Abstract
An enriched environment (EE) is an animal housing technique in which animals are given increased amounts of space, physical activity, and social interaction. Currently, researchers studying EE focus mainly on its effects within the context of neurological diseases. However, little is known about how EE affects the intestinal mucosal barrier. This study assessed the effects of EE on the intestinal mucosal barrier in rats with colorectal cancer. A rat model of 1,2-dimethylhydrazine (DMH)-induced colorectal cancer was used. The rats were housed in eight conditions for eight weeks: EE, large cages containing eight rats with stimulating objects; enlarged space and socially enriched conditions (ES), large cages containing eight rats; enlarged space and cognition enriched conditions (EC), large cages containing one rat with stimulating objects; enlarged space enriched conditions (E), large cages containing one rat; cognition and socially enriched conditions (CS), four to five rats housed in standard cages containing stimulating objects; cognition enriched conditions (C), rats housed individually in small-size cages containing stimulating objects; socially enriched conditions (S), standard cages containing four rats; and normal conditions (blank group, B). We determined the weight of each rat, measured the intestinal mucosa and plasma levels of tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), interleukin 10 (IL-10), ghrelin, corticotropin-releasing factor (CRF), occludin, bacterial translocation (BT), and secretory immunoglobulin A (SIgA), and assessed the morphology of the intestinal mucosa. On the whole, the combination of cognitive stimulus and social support was better than the combination of three factors in maintenance of the intestinal mucosal immune barrier and brain–gut peptide. The combination of all three factors and combination of cognitive training and social support were more effective than any single factor. Future studies are needed to study the effects of an EE on body weight, brain–gut peptide, and the intestinal mucosa biological barrier.
Impact statement
An enriched environment (EE) is an animal housing technique where animals are given increased amounts of space, physical activity, and social interaction. Presently, researchers studying EEs focus mainly on their effects within the context of neurological diseases. However, little is known about how EEs affect the intestinal mucosal barrier. This study assessed the effects of an EE on the intestinal mucosal barrier in rats with colorectal cancer.
Keywords: Barrier, colorectal cancer, intestinal mucosa, enriched environment
Introduction
An enriched environment (EE) is an animal housing technique consisting of three aspects: increased space, cognitive training, and social interactions among animals. Using igloos, running wheels, saucer wheels, tube mazes, and other objects, EEs have been shown to increase sensory, cognitive, motor, and social stimulation.1 In an EE, the layout of the objects in the enclosure changes regularly to present a novel environment to which the animals can adapt.2 Recent studies demonstrated that EEs decrease adiposity, increase energy expenditure, limit diet-induced obesity, and cause cancer remission and inhibition of tumor growth.2 For example, one study demonstrated that an EE affected normal mammary gland development and inhibited mammary tumor growth, resulting in a marked decrease in intratumoral cyclooxygenase-2 (COX-2) activity and an increase in the plasma ratio of adiponectin/leptin levels.3 Another study showed that an EE inhibited colon cancer growth post-MC38 implantation and suppressed intestinal tumorigenesis in ApcMin/+ mice.4
The intestinal mucosal barrier plays an important part in the occurrence and development of colorectal cancer. Interestingly, pro-inflammatory cytokines such as interferon gamma (INF-γ), interleukin 4 (IL-4), tumor necrosis factor-alpha (TNF-α), and platelet-activating factor produced by the intestinal mucosa as a result of inflammatory disorders could increase the permeability of intestinal epithelial cells.5. In addition, the intestinal mucosa requires a sufficient supply of nutrients to maintain its integrity, and poor nutrition results in deficiencies of mucosal barrier function.6 The rapid growth of malignant tumors reduces the levels of nutrients available to the mucosal barrier, leading to intestinal mucosal damage and atrophy of villi. However, intestinal mucosal barrier integrity is crucial for the wellbeing of cancer patients. Increased permeability of the intestinal mucosa not only affects nutrient absorption and utilization, but also causes bacterial translocation (BT) and Ent erogenous sepsis, triggering both bacterial systemic inflammatory response syndrome and multiple organ dysfunction syndrome.7 Therefore, intestinal mucosal barrier integrity is a key factor that affects therapeutic effects and prognosis in colorectal cancer.
Brain–gut peptides secreted by the brain–gut axis modulate hormone signaling pathways to modify gastrointestinal function. They also have distinct roles in regulating gastrointestinal motility, intestinal mucosal osmotic pressure, mechanical changes of the intestinal mucosa, intestinal bacterial colonization, visceral sensation, emotional response, and immunity.8The brain–gut axis also plays a crucial part in maintaining the integrity of the intestinal mucosal barrier during periods of stress and disease.9
In the case of experimental rats, an EE consists of a cage in which a certain number of rats (8–12) are raised within an enlarged space with some environmental stimuli. In a study of the effects of an EE on the intestinal mucosal barrier10 and the brain–gut axis,11 it was shown that the exposure of the body to a source of stress affected the intestinal microbial environment, including the population of Lactobacillus. Intestinal microorganisms are important components of the intestinal mucosal biological barrier, which is closely related to its function. A source of stress can also affect the balance of the brain. The main mechanism is the overexpression of corticotropin-releasing factor (CRF), which is involved in the stress response. Stress regulates the integrity, secretion, and sensation of the gastrointestinal tract through the hypothalamic–pituitary–adrenal (HPA) axis, which can lead to increased cortisol levels,11,12 thereby destroying the intestinal immune system and causing intestinal dysfunction.11 Social support and EE are effective ways to alleviate stress.13 In addition, some studies have shown that long-term appropriate exercise could increase the level of the brain–gut peptide13 ghrelin. Short-term exercise could also increase the level of ghrelin under certain conditions. In an EE, the body is able to exercise effectively.14 Therefore, it is conjectured that an EE can improve the intestinal microenvironment by alleviating the irritation from stress sources. It can protect the function of the intestinal mucosal barrier and maintain the balance of the brain and intestine. In addition, studies on the role of EEs in colorectal cancer have mainly focused on their effects on tumor growth inhibition. The results suggest that an EE can mediate intratumoral complex signaling pathways, such as phospho-Akt, phospho-ERK1/ERK2 and phospho-P38a, to reduce tumor weight.4 However, studies have shown that while an EE can relieve symptoms and improve physical condition, it cannot alone reduce tumor weight.15 Therefore, the role of the EE in the occurrence and development of colorectal cancer needs further study. Studies of the intestinal mucosal barrier in EE have shown that the structure of intestinal microflora can be destroyed and levels of beneficial bacteria can be reduced when the body is exposed to harmful social pressure10; however, positive pressure can increase these beneficial bacteria. The improvement of cognitive function is also associated with recovery of intestinal mucosa injury in abdominal disease,16 and exercise can change gene expression in the colon mucosa,17 thus preventing the occurrence of colorectal cancer. However, little is known about the effects of EEs in patients with colorectal cancer.
This study aimed to explore the effects of three aspects of EE conditions on the intestinal mucosal barrier and the brain–gut axis in mice with colorectal cancer. Our primary goal was to assess the role of EE during tumor development and to determine how EE might affect the rehabilitation of colorectal cancer patients.
Materials and methods
Pre-experiment
We obtained 10 four-week-old male Sprague-Dawley rats, weighing approximately 150–160 g each, from the Animal Experimental Center of Fujian Medical University. The rats were housed according to the following criteria. They were housed individually in ventilated cages with soft shavings and air-conditioning (22 ± 1°C, 50–60% humidity, 12-h light/dark cycles), and were fed ad libitum (20–25% protein, 5–10% fat, 3–5% crude fiber). Food was prepared and mixed as per the guidelines set by the Association of Analytical Communities. Prior to experimentation, the rats were fed adaptively by the criteria described above for two weeks. The animals were then monitored for agility and missing teeth. Rats that showed neither occurrence were deemed fit to proceed with experimentation. All procedures were approved by the Laboratory Animal Welfare and Ethics Committee of Fujian Medical University (certificate number 2016–06), and were performed in accordance with the National Institute of Health guidelines for the treatment of animals.
Beginning at six weeks of age, 1,2-dimethylhydrazine (DMH; Sigma Chemical Co) was injected subcutaneously (20 mg/kg) once a week for 21 weeks.
