Short abstract
Diabetic macular oedema (DMO) is a common complication of diabetic retinopathy and may lead to severe visual loss. In this review, we describe the pathophysiology of DMO and review current therapeutic options such as macular laser photocoagulation, anti-vascular endothelial growth factor agents, and steroid implants with a focus on the new fluocinolone acetonide implant, ILUVIEN®. The results of the Fluocinolone Acetonide in Diabetic Macular Edema (FAME) studies are also presented together with the results of real-world studies to support the clinical use of ILUVIEN® in achieving efficient resolution of DMO and improving vision and macular anatomy in this challenging group of patients.
Keywords: Diabetic macular oedema, steroid implant, fluocinolone acetonide, ILUVIEN, diabetes, anti-VEGF agents, diabetic retinopathy, intravitreal injections
Introduction
Diabetic retinopathy (DR), a common vascular complication of diabetes, can become vision-limiting as the severity of disease progresses in 6.5% to 7.0% of patients.1–3 Diabetic macular oedema (DMO), a manifestation of DR, is characterised by increased vascular permeability and a breakdown of the blood–retina barrier. This results in the leakage of fluid and other plasma constituents into or surrounding the macula.3,4 Approximately 14% to 25% of patients diagnosed with diabetes appear to develop DMO within 10 years of presentation.5 Clinically significant DMO, which is characterised by retinal thickening and/or hard exudates that are present or evolving within the centre of the macula, is associated with advanced visual impairment and requires urgent treatment.6
The mechanisms by which hyperglycaemia causes DMO are thought to involve four major biochemical pathways (polyol, advanced glycation end products, protein kinase C, and hexosamine). Oxidative stress and angiogenesis lead to upregulation of inflammatory mediators, contributing to the breakdown of the blood–retinal barrier.4,7 Signalling molecules such as insulin-like growth factor-1, platelet-derived growth factor, angiopoietin, and vascular endothelial growth factor (VEGF) play significant roles in microangiopathy.8 The importance of VEGF in the breakdown of the blood–retinal barrier has led to the introduction of anti-VEGF treatments in DMO; however, their use has revealed that approximately one-third of patients are resistant to intravitreal treatment (including steroid implants).9,10
Historically, the gold standard treatment for DMO has been focal/grid laser photocoagulation based on the Early Treatment Diabetic Retinopathy Study (ETDRS).6 More recently, however, several landmark clinical trials have established anti-VEGF treatment as the first-line therapy in many patients with DMO.11,12 Nevertheless, many patients respond poorly to anti-VEGF treatment with transient or incomplete resolution of fluid.4,13 According to a recent DRCR.net analysis, approximately 40% of eyes had persistent DMO after 24 weeks of monthly ranibizumab injections.14 Among these eyes, the rate of chronic persistent DMO was approximately 55% at year 2.14 Corticosteroid treatment has also been shown to be an effective treatment option because corticosteroids can not only inhibit multiple damaging pathways, including the anti-VEGF pathway, but they can also inhibit chemokines and inflammatory cytokines.4,15 The preferred method of treatment for DMO using corticosteroids is placement of sustained-release, low-dose implants in the retina in an attempt to minimalise side effects whilst maximising macular delivery and efficacy.16
Fluocinolone acetonide (FA) is a corticosteroid available as an intravitreal implant. It is small (3.5 mm in length, 0.37 mm in diameter), non-biodegradable, and designed for injection using a 25-gauge injector via the pars plana into the vitreous cavity.17 The approved implant (ILUVIEN®) contains 0.19 mg of FA that is initially released at 0.25 µg/day (average, 0.2 µg/day), and the implant lasts 36 months.18 ILUVIEN® was approved by the National Institute for Health and Care Excellence (NICE) in 2013 as a treatment option for chronic DMO in patients with pseudophakia that is insufficiently responsive to standard therapies.19 The Fluocinolone Acetonide in Diabetic Macular Edema (FAME) A and B randomised clinical trials showed the clinical effectiveness of ILUVIEN® up to 36 months.20 This remains the primary source of evidence for its efficacy; however, new data are starting to emerge from clinical practice. The present report provides an updated review of ILUVIEN® with a focus on the new data.
Methods
We performed a search of PubMed using the keywords “fluocinolone acetonide” AND (“eye” OR “ocular”) and retrieved 199 results. Only 30 of them were studies on DMO; most were focused on uveitis and the RETISERT implant (Bausch + Lomb, Rochester, NY, USA). We then used the keywords “fluocinolone acetonide” AND “intravitreal” and retrieved 161 articles. Only 2 of them were not present in our first query and were therefore added to the first 30 articles that were retrieved.
Because this is a review article, no ethics committee approval was obtained. No studies or experiments on animals or humans were conducted.
