Short abstract
Objective
To investigate the relationship between pretreatment plasma D-dimer levels and survival in Chinese patients with small cell lung cancer (SCLC).
Methods
This retrospective study enrolled 82 patients with SCLC treated at Beijing Chaoyang Hospital, Capital Medical University, from January 2012 to January 2015. All patients were followed up. Associations between pretreatment plasma D-dimer levels measured by immunoturbidimetric assay and clinical outcomes were analyzed by Kaplan–Meier and multivariate analyses, using a cut-off level of 0.55 mg/L fibrinogen equivalent units (FEU).
Results
Median progression-free survival (PFS) and overall survival (OS) were significantly higher in patients with low D-dimer levels (≤0.55 mg/L FEU; 8.0 and 17.0 months, respectively) compared with patients with high levels (>0.55 mg/L FEU; 5.0 and 9.0 months, respectively). Plasma D-dimer levels, Karnofsky performance status, N stage, TNM stage, treatment, and neuron-specific enolase (NSE) levels were significantly associated with PFS, while D-dimer levels, N stage, TNM stage, and treatment were significantly associated with OS. Multivariate analysis revealed that TNM stage, treatment, and NSE levels were independently associated with PFS, while D-dimer levels and treatment were independently associated with OS.
Conclusions
Pretreatment plasma D-dimer levels were independently associated with OS in patients with SCLC.
Keywords: D-dimer, small cell lung cancer, survival, prognosis, progression-free survival, pretreatment level
Introduction
Lung cancer is among the most common malignant tumors in humans and is associated with high mortality.1 Small cell lung cancer (SCLC) is a pulmonary neuroendocrine tumor2 that accounts for about 14% of all lung cancer cases.3 Lung cancer is the most common type of cancer in China, with an incidence of 53.6 per 10 million in 2009, largely due to the high proportion of smokers.4 Compared with non-small lung cancer (NSCLC), SCLC shows a higher degree of malignancy, shorter doubling time, early metastasis, poor prognosis, and a 5-year survival of <7%.5 The median survival without treatment is 2 to 4 months, irrespective of the clinical stage.6 Recognized prognostic factors for SCLC include performance status,7 sex,8 age,8 expression of pituitary tumor transforming gene-1,7 tumor size,8,9 tumor stage,7,8 and blood carboxyhemoglobin levels.10
Previous studies showed that coagulation disorders are often the first signs of a malignant tumor.11,12 The tumor can induce a pro-coagulant state, and the activated coagulation factors then promote tumor growth, infiltration, metastasis, and angiogenesis.11,12 D-dimer is produced by fibrin degradation, and measurement of D-dimer levels can help in the diagnosis of thrombosis.13 Plasma D-dimer levels are correlated with prognosis in patients with malignant tumors,14–16 and were shown to be independently associated with the prognosis of esophageal squamous cell carcinoma,14 operable colorectal cancer,15 lung cancer in general (mostly types other than SCLC),17 and in various other cancers.16
However, data on the prognostic value of plasma D-dimer levels in SCLC are lacking. The current study therefore aimed to investigate the relationship between pretreatment plasma D-dimer levels and survival in Chinese patients with SCLC.
Patients and methods
Patients
This was a retrospective cohort study including 82 Chinese patients with SCLC treated at Beijing Chaoyang Hospital, Capital Medical University, from January 2012 to January 2015.
The inclusion criteria were: 1) pathological diagnosis of SCLC18 (if mixed with other pathological components, the SCLC component should be predominant); 2) age >18 years; 3) Karnofsky performance status (KPS) score ≥70; 4) treated with at least four cycles of standard first-line etoposide + platinum drugs; 5) received at least two cycles of second-line chemotherapy such as irinotecan, topotecan, paclitaxel, docetaxel, or combined platinum; 6) with complete coagulation data; and 7) with complete follow-up data. The exclusion criteria were: 1) history of anticoagulant therapy; 2) history of any cancer before SCLC diagnosis; or 3) history of radiotherapy and chemotherapy prior to blood sampling for D-dimer measurement.
The study was approved by the ethics committee of our hospital, which waived the need for individual consent.
