Short abstract
Objective
This study was performed to investigate the diagnostic accuracy of frozen section (FS) of mucinous borderline ovarian tumors (mBOTs) and the diagnostic value of various risk factors for misdiagnosis.
Methods
Patients with either an FS or permanent pathologic diagnosis of mBOT were included. Optimum cut-off values for serum tumor markers and maximal tumor diameter were determined, and risk factors for underdiagnosis of mucinous malignant ovarian tumors (mMOTs) were evaluated. The sensitivity, specificity, Youden’s index, and diagnostic odds ratio of the risk factors were assessed to determine their diagnostic value for mMOTs.
Results
Of 121 included patients, 97 were diagnosed with mBOTs by FS. Relatively abnormal cancer antigen 125 (CA125), carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA) levels; bilateral tumors; and specific pathological features showed significant associations with underdiagnosis of mMOTs in the univariate analysis. The presence of specific pathological features was the only significant risk factor in the multivariate analysis. The CA125, CA19-9, and CEA levels and specific pathological features demonstrated certain diagnostic value in detecting malignant cases among FS-diagnosed mBOTs.
Conclusions
In patients with FS-diagnosed mBOT, significant predictors of malignancy were relatively higher CA125, CA19-9, and CEA levels; bilateral tumors; and tumors with specific pathological features.
Keywords: Mucinous borderline ovarian tumor, frozen section, permanent pathology, mucinous malignant ovarian tumor, misdiagnosis, risk factor
Introduction
Borderline ovarian tumors (BOTs), a kind of indolent neoplasm characterized by the presence of cellular proliferation and nuclear atypia in the absence of destructive stromal invasion, account for 10% to 20% of all epithelial ovarian tumors.1 Compared with their invasive counterparts, BOTs are characterized by an early stage at diagnosis and an excellent prognosis.2 Moreover, up to 45% of women with BOTs are younger than 40 years.1,3 However, only 29% to 69% of patients with BOTs can be correctly diagnosed preoperatively,4 and the discriminative capacity is limited even for prediction models of BOTs.5–7 Thus, an accurate intraoperative frozen section (FS) diagnosis plays a crucial role in determining the appropriate surgical procedure for BOTs.
During the past two decades, considerable efforts have been made to increase the diagnostic accuracy of FS of BOTs. However, only 62.8% to 87.0% of FS-diagnosed BOTs can be confirmed by the final pathology,8–10 and this rate is even poorer for mucinous BOTs (mBOTs) because of their large diameter and high heterogeneity.11 A misdiagnosis of BOTs may result in unnecessary surgical staging in benign cases and restaging procedures in early-stage malignant tumors; this is particularly important for young women wishing to preserve their childbearing capacity.12 Unfortunately, the diagnostic accuracy of FS of mBOTs has been poorly studied. Most studies that have explored this parameter mainly included mucinous tumors as part of an analysis of the entire group of ovarian masses or BOTs, and any diagnostic disagreement was attributed to the mBOTs.13
Thus, the primary endpoint of our retrospective study was to investigate the diagnostic accuracy of FS of mBOT. The secondary endpoint was to analyze the factors associated with misdiagnosis of FS of mBOT.
Materials and methods
After obtaining Institutional Review Board approval for the present medical record review (approval No. S-K351), patients with either an FS or permanent pathologic diagnosis of mBOT at the Peking Union Medical College Hospital from 2005 to 2015 were identified and included in this retrospective study. Verbal informed consent was obtained from all patients at their follow-up interviews, and the study was conducted in accordance with the Declaration of Helsinki Principles and regulations of our institute. Consultative specimens and patients lacking an FS analysis were excluded. We also excluded patients with recurrent mBOTs or with other coexisting carcinomas.