Pharmacodynamics
FA is a medium-potency, synthetic fluorinated glucocorticoid. It is a selective, potent glucocorticoid receptor agonist with no mineralocorticoid activity.18,21 Glucocorticoids are well-known anti-inflammatory agents, but they also reduce the intravitreal levels of VEGF by cell inhibition and gene inhibition, causing regression of active neovascularisation.18 The principle method of corticosteroid action is thought to be via the induction of lipocortins particularly phospholipase A2. Most importantly, they inhibit the release of arachidonic acid from the phospholipid membrane, a common precursor for prostaglandins and leukotrienes, which are potent inflammatory cell mediators.22,23 FA has been shown to inhibit cell migration; the release of heparin, growth factors, and angiogenic factors; and the secretion of proinflammatory cytokines, which stimulate VEGF production. In early studies of retinal degeneration in rat models, intravitreal FA was shown to reduce the levels of neuroinflammation in the anterior and posterior chambers, preserve retinal electrophysiology and morphology, inhibit cell proliferation, and reduce angiogenesis.21,24,25 Sustained-release FA was associated with suppression of retinal microgliosis compared with high-dose FA.26 Intravitreal FA was shown to have positive effects in a human retinal pigment epithelial cell line (ARPE-19) by inhibiting VEGF secretion and mRNA expression. In a chick chorioallantoic membrane assay, it was also noted to inhibit tumour necrosis factor α-induced angiogenesis.27
In phase III trials, FA at 0.2 µg/day improved best-corrected visual acuity (BCVA) and reduced foveal thickness in patients with DMO. As early as 3 weeks, patients who received FA at 0.2 µg/day showed improvement in BCVA of ≥15 letters (p < 0.05) compared with placebo.20 This improvement remained significant throughout the remainder of the trial (36 months).16 Rapid and sustained reductions in foveal thickness (mean retinal central point thickness) were seen in patients who received FA at 0.2 µg/day. The central point thickness was significantly lower (p < 0.05) in the treatment arm than in the placebo are from as early as week 1. This significant difference remained at all measured points through the trial (24 months).16,20 An earlier phase II study showed no statistically significant difference between patients receiving FA at 0.2 versus 0.5 µg/day.15,21
Pharmacokinetic profile
A phase II study assessing the aqueous levels of FA after administration of inserts/implants showed that FA was released into the aqueous humour in a sustained manner for at least 36 months in patients with DMO.28 The mean aqueous concentration of FA reached its maximum approximately 1 week after administration. The sustained-release method of delivery in the 0.2-µg/day FA implant meant that a steady-state concentration of 0.5 to 1.0 ng/mL was reached by 6 to 9 months and maintained through the remainder of the 36-month trial. Low- and high-dose inserts provide sustained-release delivery of FA for at least 12 months with little variability between the doses. The 0.5-µg/day dose resulted in significantly higher aqueous levels for the first month, but no statistically significant difference was noted after that time.29 The plasma concentrations of FA remained below the lower limit (100 pg/mL) from day 7 until completion of the trial at 36 months for both the lower- and higher-dose implants.18
Approved indications for FA
In the United Kingdom, based on a cost–benefit analysis, the guidelines stipulate the use of FA only in pseudophakic eyes with DMO that exhibits a poor response to available therapies. In the rest of Europe, the 0.2-µg/day FA implant is approved for visually impaired patients with chronic DMO that is considered insufficiently responsive to available therapies. In the United States, FA is approved for the treatment of DMO in patients who have had a poor response to corticosteroid therapy. Patients who have been previously treated with a course of corticosteroids without a clinically significant rise in intraocular pressure (IOP) are eligible for treatment.
FAME studies and real-world outcomes
Efficacy
The FAME studies were randomised, double-masked, placebo-controlled, 24- and 36-month phase III trials conducted across the United States, Canada, Europe, and India. Patients with DMO who had undergone prior macular photocoagulation treatment, had a central retinal thickness of >250 µm, and had an ETDRS BCVA of 19 to 68 letters were eligible for the trial. Patients were randomised to receive a low-dose implant (0.2 µg/day; n = 375), high-dose implant (0.5 µg/day; n = 393), or placebo (n = 185), and treatment was only administered to one eye (study eye).16,20 Approximately 28% of patients had a visual gain of 15 letters at 24 months versus 16% in the placebo group. These results were similar at 36 months with a need for more than one injection in only 25% of patients. For obvious ethical reasons, patients could benefit from laser or intravitreal injections if required. The need for additional laser treatment or injections was 50% and 100% higher, respectively, in the placebo group. The subgroup analysis highlighted a significant improvement in visual acuity, especially for patients with chronic DMO.
