Short abstract
Objective
This study was performed to compare the predictive performance of serum procalcitonin (PCT), N-terminal brain natriuretic propeptide (NT-proBNP), interleukin-6 (IL-6), prothrombin time (PT), thrombin time (TT), and Sequential Organ Failure Assessment (SOFA) score in the intensive care unit (ICU).
Methods
This retrospective cohort study enrolled 150 patients with sepsis and septic shock and 30 control patients without sepsis. Each patient was followed until death or 28 days. Correlations between variables were assessed with Spearman’s rho test. The Kruskal–Wallis and Mann–Whitney U tests were used for between-group comparisons.
Results
Receiver operating characteristic curve analysis of the SOFA score, PCT, NT-proBNP, IL-6, PT, and TT showed an area under the curve of 0.872, 0.732, 0.711, 0.706, 0.806, and 0.691, respectively, for diagnosing sepsis. Binary logistic regression demonstrated that the SOFA score was an independent predictor of 28-day mortality and septic shock. The correlation coefficient (r) between SOFA and PCT, NT-proBNP and SOFA, IL-6 and SOFA, PT and SOFA, and TT and SOFA was 0.79, 0.52, 0.57, 0.56, and 0.58, respectively.
Conclusion
While the SOFA score is the gold standard, analysis of multiple biomarkers could increase the performance capacity for diagnosis and prognosis in patients with sepsis in the ICU.
Keywords: Interleukin-6, N-terminal brain natriuretic propeptide, prothrombin time, Sequential Organ Failure Assessment score, thrombin time, sepsis, septic shock
Introduction
Sepsis is a major cause of morbidity and mortality in intensive care units (ICUs), accounting for one-quarter of all ICU deaths.1,2 The incidence of sepsis has been increasing annually by 8% to 13% during the past decade, primarily because of the increase in invasive procedures, immunosuppressive drugs, chemotherapy, and transplantations, and partly because of the lack of early identification of and prompt intervention for sepsis in the emergency department (ED).3 The social and economic impacts of sepsis are severely underestimated, and this condition consumes a significant proportion of health care resources.3,4
Several potential biomarkers and scores for timely diagnosis, risk stratification, and evaluation of the prognosis of sepsis in the ED have come into focus in the last decade.5–10 Organ failure worsens the outcome of sepsis, and the mortality rate increases with increasing severity of organ dysfunction and failure. The mortality rate in patients without organ failure is approximately 15%, but it is 70% for those with three or more dysfunctional organs. The number of organs that fail within the first 48 hours of ICU admission is an accurate predictor of mortality.8,11–13 A recent systematic review identified 178 different biomarkers of sepsis; since then, many new biomarkers have been proposed.5,14–16 However, the quality of these studies is limited, and the use of such biomarkers remains to be validated. Some of these studies were biased because of small sample sizes and inappropriate statistical tests. Another reason for the compromised quality is the inclusion of patients with systemic inflammatory response syndrome, making the study population extremely heterogeneous. The appropriate use of biomarkers allows patients with sepsis to be identified among the heterogeneous patients admitted to the ED, to identify nosocomial infection among groups of homogenous patients (e.g., patients undergoing transplantation and cardiopulmonary bypass surgery), and to determine the occurrence of sepsis among patients with systemic inflammatory response syndrome who have different types of disorders in the ICU. Moreover, age, sex, medication, and the presence of various underlying diseases can influence the diagnostic or predictive accuracy of these biomarkers. Consequently, the primary goal of sepsis treatment is to prevent new or worsening organ dysfunction.17,18 Various clinical scoring models have been developed to estimate the level of organ dysfunction in patients with sepsis. The Sequential Organ Failure Assessment (SOFA) score was designed to describe the sequential complications that occur during treatment in the ICU, but not to predict outcomes. Outcome prediction is important for the management of critically ill patients. Several biomarkers, such as procalcitonin (PCT), N-terminal brain natriuretic propeptide (NT-proBNP), interleukin-6 (IL-6), prothrombin time (PT), and thrombin time (TT), have been independently assessed for their association with the diagnosis and prognosis of sepsis. However, the diagnostic and prognostic value of these markers has not been systematically assessed in the same cohort. The goal of the present study was to determine the relationship between the inflammatory markers PCT, NT-proBNP, and IL-6; the coagulation markers PT and TT; and the SOFA score in predicting the diagnosis and prognosis of sepsis using a retrospective cohort of patients with sepsis. We hypothesized that PCT, NT-proBNP, IL-6, PT, and TT are correlated with the SOFA score in patients with sepsis.
Material and methods
Study population
A retrospective cohort study of critically ill patients admitted to the ICU of the First Affiliated Hospital of Dalian Medical University was performed. From 1 January 2014 to 30 April 2016, all admissions that fulfilled the Sepsis-3 criteria established by the Society of Critical Care Medicine/European Society of Intensive Care Medicine (SCCM/ESICM) task force were identified in the ICU database.14 Patients diagnosed with sepsis or septic shock grouped according to the SCCM/ESICM criteria14 were enrolled in this study. Age-matched non-critically ill patients hospitalized in general wards (non-acute care setting) for <3 days for observation and treatment were enrolled as the non-septic (control) group.
The patients were longitudinally monitored. The following exclusion criteria were applied: age of <18 years, traumatic brain injury, pre-existing impaired left ventricular function (left ventricular ejection fraction of ≤50%), terminal stage of disease (end-stage liver disease, malignant cancer of any type, chronic renal failure, acquired immunodeficiency syndrome), dilated cardiomyopathy, acute or chronic cor pulmonale, valve disease, and death within 8 days of ICU admission. Non-survivors were defined during a follow-up period of 28 days. This study was approved by the Dalian Hospital Ethics Committee (YJ-KY-FB-2017-17). All patients provided written informed consent.