At the time of the intraoperative consultation, FS was performed on the most suspicious areas on gross examination, and the number of FSs depended on the tumor size and degree of suspicion. All FS specimens were examined by an expert pathologist, and an expert in gynecologic pathology was always available for consultation. With respect to the final pathology, at least one section per centimeter of maximal tumor diameter was sampled, and additional sampling was performed for any suspicious areas. At our hospital, the permanent histology reports were issued by two pathologists (one was the pathologist who performed the FS), including a senior pathologist. Cases of discrepant FS and permanent pathologic diagnoses were revaluated to identify the source of misdiagnosis (errors in gross sampling or interpretation). mBOTs were diagnosed according to Ronnett et al.,14 and specific features such as microinvasion and intraepithelial carcinoma were noted.
The patients’ medical records were comprehensively reviewed, and the following 12 variables were assessed: the patient’s age, menopausal status, fertility status, preoperative serum cancer antigen 125 (CA125) level, carbohydrate antigen 19-9 (CA19-9) level, carcinoembryonic antigen (CEA) level, type of surgery (conservative or radical), presence of bilateral tumors, maximal tumor diameter, presence of ovary endometriosis, number of FSs, and specific pathological features. The FIGO 2013 staging system for epithelial ovarian tumors was used to determine the disease stage based on the operative descriptions and pathology records.15 Conservative surgery was defined as fertility-sparing wherein the uterus and at least part of one ovary were salvaged, whereas radical surgery was defined as bilateral salpingo-oophorectomy with or without hysterectomy.
We did not use the common cut-off values for serum tumor markers to reflect whether the markers were abnormal. Instead, we created new optimum cut-off values for CA125, CA19-9, and CEA by performing receiver operator characteristic (ROC) curves to distinguish relatively abnormal marker levels and relatively normal marker levels and thus classified the tumor markers. This new classification was then used in the univariate analysis and multivariate analysis. We also determined the optimum cut-off value for the quantitative maximal tumor diameter. We classified values as relatively abnormally high if they were above the optimum cut-off values. The optimum cut-off values for CA125, CA19-9, and CEA and the maximal tumor diameter were 65 U/mL, 2000 U/mL, 4.6 U/mL, and 10 cm, respectively.
To identify factors influencing the underdiagnosis of mucinous malignant ovarian tumors (mMOTs), univariate logistic regression analysis of the 12 above-mentioned variables was performed. Multivariate logistic regression analysis was then performed retaining only those variables that were significant at a level of 0.05 in the univariate analysis. For clinical parameters that were found to be significant in the univariate analysis, the sensitivity, specificity, Youden’s index, and diagnostic odds ratio (DOR) were evaluated. The DOR is a special summarizing function of specificity and sensitivity for a given diagnostic test and has been suggested as a measure of diagnostic discriminatory power. Youden’s index serves as a global measure of overall diagnostic accuracy. All data were analyzed with IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA), and p < 0.05 was considered statistically significant.
Results
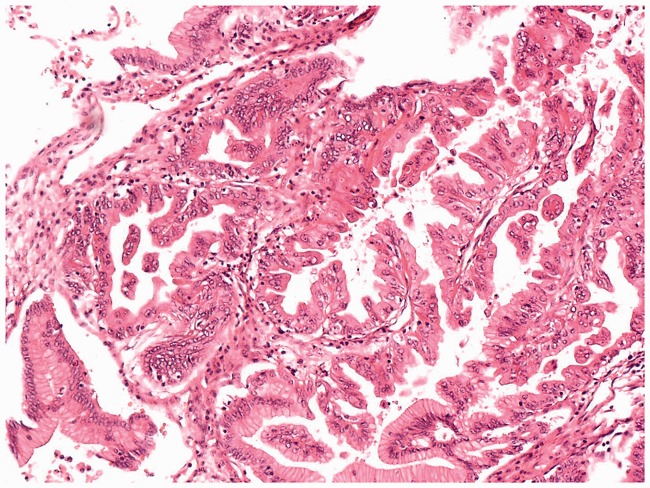
We included 121 patients. Of these patients, 109 were diagnosed with mBOTs by permanent pathology. Of the 109 patients, 86 were diagnosed with BOTs by FS, yielding a concordance rate of 78.9%; however, 1 of the 86 patients was diagnosed with a serous BOT by FS. Additionally, 22 of the mBOTs were diagnosed as benign tumors by FS, with an underdiagnosis rate of 20.2%. Only one mBOT was diagnosed as a malignant tumor by FS, with an overdiagnosis rate of 0.9%. Furthermore, of all 121 patients, 97 were diagnosed with mBOTs by FS. Of these 97 patients, 87 were diagnosed with BOTs by permanent pathology, yielding a concordance rate of 89.7%; however, 2 of the 87 patients were diagnosed with endometrioid BOT and mixed BOT by permanent pathology, respectively. One mBOT was diagnosed as a benign tumor by permanent pathology, with an overdiagnosis rate of 1.0% of FS. Nine mBOTs (see Figure 1) were diagnosed as malignant tumors by permanent pathology, with an underdiagnosis rate of 9.3% (Table 1). Figure 1 shows a photomicrograph of a malignant tumor that was interpreted as borderline by FS. Overall, 88 BOTs were accurately diagnosed as BOTs by FS, yielding a positive predictive value of 89.8% and an overall sensitivity of 79.3%.