Similar to the FAME trial, real-world studies have assessed the efficacy of ILUVIEN® by evaluating visual gain or the reduction of macular oedema. Visual gain was noted within the first 3 weeks following administration of the implant20 and persisted up to 36 months post-implantation.30,31 Visual gain in terms of EDTRS letters improved with time. At 1 year, a gain of 6 letters was achieved in most cases,32 whilst in some studies a visual gain of ≥15 EDTRS letters was achieved in approximately 18% to 22% of patients; these findings are similar to those of the FAME study (19% and 23% in patients without and with chronic macular oedema).33,34 Other studies showed that 25% of patients had a gain of 15 letters.35 Conversely, a prospective study including 14 clinical sites in the United Kingdom showed that only 20.8% of patients had a visual gain of 15 EDTRS letters at 24 months; this was lower than that reported in the FAME study (22% and 34% in patients without and with chronic macular oedema).36 BCVA was found to improve or remain stable in at least 74% of treated eyes.36,37 Among patients with visual gain, those with a 15-letter gain might constitute only one-fifth rather than one-quarter or one-third as reported in the FAME study.36 Some cases of improvement occurred among patients with chronic macular oedema (up to 20 years).38
Lens opacification may adversely affect visual gain results as described in the FAME study. Real-world studies are confirming this finding. This was particularly highlighted in the prospective RESPOND study, which showed decreased vision compared with baseline by −2.5 letters in the phakic group (versus +6.8 letters in the aphakic group) at 12 months.39 However, following cataract extraction surgery, the visual acuity improved from baseline, similar to patients with pseudophakia who have undergone cataract surgery prior to FA injection.30 A visual gain of three lines was generally achieved in >15% of the patients during the first year of treatment and in >30% during the second and third years.30 Vitrectomised eyes appeared to have a similar benefit with a visual gain of 15 letters achieved in 37.5% of patients versus 36.8% in the non-vitrectomised eyes group.40,41
As previously mentioned, the FAME study included only patients with persistent macular oedema (median of 3 years). A clear benefit was noted for patients who had had macular oedema for more than 3 years. Correspondingly, real-world studies highlighted that patients with acute macular oedema failed to show a clear benefit from treatment with ILUVIEN®.31 Interestingly, the visual acuity might have been even worse than that in the placebo group.31 Because many cytokines are involved, VEGF is no longer considered the primary factor during the chronic phase of macular oedema.42
Reduction in macular thickness was observed as soon as 7 days post-implantation and maintained for up to 3 years post-injection with a 20% to 30% decrease in macular oedema on average.32,36,39 Greater oedema (>400 µm) is associated with higher efficacy of the implant, with a reduction in thickness of up to 50% in 21% of treated eyes.43 Some studies showed significantly greater results, with a reduction from 960 to 246 µm within 3 months.44 The correlation between decreased macular thickness and improved visual acuity is controversial; in some studies, visual improvement was more limited than anatomical improvement. This is likely due to factors other than the macular thickness that affect vision in patients with diabetes, such as ischaemia, the duration of DMO, or structural changes.43,45
Aside from the measured effects on DMO, the FA implant (0.2 or 0.5 mcg/day) could also delay or reduce the rate of progression to proliferative DR. This effect was robust and maintained up to 18 months with a reduction in the severity of the DR.46
Most studies treated only one eye at a time, allowing inter-ocular comparison with the same diabetic background. A significant difference was noted between treated and untreated eyes. A gain of ≥15 EDTRS letters was achieved in 18% of treated eyes and only 4% of untreated eyes. These functional results were supported by a significant decrease in macular oedema on optical coherence tomography (−113 vs. −13 µm).47
ILUVIEN® has been shown to be effective in real-world studies in terms of improving patient vision for up to 3 years; however, practitioners should bear in mind that more than one injection or additional treatment with anti-VEGF or steroid injections is usually required. The second ILUVIEN® injection is generally administered at 12 months.32 In the FAME studies, 65.5% of patients in the low-dose group and 63.2% in the high-dose group required additional treatment.16 In the MEDISOFT study, 6.4% of eyes required additional macular laser treatment, 1.2% required bevacizumab, 13.6% required aflibercept, 17.7% required ranibizumab, 2.3% required intravitreal dexamethasone, 2.3% required intravitreal triamcinolone, and 0.53% required another FA implant within a 2-year follow-up period.36 In a recent report by Fusi-Rubiano et al.,32 34.5% of the eyes required supplementary treatment by 12 months, 60.0% by 24 months, and 83.3% by 36 months. Nevertheless, ILUVIEN® remains an affordable therapeutic option for persistent DMO.48–50
Safety
The most significant concerns in the literature are ocular hypertension and cataractogenesis. Interestingly, despite intravitreal injection of steroids, which are known to be immunosuppressive in already compromised patients (those with diabetes), only a handful of reported cases of endophthalmitis have been published in real-world studies to date.35 During the FAME trial, four cases of endophthalmitis occurred, among which two were considered secondary to ILUVIEN® injection (days 13 and 16 post-injection).36 Care should be taken if the patient has a history of herpetic or viral eye disease because induced immunosuppression might lead to a higher recurrence rate.