All rats were kept under a constant 12-h light/dark cycle for six weeks. Food and water were available ad libitum. Twenty-one weeks after injection of DMH, the rats were examined by ultrasonography (Esaote, MylabClassC, probe frequency 18–22 MHz, Italy). Tumor formation occurred in nine rats. Ten rats were dissected. The intestinal tissues and masses were taken for pathological biopsy and examined by two pathologists to reach a unified diagnosis. All the tumors in the nine rats were progressive adenocarcinomas. The pathological results for the rat with no tumor mass were normal. In addition, ultrasonography is a non-invasive means of inspection. Therefore, ultrasonography could be used to determine whether or not a tumor was present.
Animals
We obtained 70 four-week-old male Sprague-Dawley rats, weighing approximately 150–160 g each, from the Animal Experimental Center of Fujian Medical University. The rats were treated as described above.
Generation of 1,2-DMH-induced tumors
Beginning at six weeks of age, DMH (Sigma Chemical Co) was injected subcutaneously (20 mg/kg) once a week for 21 weeks.
Animal housing procedures
All rats were kept under a constant 12-h light/dark cycles for six weeks. Food and water were available ad libitum. Twenty-one weeks after injection of DMH, the rats were examined by ultrasonography (Esaote, MylabClassC, probe frequency 18–22 MHz, Italy). Tumor formation occurred in 65 rats; these animals were divided into groups of eight (except for the blank group, which consisted of nine rats) and then assigned to one of the eight housing conditions (Table 1).
Table 1.
Different housing conditions.
| Group | Housing conditions |
|---|---|
| EE | Large cages (109 × 79 × 41 cm) containing eight rats each were used; each cage contained a variety of stimulating objects such as tunnels, running wheels, labyrinths, wooden boxes and balls. |
| S | Standard cages (54.5 × 39.5 × 20 cm) containing four rats each were used, without any stimulating objects. |
| ES | Large cages containing eight rats each were used, without any stimulating objects. |
| EC | Large cages with one rat each were used; each cage contained a variety of stimulating objects. |
| E | Standard cages with one rat each were used, without any stimulating objects. |
| CS | Four to five rats were housed in standard cages containing a variety of stimulating objects. |
| C | Rats were housed individually in small-size (32 × 21 × 16 cm) cages containing various stimulating objects. |
| B | Rats were housed individually in small-size cages without any stimulating objects. |
All stimulating objects and the location of food and water were changed twice per week. After eight weeks of experimentation, two rats from the B group were euthanized owing to excessive tumor burden.
From the literature, it is clear that there is no uniform standard regarding the experimental variables used by researchers. However, we designed our experiments based on the following characteristics of EEs from the literature.1,18–20 ① The number of rats in an EE is typically 6–20 per cage. ② The spatial size of an EE has been in the range 30,000–50,000 cm3per rat. ③ The number of stimulatory objects has not been specified in the reported studies; however, it can be surmised that the number of stimulatory objects was about 1–2 per rat. ④ The types of objects included huts made of wood, walking wheels made of plastic with a diameter of 21 cm, transparent labyrinths tunnels made of acrylic with a diameter of 13 cm and various wooden toys. All of these objects were harmless to rats. The inclusion of these items is mainly based on the rodent’s mode of playing with them, such as burrowing, climbing, and often also biting, to explore their purpose. Therefore, in this study, the conditions adopted for the EE were according to the above practices. Items destroyed by the rats were replaced periodically. In addition, the positions of the stimulants, water, and food in the cages were changed twice weekly to ensure the freshness of the rats’ environment.
Western blot
Prior to obtaining tissue samples, all rats were anesthetized then sacrificed. Colon tissue (approximately 100 mg) was clipped 2 cm before the end of the cecum and washed with saline. The tissues were then placed in an Eppendorf (EP) tube and kept frozen at −80°C. About 100 mg of tissue was treated with 200 µL Protein Seeker Mammalian Cell Lysis Solution (BoKang, Hangzhou, China). The mixture was ground in ice water, shaken at 1200 r/min for 5 s and then centrifuged at 12,000–16,000 × g for 5 min. The supernatant was collected and stored at −80°C before use. The extracted proteins were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene fluoride (Pall) membrane. Western blots were performed using a polyclonal anti-ghrelin primary antibody (1:250; Abcam). The blots were then incubated with horseradish peroxidase-conjugated secondary antibodies (1:8000, ZB-2301, Zhong Shan Company, Beijing) for 1 h. Immunoreactive proteins were detected using the enhanced chemiluminescence (ECL-Plus Western Blotting Detection System, Amersham Life Sciences, Braunschweig, Germany). Image J software was used to quantify the results.
Measurement of TNF-α, IL-6, IL-10, and CRF levels
Rat intestines (approximately 400 mg of tissue per sample) were clipped and washed with saline. The samples were cut into slices, homogenized using a Dounce homogenizer (Wheaton, USA), and centrifuged at 10,000 r/min for 30 min at 4°C. The supernatants from each fraction were collected and stored at −70°C. For brain tissue samples, rat brains were isolated in an ice bath, and the hypothalami were separated and placed in EP tubes. The tissues were then frozen in liquid nitrogen for 5 min and stored at −70°C. For serum samples, blood was drawn from the inner canthus vein, and then rested for 4 h before being centrifuged at 10,000 r/min for 30 min. The serum was isolated from whole blood and stored at −70°C. All samples were later thawed and the levels of TNF-α, IL-6, IL-10, and CRF were determined by enzyme-linked immunosorbent assay (ELISA) as per the manufacturer’s instructions. The ELISA kit by Xin Bo Sheng (Shen Zhen, China) was used for TNF-α, IL-6, IL-10, and a kit from Bio Vendor (Europea) was used for CRF.
Detection of BT
Mesenteric lymph nodes, livers, and spleens were collected post-euthanasia and placed in 1 mL of cold saline. The samples were ground using a mortar and pestle, and 0.5 mL of each sample was incubated with medium containing eosin methylene blue agar at 37°C for 48 h. This medium is a standard non-inhibitory solid medium containing: (a) Broth agar (pH 7.4) (BR grade) 1000 mL, (b) 1% eosin methylene blue aqueous solution (AR grade) 10 mL, (c) lactose (AR grade) 10 g, (d) 1% rosin acid ethanol solution (AR grade) 10 mL per 1000 mL eosin methylene blue agar. Rosmaric acid only inhibited the growth of Gram-positive bacteria, but did not inhibit the growth of Gram-negative bacteria. Lactose-decomposing bacteria formed blue colonies on the medium. Escherichia coli are a Gram-negative bacterium with lactose decomposition. It can form blue colony on the medium, and the size of a single colony is about 2 mm.21 Therefore, in this study, cultured bacteria were used as enterococci of Enterobacteriaceae and more than five blue colonies were recorded as positive for bacterial culture.22 The number of bacterial colonies was counted and the number of colony-forming units per gram of tissue (CFU/g) was calculated.
Intestinal mucosa morphology
Intestinal mucosa villi length, villi width, and muscle layer thickness were measured. Rat intestines were isolated and fixed with 10% formaldehyde, dehydrated, and embedded in paraffin. Five-μm-thick sections were cut, dewaxed with xylene, hydrated with an alcohol gradient, and stained with hematoxylin for 1 min. The samples were washed with phosphate-buffered saline (PBS) and stained with eosin for 15 s, and then quickly dehydrated with an alcohol gradient. Finally, the sections were treated with xylene, mounted in neutral gum, and viewed with a light microscope. Hematoxylin and eosin staining results were evaluated using Image-Pro Plus 6.0 software, and between-group comparisons were conducted.
Immunohistochemical detection of occludin and secretory immunoglobulin A
Rat intestines were fixed in 10% formalin for 24 h and embedded in paraffin. Five-μm-thick sections were cut, mounted on slides, and incubated with anti-occludin (1:120, Thermo, USA) or anti-secretory immunoglobulin A (SIgA) antibodies (1:100, Thermo, American) for 2 h at 37°C. The slides were then washed three times with PBS and incubated with goat anti-rabbit secondary antibodies (Maixin Biological Technology Development Co, Ltd, Fuzhou, China) for 30 min. The slides were washed three times with PBS and developed using diaminobenzidine (DAB) color development solution (Fuzhou Maixin Biotechnology Development Co, Ltd) for 5 min. The slides were then stained with hematoxylin for 1 min, washed with PBS, dehydrated with an alcohol gradient, treated with xylene, mounted with neutral gum, and viewed with a light microscope (NikonSMZ645, Japan). Immunohistochemical staining results were evaluated using Image-Pro Plus 6.0 software, and between-group comparisons were conducted. The reviewer who analyzed all immunohistochemically stained slides was blinded to the group allocation of each sample.
