibiting MMP-1 and -13 expression. These results were confirmed by RT-PCR analysis, which showed that SVT attenuated transcriptional effects of SNP in articular chondrocytes by increasing mRNA of type II collagen, SOX-9, and aggrecan, and decreasing that of iNOS, MMP-1, and MMP-13 (Figure 3(d)). Analysis of type II collagen, MMP-1, and MMP-13 expression by immunocytochemistry revealed that in SVT-treated chondrocytes, staining for type II collagen was significantly stronger and that for MMP-1 and MMP-13 weaker compared to cells treated with SNP alone (Figure 4), indicating that SVT reversed the negative effects of SNP on chondrocyte differentiation. Together, these results suggest that SVT prevents the induction of MMP-1 and MMP-13 expression and subsequent loss of collagen due to SNP-induced NO generation in chondrocytes, thus protecting them against degeneration and supporting ECM homeostasis in articular cartilage.
Figure 3.
Effects of SVT on NO production and MMP-1 and -13 expression in SNP-treated chondrocytes. Cells were treated with the indicated concentrations of SVT for 1 h and then with 1 mM SNP for additional 24 h. (a) NO production in rabbit chondrocytes (*p < 0.05 compared to SNP-treated cells). (b) Type II collagen and SOX-9 in rabbit and rat chondrocytes analyzed by western blotting. (c) Expression of MMP-1 and -13 in rabbit chondrocytes analyzed by western blotting. (d) Gene transcription determined by RT-PCR. MMP: metalloproteinase; SNP: sodium nitroprusside; SVT: simvastatin; iNOS: inducible nitric oxide synthase; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
Figure 4.
SVT attenuates SNP-caused reduction of type II collagen and induction in MMP-1 and MMP-13 expression. Primary rabbit chondrocytes were added with 50 μM SVT for 1 h and then with 1 mM SNP for additional 24 h and analyzed for the expression of MMP-1 (a), MMP-13 (b), and type II collagen (a, b) by immunocytochemistry; DAPI staining was performed for counter staining. MMP: metalloproteinase; SNP: sodium nitroprusside; SVT: simvastatin; CON: control. (A color version of this figure is available in the online journal.)
SVT inhibits chondrocyte dedifferentiation caused by SNP-induced MMP-1/-13 expression
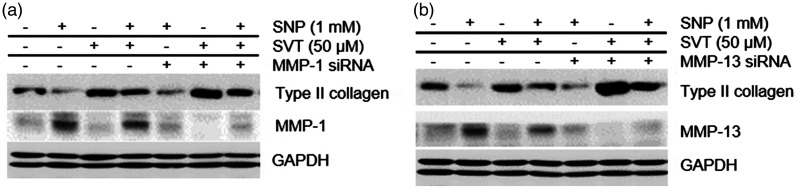
To assess the involvement of MMP-1 and -13 in the protective impacts of SVT on SNP-caused chondrocyte dedifferentiation, we performed RNA interference experiments. Western blotting analysis showed that knocking down of MMP-1 or MMP-13 with siRNA rescued the reduction of type II collagen caused by SNP, which was similar to the effect of SVT (Figure 5). These results suggest that SNP mediates dedifferentiation of chondrocytes by upregulating MMP-1 and -13 and that SVT protects articular chondrocytes from SNP toxicity through blocking of MMP-1 and -13 expression.
Figure 5.
SVT prevents SNP-induced chondrocyte dedifferentiation by downregulating MMP-1 and -13. Cells were transfected with either scrambled siRNA or (a) MMP-1- or (b) MMP-13-specific siRNA. After 24 h, cells were added with 50 μM SVT for 1 h and then with 1 mM SNP for additional 24 h and analyzed by western blotting. MMP: metalloproteinase; SNP: sodium nitroprusside; SVT: simvastatin; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
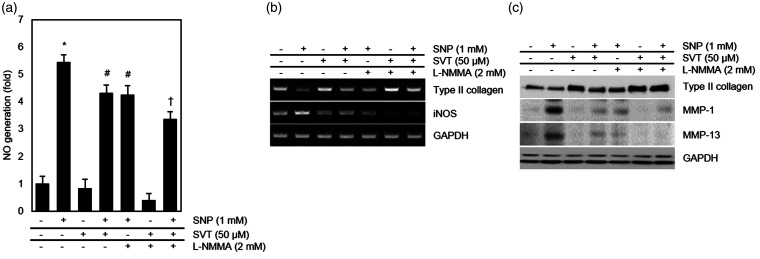
As SNP produces NO which promotes the expression of MMPs,26,27 we also examined the effects of SVT on NO generation and iNOS transcription in SNP-treated chondrocytes. The results showed that SVT reduced both NO production and iNOS mRNA expression induced by SNP in chondrocytes, which was similar to the effect of L-NG-monomethyl arginine (L-NMMA), an inhibitor of NOS (Figure 6(a) and (b)). L-NMMA also blocked the increase in MMP-1 and MMP-13 and rescued the loss of type II collagen caused by SNP (Figure 6(b) and (c)), thus confirming the role of NO in dedifferentiation of chondrocyte and OA development.