D-dimer and neuron-specific enolase levels
Fasting blood was collected from an antecubital vein into vacutainer tubes containing anticoagulant citrate buffer (1:9, buffer:blood; 3 mL of blood) and clotting agent (3.5 mL of blood), respectively, within 1 week prior to chemotherapy or radiotherapy. Plasma (for D-dimer assay) was obtained by centrifugation at 400 × g for 25 minutes. D-dimer levels were determined by immunoturbidimetric assay (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany) using a SYSMEX CA-7000 system (Sysmex Corp., Kobe, Japan). The cut-off value for plasma D-dimer levels used by the hospital laboratory was 0.55 mg/L fibrinogen equivalent units (FEU), based on the manufacturer’s recommendations. Serum (for neuron-specific enolase [NSE] assay) was obtained by centrifugation at 2860 × g for 15 minutes. Serum NSE levels were measured within 7 days before chemotherapy using a solid-phase radioimmunoassay method (SRL Inc., Tokyo, Japan). The cut-off value for plasma NSE levels was 16.3 ng/mL, based on the manufacturer’s recommendations.
Data collection
Information on age, sex, KPS score, smoking status (smoker defined as a smoking history of at least 30 packs/year; former smoker defined as having quit for at least 15 years19), TNM stage, venous thromboembolism (VTE), and treatment were extracted from the medical records.
Follow-up
The patients were followed up until 31 January 2017. The patients underwent head magnetic resonance imaging, and thoracic and abdominal computed tomography every two cycles of chemotherapy. Progression was evaluated based on RECIST 1.1.20 Progression-free survival (PFS) was measured as the time from the first cycle of chemotherapy to progression or death, and overall survival (OS) was the time from the first cycle of chemotherapy to death. Follow-up was censored at the last visit for patients who were still alive on 31 January 2017.
Statistical analysis
Continuous data are presented as mean ± standard deviation or median (range), as appropriate. Categorical data are presented as frequencies. Differences between different stages for D-dimer and fibrinogen levels were analyzed by Mann–Whitney U-tests, and categorical variables were compared using χ2 tests. Survival was analyzed using the Kaplan–Meier method and log-rank test. Multivariate analyses were performed using the Cox proportional hazards model. Factors with a P value < 0.05 in univariate analysis were assessed by multivariate analysis. Data were analyzed using IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., Armonk, NY, USA). A two-sided P value < 0.05 was considered statistically significant.
Results
Patient characteristics
The characteristics of the 82 patients are shown in Table 1. The patients’ ages ranged from 28 to 82 years (median, 60 years).
Table 1.
Demographic and clinical characteristics of the patients.
| Clinical characteristic | Category | n (%) |
|---|---|---|
| Age (years) | ||
| <60 | 36 (43.9) | |
| ≥60 | 46 (56.1) | |
| Sex | ||
| Male | 67 (81.7) | |
| Female | 15 (18.3) | |
| KPS score | ||
| 90–100 | 46 (56.1) | |
| 70–90 | 36 (43.9) | |
| Smoking status | ||
| Yes | 63 (76.8) | |
| No | 19 (23.2) | |
| D-dimer (mg/L FEU) | ||
| ≤0.55 | 29 (35.4) | |
| >0.55 | 53 (64.6) | |
| Clinical Stage | ||
| T stage | ||
| T1+T2+T3 | 43 (52.4) | |
| T4 | 39 (47.6) | |
| N stage | ||
| N0+N1+N2 | 45 (54.9) | |
| N3 | 37(45.1) | |
| TNM stage | ||
| I+II+IIIa | 19 (23.2) | |
| IIIb+IV | 63 (76.8) | |
| Brain metastases | ||
| Yes | 26 (31.7) | |
| No | 56 (68.3) | |
| VTE | ||
| Yes | 4 (4.9) | |
| No | 78 (95.1) | |
| Treatment | ||
| Chemotherapy only | 27 (32.9) | |
| Chemoradiotherapy | 55 (67.1) | |
| NSE (ng/mL) | ||
| 0–16.3 | 11 (13.4) | |
| >16.3 | 71 (86.6) |
KPS, Karnofsky performance status; TNM, tumor node metastasis; NSE, neuron-specific enolase; VTE, venous thromboembolism
Fifty-five patients received chest, bone, or brain radiation. VTE was observed in 4.9% (n = 4) of the study population during follow-up (3 deep vein thrombosis, 1 pulmonary embolism). Fifty-three patients had above-normal (0.55 mg/L FEU) plasma D-dimer levels. The pretreatment plasma D-dimer levels ranged from 0.12 to 5.27 mg/L FEU (median, 1.25 mg/L FEU).