Statistical analyses
BT (CFU/g) measured from various organs is presented as percentages. Protein expression levels are presented as means ± standard deviation. Differences among groups and interactions of various factors were assessed using one-way analysis of variance with a post hoc Bonferroni pairwise comparison. When the data were not normally distributed, a Kruskal–Wallis test with post hoc Mann–Whitney U-test for pairwise comparison was performed. For all statistical analyses, P < 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS 24.0 statistics software (SPSS Inc., Chicago, IL, USA).
Results
Microscopic morphology of intestinal mucosa
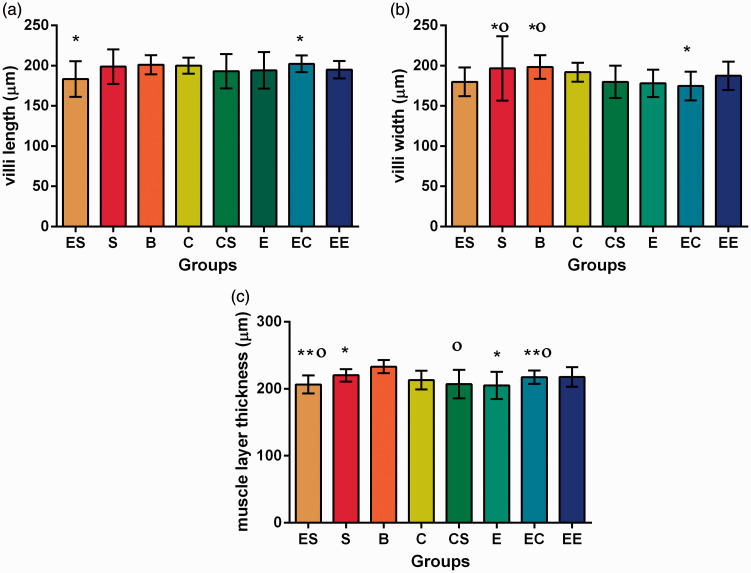
Intestinal villi length
The results showed that there was no significant interaction among the three factors or between the two two-factor combinations in the length of small intestinal villi (P > 0.05) (Table S1).
Further analyses of variance were performed. The results showed that there was a statistically significant difference between the EC and ES groups (P = 0.035) (Table S4, Figure 1).
Figure 1.
Microscopic morphology of intestinal mucosa in eight groups of rats. (a) villi length of intestinal mucosa in eight groups of rats. (b) villi width of intestinal mucosa in eight groups of rats. (c) muscle layer thickness of intestinal mucosa in eight groups of rats.
*P ≤ 0.05 compared between each other with the same symbol; **P ≤ 0.05 compared between each other with the same symbol;
Οp ≥ 0.05 compared between each other with the same symbol. (A color version of this figure is available in the online journal.)
Intestinal villi width
There was no significant interaction among the three factors or between the two two-factor combinations in the width of small intestinal villi (P > 0.05) (Table S2).
Further analysis of variance was performed. The results showed that there were significant differences between group EC and group S, and between group EC and group B. There were no significant differences between the other groups (Table S4, Figure 1).
Muscle layer thickness
The results showed that there was no significant interaction among the three factors in the muscle layer thickness (F = 0.297, P = 0.588). EC was the only two-factor combination that showed interaction (F = 14.312, P = 0.000) (Table S3).
By the simple effect analysis, there were significant differences between the C and B group, S and B group, E and B group, and S and ES group (F = −2.546, P = 0.011; t = 2.600, P = 0.022; t = 3.312, P = 0.006; t = 2.358, P = 0.033). Further analysis of variance was used. The results showed that there were significant differences between the E and S group, CS and B group, EC and B group, and ES and B group (P = 0.046; P = 0.001; P = 0.048; P = 0.001) (Table S4, Figure 1).
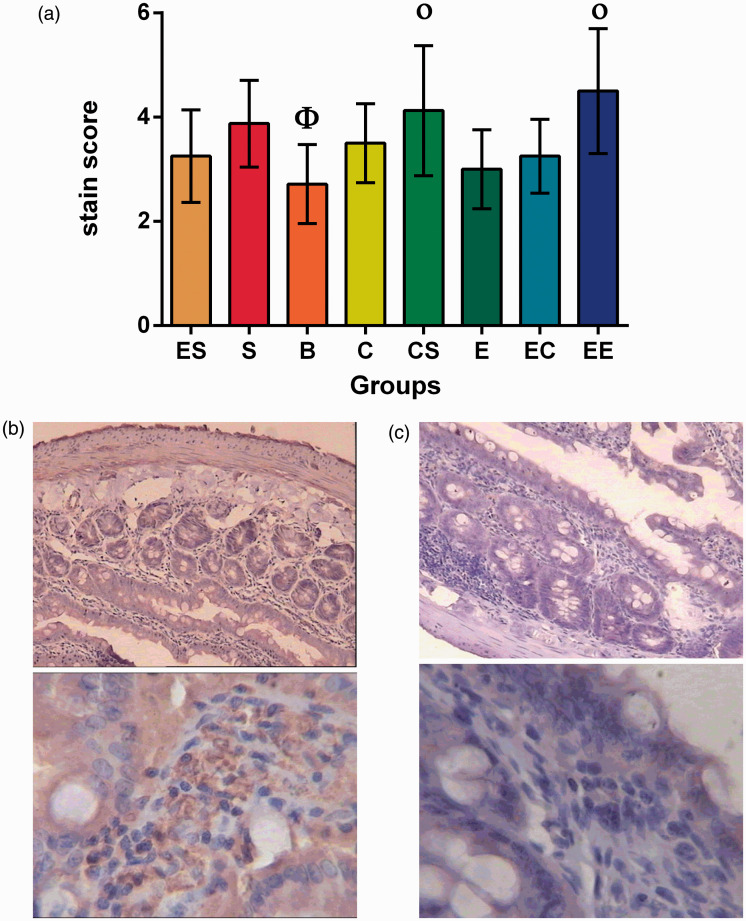
Immunohistochemical detection of occludin
The results showed that there was no interaction among the three factors (F = 2.760, P = 0.102) or between the two two-factor combinations in the occludin, and cognitive stimulation and social support had statistically significant effects on the difference in the level of occludin secretion (Table S5).
The simple effect analysis showed that there were significant differences between the ES and EE group, S and B group, and EC and EE group (F = −1.96, P = 0.05; t = −2.806, P = 0.015; t = −2.546, P = 0.023). The results of variance analysis showed that there were statistically significant differences between the EE and C group, CS and B group, and EE and B group (P = 0.033, P = 0.004, P = 0.000) (Table S6, Figure 2).
Figure 2.
Occludin level in eight groups of colorectal cancer rats. (a) Φ the lowest level group compared with all other groups.
Οp ≥ 0.05 compared between each other with the same symbol.
(b) Intestinal epithelial occludin expression of the EE group. (c) Intestinal epithelial occludin expression of the B group.