Figure 6.
Effect of SVT on NO and iNOS expression in SNP-treated articular chondrocytes. Cells were treated or not with 2 mM L-NMMA for 1 h and then incubated with 50 μM SVT or/and 1 mM SNP. (a) Production of NO determined by the NO assay (*p < 0.05 compared to control, #p < 0.05 compared to control, and †p < 0.05 compared to control). (b) mRNA expression analyzed by RT-PCR. (c) Protein expression analyzed by western blotting. MMP: metalloproteinase; SNP: sodium nitroprusside; SVT: simvastatin; L-NMMA: L-NG-monomethyl arginine; iNOS: inducible nitric oxide synthase; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
Discussion
In this study, we showed that SVT could inhibit the chondrocytes dedifferentiation through decreasing the production of NO and expression of MMP-1 and -13, which resulted in partial restoration of collagen type II and aggrecan expression, suggesting protective effects of SVT on articular cartilage.28 Given that OA is characterized by increase in the synthesis of ECM-degrading enzymes, these results indicate SVT possible as a beneficial agent in OA.28 Supplementary studies are necessary to clarify the precise mechanism of the inhibitory effect of SVT on MMP-1 and -13 expression, which should further understanding of OA pathogenesis.
The irreversible stage in OA development and progression occurs when the balance between the production and degradation of cartilage components, collagen and proteoglycans, is disturbed29 The ECM produced by normal chondrocytes mainly consists of type II collagen, a chondrogenic marker,5 and the decrease in collagen type II content is typical for cartilage degradation in OA.30
NO is a signaling molecule synthesized through conversion of L-arginine to L-citrulline by NOS.31 Because the highly level production of NO causes cell dysfunction and even apoptotic cell death, prevention of iNOS generation may have beneficial impacts on cell survival and differentiation.32 iNOS is highly expressed in various types of cells and is found more often in cells with OA compared to normal cells.33 Abnormal levels of NO are associated with cartilage damage and the inhibition of iNOS blocked the loss of glycosaminoglycan synthesis in an OA model. NO has multiple functions on chondrocytes including dedifferentiation, inflammation, and apoptosis.25,34
Chondrocytes from patients with OA exhibit NO, and a high level of nitrites/nitrates have been found in the synovial fluid and serum of patients, reflecting an increase in NO during OA disease development.35 Cedergren et al. showed improved levels iNOS in plasma and synovial fluid in the knees of OA patients.36,37
The importance of NO is well known in the progression of arthritis. Adjuvant-caused arthritis can be blocked by the NO synthase inhibitor L-NMMA. In the adjuvant-caused arthritis animals, the onset of symptoms was preceded by raised generation of nitrates and nitrites. Promotions of NO and enhancement of disease by non-selective NOS inhibitors has also been revealed in collagen-induced arthritis. The elevation of iNOS in OA patients could be attributed to inflammation-related process, including that oxidative stress are involved with OA pathogenesis.
NO is a proinflammatory second messenger playing an important role in OA,38 in particular through upregulation of cartilage-degrading MMPs39; therefore, in this study we investigated the ability of SVT to reduce MMP-1 and -13 expression in NO-stimulated chondrocytes.
