Short abstract
Objective
The growth of the older population is a great challenge for tuberculosis (TB) control in South Korea. This study was performed to investigate the clinical characteristics of and treatment outcomes among octogenarian patients with TB.
Methods
We retrospectively analyzed the medical records of 109 patients with TB (age of ≥80 years) from January 2014 to March 2017. Clinical, microbiologic, and radiologic findings were obtained.
Results
Fifty-five patients (50.5%) were male, the mean age of the patients was 83.8 years, and 75 patients (68.8%) had pulmonary TB. All patients with pulmonary TB underwent either chest X-ray or chest computed tomography examination, and the results showed that only one-third (n = 33, 39.3%) had active lesions suggestive of TB. Twenty-nine patients (26.4%) had an unfavorable outcome (21 died and 8 were lost to follow-up). Only two TB-related deaths occurred, and both were caused by respiratory failure. Among the 15 non-TB-related deaths, the progression of malignancy and sepsis were the most frequent causes of death.
Conclusions
A high mortality rate was observed in octogenarian patients with TB, and most of these deaths were non-TB-related. Among all causes of mortality, solid malignancy was a significant risk factor for death.
Keywords: Outcome, mortality, octogenarian, tuberculosis, malignancy, sepsis
Introduction
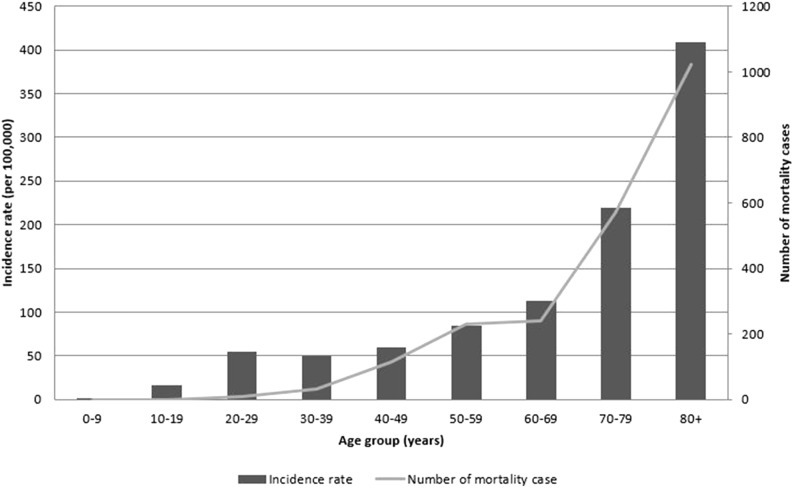
Tuberculosis (TB) is a major burden in numerous countries worldwide, including both developed and developing countries, with approximately 10.4 million cases reported in 2016.1 Despite the implementation of appropriate strategies against TB, it remains the ninth leading cause of death worldwide and the leading cause of death from a single infectious agent. In South Korea, the incidence of TB is rapidly increasing in individuals more than 60 years of age, and the incidence and mortality rates of TB in patients more than 80 years of age are significantly higher than those of any other age group in South Korea (Figure 1).2 The total healthcare cost of treating octogenarian patients with TB in South Korea is also steadily increasing.3 Moreover, owing to the limited daily activities of octogenarian people in South Korea, many of these individuals stay in long-term residential facilities such as nursing homes. Once these individuals have contracted TB, they are likely to spread the disease among the other residents of the facility and the younger caregivers. TB outbreaks among older people residing at such facilities often cause public health and social problems.
Figure 1.
Incidence rate of tuberculosis and number of tuberculosis-related deaths in South Korea (data from the annual report on the notified tuberculosis patients in Korea, 2016).
People of advanced age are more susceptible to numerous infectious diseases. They also have an increased risk of developing age-associated diseases such as cancer and cardiac disease. Changes in immune function with increasing age are considered a risk factor for infection in older individuals.4 In addition to reactivation of latent TB infection in people of advanced age,5,6 such individuals are also highly susceptible to developing TB if they are infected at an older age. Numerous studies have shown that advanced age is a risk factor for death in patients with TB.7,8
Although the clinical characteristics of older patients and the burdens of TB have been investigated, many previous studies used the chronological age of ≥65 years as the definition of elderly; the TB burden among octogenarian patients has not been fully elucidated. Therefore, the present study was performed to investigate the clinical characteristics and treatment outcomes among octogenarian patients with TB.