Figure 1.
Frozen section photomicrograph of a malignant tumor interpreted as borderline by frozen section.
Table 1.
Frozen section diagnosis compared with permanent pathologic diagnosis.
|
Permanent |
Total | ||||
|---|---|---|---|---|---|
| Frozen | Benign | mBOT | Mixed BOT/endometrioid BOT | Malignant | |
| Benign | – | 22 | – | – | 22 |
| mBOT | 1 | 85 | 2 | 9 | 97 |
| Serous BOT | – | 1 | – | – | 1 |
| Malignant | – | 1 | – | – | 1 |
| Total | 1 | 109 | 2 | 9 | 121 |
BOT, borderline ovarian tumor; mBOT: mucinous borderline ovarian tumor.
Because nine malignant tumors were interpreted as borderline by FS, we analyzed the risk factors for underdiagnosis of mMOTs. The outcomes of the univariate and multivariate analyses are shown in Table 2. In Table 2, we included 96 cases; i.e., 9 underdiagnosed cases and 87 concordant cases (cases diagnosed as BOTs by both FS and permanent pathology). The patient’s age, menopausal status, fertility status, type of surgery (conservative or radical), maximal tumor diameter, presence of ovarian endometriosis, and number of FSs showed no significant associations with underdiagnosis of mMOT. Tumors with CA125, CA19-9, and CEA levels above the cut-off values were more likely to be underdiagnosed by FS than tumors with serum tumor marker levels below the cut-off values (p = 0.006, 0.020, and 0.005, respectively), as were bilateral tumors than unilateral tumor (p = 0.026) and tumors with specific pathological features than those without (p = 0.003) in the univariate analysis. In the multivariate analysis, the presence of specific pathological features was the only significant predictor of underdiagnosis of mMOT by FS (p = 0.043).
Table 2.
Univariate and multivariate analysis results for risk factors of underdiagnosis by frozen section.