Data collection
The following data were collected from the database: patient name, age, sex, medical history, and vital signs. Within 24 hours of ICU admission, venous blood samples were collected in tubes containing heparin or ethylenediaminetetraacetate; the samples were then stored at –80°C and analyzed within 24 hours. The whole blood leukocyte count, blood gas analysis results, blood biochemistry parameters, and radiographic findings were recorded. SOFA scores were calculated based on six variables (respiratory, hepatic, cardiovascular, coagulation, renal, and neurological) on ICU admission and every 48 hours until discharge. The SOFA score was not intended to be used as a tool for patient management but as a measure of organ dysfunction in patients with sepsis. For each parameter, the worst values within the first 24 hours of ICU admission were used. Where values were missing, the values obtained immediately preceding the missing value were used.19 The SOFA score at 48 hours was recorded. The Glasgow coma scale score was used to evaluate the neurological status of unconscious patients. The Quick SOFA (qSOFA) score (1 point each for a respiratory rate of >22 breaths/minute, altered state of consciousness, and systolic blood pressure of <110 mmHg) was also calculated to identify patients at high risk of a poor outcome.
Patients with sepsis were classified into the survivor and non-survivor groups according to their 28-day mortality outcome. Within the 28-day follow-up period, patients who died from all causes were considered non-survivors.
Statistical analysis
Statistical analysis was performed using SPSS Statistics for Windows, Version 17.0 (SPSS Inc., Chicago, IL, USA). The distributions of the serum PCT, NT-proBNP, IL-6, PT, TT, and SOFA score were skewed and are thus expressed as median (25th–75th percentile). Kruskal–Wallis one-way analysis of variance was applied for multi-group comparisons, and the Mann–Whitney U test was used for two-group comparisons.20 Receiver operating characteristic (ROC) curves were constructed and the areas under the ROC curves (AUCs) were calculated to compare the predictive values of PCT, NT-proBNP, IL-6, PT, TT, and SOFA score for sepsis, septic shock, and 28-day mortality. The response variable was 28-day mortality. Logistic regression analysis was employed to determine independent predictors of sepsis, septic shock, and 28-day mortality.21 All statistical tests were two-tailed, and P < 0.05 was considered statistically significant.
Correlation coefficients were calculated to investigate the correlation between variables. In the case of normally distributed data, Pearson’s correlation coefficient was used. Spearman’s correlation coefficient was employed if there was at least one sample with a non-normal distribution. A correlation was considered statistically significant when the P-value was <0.05.
Results
Baseline data
In total, 150 patients admitted to the ICU of the First Affiliated Hospital of Dalian Medical University and 30 control patients without sepsis admitted to the general ward from 1 January 2014 to 30 April 2016 were retrospectively analyzed in this study. The patients’ characteristics, including diagnoses and associated infections, are shown in Table 1.
Table 1.
Patient characteristics.
| Control (non-septic) group | Sepsis | Septic shock | P-value | |
|---|---|---|---|---|
| Patients, n | 30 | 66 | 84 | |
| Age, years | 66.5 (25–89) | 70 (24–91) | 74.5 (24–89) | 0.24 |
| Male, % | 80 | 60.6 | 65.5 | 0.54 |
| Female, % | 20 | 39.4 | 34.5 | 0.00 |
| PCT, ng/mL | 0.05 (0.05 to 0.07) | 5.32 (1.29 to 10.45) | 3.9 (1.81 to 40.20) | 0.00 |
| NT-proBNP, pg/mL | 93.75 (20 to 118.30) | 16548.75 (190 to 29925.90) | 9005.36 (1243.97 to 35000) | 0.00 |
| IL-6, pg/mL | 89.35 (59.80 to 146.50) | 173029.8 (927.60 to 331980.21) | 20265.03 (250.10 to 449450.00) | 0.00 |
| PT | 12.30 (11 to 14) | 26.8 (14.60 to 100.0) | 20.85 (16.54 to 36.40) | 0.00 |
| TT | 13.85 (11 to 17) | 28.90 (18.60 to 102.43) | 26.55 (16.54 to 49.80) | 0.00 |
| SOFA(admission) | 6 (4 to 8) | 4 (2 to10) | 0.00 | |
| Admission department | General ward | ICU | ICU | |
| Diagnosis | ||||
| Respiratory | Respiratory medicine | Pneumonia (n = 45) | Pneumonia (n = 58) | 0.00 |
| Abdominal | General surgery | IAI (n = 8) | IAI (n = 8) | 1.00 |
| CNS | Neurology | Meningitis (n = 3) | Meningitis (n = 5) | 0.00 |
| Urinary | Urology | Pyelonephritis (n = 4) | Pyelonephritis (RM and ARF) (n = 7) | 0.00 |
| Others | Orthopedic/thoracic | Trauma (ACS) (n = 2) | Trauma (n = 6) | 0.00 |
| Cardiovascular Dept. | AMI (n = 1), cardiac surgery (n = 1) | |||
| General surgery | Non-cardiac surgery (n = 1) | |||
| Internal medicine | Skin/soft tissue infection (n = 1) | |||
| 28-Day mortality | ||||
| Survival, % | 24.24 | 42.86 | ||
| Non-survival, % | 75.76 | 57.14 | ||
| Length of ICU stay, days | 5 (3–11) | 6 (3–12) | 0.006 | |
| Length of hospital stay, days | 3 (1–5) | 11 (8–23) | 14 (9–30) | 0.00 |
| Infecting organism | ||||
| Gram-negative, % | 27.27 | 47.62 | 0.01 | |
| Gram-positive, % | 27.7 | 36.90 | 0.235 | |
| Fungal (%) | 30.3 | 21.43 | 0.21 | |
| Viral, % | 15.15 | 5.95 | 0.063 |
Data are presented as median (25th–75th percentile) unless otherwise indicated.
PCT, procalcitonin; NT-pro BNP, N-terminal brain natriuretic propeptide; IL-6, interleukin 6; PT, prothrombin time; TT, thrombin time; SOFA(admission), Sequential Organ Failure Assessment Score at admission; ICU, intensive care unit; IAI, intra-abdominal infection; CNS, central nervous system; ACS, abdominal compartment syndrome; RM, rhabdomyolysis; ARF, acute renal failure; AMI, acute myocardial infarction.