Materials and methods
Participants
All adult patients aged ≥80 years who were diagnosed with TB at Chungbuk National University Hospital, South Korea from January 2014 to March 2017 were enrolled in the study. For the diagnosis of pulmonary TB, both sputum acid-fast bacilli (AFB) smears and culture were performed simultaneously in the initial microbiological investigation of every patient with respiratory signs and symptoms. For patients with suspected TB and atypical pneumonia, nucleic acid amplification (i.e., TB-polymerase chain reaction assay) was also performed. Bronchoscopy was performed for patients unable to expectorate sputum. Pathology results were used in the diagnosis of both pulmonary and extrapulmonary TB. Active TB was diagnosed in the presence of at least one of the following three criteria: a positive microbiological test result for Mycobacterium tuberculosis, biopsy-based histological findings compatible with TB, and radiographic findings suggestive of TB.
Patients with TB underwent a 6-month treatment regimen as recommended by the National Tuberculosis Program. The regimens were modified based on each patient’s adverse drug reactions or drug resistance profile. Anti-TB drugs were self-administered with the support of trained nurses in a private–public matrix project.
Data collection
Data on the following clinical and demographic characteristics were collected: age, sex, height, body weight, initial presenting symptoms, and medical history. The results of the initial microbiological tests were also obtained. If the sputum volume was not adequate for mycobacterial testing, the results were deemed negative. The results of radiographic examinations, computed tomography (CT), and magnetic resonance imaging were classified into three categories: active lesions suggestive of TB, active lesions suggesting disease conditions other than TB, and inactive lesions. We also measured the health system delay, which was defined as the number of days that had elapsed from the first hospital visit to the administration of anti-TB drugs.9
Treatment outcomes
In the analysis of treatment outcomes, we used the definitions and recommendations of the World Health Organization (WHO)10 regarding cure, treatment completion, treatment failure, and death. Death was defined as death during treatment irrespective of cause. All deaths in patients with TB were classified into two groups according to the underlying cause of death: either TB-related or non-TB-related. We adopted the following definition of TB-related death described in a previous article11: microbiological or pathologic evidence of sole TB infection without other pathogens cultured from sterile body fluid or tissue that was collected aseptically, agreement between the reviewing physician and the underlying cause of death recorded on the death certificate or medical records by the primary care physician, and no other cause that was equally likely to result in mortality. All three criteria were required to be met for a diagnosis of TB-related death; otherwise, the mortality was classified as non-TB-related death. The outcomes of patients who were transferred to other institutions were also evaluated using a private–public matrix network.
Statistical analysis
Continuous variables are presented as mean and standard deviation or median and interquartile range (IQR), whereas discrete variables are presented as frequency or percentage. To compare differences between patients who died and patients who survived, a multivariate analysis was performed using binary logistic regression to determine the odds ratios and 95% confidence intervals. In the regression analysis, forward and backward methods were used to select variables for inclusion in the final model. A p-value of ≤0.05 was considered statistically significant. The statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki. The institutional review board of Chungbuk National University Hospital, South Korea approved the study protocol and waived the need for informed consent because no patients were at risk (IRB no. 2017-12-008).