| Characteristic | n (%) | Univariate | Multivariate |
|---|---|---|---|
| Age | 1.000 | ||
| ≤40 years | 5 (53.1) | ||
| >40 years | 45 (46.9) | ||
| Menopausal status | 1.000 | ||
| Premenopausal | 65 (67.7) | ||
| Menopausal | 31 (32.3) | ||
| Nulliparous | 1.000 | ||
| Yes | 45 (46.9) | ||
| No | 49 (51.0) | ||
| NA | 2 (2.1) | ||
| CA125 | 0.006 | 0.085 | |
| ≤65.0 U/mL | 67 (69.8) | ||
| >65.0 U/mL | 20 (20.8) | ||
| NA | 9 (9.4) | ||
| CA19-9 | 0.020 | 0.192 | |
| ≤2000.0 U/mL | 63 (65.6) | ||
| >2000.0 U/mL | 3 (3.1) | ||
| NA | 30 (31.3) | ||
| CEA | 0.005 | 0.300 | |
| ≤4.6 U/mL | 41 (42.7) | ||
| >4.6 U/mL | 4 (4.2) | ||
| NA | 51 (53.1) | ||
| Type of surgery | 0.490 | ||
| Conservative | 55 (57.3) | ||
| Radical | 41 (42.7) | ||
| Bilateral tumors | 0.026 | 0.647 | |
| Present | 8 (8.3) | ||
| Absent | 88 (91.7) | ||
| Maximal tumor diameter | 0.269 | ||
| ≤10 cm | 28 (29.2) | ||
| >10 cm | 65 (67.7) | ||
| NA | 3 (3.1) | ||
| Ovarian endometriosis | – | ||
| Absent | 90 (93.8) | ||
| Present | 6 (6.3) | ||
| Number of frozen sections | 0.486 | ||
| ≤2 | 61 (63.5) | ||
| >2 | 33 (34.4) | ||
| NA | 2 (2.1) | ||
| Specific pathological features | 0.003 | 0.043 | |
| Absent | 82 (85.4) | ||
| Present | 14 (14.6) | ||
| NA | 0 (53.1) |
CA125, cancer antigen 125; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; NA, not available.
The sensitivity, specificity, Youden’s index, and DOR for each parameter with significant differences in the univariate analysis are shown in Table 3. We also added the results of tumor markers with commonly used cut-off values (35.0 U/mL for CA125, 37.0 U/mL for CA19-9, and 5.0 U/mL for CEA). All Youden’s indices and DORs of tumor markers with commonly used cut-off values were lower than those with the optimum cut-off values. Youden’s index of CA125 (cut-off = 65.0 U/mL) was the highest, followed by CEA (cut-off = 4.6 U/mL) and specific pathological features. The DOR of CEA (cut-off = 4.6 U/mL) was highest, followed by CA19-9 (cut-off = 2000.0 U/mL).
Table 3.
Diagnostic values of parameters to increase the accuracy of frozen section of mucinous malignant ovarian tumors.
| Parameter | Sensitivity | Specificity | Youden’s index | Diagnostic odds ratio |
|---|---|---|---|---|
| CA125 (cut-off = 65.0 U/mL) | 71.4% | 81.2% | 52.6% | 10.78 |
| (cut-off = 35.0 U/mL) | 71.4% | 65.0% | 36.4% | 4.64 |
| CA19-9 (cut-off = 2000.0 U/mL) | 33.3% | 98.3% | 31.6% | 28.87 |
| (cut-off = 37.0 U/mL) | 33.3% | 61.7% | −5.0% | 0.80 |
| CEA (cut-off = 4.6 U/mL) | 50.0% | 97.4% | 47.4% | 37.46 |
| (cut-off = 5.0 U/mL) | 33.3% | 97.4% | 30.7% | 18.70 |
| Presence of bilateral tumors | 33.3% | 94.3% | 27.6% | 8.26 |
| Specific pathological features | 55.6% | 89.7% | 45.3% | 10.91 |
CA125, cancer antigen 125; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen.
Discussion
It is important to identify the accuracy of FS in diagnosing borderline tumors. Ureyen et al.16 reported that among patients with final diagnoses of BOTs, the concordance rates of FS for BOTs, serous BOTs, and mucinous BOTs were 79%, 92%, and 62%, respectively. Our study showed a concordance rate of 78.9% of FS for mBOTs, which is slightly higher than that in the above-mentioned study. One potential concern of using FS diagnosis to guide surgical decisions is the risk of inadequate staging surgery for what is believed to be a borderline lesion but which is diagnosed as invasive cancer by permanent pathology.