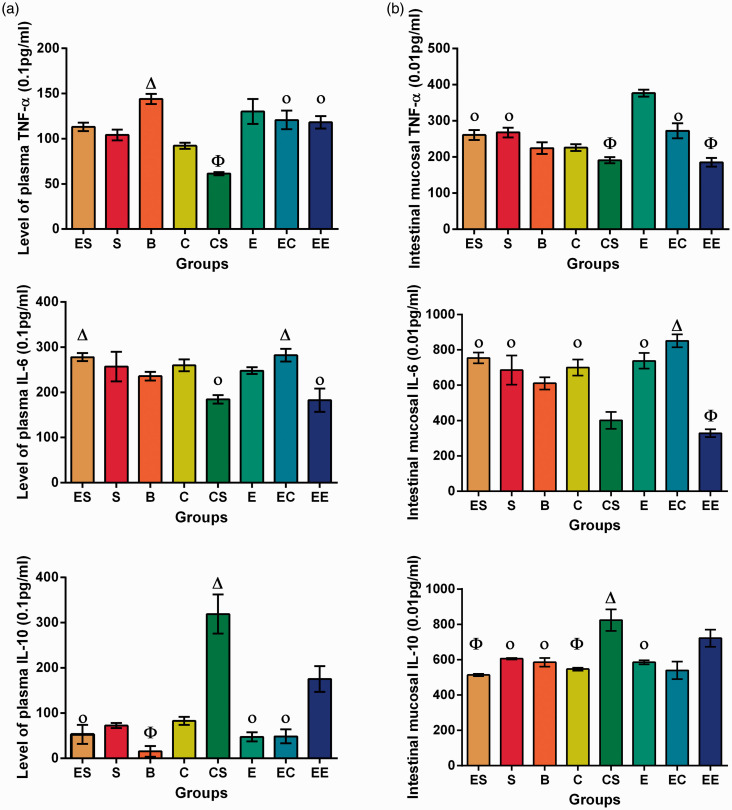
EE modulates plasma TNF-α, IL-6, and IL-10
Plasma IL-10
The results showed that there were interactions among the three factors (F = 6.914, P = 0.011) and between the two two-factor combinations in the plasma IL-10, and the three factors all had statistically significant effects on the difference in the level of plasma IL-10 (Table S7).
The simple effect test showed that the differences between the plasma IL-10 levels in the E and EC group, and the E and ES group, were not statistically significant (P > 0.05). The results of variance analysis showed that there were no statistically significant differences between the ES and EC group, or the C and S group (P > 0.05). The differences between the remaining groups were statistically significant (P < 0.05) (Table S10, Figure 3).
Figure 3.
Plasma and intestinal mucosal TNF-α, IL-6, and IL-10 levels in eight groups of rats with colorectal cancer. (a) Plasma TNF-alpfa, IL-6 and IL-10 levels in eight groups of rats with colorectal cancer. (b) Intestinal mucosal TNF-alpha, IL-6 and Il-10 levels in eight groups of rats with colorectal cancer.
Φ the lowest level group compared with all other groups.
Οp ≥ 0.05 compared between each other with the same symbol.
Δ the highest level group compared with all other groups. (A color version of this figure is available in the online journal.)
Plasma Il-6
The results showed that there was no interaction among the three factors (F = 3.526, P = 0.066). CS was the only two-factor combination that showed an interaction, and the three factors all had statistically significant effects on the difference in the level of plasma IL-6 (Table S8).
The simple effect test showed that the differences between plasma IL-6 levels of the S and EC group, and the CS and EE group, were not statistically significant (P > 0.05). Variance analysis showed that there were no statistically significant differences between the EC and ES group, S and E group, S and C group, E and B group, or E and C group (P > 0.05). The differences among the remaining groups were statistically significant (P < 0.05) (Table S10, Figure 3).
Plasma TNF-α
The results showed that there was no interaction among the three factors (F = 0.561, P = 0.457) with respect to plasma TNF-α. There were interactions between the two two-factor combinations, and the three factors all had statistically significant effects on the difference in levels of plasma TNF-α (Table S9).
The simple effect test showed that the differences between the plasma TNF-α levels in the E and EC group, and the E and ES group, were not statistically significant (P > 0.05). The variance analysis showed that there were no statistically significant differences between the EE and ES group, or the EE and EC group (P > 0.05). The differences between the remaining groups were statistically significant (P < 0.05) (Table S10, Figure 3).
EE modulates intestinal mucosal TNF-α, IL-6, and IL-10
Intestinal mucosal IL-10
The results showed that there was no interaction among the three factors (F = 0.007, P = 0.930). CS and SE were the two-factor combinations that showed interaction, and the three factors all had statistically significant effects on the difference in the level of intestinal mucosal IL-10 (Table S11).
The simple effect test showed that the differences between intestinal mucosal IL-10 levels of the E and EC group, S and B group, E and B group, and C and EC group were not statistically significant (P > 0.05). The results of variance analysis showed that there were no statistically significant differences between the C and ES group, ES and EC (P > 0.05). The differences between the remaining groups were statistically significant (P < 0.05) (Table S14, Figure 3).
Intestinal mucosal IL-6
The results showed that there were interaction among the three factors (F = 12.455, P = 0.001) and between the two two-factor combinations in the plasma IL-6, and the three factors all had statistically significant effects on the difference in the level of plasma IL-6 (Table S12).
The simple effect test showed that the difference between intestinal mucosal IL-10 level of the E and ES group was not statistically significant (P > 0.05). The results of variance analysis showed that there were no statistically significant differences between the C and S group, or the E and C group (P > 0.05). The differences between the remaining groups were statistically significant (P < 0.05) (Table S14, Figure 3).
Intestinal mucosal TNF-α
The results showed that there were interaction among the three factors (F = 61.800, P = 0.000) and between the two two-factor combinations with respect to the plasma TNF-α, and the three factors all had statistically significant effects on the difference in the level of plasma TNF-α (Table S13).
The simple effect test showed that the differences between the intestinal mucosal TNF-α levels of the E and ES group, and the CS and EE group, were not statistically significant (P > 0.05). The results of variance analysis showed that there were no statistically significant differences between the EC and ES group, or the EC and S group (P > 0.05). The differences between the remaining groups were statistically significant (P < 0.05) (Table S14, Figure 3).
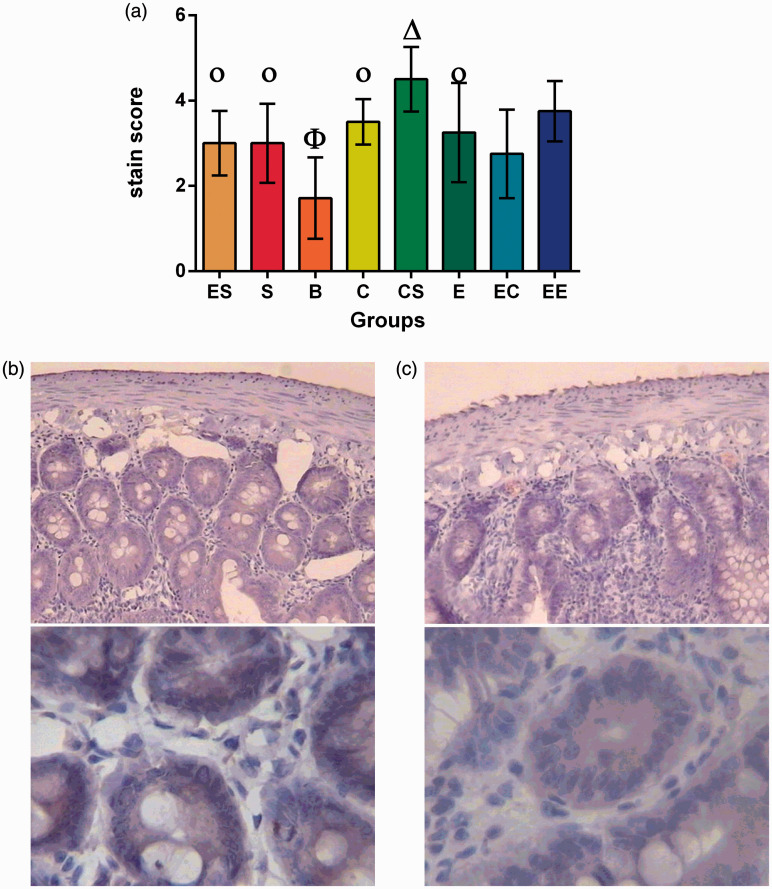
Immunohistochemical detection of SIgA
The results showed that there was no interaction among the three factors (F = 3.039, P = 0.087). EC was the only two-factor combination that showed interaction, and the cognitive stimulation and social support of three factors had statistically significant effects only on the difference in the level of SIgA (Table S15).
The simple effect test showed that there were significant differences between the SIgA level of the C and B group, CS and S group, S and B group, C and CS group, EC and EE group, and E and B group (F = −2.950, P = 0.003; F = −2.762, P = 0.006; F = −2.209, P = 0.027; t = −2.571, P = 0.010; t = −2.256, P = 0.041; F = −2.477, P = 0.013) (P < 0.05). The results of variance analysis showed that there were statistically significant differences between the B group and the other groups (P < 0.05) (Table S16, Figure 4).
