Short abstract
Objective
Delayed-onset muscle soreness (DOMS) is a symptom of exercise-induced muscle injury that is commonly encountered in athletes and fitness enthusiasts. Vibration is being increasingly used to prevent or treat DOMS. We therefore carried out a meta-analysis to evaluate the effectiveness of vibration in patients with DOMS.
Method
We searched nine databases for randomized controlled trials of vibration in DOMS, from the earliest date available to 30 May 2018. Visual analogue scale (VAS) and creatine kinase (CK) levels were set as outcome measures.
Results
The review included 10 identified studies with 258 participants. The meta-analysis indicated that vibration significantly improved the VAS at 24, 48, and 72 hours after exercise, and significantly improved CK levels at 24 and 48 hours, but not at 72 hours.
Conclusion
Vibration is a beneficial and useful form of physiotherapy for alleviating DOMS. However, further studies are needed to clarify the role and mechanism of vibration in DOMS.
Keywords: Delayed-onset muscle soreness, physiotherapy, sports, vibration, visual analogue scale, creatine kinase
Introduction
Frequent habitual exercise can reduce the risks of obesity and cardiovascular disease; however, excessive exercise or sport can also elicit temporary muscle injury, which presents as delayed-onset muscle soreness (DOMS).1 DOMS indicates subclinical muscle damage, which serves as a precursor to ancillary complications.2 Growing reports have shown that DOMS represents a type I muscle strain injury, resulting in muscle aches, pain, discomfort, and inflammation.3 DOMS is characterized by allodynia in the distal portions of skeletal muscles, peaking at around 24 to 48 hours after exercise, and thus differs from normal muscle soreness, which occurs immediately after exercise.4 DOMS usually occurs in competitive athletes or people who participate in excessive sport, and has become a major challenge in many sports.5
Numerous recovery modalities have been developed to offset the adverse effects of DOMS by promoting the recovery process after muscle injury, such as massage, cold water immersion, and vibration.6,7 Vibration treatments typically consist of local mechanical vibration (LV) administered directly to the muscle or tendon, or whole-body vibration (WBV), performed by vibrating platforms or devices fixed to resistance training machines.8 Vibration treatment is becoming more popular in the field of sports, with the aim of enhancing skeletal musculature performance and injury recovery.9 Vibration has also been shown to increase morphological functional development of muscle fibers.10,11 Moreover, both LV and WBV therapies have demonstrated beneficial preventive and therapeutic effects in sports rehabilitation.12,13
However, the efficacy of vibration for DOMS remains controversial. Two studies reported that vibration therapy was no more effective than massage or placebo in patients with DOMS,14,15 while other studies found that vibration promoted the recovery of DOMS and relieved pain.2,16,17 A previous review in 2012 indicated that WBV had potential beneficial effects for muscle recovery after exercise.18 Moreover, another systematic review in 2014 also showed benefits of vibration on DOMS,19 though this was a descriptive systematic review rather than a quantitative synthesis of the evidence. There is thus a lack of strong evidence regarding the effectiveness of vibration for the treatment of DOMS. Few randomized controlled trials (RCTs) concerning the effect of vibration on DOMS had been conducted up to 2014, though some new RCTs have since been carried out. We therefore aimed to clarify the beneficial effect of vibration in patients with DOMS by conducting a meta-analysis based on available RCT data.
Methods
Search strategy
We searched the following electronic databases: PubMed, the Cochrane Library, Embase, Web of Science, SPORTDICUS, Physiotherapy Evidence Database, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (SinoMed), and WanFang. There were no exclusions on the basis of language. Databases were searched from the earliest date available to May 30, 2018, using the terms (“vibration”) AND (“delayed onset muscle soreness”) AND (“randomized controlled trial”). Equivalent Chinese terms were used to search Chinese language databases.
Inclusion criteria
The inclusion criteria were: (1) RCTs; (2) trials that contained subjects suffering from DOMS; (3) vibration as the intervention (either WBV with subjects sitting, standing, lying on a platform, or LV to regional muscles or other local regions using with wearable devices, vibrators, cushions, insoles, or footwear); (4) controls received placebo vibration or conventional physical therapy; and (5) outcome measures were visual analogue scale (VAS) or serum creatine kinase (CK) levels.
Exclusion criteria
Studies were excluded if they were: (1) quasi-experimental studies (non-RCTs, before and after, interrupted time series, crossover trials), observational studies (prospective and retrospective), case reports, reviews or systematic literature reviews and qualitative studies, opinion pieces, editorials, comments, news, and letters; (2) the mean and standard deviation could not be obtained from the articles, and no further information was obtained from correspondence with the authors; and (3) muscle soreness was reported within 12 hours of exercise.