Median levels of PCT, NT-proBNP, IL-6, PT, TT, and SOFA score
The median PCT, NT-proBNP, IL-6, PT, TT, and SOFA score in each group are shown in Table 1. The PCT, NT-proBNP, IL-6, PT, TT, and SOFA score at ICU admission differed significantly among the groups. PCT, NT-proBNP, IL-6, PT, and TT were significantly higher in patients with sepsis and septic shock than in patients without sepsis (P < 0.001), and PCT, NT-proBNP, IL-6, and SOFA score were significantly higher in patients with sepsis than septic shock.
Clinical value of PCT, NT-proBNP, and IL-6 for diagnosing sepsis
ROC curves were constructed using raw laboratory parameters. The ROC curves of PCT, NT-proBNP, and IL-6 for diagnosing sepsis among the three groups are shown in Figure 1. The AUC was 0.732 for PCT, 0.711 for BNP, 0.706 for IL-6, 0.806 for PT, and 0.872 for the SOFA score (Table 2).
Figure 1.
ROC curves of PCT, NT-proBNP, IL-6, PT, TT, and SOFA score for diagnosing sepsis. Areas under the ROC curve are as follows: SOFA score (red line): 0.872 (95% confidence interval (CI), 0.821–0.922), P < 0.0001; PCT (blue line): 0.732 (95% CI, 0.656–0.808), P < 0.0001; NT-proBNP (green line): 0.711 (95% CI, 0.636–0.785), P < 0.0001; IL-6 (gray line): 0.706 (95% CI, 0.629–0.782), P < 0.0001; PT (purple line): 0.806 (95% CI, 0.743–0.868), P < 0.0001; TT (yellow line): 0.691 (95% CI, 0.614–0.769), P < 0.001. ROC, receiver operating characteristic; PCT, procalcitonin; NT-proBNP, N-terminal brain natriuretic propeptide; IL-6, interleukin 6; PT, prothrombin time; TT, thrombin time; SOFA, Sequential Organ Failure Assessment score.
Table 2.
Area under the curve for diagnosing sepsis.
| Variables | Area under the curve | Standard error | P-value |
95% Confidence interval |
|
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| PCT, ng/mL | 0.732 | 0.039 | 0.00 | 0.656 | 0.808 |
| NT-proBNP, pg/mL | 0.711 | 0.038 | 0.00 | 0.636 | 0.785 |
| IL-6, pg/mL | 0.706 | 0.032 | 0.00 | 0.629 | 0.782 |
| PT, s | 0.806 | 0.040 | 0.00 | 0.743 | 0.868 |
| TT, s | 0.691 | 0.039 | 0.00 | 0.614 | 0.769 |
| SOFA score | 0.872 | 0.026 | 0.00 | 0.821 | 0.922 |
PCT, procalcitonin; NT-pro BNP, N-terminal brain natriuretic propeptide; IL-6, interleukin 6; PT, prothrombin time; TT, thrombin time; SOFA, Sequential Organ Failure Assessment.
Using a PCT cut-off value of 2.31 ng/mL, the sensitivity was 77.65%, specificity was 73.17%, positive predictive value (PPV) was 85.71%, negative predictive value (NPV) was 61.22%, positive likelihood ratio (LR+) was 2.89, and negative likelihood ratio (LR−) was 0.31. Using a cut-off value of 2800 pg/mL for NT-proBNP, the sensitivity was 73.53%, specificity was 51.72%, PPV was 84.27%, NPV was 35.71%, LR+ was 1.52, and LR− was 0.51. Using a cut-off value of 1200 pg/mL for IL-6, the sensitivity was 73.89%, specificity was 51.72%, PPV was 82.27%, NPV was 36.14%, LR+ was 1.53, and LR− was 0.50. Using a cut-off value of 3 for the SOFA score, the sensitivity was 93.17%, specificity was 85.71%, PPV was 96.77%, NPV was 73.17%, LR+ was 6.52, and LR− was 0.08. Using a cut-off value of 20.00 for PT, the sensitivity was 83.54%, specificity was 65.22%, PPV was 80.49%, NPV was 69.77%, LR+ was 2.40, and LR− was 0.25. A binary logistic regression model was also built to analyze whether PCT, NT-proBNP, IL-6, PT, TT, and the SOFA score can be combined to improve the predictive accuracy. The combined model significantly improved the odds of observing the sepsis category (P < 0.001).
Comparison of median levels of PCT, NT-proBNP, IL-6, PT, TT, and SOFA score at admission in non-survival and survival groups
The median levels of PCT, NT-proBNP, IL-6, PT, TT, and SOFA score were significantly higher in the non-survival than survival group, as shown in Table 3. The median level of PCT was 5.38 ng/mL in the non-survival group and 3.08 ng/mL in the survival group (P < 0.001). The median level of NT-proBNP was higher in the non-survival than survival group (13722.35 vs. 9009.68 pg/mL, respectively). The median levels of IL-6 and PT were significantly higher in non-survival than survival group (IL-6: 178335.99 and 74187.31 pg/mL, respectively; P = 0.05 and PT: 23.9 vs. 20.95 s, respectively; P < 0.001). In contrast, TT was lower in the non-survival than survival group (26.55 vs. 26.88 s, respectively; P < 0.001). Finally, the median SOFA score was significantly higher in the non-survival than survival group (P < 0.001).
Table 3.
Comparison of median levels of PCT, NT-proBNP, IL-6, PT, and TT between the survival and non-survival groups.
| Survival | Non-survival | P-value | |
|---|---|---|---|
| Patients, n | 52 | 98 | |
| PCT | 3.08 (1.52–7.35) | 5.38 (1.29–40.20) | 0.00 |
| NT-pro BNP | 9009.68 (190–32417.33) | 13722.35 (12443.97–35000.0) | 0.128 |
| IL-6 | 74187.31 (250.10–379190.90) | 178335.99 (260.32–449450.00) | 0.05 |
| PT | 20.95 (12.22–36.20) | 23.9 (13.50–100.00) | 0.00 |
| TT | 26.55 (16.90–49.80) | 26.83 (16.54–102.43) | 0.00 |
| SOFA score | 4 (2–8) | 5.5 (3–10) | 0.00 |
Data are presented as median (25th–75th percentile)
PCT, procalcitonin; NT-pro BNP, N-terminal brain natriuretic propeptide; IL-6, interleukin 6; PT, prothrombin time; TT, thrombin time; SOFA, Sequential Organ Failure Assessment.