Figure 4.
Intestinal epithelial SIgA expression in eight groups of rats with colorectal cancer. (a) Φ the lowest level group compared with all other groups.
Οp ≥ 0.05 compared between each other with the same symbol.
Δ the highest level group compared with all other groups.
(b) Intestinal epithelial SIgA expression in the CS group. (c) Intestinal epithelial SIgA expression in the B group.
BT ratio in eight groups
The results indicated that BT occurred in 8 of 24 tissues in the EE group, 13 of 24 tissues in the EC group, 11 of 24 tissues in the S group, 16 of 24 tissues in the E group, 11 of 24 tissues in the ES group, 8 of 24 tissues in the CS group, 12 of 24 tissues in the C group, and 14 of 24 tissues in the B group. However, the differences in the BT ratios in the eight groups were not statistically significant. None of the two-factor combinations and single-factor analyses showed statistically significant differences.
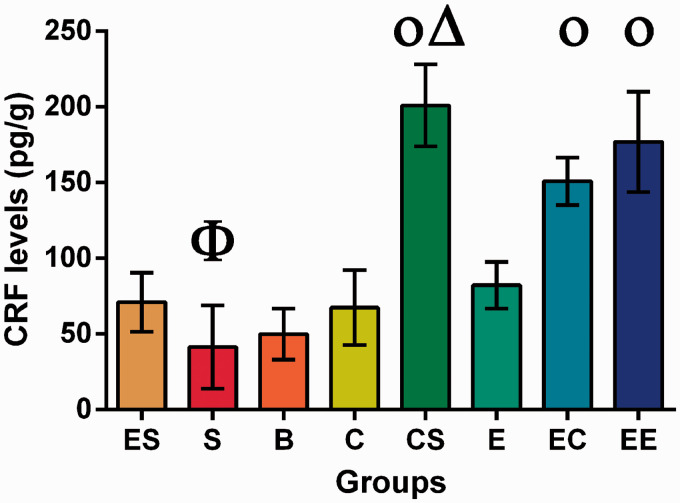
Hypothalamic CRF levels
Our results showed that the CRF levels in the hypothalamus were significantly affected by enlarged space intervention, social support intervention, and cognitive training (F = 19.728, P = 0.000). CS and ES were two-factor combinations that had significant interactions, with statistical values of F = 57.489, P = 0.000 and F = 19.728, P = 0.000, respectively (Table S17).
The simple effect test showed that the differences between the hypothalamic CRF levels of B and S group, B and C group, E and ES group, EC and EE group, and CS and EE group were not statistically significant (P > 0.05). The results of variance analysis showed that there were no statistically significant differences between the C and E group, ES and B group, or ES and C group (P > 0.05). The differences between the remaining groups were statistically significant (P < 0.05) (Table S18, Figure 5).
Figure 5.
CRF levels in the hypothalamus of rats with colorectal cancer.
Φ the lowest level group compared with all other groups.
Οp ≥ 0.05 compared between each other with the same symbol.
Δ the highest level group compared with all other groups. (A color version of this figure is available in the online journal.)
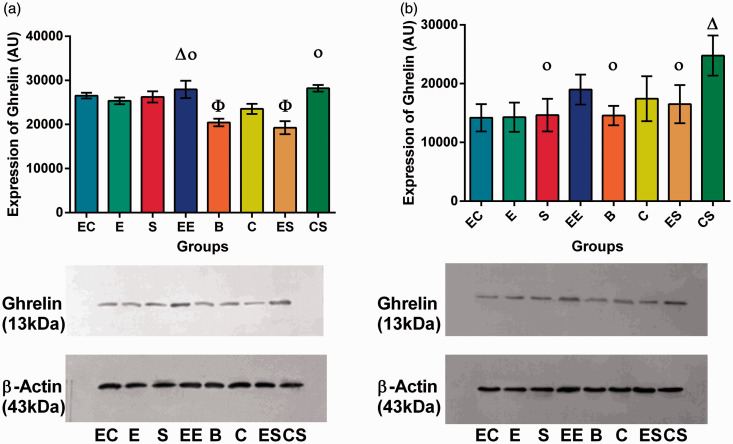
Intestinal mucosal ghrelin
Results for intestinal mucosal ghrelin secretion showed that there was significant interaction among enlarged space intervention, social support intervention, and cognitive training (F = 51.224, P = 0.000). Two-factor combinations also showed interactions (Table S19).
The simple effect test showed that the differences between the intestinal mucosal ghrelin levels of the EC and EE group, and EE and CS group, were not statistically significant (P > 0.05). The results of variance analysis showed that there were no statistically significant differences between the S and E group, EC and S group, or ES and B group (P > 0.05). The differences between the remaining groups were statistically significant (P < 0.05) (Table S21, Figure 6).
Figure 6.
Ghrelin secretion in intestinal mucosa and hypothalamus. (a) Ghrelin secretion in intestinal mucosa. (b) Ghrelin secretion in the hypothalamus. (A color version of this figure is available in the online journal.)
Hypothalamic ghrelin
Hypothalamic ghrelin secretion showed no significant interaction with respect to enlarged space intervention, social support intervention, and cognitive training (F = 2.634, P = 0.110). The two-factor combination ES showed no interaction (F = 0.022, P = 0.884), but CS (F = 11.149, P = 0.001) and EC (F = 13.471, P = 0.001) showed interactions (Table S20).
The simple effect test showed that there were significant differences between the hypothalamic ghrelin levels of the S and CS group, C and CS group, EC and EE group, and CS and EE group(F = −6.516, P = 0.000; F = −4.405, P = 0.001; F = −3.932, P = 0.002; F = 3.839, P = 0.002) (P < 0.05). The results of variance analysis showed that there were statistically significant difference between the CS group and the other groups, and between the EE and S group, EE and E group, EE and B group, C and E group, and C and EC group (P < 0.05) (Table S21, Figure 6).
Weight
There was no significant difference in body weight among the main interventions (F = 0.048, P = 0.828); however, the difference in the main effects of time had statistical significance (F = 56.491, P = 0.000).
Discussion
Previous studies have focused on various medical interventions, such as the use of antibiotics, probiotics, enteral nutrition, and traditional Chinese medicine, to protect the intestinal mucosal barrier of cancer patients and to regulate the brain–gut axis. Currently, there exist no effective nursing interventions to maintain intestinal mucosal barrier function in cancer patients. Interestingly, several rigorous studies have begun to discern the physiological mechanisms by which “soft” science can influence the development and growth of cancer.23 Many studies have begun to examine the effects of different elements of EE on the body. In the present study, we hypothesized that EE could effectively prevent damage to the intestinal mucosal barrier in rats with colorectal cancer.
Intestinal mucosal physical barrier
The basic structure of the intestinal mucosal barrier is composed of connecting intestinal epithelial cells, and the integrity of the barrier is directly related to the maintenance of the tight-junction complex. Occludin, an integral plasma membrane-associated protein, has a pivotal role in the formation and stability of tight junctions.24 Current evidence indicates that the expression levels of occludin in intestinal mucosal epithelial cells reflect the degree of deterioration of the intestinal mucosal barrier when confronted with pathogenic microorganisms and exogenous toxins under conditions of intestinal flora irregularity. Occludin levels also reflect the degree of repair of the intestinal mucosal barrier after stressful or traumatic periods, such as major surgery, severe disease, infection, burn, and ischemia–reperfusion.25,26 Therefore, we used occludin expression levels, the length and width of the small intestinal villi, and muscle layer thickness to indicate the extent of damage to the intestinal mechanical barrier.
In the study of occludin, although the three factors had no combined interaction, the secretion of EE group was beneficial to the release of occludin. In the study of intestinal morphology, there was no synergistic effect of the combination of the three factors or the two two-factor combinations on villus length and width. In the maintenance of the intestinal mucosal mechanical barrier, no single factor had any obvious protective effect on the intestinal mucosal mechanical barrier. However, the combination of social support, cognitive stimulus and enlarged space may have beneficial effects on the intestinal mucosal mechanical barrier to some extent. But this effect is only reflected in the increase of occludin secretion and has no obvious effect on the regulation of intestinal mucosal morphology. Studies have shown that proper exercise can help increase the activity of occludin protein, thereby increasing the tight junction of the intestine.27 Therefore, the exercise induced by an EE can help to strengthen the tight junction of the intestine. However, in the pathological intestinal tract, the main reason that the EE has no obvious effect on the intestinal tract may be that the EE cannot completely resist the destruction and consumption of the tumor to the normal intestinal tissue.