Results
In total, 109 patients aged ≥80 years were enrolled in this study. Table 1 summarizes their clinical characteristics. Fifty-six (50.9%) patients were men. One-fifth of the patients had a previous history of TB, and one-third were smokers. Hypertension (56.4%) was the most frequent comorbidity, followed by diabetes (24.5%) and solid malignancy (15.6%). The most common respiratory symptom was cough, and more than half of the patients complained of dyspnea at their initial hospital visit. Eighty-four (77.1%) patients had pulmonary TB. Ninety-two (84.4%) patients had received either a combination regimen of isoniazid, rifampicin, ethambutol, and pyrazinamide (HREZ) or HRE (HREZ without pyrazinamide). No clinical variables were significantly different between patients with pulmonary and extrapulmonary TB except that more patients with pulmonary TB had a productive cough. The patients in this study had symptoms of TB for a median of 7 days (IQR, 1–14 days) before the first hospital visit. The median health system delay was 8 days (IQR, 4–16 days). Twenty-eight (25.6%) patients experienced a health system delay of >14 days. Five patients had drug-resistant TB; two had multidrug-resistant TB and three had non-multidrug-resistant, isoniazid-resistant TB. One patient with multidrug-resistant TB developed a severe adverse event that resulted in the interruption of anti-TB treatment. Four other patients with drug-resistant TB completed anti-TB treatment according to the WHO guideline without extension of the treatment duration.
Table 1.
Demographic and clinical characteristics of 109 octogenarian patients with TB.
| Characteristics | |
| Age, years | 83.8 ± 3.4 |
| Male | 55 (50.5) |
| Body mass index, kg/m2 | 21.2 ± 3.5 |
| History of previous TB | 25 (22.9) |
| Current smoker | 35 (32.1) |
| Comorbidities | |
| Hypertension | 62 (56.9) |
| Diabetes mellitus | 27 (24.8) |
| Solid malignancy | 17 (15.6) |
| Chronic obstructive pulmonary disease | 14 (12.8) |
| Chronic kidney disease with dialysis | 1 (0.9) |
| Human immunodeficiency virus infection | 1 (0.9) |
| Initial symptoms | |
| Cough | 59 (54.1) |
| Sputum | 54 (49.5) |
| Fever | 28 (25.7) |
| Dyspnea | 57 (52.3) |
| Interval from symptom onset to first hospital visit, days | 7 [1–14] |
| Interval from first hospital visit to anti-TB treatment, days | 8 [4–16] |
| Anti-TB treatment regimen | |
| HREZ | 82 (75.2) |
| HRE | 10 (9.2) |
| HR | 8 (7.3) |
| Other combination regimen including second-line drugs | 6 (5.5) |
| No treatment | 3 (2.8) |
| Treatment outcome | |
| Cured | 9 (8.3) |
| Complete | 71 (65.1) |
| Death | 21 (19.3) |
| Loss to follow-up | 8 (7.3) |
Data are presented as mean ± standard deviation, n (%), or median (interquartile range).
TB, tuberculosis; HREZ, combination regimen of isoniazid, rifampicin, ethambutol, and pyrazinamide; HRE, combination regimen of isoniazid, rifampicin, and ethambutol; HR, combination regimen of isoniazid and rifampicin.
Among all 109 patients, respiratory specimens for 101 (92.7%) patients were available and were used for initial AFB smear and culture tests. Eight (7.3%) patients were both unable to expectorate an adequate sputum sample and unable to undergo bronchoscopy. A TB-polymerase chain reaction assay was also performed for 48 patients (44.0%). Although AFB smear and culture tests were performed for patients with both pulmonary and extrapulmonary TB, only patients with pulmonary TB had positive results (Table 2). Eighteen (16.5%) and 46 (42.2%) patients had a positive AFB smear and culture test result for pulmonary TB, respectively. In total, 56 (51.4%) patients had bacteriologically confirmed TB with at least one positive mycobacterial test result, and 53 (48.6%) patients had clinically diagnosed TB that did not fulfill the criteria for bacteriological confirmation but that was diagnosed as active TB by a clinician who had decided to administer a full course of TB treatment according to the WHO guidelines.12
Table 2.