We identified the risk factors associated with the underdiagnosis of mMOTs by FS. The CA125, CA19-9, and CEA levels; laterality of the tumor; and specific pathological features were found to be associated with underdiagnosis of malignant tumors, especially the specific pathological features that showed significant differences in both the univariate and multivariate analyses. CA125 is especially useful to detect serous malignant tumors; however, it may also increase preoperatively in mucinous invasive carcinomas.17–20 Seckin et al.21 evaluated patients with intraoperative FS diagnoses of mBOTs and found that CA19-9 and CA125 were significant predictors of malignancy. These findings are consistent with those of our study, in which mBOTs diagnosed by FS in patients with relatively abnormal CA125 and CA19-9 levels were more likely to be malignant by permanent pathology. CA19-9 is commonly expressed in mucinous tumors and may be a useful diagnostic marker in patients with ovarian mucinous tumors.22 Our study showed that mBOTs diagnosed by FS with an extremely high CA19-9 level were more likely to prove to be malignant by permanent pathology. Studies by Seckin et al.21 and Poncelet et al.23 also support our findings that the CA19-9 level significantly increased especially in mMOTs. Our univariate analysis showed that mBOT diagnosed by FS in patients with a CEA level above the cut-off value may be associated with an increased risk of underdiagnosis. However, the study by Seckin et al.21 showed no significant association between abnormal preoperative CEA values and underdiagnosis of mMOT by FS. Our study demonstrated that the presence of bilateral mBOTs diagnosed by FS had a high probability of being malignant by permanent pathology. This finding is supported by another similar study by Basaran et al.,24 who evaluated patients with a diagnosis of BOT either by FS or permanent pathology and reported that the presence of bilateral tumors was associated with underdiagnosis. Notably, the patients included in the latter study were different from those in our study. Additionally, very few studies have focused on mBOTs; thus, further studies are needed to prove this phenomenon. The presence of specific pathological features (i.e., microinvasion or intraepithelial carcinoma) was the only factor found to be significantly associated with underdiagnosis in both the univariate and multivariate analyses. No similar studies with which to prove or oppose our findings have been performed. However, Khunamornpong et al.25 conducted a study of 171 mBOTs and found that microinvasion and intraepithelial carcinomas were risk factors for relapse. This probably reflects the utility of special pathological features for predicting the underdiagnosis of mMOTs by FS or relapse of mBOTs. As reported in one study, adequate sampling of mucinous tumors for FS cannot always be performed because of the relatively larger dimensions of such tumors, and malignant areas can be overlooked.11 However, we did not find an increased risk of underdiagnosis of mMOTs with diameters of >10 cm; this finding is probably correlated with the skill of the pathologists. Additionally, we found no significant association between the number of FSs and underdiagnosis.
Our findings indicate that in cases diagnosed as mBOTs by FS, the CA125, CA19-9, and CEA levels and the presence of specific pathological features may be useful parameters with certain diagnostic value for the diagnosis of mMOTs. However, the presence of bilateral tumors was not a suitable parameter in patients with a low Youden’s index and DOR. Thus, these characteristics should be considered during intraoperative assessment to avoid underdiagnosis of mMOTs. In this context, sufficient doctor–patient communication is necessary to inform the patient’s relatives of the possible poor results of the final pathology. Moreover, communication between the doctor and the pathologist is also important, and more FSs are needed to improve the accuracy of FS-based diagnosis.
The strengths of our study are as follows. First, our study targeted mBOTs, which have been seldom evaluated. Additionally, we analyzed the risk factors influencing the underdiagnosis of mMOTs, which have also rarely been studied, and we provided abundant information about the concordance rate, underdiagnosis rate, and overdiagnosis rate of FS in patients with an FS-based diagnosis of mBOTs and patients with a permanent pathologic diagnosis of mBOTs. Second, we selected the optimum cut-off points as the thresholds by which to distinguish relatively abnormal and relatively normal levels of the variables by performing ROC curves for CA125, CA19-9, CEA, and the maximal tumor diameter with the aim of improving the diagnostic values of these parameters. We established new optimum cut-off values for the above-mentioned serum tumor markers and proved the diagnostic values of these cut-off values; this is never been performed in previous studies. Third, we analyzed 12 possible factors and finally identified 5 risk factors for underdiagnosis of mMOTs. Two of these risk factors (i.e., laterality of the tumor and specific pathological features) have never been investigated in mBOTs. Fourth, we evaluated the diagnostic value of the above five risk factors using multiple indicators (sensitivity, specificity, Youden’s index, and DOR) and found four valuable parameters than can contribute to the detection of mMOTs. However, the present study also has several limitations. First, the sample size of our study was small, which may have caused false-negative results. Second, values were missing for several clinical parameters, such as the CA19-9 and CEA levels, which probably influenced the actual associations between the serum tumor marker levels and underdiagnosis of mMOTs. Third, the presence of specific pathological features was the only parameter showing a significant association with underdiagnosis in both the univariate analysis and multivariate analysis. The actual associations between the other parameters and underdiagnosis still need to be proven through future studies.