PCT, NT-proBNP, IL-6, PT, TT, and SOFA score as independent predictors of mortality in patients with sepsis and septic shock
Univariate logistic analysis of age, sex, blood group, and SOFA score was performed to assess the association of each parameter with 28-day mortality. Linear regression indicated that age, sex, and blood group were not significantly associated with 28-day mortality (P = 0.09, 0.574, and 0.293, respectively). However, the SOFA score was significantly associated with 28-day mortality (P < 0.001).
The chi-square test was also performed to assess the association of age, sex, and blood group with sepsis. Neither sex nor blood group was significantly associated with sepsis; however, age was associated with sepsis (P < 0.001).
Univariate logistic analysis of PCT, NT-proBNP, IL-6, PT, TT, and SOFA score was performed within the sepsis and non-sepsis groups. Binary logistic regression to predict septic shock in patients with sepsis showed that among PCT (B = −0.01, odds ratio (OR) = 0.99), NT-proBNP (B = −0.00, OR = 1), IL-6 (B = −0.00, OR= 1.00), PT (B = −0.12, OR = 0.88, P < 0.001), TT (B = 0.00, OR = 1.00), and SOFA score (B= −0.76, OR = 0.47, P < 0.001), the PT and SOFA score were strong predictors of septic shock (Table 4). The SOFA score was an independent predictor of septic shock in patients with sepsis (Table 5). The ROC curves of PCT, NT-proBNP, IL-6, PT, TT, and SOFA score for predicting septic shock in patients with sepsis also confirmed these results (Figure 2). The AUCs of PCT, NT-proBNP, IL-6, PT, and SOFA score for predicting sepsis were 0.636 (0.541–0.732), 0.607 (0.515–0.699), 0.60 (0.507–0.694), 0.736 (0.657–0.816), and 0.826 (0.760–0.892), respectively (Table 6).
Table 4.
Independent factors for predicting septic shock and 28-day mortality.
| Variables | B | Standard error | Wald | Degree of freedom | P-value | Odds ratio |
95% Confidence interval |
||
|---|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||||
| Septic shock | PCT | −0.01 | 0.10 | 0.105 | 1 | 0.746 | 0.99 | 0.920 | 1.062 |
| NT-proBNP | −0.00 | 0.00 | 3.382 | 1 | 0.06 | 1.00 | 1.00 | 1.00 | |
| IL-6 | −0.00 | 0.01 | 1.319 | 1 | 0.25 | 1.00 | 1.00 | 1.00 | |
| PT | −0.01 | 0.03 | 20.24 | 1 | 0.00 | 0.99 | 0.967 | 1.007 | |
| SOFA | −0.76 | 0.13 | 31.905 | 1 | 0.00 | 0.47 | 0.360 | 0.609 | |
| TT | −0.06 | 0.02 | 3.99 | 1 | 0.00 | 0.95 | 0.992 | 1.015 | |
| Constant | 0.241 | 0.164 | 2.15 | 1 | 0.143 | 1.273 | |||
| 28-Day mortality | PCT | −0.76 | 0.146 | 27.115 | 1 | 0.00 | 0.47 | 1.606 | 2.846 |
| NT-proBNP | −0.00 | 0.000 | 1.90 | 1 | 0.164 | 1.00 | 1.00 | 1.00 | |
| IL-6 | 0.00 | 0.00 | 3.52 | 1 | 0.061 | 1.02 | 1.00 | 1.00 | |
| PT | 0.01 | 0.01 | 0.279 | 1 | 0.242 | 1.02 | 0.995 | 1.038 | |
| SOFA | −1.12 | 0.21 | 29.79 | 1 | <0.001 | 0.33 | 2.211 | 4.710 | |
| TT | 0.01 | 0.02 | 3.99 | 1 | 0.007 | 1.04 | 1.001 | 1.024 | |
| Constant | 0.634 | 0.172 | 13.644 | 1 | 0.00 | 1.885 | |||
PCT, procalcitonin; NT-proBNP, N-terminal brain natriuretic propeptide; IL-6, interleukin 6; PT, prothrombin time; TT, thrombin time; SOFA, Sequential Organ Failure Assessment score.
Table 5.
Performance of multivariable models for predicting septic shock and 28-day mortality in patients with sepsis.
| Variables | Cut-off | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Disease prevalence, % | LR+ | LR− | |
|---|---|---|---|---|---|---|---|---|---|
| Septic shock | PCT | 4.22 | 68.85 | 71.74 | 76.36 | 63.46 | 57.01 | 2.44 | 0.43 |
| NT-proBNP | 16485.91 | 81.55 | 66.00 | 71.19 | 77.65 | 50.74 | 2.40 | 0.28 | |
| IL-6 | 89418.75 | 80.00 | 70.97 | 75.68 | 75.86 | 53.03 | 2.76 | 0.28 | |
| PT | 22.32 | 84.00 | 61.68 | 67.20 | 80.49 | 48.31 | 2.19 | 0.26 | |
| SOFA | 5 | 85.71 | 74.16 | 78.50 | 82.50 | 52.41 | 3.32 | 0.19 | |
| TT | 29.70 | 68.85 | 71.74 | 76.36 | 63.46 | 57.01 | 2.48 | 0.42 | |
| 28-Day mortality | PCT | 4.70 | 85.22 | 72.22 | 83.05 | 75.36 | 61.50 | 3.07 | 0.20 |
| NT-proBNP | 4106.46 | 71.02 | 38.8 | 75.38 | 96.30 | 54.35 | 1.16 | 0.75 | |
| IL-6 | 16331.18 | 72.06 | 66.67 | 79.03 | 57.78 | 63.55 | 2.16 | 0.42 | |
| PT | 19.80 | 52.13 | 65.82 | 78.40 | 36.62 | 70.41 | 1.53 | 0.73 | |
| SOFA | 4 | 90.74 | 73.24 | 83.76 | 83.87 | 60.34 | 3.39 | 0.31 | |
| TT | 26.80 | 50.00 | 51.49 | 66.67 | 34.67 | 65.99 | 1.03 | 0.97 |
PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR–, negative likelihood ratio; PCT, procalcitonin; NT-proBNP, N-terminal brain natriuretic propeptide; IL-6, interleukin 6; PT, prothrombin time; TT, thrombin time; SOFA, Sequential Organ Failure Assessment score.