Study selection and data extraction
Two of the authors independently screened the literature using the above predetermined inclusion criteria and extracted the following data from the trials: study design, participant characteristics, intervention and outcome data, adverse effects, and methodological quality. If the data were incomplete, we attempted to contact the authors to obtain additional details. Disagreements about study inclusion and extracted data were resolved by consensus between the two coauthors. If disagreements persisted, the coauthors consulted with a third author.
Risk of bias
Risk of bias was assessed according to the evaluation criteria provided by the Cochrane Handbook for Systematic Reviews of Interventions and by examining the random sequence generation, allocation concealment, incomplete outcome data, blinding (participants, personnel, and an outcome assessment), selective reporting, and other biases. Two review authors independently assessed the risk of bias of the included studies, and judged each domain as having bias, a high risk of bias, or an unclear risk of bias, respectively.
Subgroup analysis and sensitivity analysis
Subgroup and sensitivity analyses were performed to explore the possible reasons for statistical heterogeneity when I2 > 50%. Sensitivity analysis was performed by omitting studies one at a time. Subgroup analyses in relation to the primary outcomes were performed to compare different vibrations, and types of control interventions (i.e., vibration before or after exercise, vibration frequency, and duration of vibration). Some data, such as participants’ mean age and medical history, were not obtained or were missing for some studies and no subgroup analyses of these variables were therefore performed.
Publication bias
Asymmetry and potential publication bias were investigated visually by Funnel plots and quantitatively by Egger’s test for at least 10 studies.
Statistical analysis
Data analysis was carried out using Review Manager software (Revman, Version 5.3) provided by the Cochrane Collaboration, and STATA 14.0 (StataCorp LP, College Station, TX, USA). Continuous variables were analyzed by calculating the standardized mean difference (SMD) and 95% confidence interval (CI). We conducted tests of heterogeneity for each outcome using the χ2 test and I2 statistic. The meta-analysis was carried out using a fixed-effects model if no significant heterogeneity was observed (P > 0.05 and I2 < 50%), and a random-effects model if heterogeneity was detected (P < 0.05 and I2 ≥ 50%).
Results
Literature search
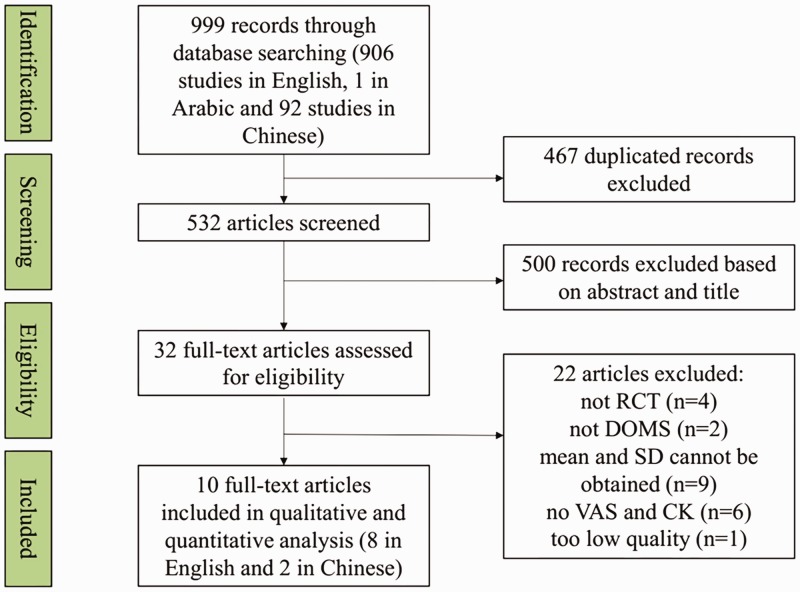
The preliminary search identified 999 studies, comprising 906 studies in English, one in Arabic, and 92 in Chinese. After excluding 467 duplicated studies, the titles and abstracts of the remaining 532 studies were inspected; 500 studies were excluded based on title and abstract criteria, and the remaining 32 studies were screened by full-text review. Among these 32 studies, one was not a RCT,20 three were crossover trials,21–23 and two studies did not investigate the curative effect of vibration on DOMS.24,25 Furthermore, some studies only presented the mean and standard deviation in figures, and no further information was obtained by attempted correspondence with the authors.5,15,16,26–31 Five studies did not include information on VAS (primary outcome) or CK (secondary outcome),2,31–34 and only one trial demonstrated the magnitudes of changes in VAS and CK.17 One abstract was deemed to be too low quality, with confusion between the groups and a lack of units for CK levels.35 Ten studies were finally included after consideration of the inclusion and exclusion criteria and after careful reading of the full texts. The literature screening process and results are shown in the attached flow diagram (Figure 1).