Survival
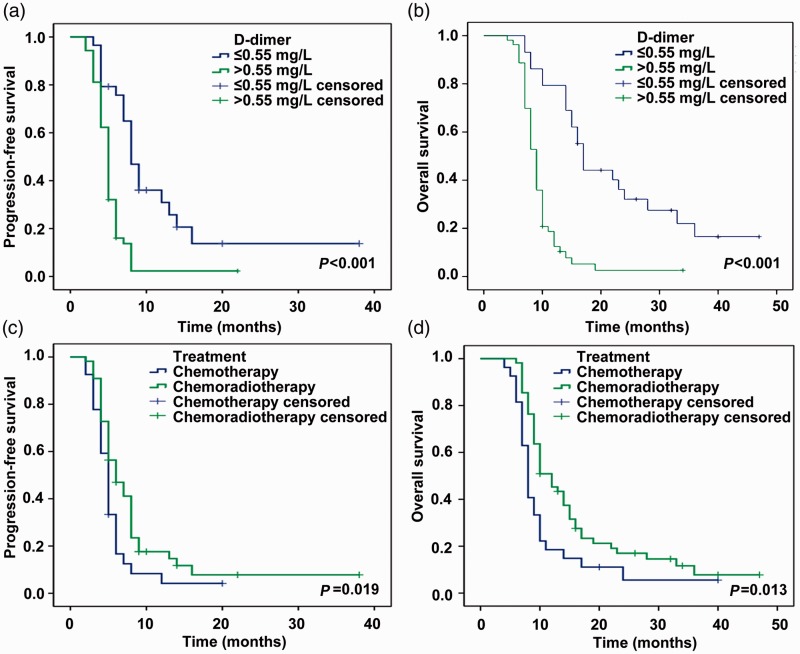
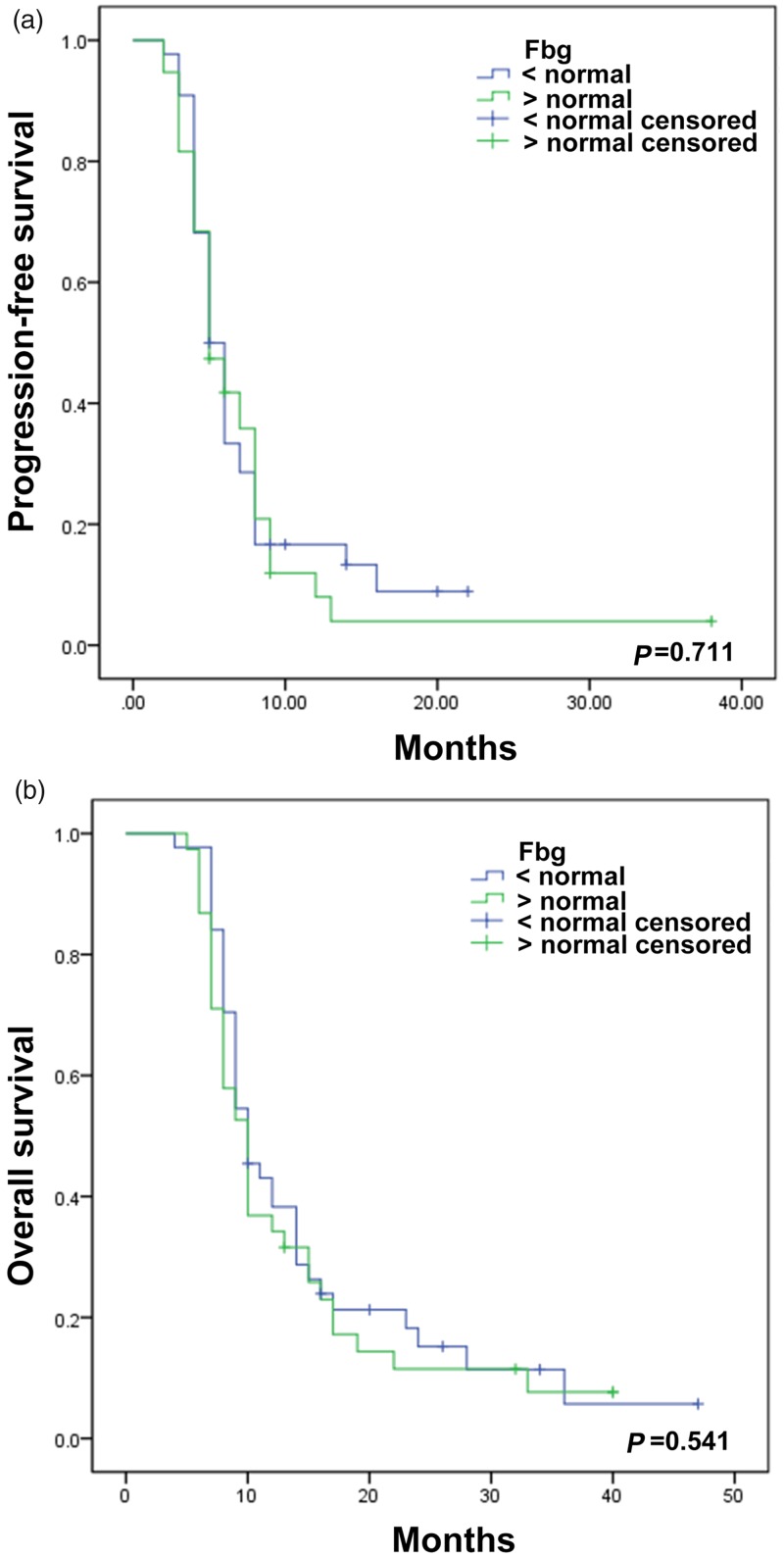
The median PFS and OS according to Kaplan–Meier analysis were 8.0 and 17.0 months, respectively, in patients with low D-dimer levels (≤0.55 mg/L FEU), and 5.0 and 9.0 months in patients with high D-dimer levels (>0.55 mg/L FEU) (PFS: P < 0.001; OS: P < 0.001) (Figure 1). Median PFS and OS were 5 and 8 months, respectively, in patients with chemotherapy only compared with 6 and 10 months in patients treated with chemoradiotherapy (PFS: P = 0.019; OS: P = 0.013) (Figure 1). Fibrinogen levels were not significantly associated with OS or PFS (Figure 2).
Figure 1.
Kaplan–Meier curves for progression-free survival and overall survival in patients with small cell lung cancer according to pretreatment plasma D-dimer levels (A and B) and treatment (C and D)
Figure 2.
Kaplan–Meier curves for progression-free survival (A) and overall survival (B) in patients with small cell lung cancer according to plasma fibrinogen level. The differences were not statistically significant. Fbg, plasma fibrinogen
Univariate analysis
KPS score (P = 0.047), plasma D-dimer level (P < 0.001), N stage (P = 0.003), TNM stage (P < 0.001), treatment (P = 0.038), and NSE level (P = 0.032) were significantly associated with PFS, while D-dimer level (P < 0.001), N stage (P < 0.001), TNM stage (P < 0.001), and treatment (P = 0.021) were significantly associated with OS (Table 2).
Table 2.
Univariate analyses of associations between clinical characteristics and OS.