Microbiological, radiological, and pathological findings of 109 octogenarian patients with TB.
| Characteristics | All(n = 109) | Pulmonary TB (n = 84) | Extrapulmonary TB (n = 25) | p-value |
|---|---|---|---|---|
| Microbiological findings | ||||
| Positive AFB smear | 18 (16.5) | 18 (21.4) | 0 (0.0) | 0.011 |
| Positive AFB culture | 46 (42.2) | 46 (54.8) | 0 (0.0) | 0.000 |
| Positive TB-PCR assay | 20 (18.3) | 20 (23.8) | 0 (0.0) | 0.003 |
| At least one positive mycobacterial test result | 56 (51.4) | 56 (66.7) | 0 (0.0) | 0.000 |
| Radiologic findings | ||||
| Active lesions suggestive of TB | 35 (32.1) | 33 (39.3) | 2 (8.0) | 0.013 |
| Active lesions suggestive of conditions other than TB§ | 57 (52.3) | 39 (46.4) | 18 (72.0) | |
| Inactive lesions¶ | 17 (15.6) | 12 (14.3) | 5 (20.0) | |
| Radiologically confirmed TB cases | 57 (52.3) | 51 (46.8) | 6 (24.0) | 0.001 |
| Pathological findings consistent with TB | 21 (19.3) | 15 (17.8) | 6 (24.0) | 0.494 |
Data are presented as n (%).
SD, standard deviation; TB, tuberculosis; AFB, acid-fast bacilli; PCR, polymerase chain reaction.
§Lesions suggestive of infection, malignant mass, lung nodule, or pleural effusion.
¶Lesions such as fibrotic changes of lung parenchyma or atelectasis.
All patients with pulmonary TB initially underwent either chest X-ray or chest CT examinations. The results showed that only about one-third of patients (n = 33, 39.3%) had active lesions suggestive of TB. Among the other 51 patients with pulmonary TB who had no evidence of active TB lesions on chest imaging, 24 had suspected bacterial pneumonia, 9 had suspected lung cancer, 4 had pleural effusion, and 12 had only inactive TB lesions on chest imaging. All patients with extrapulmonary TB underwent additional radiographic examinations, including CT or magnetic resonance imaging of other affected organs, the results of which showed that nearly three-quarters (n = 18, 72.0%) had active lesions suggestive of disease conditions other than TB, such as malignancy. The most common site of extrapulmonary TB was the pleura, followed by the bone. Twenty-one patients had positive biopsy results compatible with TB. Because other diseases such as lung cancer were initially suspected, 15 patients with pulmonary TB underwent either percutaneous needle aspiration biopsy or transbronchial biopsy in addition to radiographic examination. These biopsies comprised 11 lung tissue specimens, 3 bronchial tissue specimens, and 1 lymph node tissue specimen.
Twenty-nine patients (26.4%) had unfavorable outcomes (21 died and 8 were lost to follow-up). TB-related death occurred in only two patients, both of whom died of respiratory failure (Table 3). Among the 15 non-TB-related deaths, the most frequent causes of death were progression of malignancy and sepsis, followed by respiratory failure and heart failure. Among the eight patients who were lost to follow-up, two developed adverse effects to the anti-TB drugs and had to stop anti-TB treatment, and six patients either refused to undergo treatment or did not visit the hospital.
Table 3.
Causes of death among 21 octogenarian patients with TB.
| Cause of death | |
| TB-related death (n = 2) | |
| Respiratory failure | 2 (9.5) |
| Non-TB-related death (n = 15) | |
| Malignancy | 5 (23.8) |
| Sepsis or septic shock | 5 (23.8) |
| Pneumonia with respiratory failure | 3 (14.3) |
| Acute exacerbation of chronic respiratory disease | 1 (4.8) |
| Heart failure | 1 (4.8) |
| Unknown cause of death (n=4) |
Data are presented as n (%).
TB, tuberculosis.
The median interval from TB diagnosis to mortality was 30 days (IQR, 11–112 days). Among the 21 patients who died, 9 (42.9%) died within 30 days of diagnosis and 12 (57.1%) died during the intensive phase of anti-TB treatment. There was no significant difference in the health system delay between the patients who died and those who survived. Multivariate analysis using binary logistic regression was performed to identify risk factors for mortality, and the results showed that solid malignancy was an independent risk factor for death (Table 5).
Table 5.