We look forward to future studies with larger sample sizes in which more additional clinical parameters with higher diagnostic values can be discovered and integrated into clinical practice. At this time, we can conclude that CA125, CA19-9, and CEA levels above the optimal cut-off values, the presence of bilateral tumors, and tumors with specific pathological features are probably risk factors for underdiagnosis of mMOTs. In particular, the presence of specific pathological features was significantly associated with underdiagnosis of mMOTs in both the univariate and multivariate analyses. Given the diagnostic values of these parameters, the CA125, CA19-9, and CEA levels and the presence of specific pathological features should be used to guide intraoperative treatment planning and communication between doctors and patients/pathologists.
Acknowledgment
We thank all of the patients for participating in our study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National Key R&D Program of China (2017YFC1001200).
References
- 1.Cadron I, Leunen K, Van Gorp T, et al. Management of borderline ovarian neoplasms. J Clin Oncol 2007; 25: 2928–2937. [DOI] [PubMed] [Google Scholar]
- 2.Guvenal T, Dursun P, Hasdemir PS, et al. Effect of surgical staging on 539 patients with borderline ovarian tumors: a Turkish Gynecologic Oncology Group study. Gynecol Oncol 2013; 131: 546–550. [DOI] [PubMed] [Google Scholar]
- 3.Skirnisdottir I, Garmo H, Wilander E, et al. Borderline ovarian tumors in Sweden 1960–2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int J Cancer 2008; 123: 1897–1901. [DOI] [PubMed] [Google Scholar]
- 4.Fischerova D, Zikan M, Dundr P, et al. Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist 2012; 17: 1515–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Calster B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ 2014; 349: g5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaijser J, Sayasneh A, Van Hoorde K, et al. Presurgical diagnosis of adnexal tumours using mathematical models and scoring systems: a systematic review and meta-analysis. Hum Reprod Update 2014; 20: 449–462. [DOI] [PubMed] [Google Scholar]
- 7.Sayasneh A, Ferrara L, De Cock B, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model: a multicentre external validation study. Br J Cancer 2016; 115: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tempfer CB, Polterauer S, Bentz EK, et al. Accuracy of intraoperative frozen section analysis in borderline tumors of the ovary: a retrospective analysis of 96 cases and review of the literature. Gynecol Oncol 2007; 107: 248–252. [DOI] [PubMed] [Google Scholar]
- 9.Song T, Choi CH, Kim HJ, et al. Accuracy of frozen section diagnosis of borderline ovarian tumors. Gynecol Oncol 2011; 122: 127–131. [DOI] [PubMed] [Google Scholar]
- 10.Shih KK, Garg K, Soslow RA, et al. Accuracy of frozen section diagnosis of ovarian borderline tumor. Gynecol Oncol 2011; 123: 517–521. [DOI] [PubMed] [Google Scholar]
- 11.Gol M, Baloglu A, Yigit S, et al. Accuracy of frozen section diagnosis in ovarian tumors: is there a change in the course of time? Int J Gynecol Cancer 2003; 13: 593–597. [DOI] [PubMed] [Google Scholar]
- 12.Ganesan R, Brown LJ, Kehoe S, et al. The role of frozen sections in gynaecological oncology: survey of practice in the United Kingdom. Eur J Obstet Gynecol Reprod Biol 2013; 166: 204–208. [DOI] [PubMed] [Google Scholar]