| Variable |
Progression-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | 0.926 | 0.577–1.486 | 0.749 | 1.040 | 0.648–1.670 | 0.870 |
| Sex | 0.871 | 0.467–1.624 | 0.664 | 0.849 | 0.456–1.579 | 0.605 |
| KPS score | 1.620 | 1.006–2.608 | 0.047 | 1.611 | 0.998–2.602 | 0.051 |
| Smoking status | 0.815 | 0.465–1.427 | 0.474 | 0.909 | 0.520–1.590 | 0.738 |
| D-dimer | 3.049 | 1.765–5.265 | <0.001 | 4.239 | 2.395–7.503 | <0.001 |
| T stage | 1.391 | 0.870–2.224 | 0.169 | 1.294 | 0.812–2.060 | 0.278 |
| N stage | 2.117 | 1.286–3.484 | 0.003 | 2.626 | 1.570–4.392 | <0.001 |
| TNM stage | 3.799 | 2.027–7.120 | <0.001 | 4.096 | 2.200–7.626 | <0.001 |
| Brain metastases | 1.345 | 0.821–2.204 | 0.240 | 1.452 | 0.889–2.374 | 0.136 |
| VTE | 0.868 | 0.273–2.764 | 0.811 | 0.902 | 0.283–2.872 | 0.861 |
| Treatment | 0.593 | 0.361–0.972 | 0.038 | 0.563 | 0.345–0.918 | 0.021 |
| NSE | 2.398 | 1.079–5.328 | 0.032 | 1.607 | 0.766–3.373 | 0.210 |
HR, hazard ratio; 95%CI, 95% confidence interval; KPS, Karnofsky performance status; TNM, tumor node metastasis; NSE, neuron-specific enolase; VTE, venous thromboembolism
Multivariate analysis
Multivariate analysis revealed that TNM stage (hazard ratio [HR] = 2.65, 95% confidence interval [CI]: 1.16–6.07, P = 0.021), treatment (HR = 0.59, 95%CI: 0.35–0.98, P = 0.043), and NSE level (HR = 2.60, 95%CI: 1.12–6.07, P = 0.027) were independently associated with PFS, while D-dimer level (HR = 2.73, 95%CI: 1.33–5.63, P = 0.006) and treatment (HR = 0.56, 95%CI: 0.34–0.93, P = 0.024) were independently associated with OS (Table 3).
Table 3.
Multivariate Cox proportional hazards regression analysis of factors associated with survival in patients with small cell lung cancer.
| Variable |
Progression-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| KPS score | 1.160 | 0.597–2.255 | 0.661 | – | – | – |
| D-dimer | 1.878 | 0.910–3.877 | 0.088 | 2.733 | 1.326–5.630 | 0.006 |
| N stage | 0.793 | 0.373–1.685 | 0.547 | 1.236 | 0.699–2.186 | 0.467 |
| TNM stage | 2.649 | 1.157–6.066 | 0.021 | 2.116 | 0.970–4.615 | 0.060 |
| Treatment | 0.589 | 0.352–0.983 | 0.043 | 0.560 | 0.339–0.925 | 0.024 |
| NSE | 2.603 | 1.116–6.072 | 0.027 | – | – | – |
HR, hazard ratio; 95%CI, 95% confidence interval; KPS score, Karnofsky performance status score of either 90–100 or 70–90; TNM, tumor node metastasis; NSE, neuron-specific enolase
Correlations between D-dimer level and tumor stage
High D-dimer levels were associated with T4 stage (76.9% vs. 53.5%, P = 0.03 vs. T1+T2+T3), N3 stage (94.6% vs. 40.0%, P < 0.001 vs. N0+N1+N2), and IIIb+IV stage (82.5% vs. 5.4%, P < 0.001 vs. I+II+IIIa) (Table 4). Fibrinogen levels were not associated with TNM stage.
Table 4.
Correlations between clinical TNM stage and D-dimer and fibrinogen levels.