Intestinal mucosal immunological barrier
Cytokines are major regulators of mucosal immunity and have important roles in intestinal immune defense. Throughout the course of various conditions of stress and disease, T cells are activated and release TNF-α, which can induce IL-1 and IL-6 production, leading to the deterioration of the intestinal mucosal barrier and thereby increasing intestinal mucosal permeability to promote BT.28 IL-10 is an anti-inflammatory cytokine that can inhibit the secretion of TNF-α, IL-6, and several chemokines by macrophages.29,30
In the study of plasma TNF-α, IL-6, and IL-10, only the synergistic effects produced by the cognitive stimulus and social support combination had beneficial effects on the organism. In comparison with each factor individually, the combination of enlarged space and cognitive stimulus, the combination of enlarged space and social support had no significant effects on the secretion of TNF-α and IL-10. With regard to IL-6 secretion, only the synergy produced by the combination of cognitive stimulus and social support had beneficial effects on the organism. In the analysis of single factors, all three single factors had a negative effect on TNF-α and a positive effect on IL-10, which represents a beneficial effect on the organism. Therefore, except for IL-6, the effect of each single factor was better than that of the blank group. Cognitive stimulus plays the most significant part in the secretion of TNF-α and IL-10, and has a beneficial effect on the body. However, in the secretion of IL-6, cognitive stimulus has a negative effect on the body. For the secretion of TNF-α and IL-10, addition of enlarged space to cognitive stimulus or social support did not produce statistically significant differences. The results showed that enlarged space had a weakening effect on cognitive stimulus and social support, and the combination of cognitive stimulus and social support played the most important part in regulating serum cytokines. Further analysis revealed a statistically significant difference in low IL-6 and high IL-10 levels in the EE group compared with the other groups. In the CS group, the levels of IL-6 and TNF-α were lower, and the level of IL-10 was higher, and the difference was statistically significant. Therefore, in the regulation of secretion of serum inflammatory factors, the combination of cognitive stimulus and social support is the most important factor, even compared with the EE. Cognitive stimulus was the most significant factor in the single-factor analysis. Cognitive stimulus played the most significant part of any single factor, but had different effects on different cytokines. Therefore, the effects of single factors on cytokines are not yet certain.
In the study of intestinal mucosal TNF-α, IL-6, and IL-10, the results showed that the three factors showed an interaction in the secretion of TNF-α and IL-6 which could reduce the secretion of TNF-α and IL-6, with a beneficial effect on the body. In the analysis of two two-factor combination, only the cognitive stimulus and social support combination could reduce the secretion of TNF-α and IL-6, which have beneficial effects on the body. However, the combination of enlarged space and cognitive stimulus, enlarged space and social support had an effect on the body. In the secretion of IL-10, only the combination of cognitive stimulus and social support, enlarged space and social support was significant. However, only the cognitive stimulus and social support combination increased IL-10 secretion, which has a beneficial effect on the body. The combination of enlarged space and social support also has an effect on the body. In the analysis of individual factors, three single factors had roles in the regulation of IL-10. But through further analysis, compared with the blank group, for the secretion adjustment of IL-10 in any group, except for the enlarged space and social support, and the rest had a significant difference. However, compared with the blank group, only social support and enlarged space had beneficial effects on the body via the regulation of IL-10. Moreover, social support had a greater effect than enlarged space, but these two factors were weaker than the CS group and EE group. The regulation of cytokines by the other single factors also had an effect on the body. Through further two–two comparison analysis, we found that the differences between the EE group, the CS group and other groups in the regulation of three kinds of intestinal mucosal cytokines were statistically significant, which were beneficial to the secretion of IL-10 and inhibited the secretion of TNF-α and IL-6. It can be concluded that the combination of cognitive stimulus and social support, and combination of three factors, can play a part in the regulation of three intestinal mucosal cytokines and have a beneficial effect on the body. Therefore, the combination of cognitive stimulus and social support or combination of three factors has a positive role in regulating the immune regulation of the intestinal mucosal barrier. In the single-factor analysis, only social support and enlarged space regulation of IL-6 had more beneficial effects on the body, and their effects is weaker than those observed in the CS group and EE group. Therefore, the effects of single factors on intestinal mucosal cytokines were not significant.
SIgA is the primary immunoglobulin residing on the surface of the intestinal mucosa, where it helps to resist intestinal lumen hydrolysis without activating inflammation. It is considered as the first line of immunological defense that protects the mucosa and helps resist pathogenic infection. Consistent with this, SIgA has been shown to prevent adherence of bacteria to the surface of epithelial cells.28,31 In the study of SIgA, three factors were found to have no interaction with each other in SIgA secretion. In the two-factor combination analysis, the combination of enlarged space and cognitive stimulus could produce an interaction, but there were no significant differences between them and social support factors. In single-factor analysis, cognitive stimulus and social support were beneficial to the secretion of SIgA. Based on 22 comparative analyses, the combination of enlarged space and cognitive stimulus produces a mutual weakening effect and does not produce a significant influence compared with any single factor. Further comparison showed that the levels on SIgA in the EE and CS groups were significantly higher than in the other groups, while the effect in the EE group was similar to that in the CS group. Therefore, cognitive stimulus, social support, and enlarged space had no synergistic effect, but could increase the secretion of SIgA. Cognitive stimulus and social support had no synergistic effect, but they were beneficial to the secretion of SIgA. These results show that among all the factors, the combination of three factors and cognitive stimulus and social support had the strongest effect. In the single-factor analysis, each single factor was beneficial to the secretion of SIgA, but it is not known which factor has the greatest effect. Therefore, it can be concluded that EE intervention can enhance the secretion of SIgA. For each single factor, further studies are needed to analyze which factors are more conducive to SIgA secretion.
In this study, we also found negative effects on the body in the ES, C and EC groups with respect to the secretion of serum IL-6. There were negative effects on the body in the ES and C groups on the secretion of IL-10 in the intestinal mucosa. An influence on the secretion of IL-6 of the intestinal mucosa was observed in all groups except for the CS and EE groups. The negative effects of group C on the secretion of TNF-α in the intestinal mucosa had a negative effect on the body. The reason for this may be that social support, cognitive stimulus and enlarged space in an EE can produce certain physical movements.
The stimulation produced by cognitive stimuli was stronger than that of social support in terms of persistence, distance, and frequency. Therefore, cognitive stimulus plays a more important part in sports than social support, and is the most important factor in enriching the environment.14
Studies have shown that the inflammatory response caused by intestinal diseases can be changed through the external housing environment.32 Three factors in the EE could interact and have a beneficial effect on the organism.33 The results of this study are consistent with this conclusion. The combined effect of social support, cognitive stimulus, and body movement produced by the enhanced external housing conditions may have beneficial effects on the secretion of cytokines in serum and in the intestinal mucosa to protect the intestinal mucosal immune barrier. However, in the single-factor study, the regulatory role of single factors on intestinal immune barrier was not clear and needs further study.
Intestinal mucosal biological barrier
Generally, BT is used to evaluate intestinal biological barrier function, which refers to the translocation of intestinal bacteria from the intestinal lumen to the mesentery or other organs. Under normal conditions, intestinal BT does not easily occur, owing to tight intestinal mucosal epithelial connections that provide an efficient surface for bacterial clearance. BT increases during bacterial pathogenesis occurring in the intestinal tract or during periods of stress. We therefore used BT to evaluate the permeability of the intestinal mucosal barrier.34 In this study, although we observed no statistically significant differences between any of the experimental groups, BT ratios from the CS and EE groups were, in general, lower than those from the other groups, suggesting a trend towards the combination of cognitive stimulus and social support reducing intestinal barrier permeability during tumorigenesis. The reason there was no significant difference may be related to the intervention time. Therefore, the effect of an EE on translocation rate of bacteria needs further study. Moreover, BT occurred in the intestinal tract of all groups, suggesting that intestinal permeability increased during the development of malignant tumors, which could easily lead to intestinal flora disorder and intestinal biological barrier damage. But how the intestinal flora changes also needs further study.