Ocular hypertension is one of the most common adverse events associated with the use of intraocular steroids. Up to 13% of patients might have an IOP of >30 mmHg36 (range of occurrence, 7%–50%).32,37,49 No ocular hypertension was present in the fellow untreated eye when specifically assessed.47 In the FAME study, IOP increased in 37.1% of patients who received the 0.2-µg/day FA implant (n = 375) and in 11.9% of those who received placebo (n = 185). A >30-mmHg rise in IOP was more frequent in patients who received the 0.2-µg/day FA implant (p < 0.001) than in those who received placebo in the overall population and in those without prior ocular corticosteroid exposure.16,20 A post-hoc analysis showed that glaucomatous optic nerve changes were not dissimilar between the patients in the two arms of the trial.51 A recent multicentre study from three European countries (United Kingdom, Germany, and Portugal) published by the IRISS group confirmed the results of the FAME study: about 23% of patients required IOP-lowering medication without clinically significant changes in the cup-to-disc ratio (CDR).52 However, a small percentage of patients in IRISS (5.2%) had a baseline IOP of >21 mmHg, which was an exclusion criterion in the FAME trials.
The need for glaucoma drops varied from 0% to 15%, with some larger series having an even higher rate.33,36,49,52 These higher rates are more in accordance with the FAME study, in which 26% of patients required glaucoma drops. A need for glaucoma surgery despite appropriate topical treatment was seen in up to 14.3% of cases.49 Careful patient selection remains critical to avoid complications related to ocular hypertension.
Intraocular hypertension in vitrectomised eyes was assessed by Meireles et al.41 in a retrospective study of 26 eyes with a mean follow-up of 255 days. A mean IOP change of 1.4 mmHg was found between baseline and the last visit (range, −9.0 to +8.0 mmHg), with eight eyes (30.7%) initiating or continuing anti-glaucoma drops. Pessoa et al.40 performed a retrospective study of 43 eyes (24 vitrectomised and 19 non-vitrectomised eyes) with a mean follow-up of 8.5 months and reported no significant difference in the IOP changes between the two groups; however, vitrectomised eyes exhibited a higher mean IOP elevation (1.6 vs. 0.8 mmHg).
Use of the FA implant is contraindicated in the presence of pre-existing glaucoma,53 and it is not approved for use in steroid responders in the United States. Safety could be improved by introducing a steroid provocation test. Whilst such a test could not absolutely predict the absence of ocular hypertension, it would highlight patients who may require surgical intervention so that they could be excluded from the treatment.54 In the FAME study, 6.1% of steroid-naïve patients required IOP-lowering surgery (n = 18), highlighting the importance of knowing whether patients have a strong IOP response to corticosteroid therapy.54 No increase in the CDR was detected with a 0.2-µg/day dose after 36 months, whereas the CDR increased by 0.1 in the 0.5-µg/day group.51 Therefore, a 0.2-µg/day dose is the implant used worldwide, but careful long-term follow-up focusing on IOP is required.
Another important issue is the occurrence of cataract, a well-known adverse effect of intraocular corticosteroids. Cataracts usually developed during the first year of treatment and in almost all of treated patients after 3 years of follow-up.30 The cataract surgery rate was 46% and 55% in patients with and without chronic macular oedema, respectively, whereas it was 21% and 11% in the matched placebo group.31 At the 36-month follow-up in the FAME study, among patients who were phakic at baseline, cataracts developed in 81.7% of those who received the 0.2-µg/day implant (n = 235) and 50.4% of those who received placebo (n = 121).16,20 A higher percentage of patients developed cataracts in the treatment arm; however, the visual outcome following extraction was similar if not better than that in patients with pseudophakia at baseline16,20,30 These results demonstrate that patients with chronic DMO are more likely to gain a ≥15-letter improvement than those without DMO following cataract extraction.16,20
As with all intravitreal injections, adverse events may occur even if they are not currently reported in the literature. Such adverse events include vitreous haemorrhage, transient ocular hypertension, and retinal tears or detachment.35
The presence of the implant inside the eye may also cause problems in patients who undergo vitreo-retinal surgery because it might dislodge into the infusion cannula and cause globe pressurisation difficulties during surgery;55 alternatively, it might migrate to the anterior chamber.56
ILUVIEN® is associated with a non-negligible rate of adverse events; therefore, the benefit–risk ratio should be clearly taken into account, even if some studies advocate safe bilateral use.57–59
A summary of the most important studies involving this implant is depicted in Table 1.
Table 1.