MMPs are present in the articular joint where they degrade all types of collagen, proteoglycans, and other ECM components, thus playing an important role in the degeneration of articular cartilage. Among MMPs, MMP-1 and -13 are probably the most important players in OA as they have substrate specificity for fibrillar collagen type II, the principal component of articular cartilage. ProMMP-1 and -13 have been shown to activate directly by peroxynitrite or nitro-thiol compounds without proteolysis of proMMP-1 and -13, explaining an association between MMP and iNOS expression.40–42 Pro-inflammatory cytokines that stimulate MMPs contribute to the catabolic direction43 and also initiate the production of NO in the joints.44
Our findings indicate that SVT could reduce MMP-1 and -13 expression induced in rabbit articular chondrocytes by NO-producing SNP; furthermore, it can suppress NO generation in chondrocytes, presumably through downregulation of iNOS. These effects of SVT are likely responsible for the restoration of collagen type II levels and transcriptional upregulation of aggrecan, the main cartilage proteoglycan, suggesting that application of SVT may have beneficial effects in OA.
Statins are known for their anti-inflammatory properties, and efforts have been made to investigate whether their activity could be used in the treatment of OA. Thus, mevastatin was shown to suppress inflammation, downregulate MMP expression, and reduce cartilage degradation in an in vivo model of OA,19,45 whereas pravastatin reduced the expression of MMP-3 and MMP-9 in IL-1β-stimulated chondrocytes,46 and atorvastatin decreased MMP-13 in OA chondrocytes.47 Belcher has showed that increasing concentration of statins use and larger statin concentration increases were associated with a decrease in clinical OA in comparison with non-statin users.48 While there are the reasonable effects by which statins may have helpful effects in OA joints, the mechanism for a useful effect of statins in human OA is remains.
These and our findings indicate that statins may stop OA progression by inhibiting the expression of MMPs in articular chondrocytes and restoring ECM composition of articular joints, which may provide a framework for the development of drugs and treatment strategies to prevent or limit cartilage breakdown in OA.28,39
Conclusion
Our data demonstrate that SVT inhibits dedifferentiation of primary articular chondrocytes caused by NO by reducing the expression of MMP-1 and -13 as well as iNOS, which prevented the decrease of type II collagen and proteoglycans, suggesting SVT as a candidate therapeutic agent for treating OA.
Authors’ contributions
SMY and SJK planned experiments, performed research, and wrote manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (no. 2015R1C1A2A01055015 and 2017R1D1A3B03033401).
References
- 1.Zhong HM, Ding QH, Chen WP, Luo RB. Vorinostat, a HDAC inhibitor, showed anti-osteoarthritic activities through inhibition of iNOS and MMP expression, p38 and ERK phosphorylation and blocking NF-kappaB nuclear translocation. Int Immunopharmacol 2013; 17:329–35 [DOI] [PubMed] [Google Scholar]
- 2.Tetsunaga T, Nishida K, Furumatsu T, Naruse K, Hirohata S, Yoshida A, Saito T, Ozaki T. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthritis Cartilage 2011; 19:222–32 [DOI] [PubMed] [Google Scholar]
- 3.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum 2000; 43:1916–26 [DOI] [PubMed] [Google Scholar]
- 4.Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum 1997; 40:2065–74 [DOI] [PubMed] [Google Scholar]
- 5.Maldonado M, Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int 2013; 2013:284873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed S, Rahman A, Hasnain A, Goldberg VM, Haqqi TM. Phenyl N-tert-butylnitrone down-regulates interleukin-1 beta-stimulated matrix metalloproteinase-13 gene expression in human chondrocytes: suppression of c-Jun NH2-terminal kinase, p38-mitogen-activated protein kinase and activating protein-1. J Pharmacol Exp Ther 2003; 305:981–8 [DOI] [PubMed] [Google Scholar]
- 7.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res 2002; 4:157–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borden P, Solymar D, Sucharczuk A, Lindman B, Cannon P, Heller RA. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J Biol Chem 1996; 271:23577–81 [DOI] [PubMed] [Google Scholar]