| Clinical stage | P value | ||
|---|---|---|---|
| T stage | T1+T2+T3 | T4 | |
| D-dimer (mean ± SD)Median (min, max) | 1.14 ± 1.16 | 1.37 ± 1.38 | 0.183$ |
| 0.62 (0.12, 5.27) | 0.91 (0.25, 5.27) | ||
| >0.55 mg/L (n, %) | 23, 53.5% | 30, 76.9% | 0.027 |
| Fibrinogen (mean ± SD) Median (min, max) | 378.35 ± 113.72 | 391.34 ± 106.37 | 0.504$ |
| 384 (236.0, 783.0) | 397 (203.0, 666.0) | ||
| >Normal | 20, 46.5% | 18, 46.2% | 0.974 |
| N stage | N0+N1+N2 | N3 | |
| D-dimer (mean ± SD)Median (min, max) | 0.83 ± 0.96 | 1.77 ± 1.41 | <0.001$ |
| 0.50 (0.12, 5.27) | 1.20 (0.28, 5.27) | ||
| >0.55 mg/L (n, %) | 18, 40.0% | 35, 94.6% | <0.001 |
| Fibrinogen (mean ± SD)Median (min, max) | 386.18 ± 112.83 | 382.52 ± 107.51 | 0.915$ |
| 397.0 (203.0, 783.0) | 379.0 (236.0, 666.0) | ||
| >Normal | 21, 46.7% | 17, 46.0% | 0.948 |
| TNM stage | I+II+IIIa | IIIb+IV | |
| D-dimer (mean ± SD)Median (min, max) | 0.57 ± 0.57 | 1.46 ± 1.35 | <0.001$ |
| 0.42 (0.15, 2.81) | 0.91 (0.12, 5.27) | ||
| >0.55 mg/L (n, %) | 1, 5.4% | 52, 82.5% | <0.001 |
| Fibrinogen (mean ± SD)Median (min, max) | 371.02 ± 103.67 | 388.60 ± 112.06 | 0.370$ |
| 358.0 (253.0, 623.0) | 397 (203.0, 783.0) | ||
| >Normal | 8, 42.1% | 30, 47.6% | 0.673 |
SD, standard deviation. $, Mann–Whitney test
Discussion
Coagulation activation is common in cancer patients and is associated with a poor prognosis.11,12 However, information on D-dimer levels in patients with SCLC is scarce. We therefore measured plasma D-dimer levels in Chinese patients with SCLC, and showed that pretreatment plasma D-dimer levels were independently associated with OS.
Activation of the hemostatic system is involved in tumor development, dissemination, and metastasis,17,21 and activation of fibrinolysis secondary to coagulation activation is a well-recognized complication in patients with lung cancer.22 Fibrin deposits in lung cancer tissues have been suggested to promote the proliferation of cancer cells as well as the development of new blood vessels, while these deposits could also protect the tumor cells from immune cells or chemotherapeutic agents and promote their capillary implantation.23
D-dimer is a product of cross-linked fibrin degradation by plasmin-induced fibrinolytic activity, and D-dimer levels are a biomarker of global hemostasis and fibrinolysis.24 High D-dimer levels were associated with poor prognoses in patients with breast,25–27 colon and rectum,28,29 and lung30–32 cancers. Furthermore, Chen et al.32 found that high plasma D-dimer levels were significantly predictive of shorter survival in patients with SCLC, while others studies17,33 also concluded that plasma D-dimer levels may predict survival in patients with SCLC and NSCLC. However, most of these previous studies, apart from Chen et al.32 (393 patients), only included small numbers of patients with SCLC. Furthermore, the current study included more patients with lower KPS scores compared with Chen et al.32 (KPS 90–100, n = 46 [56.1%]; KPS 70–90, n = 36 [43.9%]). Given that patients with SCLC are generally diagnosed at a relatively advanced stage, the present study should provide more evidence for patients with low KPS scores. Accordingly, 63 patients (76.8%) in this study were stage IIIb+IV. The present study thus provides more reference values for patients with later-stage tumors and poorer overall condition.
SCLC is a highly invasive tumor with a poor prognosis,18 with different biological characteristics from NSCLC.18 Valid biomarkers are therefore needed to determine the prognosis of SCLC. In the present study, pretreatment plasma D-dimer levels were independently associated with OS in patients with SCLC. This conclusion is supported by previous studies of lung cancer in general (both SCLC and NSCLC).17,22,30,31,33 However, many factors are known to influence the survival of cancer patients and could potentially confound the association between D-dimer levels and survival. Nevertheless, the results showed that D-dimer was associated with survival independently of treatment and TNM stage. Treatment modality (chemotherapy alone vs. chemoradiotherapy) was also independently associated with PFS and OS, in accord with previous results indicating that radiotherapy improved the prognosis of patients with SCLC.18 In the current study, TNM staging was also independently associated with PFS, but not with OS. This could be because patients with early-stage cancer would also have a good KPS score and could receive multi-line chemotherapy, thoracic perfusion chemotherapy, or radiotherapy of the chest, brain, and bone. Interestingly, the present study indicated that plasma NSE levels were independently associated with PFS, but not with OS. Several studies have identified plasma NSE levels as a useful tumor marker for SCLC.34–37 However, the present study only included 11 patients with normal NSE levels, which may have affected the validity of the statistical analysis of NSE levels.