Summary of the most important studies of ILUVIEN®.
| Study | Year | Design | Number of patients | Key results |
|---|---|---|---|---|
| FAME study group16,20 | 2011 and 2012 | Two parallel, prospective, randomised, sham injection-controlled, double-masked, multicentre clinical trials24- and 36-month follow-ups | Sham, n = 1850.2 µg/day, n = 3750.5 µg/day, n = 393 | 1. Visual gain of >15 ETDRS letters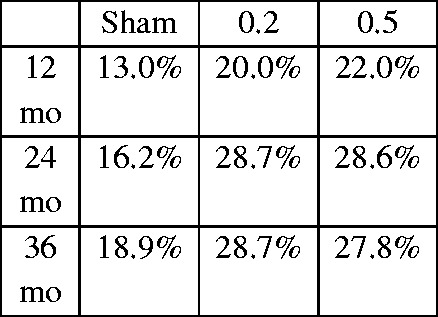
|
| 2. BCVA letter score gain at 24 monthsSham: 1.70.2 μg: 4.40.5 μg: 5.43. Glaucoma surgerySham: 0.5%0.2 µg: 3.7%0.5 μg: 7.6 % | ||||
| ICE-UK33 | 2017 | Retrospective study involving 13 ophthalmology centresApril 2013–2015, 12-month follow-up | 208 patients (233 eyes) | 1. Visual gain of >15 ETDRS letters: 18% at 12 months 2. Glaucoma surgery: 0% 3. Glaucoma therapy: 15% |
| Medisoft Audit Group36 | 2017 | 14 clinical centres in the UK, electronic pseudo-anonymised medical record system, retrospective | 305 patients (345 eyes) | 1. Visual gain of >15 ETDRS letters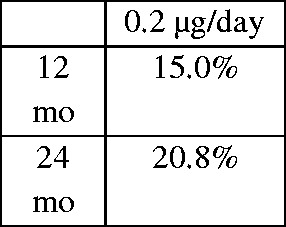
|
| 2. Letter gain at 18 and 24 months: 4.5 and 5.33. Requirement for emergent IOP-lowering medication in 22.0% and requirement for glaucoma surgery in 0.8% with a glaucoma diagnosis rate of 2.4% | ||||
| Pessoa et al.40 | 2018 | Retrospective comparative, vitrectomised vs. non-vitrectomised eyes | 43 eyes (24 vitrectomised, 19 non-vitrectomised) | 1. Visual gain of >15 ETDRS letters 8.5 months
|
| El-Ghrably et al.34 | 2017 | Observational, prospective, multicentre study | 57 patients | Gain of 5.1 EDTRS letters at month 12; 22% of patients gained ≥15 EDTRS letters |
| IRISS52 | 2018 | Prospective, observational, multicentre study | 563 patients (593 eyes) | 1. 23.3% of patients required IOP-lowering drops 2. 2.3% of patients required glaucoma surgery |
ETDRS, Early Treatment Diabetic Retinopathy Study; BCVA, best-corrected visual acuity; IOP, intraocular pressure.
Cost-effectiveness
DΜΟ is a sight-threatening condition that can lead to blindness with significant financial impact. Ιn the United Κingdom alone, the annual cost of blindness is more than GBP 5 billion.60
Ιn a recent study conducted in the Νational Ηealth Service in England, Quhill and Βeiderbeck50 analysed the 3-year cost of treating chronic DΜΟ with either a single FΑ implant or with 14 ranibizumab injections in both phakic and pseudophakic eyes. Τhe research model included the costs of drugs or drug administration, monitoring patients, additional interventions required, and management of adverse events.50 Τhe authors reported that use of the implant resulted in a total 3-year cost saving of GBP 6068 per pseudophakic eye and GBP 5341 per phakic eye, indicating that a single injection of FΑ implant can be a considerable cost-saving option compared with ranibizumab (assuming that 14 injections will be required during a 3-year period).
Ιn another study conducted in the United States, Cutino et al.61 used a Μarkov model to compare the healthcare and productivity costs with health outcomes from treatment. When 40% of patients received a unilateral FΑ implant, the expected incremental cost-effectiveness ratio was USD 38,763, suggesting that the FΑ implant in patients with DΜΟ is a cost-effective therapeutic option.
In a recent systematic literature review, a short-term cost-cost model was used with a 3-year time horizon to compare ranibizumab, aflibercept, an FΑ implant, and a dexamethasone implant (Οzurdex®) for the treatment of insufficiently anti-VΕGF responding foveas in patients with DΜΟ in Germany.48 Τhe model considered drug costs as the predominant cost component, followed by injections and optical coherence tomography costs. The total costs were EUR 17,542 for ranibizumab, EUR 15,896 for aflibercept, EUR 10,826 for the FΑ implant, and EUR 12,365 for the dexamethasone implant, suggesting that a single injection of FΑ implant is the most cost-effective (in-label) therapeutic option.
Conclusion
The FAME studies showed the efficacy of FA implants for chronic DMO that is resistant to conventional treatment. Following these trials, ILUVIEN® was approved by respective authorities in many countries for the treatment of chronic DMO.