Brain–gut peptides
CRF is one of the main hormonal regulators of the central nervous system. Under stress, CRF expression becomes elevated to modulate gastrointestinal dynamics, secretion, and sensation through the HPA.12 Our results show that CRF secretion in the CS, EE, and EC groups was the highest compared with all the other groups. There were significant differences in single-factor effects of cognitive stimulus, social support, and enlarged space on CRF secretion. Synergy occurred when cognitive stimulus, social support, and enlarged space were combined. Some studies have shown that enlarged space could increase animal movement by extending the distance between animals, food, and the stimuli. In addition, the amount of exercise was increased by the interaction of cognitive stimuli and communication among rats.35 The results suggest that the movement generated by an enlarged space could promote the secretion of CRF, but only the combination of cognitive stimulus, social support, and enlarged space could produce a more significant impact on the secretion of CRF. The combinations in the CS and EE groups showed a synergistic effect, and the results were better than those for the other groups. As the three single factors could increase the amount of exercise, we speculate based on this study that exercise is essential for CRF secretion, and that it may be an important way for the EE to influence CRF.
Ghrelin, another factor that was modified by EE, is a small endogenous brain–gut peptide.36 Binding of ghrelin to its receptor results in a wide range of biological effects, such as stimulation of growth hormone secretion, regulation of food intake and energy metabolism, and modulation of immunity. Ghrelin can also protect the gastrointestinal mucosa, regulate gastrointestinal motility, promote gastric secretion, control the proliferation of gastrointestinal tumor cells, and improve gastrointestinal dysfunction.37 Furthermore, it is a critical regulator of cognition and emotion within the brain–gut axis.37 This study found that the three factors investigated had an interactive effect on intestinal mucosal ghrelin secretion that was greater than the effect of any of the factors individually. The two two-factor combinations had an effect on intestinal mucosal ghrelin secretion. However, the enlarged space and social support combination had an adverse effect on intestinal mucosal ghrelin secretion. In a single-factor analysis, cognitive stimulus and social support were statistically significant in promoting intestinal mucosal ghrelin secretion. There was no statistically significant difference in ghrelin secretion of enlarged space on intestinal mucosa. The possible reason is that the enlarged space is simpler than that of social support and cognitive stimulus. The single movement mode could not produce a significant effect on secretion of intestinal gut peptide. However, its combination with social support has inhibited the secretion of ghrelin in intestinal mucosa, and its mechanism still needs to be explored. Therefore, the combination of enlarged space and social support was not conducive to the secretion of intestinal ghrelin. Only when a cognitive stimulus was added could a beneficial effect on ghrelin secretion be observed. In the two two-factor combinations, both the EC and CS groups contained the factor of cognitive stimulus. However, when cognitive stimulus was tested as a separate factor, its role was not as important as that of social support. Therefore, the effect of cognitive stimulus on intestinal mucosal ghrelin secretion was more significant only when it was combined with other factors, whereas as a single factor social support was better than cognitive stimulus. Therefore, it appears that social support is especially important in intestinal mucosal ghrelin secretion. The reason may be that social support could generate a positive influence to regulate the hypothalamic sympathetic nerve cell axis, thereby reducing intestinal leptin and increasing ghrelin secretion.21 The combination of social support and cognitive stimulus, or the combination of enlarged space and cognitive stimulus, or the combination of three factors, is more beneficial to the secretion of ghrelin in the intestinal mucosa, and the combined effect is superior to that of any single factor.
In hypothalamic ghrelin secretion, the combination of the three factors may have a significant effect on the secretion of ghrelin in the intestinal mucosa, but have no significant effect on the secretion of ghrelin in the hypothalamus, while the combination of cognitive stimulus and social support can produce synergy. Group CS had the most significant effect on the secretion of ghrelin in the intestinal mucosa and hypothalamus of rats, which increased the secretion of the brain–gut peptide and its positive effects on the maintenance of the gastrointestinal mucosa and gastrointestinal function in colorectal cancer. However, the effect was weaker than that of the three factors combined and the combination of cognitive stimulus and social support, if cognitive stimulus, social support, and enlarged space factors are independent. From the above results, it is conjectured that in the eight-week intervention, the effects of the EE on intestinal ghrelin were greater than those on hypothalamic ghrelin and CRF, and that the combination of cognition and social support was best. Studies have shown that short-term exercise (less than 12 weeks) does not produce obvious hypothalamic ghrelin secretion,38 which is consistent with the findings of this study. Therefore, the effects of various factors of the EE, especially the duration of exercise, on hypothalamic ghrelin secretion have not yet been determined, and the mechanism also remains unknown. Future studies could vary the length of the intervention for further analysis.
Body weight
In the analysis of the changes in body weight of the rats, the effects of the intervention factors that were observed were not statistically significant. Time was the main cause of the difference in body weight between groups. In addition, we did not measure the weight of the tumor in rats. The effect of EE on the body weights of rats needs further study.
In conclusion, we found that EE and the combination of cognitive stimulus and social support increased brain–gut peptide expression (especially ghrelin secretion) and enhanced intestinal mucosal immunologic functions, thus ameliorating intestinal dysfunction and maintaining the integrity of the intestinal mucosal barrier. The effect of combination of three factors, cognitive stimulus and social support was greater than those of any single factor. The combination of cognitive stimulus and social support can produce interaction, which may weaken the reaction of body movement of single factor cognition to the body to a certain extent. The interaction between positive cognitive stimulation and strong social support in nursing may be of great significance in recovery from colorectal cancer, as a benign external environment can influence the internal microenvironment of an organism. Moreover, simply expanding the housing environment without social support and cognitive stimulation has no significant effect on cancer rehabilitation. The reason may be that in an enlarged solitary environment without any stimuli, enlarged space sometimes even increases the body's sense of loneliness, thereby affecting the body through the neuroendocrine system and so on. Based on this understanding, in the future setting of rehabilitation environment for patients, we should not simply consider the increase of environmental areas. The role of humanistic care and cognitive stimulation should be the focus of rehabilitation for patients with colorectal cancer. Nurses should pay attention to and encourage benign social interaction and social activities of colorectal cancer patients during convalescence. However, we should also pay attention to the intensity, frequency, and duration of social activities. For example, too prolonged or too intense exercise may have an adverse effect on the body. Therefore, future research should further investigate the effects of intervention duration and intensity, and combine nursing practice with other methods to study the effects of an EE on rat body weight, hypothalamic ghrelin secretion, and translocation of bacteria, as well as the effects of single factors on the intestinal mucosal barrier. In addition, we should further study the specific mechanisms and the pathways of the combination of various factors of an EE on the intestinal mucosa.
Supplemental Material
Supplemental Material for Enriched environment on the intestinal mucosal barrier and brain–gut axis in rats with colorectal cancer by Dun Liu, Xiao-Ying Jiang and Lan-Shu Zhou in Experimental Biology and Medicine
Acknowledgements
We thank Mr Xiang-xin Wu for technical assistant. We also grateful to prof Cai-hua Huang for her advices on EE housing setting up.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; Jiang Xiao-Ying and Zhou Lan-Shu conducted the experiments, Liu Dun supplied critical reagents and animals, Liu Dun and Jiang Xiao-Ying wrote the manuscript,
Declaration of conflicting interestS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded in full by the Fujian Province Youth Teacher Education Research Project of China (grant no. JA14143) and Fujian Provincial Natural Science Foundation (grant no. 2009J01151). All procedures of this subject are approved by the laboratory animal welfare and ethics committee of Fujian Medical University (certificate number 2016–06).