Figure 2.
ROC curves of PCT, NT-proBNP, IL-6, PT, TT, and SOFA score for predicting septic shock. Areas under the ROC curve are as follows: SOFA score (red line): 0.826 (95% confidence interval (CI), 0.760–0.892), P < 0.0001; PCT (blue line): 0.636 (95% CI, 0.541–0.732), P = 0.004; NT-proBNP (green line): 0.607 (95% CI, 0.515–0.699), P = 0.025; IL-6 (yellow line): 0.600 (95% CI, 0.507–0.694), P = 0.035; SOFA48h (sky blue line): 0.848 (95% CI, 0.786–0.910), P < 0.001; PT (gray line): 0.738 (95% CI, 0.657–0.816), P < 0.001; TT (yellow line): 0.600 (95% CI, 0.526–0.711), P = 0.13. ROC, receiver operating characteristic; PCT, procalcitonin; NT-proBNP, N-terminal brain natriuretic propeptide; IL-6, interleukin 6; PT, prothrombin time; TT, thrombin time; SOFA, Sequential Organ Failure Assessment score; SOFA48h, Sequential Organ Failure Assessment score at 48 hours.
Table 6.
Area under the curve for predicting septic shock.
| Variables | Area under the curve | Standard error | P-value |
95% Confidence interval |
|
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| PCT | 0.636 | 0.049 | 0.004 | 0.541 | 0.732 |
| NT-proBNP | 0.607 | 0.047 | 0.025 | 0.515 | 0.699 |
| PT | 0.736 | 0.041 | 0.000 | 0.657 | 0.816 |
| IL-6 | 0.600 | 0.048 | 0.035 | 0.507 | 0.694 |
| TT | 0.618 | 0.047 | 0.013 | 0.526 | 0.711 |
| SOFA | 0.826 | 0.034 | 0.000 | 0.760 | 0.892 |
| SOFA48h | 0.848 | 0.032 | 0.000 | 0.786 | 0.910 |
PCT, procalcitonin; NT-proBNP, N-terminal brain natriuretic propeptide; IL-6, interleukin 6; PT, prothrombin time; TT, thrombin time; SOFA, Sequential Organ Failure Assessment score; SOFA48h, Sequential Organ Failure Assessment score at 48 hours.
Univariate logistic regression of the SOFA score and 28-day mortality was performed, and the resultant linear regression model was statistically significant [F (1,178) = 151.27, P < 0.001, R2 = 0.46], indicating that the SOFA score is an independent predictor of 28-day mortality.
Binary logistic regression was conducted to examine whether PCT, NT-proBNP, PT, TT, IL-6, and SOFA score were significantly associated with the odds of non-survival. The overall model indicated that PCT (χ2(1) = 42.58, P < 0.001) and the SOFA score (χ2(1) = 56.84, P < 0.001) were significant predictors of 28-day mortality (P < 0.001). However, PT (χ2(1) = 1.37), NT-proBNP (χ2(1) = 1.94), and IL-6 (χ2(1) = 3.65) were not significant predictors of 28-day mortality, while TT (χ2(1) = 7.36, P = 0.007) was a significant predictor (Table 5). These findings suggest that PCT, the SOFA score, and TT have a significant effect on the odds of non-survival. ROC curve analysis of PCT, BNP, IL-6, PT, TT, and SOFA score also illustrated that the SOFA score and PCT were significant predictors of 28-day mortality (P < 0.001).
For further confirmation, we also analyzed the capacity of the SOFA score at 48 hours after ICU admission to predict 28-day mortality. The AUC was 0.821 (0.753–0.888), indicating that the SOFA score was a significant predictor of 28-day mortality. The AUCs of the SOFA score, PCT, NT-proBNP, IL-6, PT, and TT were 0.841 (0.776–0.905), 0.805 (0.754–0.876), 0.576 (0.477–0.675), 0.542 (0.446–0.638), 0.589 (0.498–0.682), and 0.597 (0.502–0.692), respectively (Figure 3, Table 7). ROC curve analysis of the SOFA score upon admission to the ICU and the qSOFA score also indicated that the initial SOFA score was an independent predictor of 28-day mortality (Figure 2). The AUCs of the SOFA score and qSOFA score were 0.841 and 0.698, respectively, indicating that the SOFA score was a significant predictor of 28-day mortality.
Figure 3.

ROC curves of PCT, NT-proBNP, IL-6, PT, TT, and SOFA score for predicting 28-day mortality in patients with sepsis. Areas under the ROC curve are as follows: SOFA score (red line): 0.841 (95% confidence interval (CI), 0.776–0.905), P < 0.0001; PCT (blue line): 0.805 (95% CI, 0.734–0.876), P < 0.001; NT-proBNP (green line): 0.576 (95% CI, 0.477–0.675), P < 0.128; IL-6 (purple line): 0.597 (95% CI, 0.502–0.602), P = 0.05; PT (gray line): 0.542 (95% CI, 0.446–0.638), P = 0.401; TT (yellow line): 0.589 (95% CI, 0.496–0.682), P = 0.074; SOFA score at 48 h (sky blue line): 0.886 (95% CI, 0.839–0.933), P < 0.0001; qSOFA score: 0.698 (95% CI, 0.616–0.781), P < 0.001. ROC, receiver operating characteristic; PCT, procalcitonin; NT-proBNP, N-terminal brain natriuretic propeptide; IL-6, interleukin 6; PT, prothrombin time; TT, thrombin time; SOFA, Sequential Organ Failure Assessment score; SOFA48h, Sequential Organ Failure Assessment score at 48 hours
Table 7.