Plasma D-dimer is an indicator of the body's blood coagulation fibrinolysis state. It can be detected simply and rapidly, and can thus provide reliable information to inform immediate clinical decisions. Dynamic monitoring of plasma D-dimer levels in cancer patients could thus help to estimate the evolution of the illness, determine prognosis and the risk of recurrence, and indicate if anticoagulant treatment could reduce complications and mortality. Future clinical studies could be carried out to determine if anticoagulant therapy with prophylactic low-molecular-weight heparin could improve the prognosis of SCLC patients with abnormally elevated D-dimer levels and no bleeding tendency.
The main limitation of this study was the small sample size, which reflects the generally low incidence of SCLC. Further, multicenter studies could help to increase the sample size. In addition, the available data were limited owing to the retrospective nature of the study. The relationships between complications of anti-cancer treatment and PFS and OS could not be examined because the patients might attend any hospital or clinic in the event of complications, which would introduce underreporting or recall bias. Prospective studies with a broader coagulation-test panel could provide additional clues regarding the role of coagulation in the prognosis of SCLC. Furthermore, the current study was conducted in Chinese patients, and further studies in different ethnic groups are needed to determine the generalizability of the results.
In conclusion, high pretreatment plasma D-dimer levels were associated with shorter OS in patients with SCLC. D-dimer levels could be used as a non-invasive and convenient clinical prognostic biomarker in patients with SCLC.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–1260. [DOI] [PubMed] [Google Scholar]
- 3.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015; 121: 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Cheng Y, Li H, Zhang S. Current status of small cell lung cancer in China. J Cancer Biol Res 2014; 2: 1032. [Google Scholar]
- 5.Xiao Y, Yu H, Liu S, et al. Imaging findings and progress in treatment of small cell lung cancer. Int J Med Radio 2017; 40: 152–156. DOI: 10.19300/j.2017.Z4398 [Google Scholar]
- 6.Ries LAG, Young JL, Keel GE, et al. SEER Survival Monograph: Cancer survival among adults: U.S. SEER program, 1988-2001, patient and tumor characteristics. Pub No. 07-6215. Bethesda: National Cancer Institute, 2007. [Google Scholar]
- 7.Rehfeld N, Geddert H, Atamna A, et al. The influence of the pituitary tumor transforming gene-1 (PTTG-1) on survival of patients with small cell lung cancer and non-small cell lung cancer. J Carcinog 2006; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011; 378: 1741–1755. [DOI] [PubMed] [Google Scholar]
- 9.Henschke CI, Yankelevitz DF, Miettinen OS, et al. Computed tomographic screening for lung cancer: the relationship of disease stage to tumor size. Arch Intern Med 2006; 166: 321–325. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda H, Yamaya M, Nakayama K, et al. Arterial carboxyhemoglobin concentrations as a predictor of chemosensitivity in elderly patients with advanced lung cancer. J Am Geriatr Soc 2006; 54: 373–375. [DOI] [PubMed] [Google Scholar]
- 11.Kvolik S, Jukic M, Matijevic M, et al. An overview of coagulation disorders in cancer patients. Surg Oncol 2010; 19: e33–e46. [DOI] [PubMed] [Google Scholar]
- 12.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost 2013; 11: 223–233. [DOI] [PubMed] [Google Scholar]
- 13.Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood 2009; 113: 2878–2887. [DOI] [PubMed] [Google Scholar]
- 14.Feng JF, Yang X, Chen S, et al. Prognostic value of plasma D-dimer in patients with resectable esophageal squamous cell carcinoma in China. J Cancer 2016; 7: 1663–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Huh SJ, Oh SY, et al. Clinical significance of coagulation factors in operable colorectal cancer. Oncol Lett 2017; 13: 4669–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012; 97: 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komurcuoglu B, Ulusoy S, Gayaf M, Guler A, Ozden E. Prognostic value of plasma D-dimer levels in lung carcinoma. Tumori 2011; 97: 743–748. [DOI] [PubMed] [Google Scholar]