Comparison of risk factors for mortality among 109 patients with TB.
| Variables | Survival(n = 88) | Death(n = 21) | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| Age, years | 83.8 ± 3.4 | 84.0 ± 3.6 | 1.02 (0.89–1.17) | 0.815 | 1.07 (092–1.25) | 0.389 |
| Male | 40 (45.5) | 15 (71.4) | 3.00 (1.07–8.45) | 0.038 | 2.15 (0.58–7.99) | 0.254 |
| Body mass index, kg/m2 | 21.3 ± 3.4 | 20.5 ± 4.0 | 0.92 (0.80–1.07) | 0.281 | ||
| History of previous TB | 17 (19.3) | 8 (38.1) | 2.57 (0.92–7.18) | 0.072 | 2.96 (0.90–9.75) | 0.074 |
| Current smoker | 26 (29.5) | 9 (42.9) | 1.64 (0.62–4.39) | 0.320 | 1.35 (0.38–4.74) | 0.643 |
| Diabetes mellitus | 21 (23.9) | 6 (28.6) | 1.28 (0.44–3.71) | 0.654 | ||
| Solid malignancy | 9 (10.2) | 8 (38.1) | 5.40 (1.77–16.53) | 0.003 | 6.72 (1.89–23.90) | 0.003 |
| Chronic obstructive pulmonary disease | 11 (12.5) | 3 (14.3) | 1.18 (0.30–4.62) | 0.826 | ||
| Fever | 23 (26.1) | 5 (23.8) | 0.88 (0.29–2.68) | 0.827 | ||
| Dyspnea | 46 (69.7) | 11 (52.4) | 1.00 (0.39–2.61) | 0.993 | ||
| Positive AFB smear test result | 14 (15.9) | 4 (19.0) | 1.24 (0.36–4.26) | 0.728 | ||
| Positive AFB culture test result | 40 (45.5) | 6 (28.6) | 0.48 (0.17–1.35) | 0.165 | ||
| Radiologic findings | ||||||
| Inactive lesions | 16 (18.2) | 1 (4.8) | ||||
| Active lesions suspicious of TB | 32 (36.4) | 4 (19.0) | 2.07 (0.21–20.04) | 0.532 | ||
| Active lesions other than TB | 41 (46.6) | 16 (76.2) | 6.24 (0.76–51.05) | 0.088 | ||
Data in survival and death groups are presented as mean ± standard deviation or n (%).
OR, odds ratio; CI, confidence interval; TB, tuberculosis; AFB, acid-fast bacilli.
Among the 17 patients with solid malignancy, the most frequent type of malignancy was lung cancer (n = 6, 35.3%), followed by gastric cancer and colorectal cancer (Table 4). One patient was simultaneously diagnosed with lung cancer and pulmonary TB, and three patients were diagnosed with solid malignancy during anti-TB treatment. Eight of the 17 patients with cancer died during anti-TB treatment (mortality rate of 47.1%); 7 with cancer who were receiving anti-TB treatment but died had advanced cancer, and 1 patient with skin cancer undergoing concurrent radiotherapy died during anti-TB treatment. Five patients with cancer died during the intensive phase of anti-TB treatment.
Table 4.
Cancer treatment and anti-TB treatment of 17 patients with TB and malignancy.
| Age/Sex | TB site | Cancer site | Interval between cancer diagnosis and ATT | Treatment related to cancer before diagnosis of TB | Outcome |
|---|---|---|---|---|---|
| 82/M | Lung | Lung | 3 mo | Only supportive care | Death |
| 82/M | Lung | Lung | 35 mo | Cancer progression after chemotherapy | Death |
| 81/M | Lung | Lung | 1 mo | Hospice care without chemotherapy | Death |
| 82/M | Lung | Lung | During ATT | Simultaneous diagnosis of TB and cancer | Death |
| 84/F | Lung | Colon | 5 mo | Cancer progression after chemotherapy | Death |
| 85/F | Lung | Colon | 13 mo | Conservative care after surgical resection | Death |
| 85/M | Lung | Prostate | 48 mo | Palliative therapy after chemotherapy | Death |
| 80/M | Lung | Skin | 2 mo | Surgery followed by radiotherapy | Death |
| 81/M | Lung | Lung | During ATT | Only supportive care | Complete |
| 81/M | Lung | Lung | During ATT | Surgical resection | Complete |
| 82/F | Lung and bone | Stomach | 22 mo | Endoscopic resection | Complete |
| 84/F | CNS | Stomach | 8 mo | Surgical resection | Complete |
| 80/F | Lung | Rectum | During ATT | Surgical resection | Complete |
| 91/M | Lung | Stomach | 36 mo | Surgical resection | Cure |
| 82/M | Lung | Stomach | 60 mo | Surgical resection | Cure |
| 83/M | Lung | Liver | 40 mo | Transarterial chemoembolization | F/U loss |
M, male; F, female; mo, months; CNS, central nervous system; ATT, anti-tuberculosis treatment; TB, tuberculosis; F/U, follow-up.