Anti-VEGF agents remain the first-line treatment for this group of patients; however, the FA implant has mainly been reserved for patients with persistent or recurrent DMO despite previous multiple anti-VEGF injections. Real-world studies support the clinical use of the FA implant in achieving resolution of persistent DMO and improving vision whilst simultaneously reducing the frequency of injections and hospital visits. However, most of these studies are retrospective and have either a small number of patients or a short follow-up period. Another important question that needs to be answered is the long-term efficacy of the implant and the need for supplementary treatment because we do not have enough data to make safe conclusions regarding the number of additional injections/laser sessions required, the timing of supplementary treatment initiation, and the efficiency of the FA after a 3-year period. Moreover, the efficacy of the FA implant in different ethnic populations is worthy of study because it is already documented that sight-threatening DR is significantly more prevalent in African-Caribbeans and South Asians than in white Europeans.62
A recent report revealed the efficacy of the FA implant in a patient with persistent non-infectious uveitis with macular oedema.63 Further studies are necessary to assess the long-term safety and efficacy of ILUVIEN® in patients with chronic non-infectious intraocular inflammation or macular oedema in relation to other conditions such as retinal vein occlusion.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Butt F, Khan K, Chaudhry S, et al. Electronic Patient records to identify patients in the united kingdom with diabetic macular oedema suitable for ILUVIEN((R)) (fluocinolone acetonide). Ophthalmol Ther 2016; 5: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobo C, Pires I and Cuncha-Vaz J. Diabetic macular edema. In: Bernardes R, Cuncha-Vaz J (eds) Optical Coherence Tomography: A Clinical and Technical Update. Berlin: Spinger Verlag, 2012, pp.1–21. [Google Scholar]
- 3.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology 2015; 122: 1375–1394. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Moss SE, et al. The wisconsin epidemiologic study of diabetic retinopathy. XV. The long-term incidence of macular edema. Ophthalmology 1995; 102: 7–16. [DOI] [PubMed] [Google Scholar]
- 6.Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Early treatment diabetic retinopathy study research group. Arch Ophthalmol 1985; 103: 1796–1806. [PubMed] [Google Scholar]
- 7.Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, et al. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res 2016; 2016: 2156273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powers M, Greven M, Kleinman R, et al. Recent advances in the management and understanding of diabetic retinopathy. F1000Res 2017; 6: 2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015; 372: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetic Retinopathy Guidelines, “The Royal College of Ophthalmologists”, 2012.
- 11.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012; 119: 789–801. [DOI] [PubMed] [Google Scholar]
- 12.Triantafylla M, Massa HF, Dardabounis D, et al. Ranibizumab for the treatment of degenerative ocular conditions. Clin Ophthalmol 2014; 8: 1187–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jampol LM, Bressler NM, Glassman AR. Revolution to a new standard treatment of diabetic macular edema. JAMA 2014; 311: 2269–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bressler NM, Beaulieu WT, Glassman AR, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol 2018; 136: 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syed YY. Fluocinolone acetonide intravitreal implant 0.19 mg (ILUVIEN((R))): a review in diabetic macular edema. Drugs 2017; 77: 575–583. [DOI] [PubMed] [Google Scholar]
- 16.Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology 2012; 119: 2125–2132. [DOI] [PubMed] [Google Scholar]
- 17.Schmit-Eilenberger VK, Augustin AJ. Early experience with Iluvien for the treatment of chronic DME. Retina Today 2013: 34–37. http://retinatoday.com/2013/08/early-experience-with-iluvien-for-the-treatment-of-chronic-dme/ [Google Scholar]
- 18.Alimera Sciences Inc. Iluvien (fluocinolone acetonide intravitreal implant) 0.19 mg for intravitreal injection: US prescribing information., 2014.
- 19.National Institute for Health and Care Excellence. Fluocinolone Acetonide Intravitreal Implant for Treating Chronic Diabetic Macular Oedema After an Inadequate Response to Prior Therapy. London: NICE, 2014. [Google Scholar]
- 20.Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011; 118: 626–635 e2. [DOI] [PubMed] [Google Scholar]
- 21.Medicine and Healthcare products Regulatory Agency. Iluvien 190 micrograms intravitreal implant in applicator (fluocinolone acetonide): public assessment report., 2016.