- 9.Vincenti MP, Coon CI, Mengshol JA, Yocum S, Mitchell P, Brinckerhoff CE. Cloning of the gene for interstitial collagenase-3 (matrix metalloproteinase-13) from rabbit synovial fibroblasts: differential expression with collagenase-1 (matrix metalloproteinase-1). Biochem J 1998; 331:341–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borden P, Heller RA. Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. Crit Rev Eukar Gene Expr 1997; 7:159–78 [DOI] [PubMed] [Google Scholar]
- 11.Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 2001; 108:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum 2000; 43:801–11 [DOI] [PubMed] [Google Scholar]
- 13.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest 1996; 97:761–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li NG, Shi ZH, Tang YP, Wang ZJ, Song SL, Qian LH, Qian DW, Duan JA. New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Curr Med Chem 2011; 18:977–1001 [DOI] [PubMed] [Google Scholar]
- 15.Burrage PS, Brinckerhoff CE. Molecular targets in osteoarthritis: metalloproteinases and their inhibitors. Curr Drug Targets 2007; 8:293–303 [DOI] [PubMed] [Google Scholar]
- 16.Cao M, Westerhausen-Larson A, Niyibizi C, Kavalkovich K, Georgescu HI, Rizzo CF, Hebda PA, Stefanovic-Racic M, Evans CH. Nitric oxide inhibits the synthesis of type-II collagen without altering Col2A1 mRNA abundance: prolyl hydroxylase as a possible target. Biochem J 1997; 324: Pt 1;305–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest 1997; 100:2417–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelletier JP, Jovanovic D, Fernandes JC, Manning P, Connor JR, Currie MG, Di Battista JA, Martel-Pelletier J. Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum 1998; 41:1275–86 [DOI] [PubMed] [Google Scholar]
- 19.Akasaki Y, Matsuda S, Nakayama K, Fukagawa S, Miura H, Iwamoto Y. Mevastatin reduces cartilage degradation in rabbit experimental osteoarthritis through inhibition of synovial inflammation. Osteoarthritis Cartilage 2009; 17:235–43 [DOI] [PubMed] [Google Scholar]
- 20.Han Y, Kim SJ. Simvastatin induces differentiation of rabbit articular chondrocytes via the ERK-1/2 and p38 kinase pathways. Exp Cell Res 2016; 346:198–205 [DOI] [PubMed] [Google Scholar]
- 21.Yoon YM, Kim SJ, Oh CD, Ju JW, Song WK, Yoo YJ, Huh TL, Chun JS. Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J Biol Chem 2002; 277:8412–20 [DOI] [PubMed] [Google Scholar]
- 22.Yu SM, Yeo HJ, Choi SY, Kim SJ. Cytokine-induced apoptosis inhibitor-1 causes dedifferentiation of rabbit articular chondrocytes via the ERK-1/2 and p38 kinase pathways. Int J Biochem Cell Biol 2016; 80:10–8 [DOI] [PubMed] [Google Scholar]
- 23.Yu SM, Kim SJ. Production of reactive oxygen species by withaferin A causes loss of type collagen expression and COX-2 expression through the PI3K/Akt, p38, and JNK pathways in rabbit articular chondrocytes. Exp Cell Res 2013; 319:2822–34 [DOI] [PubMed] [Google Scholar]
- 24.Yu SM, Kim SJ. Endoplasmic reticulum stress (ER-stress) by 2-deoxy-D-glucose (2DG) reduces cyclooxygenase-2 (COX-2) expression and N-glycosylation and induces a loss of COX-2 activity via a Src kinase-dependent pathway in rabbit articular chondrocytes. Exp Mol Med 2010; 42:777–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SJ, Hwang SG, Kim IC, Chun JS. Actin cytoskeletal architecture regulates nitric oxide-induced apoptosis, dedifferentiation, and cyclooxygenase-2 expression in articular chondrocytes via mitogen-activated protein kinase and protein kinase C pathways. J Biol Chem 2003; 278:42448–56 [DOI] [PubMed] [Google Scholar]
- 26.Zaragoza C, Lopez-Rivera E, Garcia-Rama C, Saura M, Martinez-Ruiz A, Lizarbe TR, Martin-de-Lara F, Lamas S. Cbfa-1 mediates nitric oxide regulation of MMP-13 in osteoblasts. J Cell Sci 2006; 119:1896–902 [DOI] [PubMed] [Google Scholar]
- 27.Shin CY, Lee WJ, Choi JW, Choi MS, Ryu JR, Oh SJ, Cheong JH, Choi EY, Ko KH. Down-regulation of matrix metalloproteinase-9 expression by nitric oxide in lipopolysaccharide-stimulated rat primary astrocytes. Nitric Oxide 2007; 16:425–32 [DOI] [PubMed] [Google Scholar]