- 13.Pongsuvareeyakul T, Khunamornpong S, Settakorn J, et al. Accuracy of frozen-section diagnosis of ovarian mucinous tumors. Int J Gynecol Cancer 2012; 22: 400–406. [DOI] [PubMed] [Google Scholar]
- 14.Ronnett BM, Kajdacsy-Balla A, Gilks CB, et al. Mucinous borderline ovarian tumors: points of general agreement and persistent controversies regarding nomenclature, diagnostic criteria, and behavior. Hum Pathol 2004; 35: 949–960. [DOI] [PubMed] [Google Scholar]
- 15.Pereira A, Perez-Medina T, Magrina JF, et al. International Federation of gynecology and obstetrics staging classification for cancer of the ovary, fallopian tube, and peritoneum: estimation of survival in patients with node-positive epithelial ovarian cancer. Int J Gynecol Cancer 2015; 25: 49–54. [DOI] [PubMed] [Google Scholar]
- 16.Ureyen I, Turan T, Cirik DA, et al. Frozen section in borderline ovarian tumors: is it reliable? Eur J Obstet Gynecol Reprod Biol 2014; 181: 115–118. [DOI] [PubMed] [Google Scholar]
- 17.Tamakoshi K, Kikkawa F, Shibata K, et al. Clinical value of CA125, CA19-9, CEA, CA72-4, and TPA in borderline ovarian tumor. Gynecol Oncol 1996; 62: 67–72. [DOI] [PubMed] [Google Scholar]
- 18.Gotlieb WH, Soriano D, Achiron R, et al. CA 125 measurement and ultrasonography in borderline tumors of the ovary. Am J Obstet Gynecol 2000; 183: 541–546. [DOI] [PubMed] [Google Scholar]
- 19.Zanetta G, Rota S, Lissoni A, et al. Ultrasound, physical examination, and CA 125 measurement for the detection of recurrence after conservative surgery for early borderline ovarian tumors. Gynecol Oncol 2001; 81: 63–66. [DOI] [PubMed] [Google Scholar]
- 20.Bozkurt M, Yumru AE, Aral I. Evaluation of the importance of the serum levels of CA-125, CA15-3, CA-19-9, carcinoembryonic antigen and alpha fetoprotein for distinguishing benign and malignant adnexal masses and contribution of different test combinations to diagnostic accuracy. Eur J Gynaecol Oncol 2013; 34: 540–544. [PubMed] [Google Scholar]
- 21.Seckin KD, Karsli MF, Yucel B, et al. The utility of tumor markers and neutrophil lymphocyte ratio in patients with an intraoperative diagnosis of mucinous borderline ovarian tumor. Eur J Obstet Gynecol Reprod Biol 2016; 196: 60–63. [DOI] [PubMed] [Google Scholar]
- 22.Kelly PJ, Archbold P, Price JH, et al. Serum CA19.9 levels are commonly elevated in primary ovarian mucinous tumours but cannot be used to predict the histological subtype. J Clin Pathol 2010; 63: 169–173. [DOI] [PubMed] [Google Scholar]
- 23.Poncelet C, Fauvet R, Yazbeck C, et al. Impact of serum tumor marker determination on the management of women with borderline ovarian tumors: multivariate analysis of a French multicentre study. Eur J Surg Oncol 2010; 36: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 24.Basaran D, Salman MC, Calis P, et al. Diagnostic accuracy of intraoperative consultation (frozen section) in borderline ovarian tumours and factors associated with misdiagnosis. J Obstet Gynaecol 2014; 34: 429–434. [DOI] [PubMed] [Google Scholar]
- 25.Khunamornpong S, Settakorn J, Sukpan K, et al. Mucinous tumor of low malignant potential (“borderline” or “atypical proliferative” tumor) of the ovary: a study of 171 cases with the assessment of intraepithelial carcinoma and microinvasion. Int J Gynecol Pathol 2011; 30: 218–230. [DOI] [PubMed] [Google Scholar]