- 22.Sarao V, Veritti D, Boscia F, et al. Intravitreal steroids for the treatment of retinal diseases. ScientificWorldJournal 2014; 2014: 989501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang BS, Chung EY, Yun YP, et al. Inhibitory effects of anti-inflammatory drugs on interleukin-6 bioactivity. Biol Pharm Bull 2001; 24: 701–703. [DOI] [PubMed] [Google Scholar]
- 24.Holmquist F, Lundin S, Larsson B, et al. Studies on binding sites, contents, and effects of AVP in isolated bladder and urethra from rabbits and humans. Am J Physiol 1991; 261: R865–R874. [DOI] [PubMed] [Google Scholar]
- 25.Glybina IV, Kennedy A, Ashton P, et al. Photoreceptor neuroprotection in RCS rats via low-dose intravitreal sustained-delivery of fluocinolone acetonide. Invest Ophthalmol Vis Sci 2009; 50: 4847–4857. [DOI] [PubMed] [Google Scholar]
- 26.Glybina IV, Kennedy A, Ashton P, et al. Intravitreous delivery of the corticosteroid fluocinolone acetonide attenuates retinal degeneration in S334ter-4 rats. Invest Ophthalmol Vis Sci 2010; 51: 4243–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayalasomayajula SP, Ashton P, Kompella UB. Fluocinolone inhibits VEGF expression via glucocorticoid receptor in human retinal pigment epithelial (ARPE-19) cells and TNF-alpha-induced angiogenesis in chick chorioallantoic membrane (CAM). J Ocul Pharmacol Ther 2009; 25: 97–103. [DOI] [PubMed] [Google Scholar]
- 28.Campochiaro PA, Nguyen QD, Hafiz G, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology 2013; 120: 583–587. [DOI] [PubMed] [Google Scholar]
- 29.Campochiaro PA. Pharmacokinetics of sustained-delivery fluocinolone acetonide for DME. Retina Today 2009; 55–56. http://retinatoday.com/2009/06/0609_12.php/ [Google Scholar]
- 30.Yang Y, Bailey C, Holz FG, et al. Long-term outcomes of phakic patients with diabetic macular oedema treated with intravitreal fluocinolone acetonide (FAc) implants. Eye (Lond) 2015; 29: 1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunha-Vaz J, Ashton P, Iezzi R, et al. Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology 2014; 121: 1892–1903. [DOI] [PubMed] [Google Scholar]
- 32.Fusi-Rubiano W, Mukherjee C, Lane M, et al. Treating diabetic macular oedema (DMO): real world UK clinical outcomes for the 0.19mg fluocinolone acetonide intravitreal implant (Iluvien) at 2 years. BMC Ophthalmol 2018; 18: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holden SE, Currie CJ, Owens DR. Evaluation of the clinical effectiveness in routine practice of fluocinolone acetonide 190 microg intravitreal implant in people with diabetic macular edema. Curr Med Res Opin 2017; 33: 5–17. [DOI] [PubMed] [Google Scholar]
- 34.El-Ghrably I, Steel DHW, Habib M, et al. Diabetic macular edema outcomes in eyes treated with fluocinolone acetonide 0.2 microg/d intravitreal implant: real-world UK experience. Eur J Ophthalmol 2017; 27: 357–362. [DOI] [PubMed] [Google Scholar]
- 35.Alfaqawi F, Lip PL, Elsherbiny S, et al. Report of 12-months efficacy and safety of intravitreal fluocinolone acetonide implant for the treatment of chronic diabetic macular oedema: a real-world result in the United Kingdom. Eye (Lond) 2017; 31: 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey C, Chakravarthy U, Lotery A, et al. Real-world experience with 0.2 mug/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye (Lond) 2017; 31: 1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmit-Eilenberger VK. A novel intravitreal fluocinolone acetonide implant (Iluvien((R))) in the treatment of patients with chronic diabetic macular edema that is insufficiently responsive to other medical treatment options: a case series. Clin Ophthalmol 2015; 9: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertelmann T, Schulze S. Long-term follow-up of patient with diabetic macular edema receiving fluocinolone acetonide intravitreal implant. Ophthalmol Ther 2015; 4: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figueira J, Henriques J, Amaro M, et al. A nonrandomized, open-label, multicenter, phase 4 pilot study on the effect and safety of ILUVIEN(R) in chronic diabetic macular edema patients considered insufficiently responsive to available therapies (RESPOND). Ophthalmic Res 2017; 57: 166–172. [DOI] [PubMed] [Google Scholar]
- 40.Pessoa B, Coelho J, Correia N, et al. Fluocinolone acetonide intravitreal implant 190 mug (ILUVIEN(R)) in vitrectomized versus nonvitrectomized eyes for the treatment of chronic diabetic macular edema. Ophthalmic Res 2018; 59: 68–75. [DOI] [PubMed] [Google Scholar]
- 41.Meireles A, Goldsmith C, El-Ghrably I, et al. Efficacy of 0.2 mug/day fluocinolone acetonide implant (ILUVIEN) in eyes with diabetic macular edema and prior vitrectomy. Eye (Lond) 2017; 31: 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quhill H, Quhill F. Real-life ILUVIEN (fluocinolone acetonide) case study: rapid drying of the macula and improved vision within 2 years after therapy initiation. Case Rep Ophthalmol 2016; 7: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Currie CJ, Holden SE, Owens DR. Patterns of retinal thickness prior to and following treatment with fluocinolone acetonide 190 microg intravitreal implant for diabetic macular edema. Curr Med Res Opin 2017; 33: 33–43. [DOI] [PubMed] [Google Scholar]