Area under the curve for predicting 28-day mortality.
| Variables | Area under the curve | Standard error | P-value |
95% Confidence interval |
|
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| SOFA score | 0.841 | 0.033 | 0.000 | 0.776 | 0.905 |
| PCT | 0.805 | 0.036 | 0.000 | 0.734 | 0.876 |
| NT-proBNP | 0.576 | 0.051 | 0.128 | 0.477 | 0.675 |
| PT | 0.542 | 0.049 | 0.401 | 0.446 | 0.638 |
| TT | 0.589 | 0.047 | 0.074 | 0.496 | 0.682 |
| IL-6 | 0.597 | 0.048 | 0.051 | 0.502 | 0.692 |
| SOFA48h | 0.821 | 0.034 | 0.000 | 0.753 | 0.888 |
PCT, procalcitonin; NT-proBNP, N-terminal brain natriuretic propeptide; IL-6, interleukin 6; PT, prothrombin time; TT, thrombin time; SOFA, Sequential Organ Failure Assessment score; SOFA48h, Sequential Organ Failure Assessment score at 48 hours.
Correlation of PCT, NT-proBNP, IL-6, PT, TT, and SOFA score in patients with sepsis
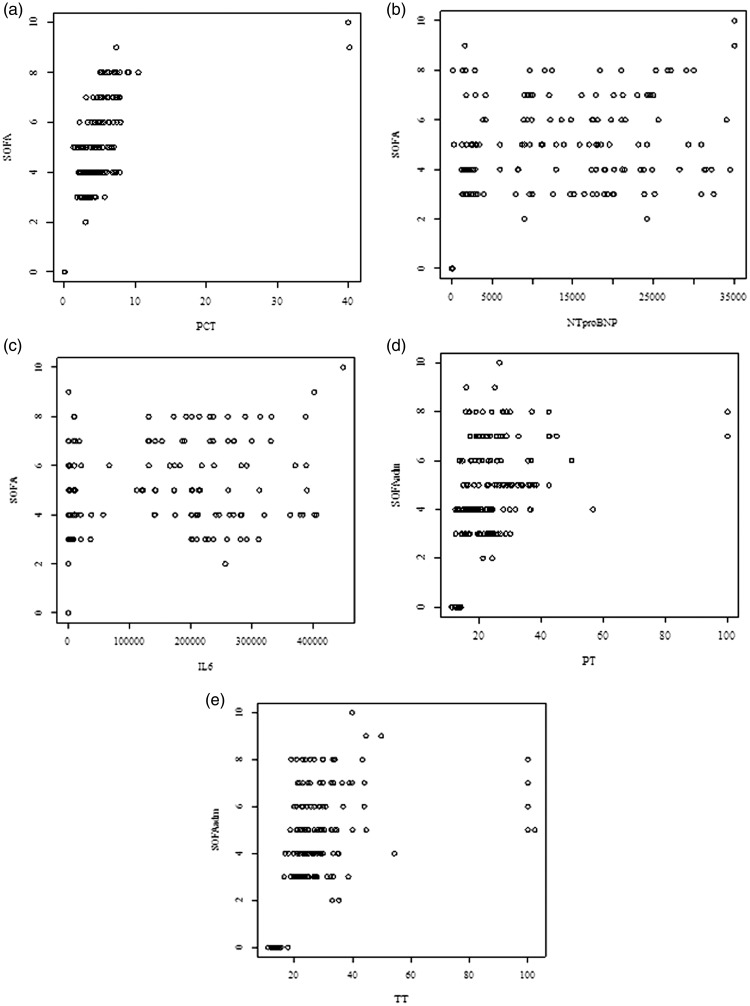
PCT was significantly and positively correlated with the SOFA score (r = 0.79, P < 0.001). The correlation coefficient of PCT and the SOFA score was 0.79, indicating a large effect size. As shown in Figure 4(a)–(c), NT-proBNP and IL-6 were significantly and positively correlated with the SOFA score (r = 0.52, P < 0.001, Figure 4(b) and r = 0.57, P < 0.001, Figure 4(c)). PT was also significantly and positively correlated with the SOFA score (r = 0.56, P < 0.001). Figure 4(d) shows the correlation coefficient between TT and the SOFA score (r = 0.58, P < 0.001), indicating a moderate effect size (Figure 4(e)).
Figure 4.
Correlation of PCT, NT-proBNP, IL-6, PT, TT, and SOFA score in patients with sepsis. (a) There was a significant positive correlation between PCT and SOFA score (r = 0.79, P < .001). (b) The correlation coefficient was a significant positive correlation between NT-pro BNP and SOFA score (r = 0.52, P <. 001). (c) The correlation coefficient was a significant positive correlation between IL-6 and SOFA score (r = 0.57, P <.001). (d) There was a significant positive correlation between PT and SOFA (r = 0.56, P < .001). (e) The correlation coefficient between TT and SOFA was r = 0.58, P <.001) indicating a moderate effect size.
Discussion
In this study, we observed that the use of individual biomarkers (PCT, NT-proBNP, IL-6, PT, and TT) with the SOFA score could facilitate diagnosis and aid prognostication of patients with sepsis and septic shock.
We found several biomarkers (PCT, NT-proBNP, IL-6, PT, and TT) to be significantly associated with the SOFA score, indicating that these biomarkers may facilitate the evaluation of sepsis progression and effective risk stratification. Furthermore, the use of these novel biomarkers can inform prompt diagnosis, which will improve the accuracy of the evaluation of patients with sepsis in the ICU.