- 28.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002; 39:237–46 [PubMed] [Google Scholar]
- 29.Santoro A, Conde J, Scotece M, Abella V, Lois A, Lopez V, Pino J, Gomez R, Gomez-Reino JJ, Gualillo O. SERPINE2 inhibits IL-1alpha-induced MMP-13 expression in human chondrocytes: involvement of ERK/NF-kappaB/AP-1 pathways. PLoS One 2015; 10:e0135979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi S, Man Z, Li W, Sun S, Zhang W. Silencing of Wnt5a prevents interleukin-1beta-induced collagen type II degradation in rat chondrocytes. Exp Ther Med 2016; 12:3161–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 2012; 33:829–37 837a–d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madonna R, Di Napoli P, Massaro M, Grilli A, Felaco M, De Caterina A, Tang D, De Caterina R, Geng YJ. Simvastatin attenuates expression of cytokine-inducible nitric-oxide synthase in embryonic cardiac myoblasts. J Biol Chem 2005; 280:13503–11 [DOI] [PubMed] [Google Scholar]
- 33.Chin K, Kurashima Y, Ogura T, Tajiri H, Yoshida S, Esumi H. Induction of vascular endothelial growth factor by nitric oxide in human glioblastoma and hepatocellular carcinoma cells. Oncogene 1997; 15:437–42 [DOI] [PubMed] [Google Scholar]
- 34.Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK, Kim JH, Yoo YJ, Bang OS, Kang SS, Chun JS. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem 2002; 277:1332–9 [DOI] [PubMed] [Google Scholar]
- 35.Ersoy Y, Ozerol E, Baysal O, Temel I, MacWalter RS, Meral U, Altay ZE. Serum nitrate and nitrite levels in patients with rheumatoid arthritis, ankylosing spondylitis, and osteoarthritis. Ann Rheum Dis 2002; 61:76–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cedergren J, Forslund T, Sundqvist T, Skogh T. Inducible nitric oxide synthase is expressed in synovial fluid granulocytes. Clin Exp Immunol 2002; 130:150–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karan A, Karan MA, Vural P, Erten N, Tascioglu C, Aksoy C, Canbaz M, Oncel A. Synovial fluid nitric oxide levels in patients with knee osteoarthritis. Clin Rheumatol 2003; 22:397–9 [DOI] [PubMed] [Google Scholar]
- 38.Rediske JJ, Koehne CF, Zhang B, Lotz M. The inducible production of nitric oxide by articular cell types. Osteoarthritis Cartilage 1994; 2:199–206 [DOI] [PubMed] [Google Scholar]
- 39.Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol 1995; 146:75–85 [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem 2001; 276:29596–602 [DOI] [PubMed] [Google Scholar]
- 41.Okamoto T, Akaike T, Suga M, Tanase S, Horie H, Miyajima S, Ando M, Ichinose Y, Maeda H. Activation of human matrix metalloproteinases by various bacterial proteinases. J Biol Chem 1997; 272:6059–66 [DOI] [PubMed] [Google Scholar]
- 42.Zaragoza C, Balbin M, Lopez-Otin C, Lamas S. Nitric oxide regulates matrix metalloprotease-13 expression and activity in endothelium. Kidney Int 2002; 61:804–8 [DOI] [PubMed] [Google Scholar]
- 43.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (Part II). J Natl Cancer Inst 1998; 90:1609–20 [DOI] [PubMed] [Google Scholar]
- 44.Vuolteenaho K, Moilanen T, Al-Saffar N, Knowles RG, Moilanen E. Regulation of the nitric oxide production resulting from the glucocorticoid-insensitive expression of iNOS in human osteoarthritic cartilage. Osteoarthritis Cartilage 2001; 9:597–605 [DOI] [PubMed] [Google Scholar]
- 45.Akasaki Y, Matsuda S, Iwamoto Y. Progress of research in osteoarthritis. The anti-inflammatory effects of intra-articular injected statin on experimental osteoarthritis. Clin Calcium 2009; 19:1653–62 [PubMed] [Google Scholar]
- 46.Baker JF, Walsh PM, Byrne DP, Mulhall KJ. Pravastatin suppresses matrix metalloproteinase expression and activity in human articular chondrocytes stimulated by interleukin-1beta. J Orthopaed Traumatol 2012; 13:119–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simopoulou T, Malizos KN, Poultsides L, Tsezou A. Protective effect of atorvastatin in cultured osteoarthritic chondrocytes. J Orthop Res 2010; 28:110–5 [DOI] [PubMed] [Google Scholar]
- 48.Kadam UT, Blagojevic M, Belcher J. Statin use and clinical osteoarthritis in the general population: a longitudinal study. J Gen Intern Med 2013; 28:943–9 [DOI] [PMC free article] [PubMed] [Google Scholar]