- 44.Elaraoud I, Andreatta W, Kidess A, et al. Use of flucinolone acetonide for patients with diabetic macular oedema: patient selection criteria and early outcomes in real world setting. BMC Ophthalmol 2016; 16: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Massin P, Erginay A, Dupas B, et al. Efficacy and safety of sustained-delivery fluocinolone acetonide intravitreal implant in patients with chronic diabetic macular edema insufficiently responsive to available therapies: a real-life study. Clin Ophthalmol 2016; 10: 1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wykoff CC, Chakravarthy U, Campochiaro PA, et al. Long-term effects of intravitreal 0.19 mg fluocinolone acetonide implant on progression and regression of diabetic retinopathy. Ophthalmology 2017; 124: 440–449. [DOI] [PubMed] [Google Scholar]
- 47.Currie CJ, Holden SE, Berni E, et al. Evaluation of the clinical effectiveness of fluocinolone acetonide 190 microg intravitreal implant in diabetic macular edema: a comparison between study and fellow eyes. Curr Med Res Opin 2017; 33: 19–31. [DOI] [PubMed] [Google Scholar]
- 48.Neubauer AS, Haritoglou C, Ulbig MW. [Cost Comparison of Licensed Intravitreal Therapies for Insufficiently Anti-VEGF Responding Fovea Involving Diabetic Macular Edema in Germany]. Klin Monbl Augenheilkd 2018. [DOI] [PubMed]
- 49.Ch'ng SW, Brent AJ, Empeslidis T, et al. Real-world cost savings demonstrated by switching patients with refractory diabetic macular edema to intravitreal fluocinolone acetonide (Iluvien): a retrospective cost analysis study. Ophthalmol Ther 2018; 7: 75–82. doi: 10.1007/s40123-017-0114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quhill F, Beiderbeck A. Cost advantage of fluocinolone acetonide implant (ILUVIEN®) versus ranibizumab in the treatment of chronic diabetic macular oedema. Glob Reg Heal Technol Assess 2017; 4: e155–e164. [Google Scholar]
- 51.Parrish RK, 2nd, Traverso CE, Green K, et al. Quantitative assessment of optic nerve changes in patients with diabetic macular edema treated with fluocinolone acetonide vitreous implants. Ophthalmic Surg Lasers Imaging Retina 2016; 47: 418–425. [DOI] [PubMed] [Google Scholar]
- 52.Chakravarthy U, Taylor SR, Koch FHJ, et al. Changes in intraocular pressure after intravitreal fluocinolone acetonide (ILUVIEN): real-world experience in three European countries. Br J Ophthalmol 2018. Sep 21. pii: bjophthalmol-2018-312284. doi: 10.1136/bjophthalmol-2018-312284. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.ILUVIEN. Summary of Product Characteristics. Available from: https://www.medicines.org.uk/emc/medicine/27636.
- 54.Parrish RK, 2nd, Campochiaro PA, Pearson PA, et al. Characterization of intraocular pressure increases and management strategies following treatment with fluocinolone acetonide intravitreal implants in the FAME trials. Ophthalmic Surg Lasers Imaging Retina 2016; 47: 426–435. [DOI] [PubMed] [Google Scholar]
- 55.Andreatta W, Elaraoud I, Mitra A. Dislodgement of fluocinolone acetonide intravitreal implant into the infusion cannula during vitrectomy for retinal detachment. Retin Cases Brief Rep 2017. DOI: 10.1097/ICB.0000000000000678 [DOI] [PubMed] [Google Scholar]
- 56.Papastavrou VT, Zambarakji H, Dooley I, et al. Observation: fluocinolone acetonide (Iluvien) implant migration into the anterior chamber. Retin Cases Brief Rep 2017; 11: 44–46. [DOI] [PubMed] [Google Scholar]
- 57.de Oliveira Dias JR, Nunes RP, Goldhardt R. New drugs and new posterior delivery methods in CME. Curr Ophthalmol Rep 2017; 5: 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elaraoud I, Quhill H, Quhill F. Case series investigating the efficacy and safety of bilateral fluocinolone acetonide (ILUVIEN((R))) in patients with diabetic macular edema: 10 eyes with 12 months follow-up. Ophthalmol Ther 2016; 5: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elaraoud I, Attawan A, Quhill F. Case series investigating the efficacy and safety of bilateral fluocinolone acetonide (ILUVIEN((R))) in patients with diabetic macular edema. Ophthalmol Ther 2016; 5: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Royal National Institute of Blind People (UK). Sight loss UK 2013 The latest evidence (2013)., 2013.
- 61.Cutino A, Green K, Kendall R, et al. Economic evaluation of a fluocinolone acetonide intravitreal implant for patients with DME based on the FAME study. Am J Manag Care 2015; 21: S63–S72. [PubMed] [Google Scholar]
- 62.Sivaprasad S, Gupta B, Gulliford MC, et al. Ethnic variations in the prevalence of diabetic retinopathy in people with diabetes attending screening in the United Kingdom (DRIVE UK). PLoS One 2012; 7: e32182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reddy AK, Burkholder BM, Khan IR, et al. Iluvien implantation for uveitis and uveitic macular edema. Ocul Immunol Inflamm 2018; 26: 315–316. [DOI] [PubMed] [Google Scholar]