Discussion
To the best of our knowledge, no previous study has been performed to evaluate >80-year-old patients with TB. The present study revealed a high rate of unfavorable outcomes and a mortality rate associated with solid malignancy. The management of older patients with TB is challenging. The diagnosis of TB may be delayed because of nonspecific physical symptoms and atypical radiographic findings, delaying the start of treatment.6,13 Several studies have revealed that older patients with pulmonary TB have a high rate of middle and lower lobe involvement that is often accompanied by pleural effusion and mass-like lesions or nodules that are not easily distinguishable from malignant disease or bacterial pneumonia. In addition, older adults have a reduced ability to produce a high-quality sputum specimen. Because many of the octogenarian patients in our study also had nonspecific respiratory symptoms and test results that were not suggestive of TB, we hypothesized that the health system delay in these patients is an important issue because physicians might miss and delay the diagnosis of TB. However, the delay in the present study was not associated with mortality, similar to other studies.11,14
The number of advanced-age patients in South Korea has steadily increased during the last three decades, and South Korea has thus become an aging society. The epidemiologic trend within the past few decades in South Korea generally changed from infectious diseases to chronic diseases such as diabetes, causing individuals (particularly older individuals) to be at greater risk of developing TB. People in South Korea who were born at the time when TB was highly prevalent, before the period of economic growth, have now become part of the older population, and TB recurrence among this population is expected to increase, making TB more difficult to control.15 The number of older people living in nursing homes has begun to increase in South Korea, and older patients with TB might pose a risk to others in the same long-term care facilities. The epidemiology of TB in the older population must be investigated to achieve TB elimination in South Korea.
In the present study, only two TB-related deaths occurred among octogenarian patients with TB. The older population generally has a higher prevalence of comorbidities than younger populations, which might explain the high rate of non-TB-related death in our study. The importance of identifying modifiable risk factors for mortality and developing interventions should be emphasized16 because such actions might provide an opportunity to reduce mortality during anti-TB treatment.
TB has been considered a risk factor for malignancy, particularly lung cancer,17 which is characterized by local and systemic chronic inflammation and the production of carcinogenic molecules. In contrast, TB might be an indicator of occult cancer because this condition may lead to locally reduced infection barriers and generalized immunosuppression. A recent systematic review and meta-analysis revealed that patients with cancer are at significantly increased risk of developing TB compared with the general population.18 There are several challenges in the diagnosis and treatment of TB among older patients with malignancy, such as similar clinical and radiographic presentation between the two diseases.19 Some experts have recommend screening patients born in countries that are endemic for TB before starting radiotherapy or chemotherapy.20 However, systematic screening for TB infection has its disadvantages because the cumulative lifetime risk of TB is reduced due to a decrease in the life expectancy among adults with solid malignancy.18 Future studies must be conducted to establish guidelines for the management of TB among patients with solid malignancy.
This study had several limitations. First, other variables, such as socioeconomic status, unemployment, and alcohol abuse, were not evaluated.7,16 Because we were unable to use such variables for adjustment, residual confounding might have affected the analysis, especially that related to mortality. Second, only a small number of patients, all of whom visited a single tertiary hospital, were enrolled in the study, thus limiting the generalizability of our findings in South Korea. Third, the study was subjected to selection bias because the data were retrospectively obtained from medical records.