- 18.Kalemkerian GP, Loo BW, Akerley W, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Small Cell Lung Cancer. version 3.2017. Fort Washington: National Comprehensive Cancer Network, 2017. [Google Scholar]
- 19.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst 2010; 102: 1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishino M, Jagannathan JP, Ramaiya NH, et al. Revised RECIST guideline version 1.1: what oncologists want to know and what radiologists need to know. AJR Am J Roentgenol 2010; 195: 281–289. [DOI] [PubMed] [Google Scholar]
- 21.Chen WH, Tang LQ, Wang FW, et al. Elevated levels of plasma D-dimer predict a worse outcome in patients with nasopharyngeal carcinoma. BMC Cancer 2014; 14: 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taguchi O, Gabazza EC, Yasui H, et al. Prognostic significance of plasma D-dimer levels in patients with lung cancer. Thorax 1997; 52: 563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasic GJ, Tuszynski GP, Gorelik E. Interaction of the hemostatic and immune systems in the metastatic spread of tumor cells. Int Rev Exp Pathol 1986; 29: 173–212. [PubMed] [Google Scholar]
- 24.Fukumoto K, Taniguchi T, Usami N, et al. Preoperative plasma D-dimer level is an independent prognostic factor in patients with completely resected non-small cell lung cancer. Surg Today 2015; 45: 63–67. [DOI] [PubMed] [Google Scholar]
- 25.Blackwell K, Haroon Z, Broadwater G, et al. Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol 2000; 18: 600–608. [DOI] [PubMed] [Google Scholar]
- 26.Dirix LY, Salgado R, Weytjens R, et al. Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer. Br J Cancer 2002; 86: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batschauer AP, Figueiredo CP, Bueno EC, et al. D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann Oncol 2010; 21: 1267–1272. [DOI] [PubMed] [Google Scholar]
- 28.Blackwell K, Hurwitz H, Lieberman G, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer 2004; 101: 77–82. [DOI] [PubMed] [Google Scholar]
- 29.Oya M, Akiyama Y, Okuyama T, et al. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol 2001; 31: 388–394. [DOI] [PubMed] [Google Scholar]
- 30.Altiay G, Ciftci A, Demir M, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol (R Coll Radiol) 2007; 19: 494–498. [DOI] [PubMed] [Google Scholar]
- 31.Buccheri G, Torchio P, Ferrigno D. Plasma levels of D-dimer in lung carcinoma: clinical and prognostic significance. Cancer 2003; 97: 3044–3052. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Yu H, Wu C, et al. Prognostic value of plasma D-dimer levels in patients with small-cell lung cancer. Biomed Pharmacother 2016; 81: 210–217. [DOI] [PubMed] [Google Scholar]
- 33.Antoniou D, Pavlakou G, Stathopoulos GP, et al. Predictive value of D-dimer plasma levels in response and progressive disease in patients with lung cancer. Lung Cancer 2006; 53: 205–210. [DOI] [PubMed] [Google Scholar]
- 34.Wojcik E, Kulpa JK, Sas-Korczynska B, et al. ProGRP and NSE in therapy monitoring in patients with small cell lung cancer. Anticancer Res 2008; 28: 3027–3033. [PubMed] [Google Scholar]
- 35.Huang Z, Xu D, Zhang F, et al. Pro-gastrin-releasing peptide and neuron-specific enolase: useful predictors of response to chemotherapy and survival in patients with small cell lung cancer. Clin Transl Oncol 2016; 18: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen LG, Osterlind K, Hansen HH, et al. Serum neuron specific enolase (NSE) is a determinant of response duration in small cell lung cancer (SCLC). Br J Cancer 1992; 66: 594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibayama T, Ueoka H, Nishii K, et al. Complementary roles of pro-gastrin-releasing peptide (ProGRP) and neuron specific enolase (NSE) in diagnosis and prognosis of small-cell lung cancer (SCLC). Lung Cancer 2001; 32: 61–69. [DOI] [PubMed] [Google Scholar]