Previous studies have shown that PCT and NT-proBNP are strongly correlated with various scoring systems, including the Acute Physiology and Chronic Health Evaluation II score, SOFA score,5,22 Simplified Acute Physiology Score II, and Mortality in Emergency Department Sepsis score.23–25 The present study further showed that the PCT and NT-proBNP levels are correlated with the SOFA score and that IL-6, PT, and TT are significantly correlated with the SOFA score. Various studies have suggested that the IL-6 level is proportional to the intensity of the pathogen insult; thus the IL-6 level is correlated with the length of fever, severity of organ dysfunction, length of hospital stay, clinical severity scores (Simplified Acute Physiology Score II and SOFA scores), and organ dysfunction.26
In this study, we found PT and TT to be significantly correlated with the SOFA score. PT is the most widely used test in clinical practice, but its relationship with the SOFA score was unknown. We herein successfully identified a significant correlation between PT and the SOFA score. TT in patients with sepsis has rarely been studied, probably because in these patients, coagulation function and thrombin generation are more impaired than fibrinolysis. However, we included PT in the present study and found it to be significantly correlated with the SOFA score. We thought that this correlation may be associated with microcirculatory hypoxia and resultant microcirculation thrombosis in patients with septic shock.
PCT is the prohormone of calcitonin, which is normally produced in the C cells of the thyroid glands. PCT is cleaved to calcitonin, and only <0.1 ng/mL is measured in the blood of healthy humans. PCT regulation evidently changes during infection. In sepsis, PCT is released into the bloodstream for the first 4 to 12 hours of infection depending on severity.15 In the present study, the optimal PCT level for diagnosing sepsis was 2.3 ng/mL, which is higher than the level of 1.1 ng/mL cited previously.15 Two possible reasons for these different values are as follows. First, patients were transferred to the ICU when their disease severity deteriorated; thus, acutely ill patients were included in the sepsis group and critically ill patients were included in the septic shock group. Second, the PCT levels were obtained at ICU admission for patients with sepsis, not from various floor wards.
The ROC analysis of PCT for predicting septic shock showed an AUC of 0.636, which was significantly higher than of NT-proBNP, IL-6, and PT (P < 0.01). The AUC of PCT was 0.636 for the septic shock group, which was lower than the AUC of 0.732 for the sepsis group. Several meta-analyses have shown that PCT guidance is associated with reduced antibiotic exposure in all patients, with no adverse effect on either outcomes or treatment failure.27–29 However, no difference in short-term mortality was observed. Meanwhile, although PCT was not found to be an independent predictor of septic shock, it was an independent predictor of 28-day mortality (P < 0.001) in the logistic regression models.
Various studies have shown that NT-proBNP is a useful predictor of the clinical outcomes of sepsis and septic shock.30 In the present study, NT-proBNP showed predictive value for diagnosing sepsis. Meanwhile, the logistic regression model showed that NT-proBNP was not independently associated with septic shock. This lack of association might have occurred because the NT-proBNP values were significantly decreased in patients after receiving treatment, and an increase was observed in patients without clinical improvement. Additionally, this study included a substantial number of patients with obesity, acute renal failure, acute coronary syndrome, and type 2 diabetes. These factors may be associated with subclinical left ventricular abnormalities, affecting the circulating levels of NT-proBNP.
A few studies have shown that NT-proBNP is correlated with age, but not with sex.31,32 The ROC analysis of NT-proBNP for predicting 28-day mortality and the logistic regression showed that the NT-proBNP level was significantly associated with 28-day mortality, indicating that NT-proBNP can be a helpful factor for diagnosis and prediction of 28-day mortality in patients with sepsis.
ROC curve analysis showed that IL-6 was of limited value in differentiating septic shock from sepsis or predicting 28-day mortality. In the logistic regression model, IL-6 was not significantly associated with septic shock or 28-day mortality. These results indicated that IL-6 can be helpful in early diagnosis of sepsis but not in predicting 28-day mortality in patients with sepsis in the ICU.
Many inflammatory mediators are involved in the activation of coagulation. However, few studies have reported the value of coagulopathy in determining the severity of sepsis.33,34 In this study, we showed that the median PT and TT did not differ significantly between the two groups. PT and TT were useful in differentiating sepsis and septic shock (P < 0.001) but not in predicting 28-day mortality in patients with sepsis.
Finally, in this study, we found that the SOFA score had an AUC of 0.841 for predicting sepsis and an AUC of 0.826 for predicting septic shock; these AUCs were significantly higher than those for PCT, NT-proBNP, IL-6, PT, and TT (P < 0.001). However, the qSOFA score (assessed at the time of admission to the ICU) showed an AUC of 0.698. Meanwhile, the logistic regression analysis indicated that the SOFA score was significantly associated with septic shock (P < 0.001) and 28-day mortality (P < 0.001). This result is consistent with previous studies indicating that the SOFA score is associated with clinical outcomes for patients with sepsis.7 The SOFA score was developed to assess the severity of illness for patients with sepsis and has been included for the definition of sepsis in the latest Sepsis-3 definition.9,35
Limitations
This single-center study included a small number of patients and did not compare the performance of the proposed biomarkers with other novel biomarkers. Moreover, the study cohort was retrospective and employed an existing database in which patients were categorized into those with sepsis, severe sepsis, and septic shock according to the American College of Chest Physicians/SCCM criteria.36 However, these patients were re-categorized into two groups: those with sepsis and those with septic shock following the SCCM/ESICM criteria (Sepsis-3),14 which may have introduced some biases. The criteria of sepsis were updated in a recent report, and the old criteria (SCCM/ESICM criteria) were found to be less accurate.14
Conclusion
This study demonstrated a strong relationship between PCT, NT-proBNP, IL-6, PT, TT, and the SOFA score. These biomarkers (PCT, NT-proBNP, IL-6, PT, TT) may have a supporting role in diagnosing early sepsis. While there is no single specific biomarker capable of predicting multiple organ dysfunction, selected biomarkers were associated with 28-day mortality and may therefore be beneficial in predicting clinical outcomes in ICU patients with sepsis.