Aging is associated with unfavorable outcomes and high mortality, particularly for patients with underlying malignancy. In South Korea, the incidence of TB among people of advanced age has increased along with demographic changes, which negatively impacts the national TB control program. Targeted efforts to improve case detection and treatment outcomes in this population are necessary. Further epidemiological studies of older patients with TB must be conducted to establish appropriate interventions and improve the treatment outcomes of TB.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2016E4600302).
References
- 1.Global Tuberculosis Report. Geneva, Switzerland: World Health Organization, 2017.
- 2.Annual Report on the Notified Tuberculosis in Korea. Osong, South Korea: Korea Centers for Disease Control & Prevention, 2016.
- 3.National Health Insurance Service. Pulmonary tuberculosis, most common in the elderly aged 70 or older. https://www.nhis.or.kr/bbs7/boards/B0039/25734 (2018, accessed 9 Aug 2018).
- 4.Piergallini TJ, Turner J. Tuberculosis in the elderly: why inflammation matters. Exp Gerontol 2018; 105: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajagopalan S. Tuberculosis and aging: a global health problem. Clin Infect Dis 2001; 33(7): 1034–1039. [DOI] [PubMed] [Google Scholar]
- 6.Negin J, Abimbola S, Marais BJ. Tuberculosis among older adults – time to take notice. Int J Infect Dis 2015; 32: 135–137. [DOI] [PubMed] [Google Scholar]
- 7.Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis 2011; 15: 871–885. [DOI] [PubMed] [Google Scholar]
- 8.Lefebvre N, Falzon D. Risk factors for death among tuberculosis cases: analysis of European surveillance data. Eur Respir J 2008; 31: 1256–1260. [DOI] [PubMed] [Google Scholar]
- 9.Yimer S, Bjune G, Alene G. Diagnostic and treatment delay among pulmonary tuberculosis patients in Ethiopia: a cross sectional study. BMC Infect Dis 2005; 5: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Tuberculosis Programme: framework for effective tuberculosis control. Geneva, Switzerland: World Health Organization, 1994.
- 11.Lin CH, Lin CJ, Kuo YW, et al. Tuberculosis mortality: patient characteristics and causes. BMC Infect Dis 2014; 14: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Definitions and reporting framework for tuberculosis – 2013 revision. Geneva, Switzerland: World Health Organization, 2013.
- 13.Tatar D, Senol G, Alptekin S, et al. Tuberculosis in older adults. Eur Geriatr Med 2013; 4: 15–19. [Google Scholar]
- 14.Oursler KK, Moore RD, Bishai WR, et al. Survival of patients with pulmonary tuberculosis: clinical and molecular epidemiologic factors. Clin Infect Dis 2002; 34: 752–759. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Yim JJ. Achievements in and challenges of tuberculosis control in South Korea. Emerg Infect Dis 2015; 21(11): 1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterling TR, Zhao Z, Khan A, et al. Mortality in a large tuberculosis treatment trial: modifiable and non-modifiable risk factors. Int J Tuberc Lung Dis 2006; 10: 542–549. [PubMed] [Google Scholar]
- 17.Kim HR, Hwang SS, Ro YK, et al. Solid-organ malignancy as a risk factor for tuberculosis. Respirology 2008; 13(3): 413–419. [DOI] [PubMed] [Google Scholar]
- 18.Dobler CC, Cheung K, Nguyen J, et al. Risk of tuberculosis in patients with solid cancers and haematological malignancies: a systematic review and meta-analysis. Eur Respir J 2017; 50: 1700157. [DOI] [PubMed] [Google Scholar]
- 19.Falagas ME, Kouranos VD, Athanassa Z, et al. Tuberculosis and malignancy. QJM 2010; 103: 461–487. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs REA, Gu P, Chachoua A. Reactivation of pulmonary tuberculosis during cancer treatment. Int J Mycobacteriol 2015; 4: 337–340. [DOI] [PubMed] [Google Scholar]