References
- 1.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 2006; 7:697–709 [DOI] [PubMed] [Google Scholar]
- 2.Slater AM, Cao L. A protocol for housing mice in an enriched environment. J Visual Exp 2015; 100:e52874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nachat-Kappes R, Pinel A, Combe K, Lamas B, Farges MC, Rossary A, Goncalves-Mendes N, Caldefie-Chezet F, Vasson MP, Basu S. Effects of enriched environment on COX-2, leptin and eicosanoids in a mouse model of breast cancer. PLoS One 2012; 7:e51525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, Lin B, During MJ. Environmental and genetic activation of a brain-adipocyte BDNF/Leptin axis causes cancer remission and inhibition. Cell 2010; 142:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Z, Wang X, Deng X, Lasson A, Soltesz V, Borjesson A, Andersson R. Beneficial effects of lexipafant, a PAF antagonist on gut barrier dysfunction caused by intestinal ischemia and reperfusion in rats. Dig Surg 2000; 17:57–65 [DOI] [PubMed] [Google Scholar]
- 6.Wacha H, Hau T, Dittmer R, Ohmann C. Risk factors associated with intraabdominal infections: a prospective multicenter study. Peritonitis Study Group. Langenbecks Arch Surg 1999; 384:24–32 [DOI] [PubMed] [Google Scholar]
- 7.Gill HS, Rutherfurd KJ, Prasad J, Gopal PK. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br J Nutr 2000; 83:167–76 [DOI] [PubMed] [Google Scholar]
- 8.He D, Wang HY, Feng JY, Zhang MM, Zhou Y, Wu XT. Use of pro-/synbiotics as prophylaxis in patients undergoing colorectal resection for cancer: a meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol 2013; 37:406–15 [DOI] [PubMed] [Google Scholar]
- 9.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013; 144:36–49 [DOI] [PubMed] [Google Scholar]
- 10.Galley JD, Nelson MC, Yu Z, owd SE, Walter J, Kumar PS, Lyte M, Bailey MT. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol 2014; 14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pigrau M, Rodino-Janeiro BK, Casado-Bedmar M, Lobo B, Vicario M, Santos J, Alonso-Cotoner C. The joint power of sex and stress to modulate brain–gut–microbiota axis and intestinal barrier homeostasis: implications for irritable bowel syndrome. Neurogastroenterol Motil 2015; 10:1–24 [DOI] [PubMed] [Google Scholar]
- 12.Marcelo F, Lopez A, Laber K. Impact of social isolation and enriched environment during adolescence on voluntary ethanol intake and anxiety in C57BL/6J mice. Physiol Behav 2015; 148:151–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li JB, Asakawa A, Li Y, Cheng K, Inui A. Effects of exercise on the levels of peptide YY and ghrelin. Exp Clin Endocrinol Diab 2011; 119:163–6 [DOI] [PubMed] [Google Scholar]
- 14.Xie H, Wu Y, Jia J, Liu G, Zhang Q, Yu K, Guo Z, Shen L, Hu R. Enrichment-induced exercise to quantify the effect of different housing conditions: a tool to standardize enriched environment protocols. Behav Brain Res 2013; 249:81–9 [DOI] [PubMed] [Google Scholar]
- 15.Jennifer AW, Phillip KD, Michael HK. Environmental enrichment does not impact on tumor growth in mice. F1000Res 2013; 2:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichtwark IT, Newnham ED, Robinson SR, Shepherd SJ, Hosking P, Gibson PR, Yelland GW. Cognitive impairment in coeliac disease improves on a glutenfree diet and correlates with histological and serological indices of disease severity. Aliment Pharmacol Ther 2014; 40:160–70 [DOI] [PubMed] [Google Scholar]
- 17.Buehleyer K, Doering F, Daniel H, Kindermann B, Schulz T, Michna H. Alteration of gene expression in rat colon mucosa after exercise. Ann Anat 2008; 190:71–80 [DOI] [PubMed] [Google Scholar]
- 18.He ZM, Li GP, Zhu DS, Lu SM. Guidelines for the management and use of experimental animals. ( in Chinese: ). Beijing: Science Press, 2016 [Google Scholar]
- 19.Dellen A, Blakemore C, Deacon R, York D, Hannan AJ. Delaying the onset of Huntington’s in mice. Nature 2000; 404:721–2 [DOI] [PubMed] [Google Scholar]
- 20.Spires TL, Grote HE, Varshney NK, Cordery PM, van DA, Blakemore C, Hannan AJ. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci 2004; 24:2270–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang QS, Zhou ZM, Gao Y. Handbook of practical medical medium. Beijing: People's Military Medical Publishing House, 1999 [Google Scholar]
- 22.Chiva M, Soriano G, Rochat I, Peralta C, Rochat F, Llovet T, Mirelis B, Schiffrin EJ, Guarner C, Balanzo J. Effects of Lactobacillus johnsonii La1 and antioxidants in intestinal flora and bacterial translocation in rats with experimental cirrhosis. J Hepatol 2002; 37:456–62 [DOI] [PubMed] [Google Scholar]
- 23.Cao L, During MJ. What is the brain-cancer connection? Annu Rev Neurosci 2012; 35:331–45 [DOI] [PubMed] [Google Scholar]
- 24.Parks RW, Clements WD. Bacterial translocation and gut microflora in obstructive jaundice. J Anat 1996; 189:561–5 [PMC free article] [PubMed] [Google Scholar]
- 25.Yang SC, Chen JY, Shang HF, Cheng TY, Tsou SC, Chen JR. Effect of synbiotics on intestinal microflora and digestive enzyme activities in rats. World J Gastroenterol 2005; 11:7413–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, Klinder A, O'Riordan M, O'Sullivan GC, Pool-Zobel B, Rechkemmer G, Roller M, Rowland I, Salvadori M, Thijs H, Van Loo J, Watzl B, Collins JK. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr 2007; 85:488–96 [DOI] [PubMed] [Google Scholar]
- 27.Zuhl M, Schneider S, Lanphere K, Conn C, Dokladny K, Moseley P. Exercise regulation of intestinal tight junction proteins. Br J Sports Med 2014; 48:980–6 [DOI] [PubMed] [Google Scholar]
- 28.Fujita T, Hara A, Yamazaki Y. The value of acute phase protein measurements after curative gastric cancer surgery. Arch Surg 1999; 134:73–5 [DOI] [PubMed] [Google Scholar]
- 29.Melgar S, Yeung MM, Bas A, Forsberg G, Suhr O, Oberg A, Hammarstrom S, Danielsson A, Hammarstrom ML. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol 2003; 134:127–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao CX. Medical immunology. 6th ed. Beijing: People's Medical Publishing House, 2013 [Google Scholar]
- 31.Hu RH, Lee PH, Yu SC. Secretion of acute phase proteins before and after hepatocellular carcinoma resection. J Formosan Med Assoc 1999; 98:85–91 [PubMed] [Google Scholar]
- 32.Reichmann F, Painsipp E, Holzer P. Environmental enrichment and gut inflammation modify stress-induced c-Fos expression in the mouse corticolimbic system. PLoS One 2013; 8:e54811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anne FG, Giulia P, Daniele Della S, Riccardo P, Marco AEM, Francesca F, Carli G, Joosten EA. Enriched environment and the recovery from inflammatory pain: social versus physical aspects and their interaction. Behav Brain Res 2010; 208:90–5 [DOI] [PubMed] [Google Scholar]
- 34.Lv YH, Shu JC, Song W. Intestinal mucosal barrier and enteral nutrition. Xi'an: The Fourth Military Medical University Publishing House, 2008, pp.242–9 [Google Scholar]
- 35.Caracciolo B, Xu W, Collins S, Fratiglioni L. Cognitive decline, dietary factors and gut–brain interactions. Mech Age Develop 2014; 136–137:59–69 [DOI] [PubMed] [Google Scholar]
- 36.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth- hormone- releasing acylated peptide from stomach. Nature 1999; 402:656–60 [DOI] [PubMed] [Google Scholar]
- 37.Ai LW. From the brain-gut axis way to explore the mechanism of the influence on gastrointestinal motility disorders and paresthesia in rats with functional gastrointestinal disorders and syndrome of liver stagnation and spleen deficiency with herbal-cake-separated moxibustion. Doctoral Thesis (in Chinese), Hunan University of Chinese Medicine, 2013
- 38.Lim CC, Ferguson LR, Tannock GW. Dietary fibres as “prebiotics”: implications for colorectal cancer. Mol Nutr Food Res 2005; 49:609–19 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Enriched environment on the intestinal mucosal barrier and brain–gut axis in rats with colorectal cancer by Dun Liu, Xiao-Ying Jiang and Lan-Shu Zhou in Experimental Biology and Medicine