Abbreviations
AUC, area under the receiver operating characteristic curve; ED, emergency department; ESICM, European Society of Intensive Care Medicine; IL-6, interleukin-6; ICU, intensive care unit; LR–, negative likelihood ratio; LR+, positive likelihood ratio; NT-proBNP, N-terminal brain natriuretic propeptide; NPV, negative predictive value; PCT, procalcitonin; PT, prothrombin time; PPV, positive predictive value; qSOFA, Quick Sequential (Sepsis-Related) Organ Failure Assessment; ROC, receiver operating characteristic; SCCM, Society of Critical Care Medicine; SOFA, Sequential Organ Failure Assessment; TT, thrombin time.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014; 311: 1308–1316. [DOI] [PubMed] [Google Scholar]
- 2.Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit. Care Med 2013; 41: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 3.Esper AM, Martin GS. Extending international sepsis epidemiology: the impact of organ dysfunction. Crit Care 2009; 13: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC case mix programme database. Crit Care 2006; 10: R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biron BM, Ayala A, Lomas-Neira JL. Biomarkers for Sepsis: What is and What Might Be? Biomarker Insights. London, England: SAGE Publications Sage UK, 2015; 10s4: BMI.S29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent JL, Moreno R. Clinical review: scoring systems in the critically ill. Crit Care 2010; 14: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Hong Y. Development of a novel score for the prediction of hospital mortality in patients with severe sepsis: the use of electronic healthcare records with LASSO regression. Oncotarget 2017; 8: 49637–49645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khwannimit B. A comparison of three organ dysfunction scores: MODS, SOFA and LOD for predicting ICU mortality in critically ill patients. J Med Assoc Thai 2007; 90: 1074–1081. [PubMed] [Google Scholar]
- 9.Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: a systematic review. Crit Care 2008; 12:R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit. Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 11.Vincent JL, De Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine . Crit. Care Med 1998; 26: 1793–800. [DOI] [PubMed] [Google Scholar]
- 12.Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA Score, SIRS Criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA 2017; 317: 290–300. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 14.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabral L, Afreixo V, Almeida L, et al. The use of procalcitonin (PCT) for diagnosis of sepsis in burn patients: a meta-analysis. PLoS One 2016; 11: e0168475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorgis N, Asselin JM, Fontana C, et al. Evaluation of the association of early elevated lactate with outcomes in children with severe sepsis or septic shock. Pediatr Emerg Care 2017. Doi: 10.1097/PEC.0000000000001021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold RC, Sherwin R, Shapiro NI, et al. Multicenter observational study of the development of progressive organ dysfunction and therapeutic interventions in normotensive sepsis patients in the emergency department. Acad Emerg Med 2013; 20: 433–440. [DOI] [PubMed] [Google Scholar]
- 18.Machado FR, Salomão R, Rigato O, et al. Late recognition and illness severity are determinants of early death in severe septic patients. Clinics (Sao Paulo) 2013; 68: 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z. Missing data imputation: focusing on single imputation. Ann Transl Med 2016; 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med 2016; 4: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z. Model building strategy for logistic regression: purposeful selection. Ann Transl Med 2016; 4: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castelli GP, Pognani C, Cita M, et al. Procalcitonin, C-reactive protein, white blood cells and SOFA score in ICU: diagnosis and monitoring of sepsis. Minerva Anestesiol 2006; 72: 69–80. [PubMed] [Google Scholar]
- 23.Wang S, Chen D. [The correlation between procalcitonin, C-reactive protein and severity scores in patients with sepsis and their value in assessment of prognosis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015; 27: 97–101 [in Chinese, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Wu Y, Tang L, et al. Brain natriuretic peptide for prediction of mortality in patients with sepsis: a systematic review and meta-analysis. Crit Care 2012; 16: R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Zhang Z, Xue Y, et al. Prognostic value of B-type natriuretic peptide (BNP) and its potential role in guiding fluid therapy in critically ill septic patients. Scand J Trauma Resusc Emerg Med 2012; 20: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haasper C, Kalmbach M. Prognostic value of procalcitonin (PCT) and/or interleukin-6 (IL-6) plasma levels after multiple trauma for the development of multi organ dysfunction syndrome (MODS) or sepsis. Technol Health Care 2010; 18: 89–100. [DOI] [PubMed] [Google Scholar]
- 27.Lam SW, Bauer SR, Fowler R, et al. Systematic review and meta-analysis of procalcitonin-guidance versus usual care for antimicrobial management in critically ill patients: focus on subgroups based on antibiotic initiation, cessation, or mixed strategies. Crit Care Med 2018; 46: 684–690. [DOI] [PubMed] [Google Scholar]
- 28.Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18: 95–107. [DOI] [PubMed] [Google Scholar]
- 29.Iankova I, Thompson-Leduc P, Kirson NY, et al. Efficacy and safety of procalcitonin guidance in patients with suspected or confirmed sepsis: a systematic review and meta-analysis. Crit Care Med 2018; 46: 691–698. [DOI] [PubMed] [Google Scholar]
- 30.Guaricci AI, Santoro F, Paoletti Perini A, et al. Correlations between NT-proBNP, outcome and haemodynamics in patients with septic shock. Acta Cardiol 2015; 70: 545–552. [DOI] [PubMed] [Google Scholar]
- 31.Burri E, Hochholzer K, Arenja N, et al. B-type natriuretic peptide in the evaluation and management of dyspnoea in primary care. J. Intern. Med 2012; 272: 504–513. [DOI] [PubMed] [Google Scholar]
- 32.Schneider HG, Lam L, Lokuge A, et al. B-type natriuretic peptide testing, clinical outcomes, and health services use in emergency department patients with dyspnea: a randomized trial. Ann. Intern. Med 2009; 150: 365–371. [DOI] [PubMed] [Google Scholar]
- 33.Moreno R, Vincent JL, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working group on sepsis related problems of the ESICM. Intensive Care Med 1999; 25: 686–696. [DOI] [PubMed] [Google Scholar]
- 34.Samuels JM, Moore HB, Moore EE. Coagulopathy in severe sepsis: interconnectivity of coagulation and the immune system. Surg Infect (Larchmt) 2018; 19: 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Smischney NJ, Zhang H, et al. AME evidence series 001-the society for translational medicine: clinical practice guidelines for diagnosis and early identification of sepsis in the hospital. J Thorac Dis 2016; 8: 2654–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101: 1644–1655. [DOI] [PubMed] [Google Scholar]