Figure 1.
Flow diagram of literature search and results
Description of included studies
Ten studies involving 258 participants were obtained for analysis, including two in Chinese36,37 and eight in English. The studies were performed in Australia,12 Spain,38,39 Korea,40,41 Iran,42,43 New Zealand,8 and China,36,37 respectively. Five studies included only male subjects,8,14,37,39,41 three studies included both male and female subjects,40,42,43 and two studies did not report the sex of the participants.36,38 Most of the studies used no intervention in the control group,8,36,38,40–43 one used standard massage as a conventional physiotherapy control,14 and two studies used static stretching as a control and a combination of static stretching and vibration as the intervention.36,39 An additional description of the other data is indicated in Table 1. Information on the VAS and CK levels at 24, 48, and 72 hours after exercise was extracted for this analysis.
Table 1.
Descriptions of 10 included studies
| Author | Year | Study design | Participants | Method inducing DOMS | Groups | Vibration intervention | Outcomes | Data time point |
|---|---|---|---|---|---|---|---|---|
| Shen37 | 2017 | RCT | 30 Male students from Beijing Sports University | Maximum force used for frog-leaping exercise (15 or 8 per group). Rest time 3 minutes | 1. Control group (n=6); 2. No-intervention group (n=6); 3. Vibration group (n=6);4. Static stretch group (n=6);5. Complex training group (n=6) | WBV using a Power Plate Pro5; vibration applied for 3 consecutive days from first day after exercise for 60 s, 35 Hz, amplitude 2 mm, repeated twice for each training | VAS | Before and 12, 24, 48, 72, and 96 hours after exercise |
| Song and Liu36 | 2017 | RCT | 27 Soccer students | Downhill running for 30 minutes | 1. Control group (n=9);2. Vibration combined with static stretch group (n=9);3. Static stretch group (n=9) | WBV using a Power Plate administered after exercise for 60 s, 30 Hz, amplitude 1.5 mm, repeated 3 times | VAS, CK, LDH, ROM | Before and 24, 48, and 72 hours after exercise |
| Bakhtiary et al.42 | 2007 | RCT | 50 Non-athletic volunteers (25 females, 25 males) | Downhill walking on a declined treadmill at 4 km/h for 30 minutes | 1. Vibration group (n=25);2. Non-vibration group (n=25) | LV applied to mid-line of left and right quadriceps, hamstring, and calf muscles using a vibrator apparatus (Model VR-7N, ITO) after downhill treadmill walking for 1 minute at 50 Hz | Isometric maximum voluntary contraction, PPT, VAS, CK | Before and 24 hours after exercise |
| Fuller et al.14 | 2015 | RCT | 50 Untrained men | 100 Maximal eccentric muscle actions of knee extensor muscles of the right leg | 1. Stretching and sports massage group (n=25);2. Vibration group (n=25) | LV applied to under the right thigh using a cycloidal vibration cushion, twice daily after exercise for 20 minutes with 73 Hz, 0.5 mm | PIT, VAS, CK, C-reactive protein, myoglobin | Before, and immediately, 24, 48, 72, and 168 hours after exercise |
| Timon et al.38 | 2016 | RCT | 20 University students | Eccentric strength training consisting of 5-minute warm up (30% 1 RM) and 4 sets of 5 repetitions at 120% 1RM, with 4 minutes rest between sets, with quadriceps leg extension | 1. Control group (n=10);2. Vibration group (n=10) | WBV using a vibratory platform (Galileo Fitness) administered after exercise for 60 s, 12 Hz, amplitude 4 mm, repeated 3 times with 30-s intervals | CK, blood urea nitrogen, VAS, PIT | Before and immediately and 24 and 48 hours after exercise |
| Kim et al.40 | 2011 | RCT | 21 University students (men and women) | Centrifugal contraction exercise conducted on biceps with 70% maximum isometric muscular strength | 1. Control group (n=7);2. Vibration group (n=7);3. Ultrasound group (n=7) | WBV using a sonic vibrator at 26 Hz for 11 minutes | VAS, PPT | Before and 24, 48, and 72 hours after exercise |
| Cochrane8 | 2017 | RCT | 26 Arms (Male) | 10 Sets of 6 maximal voluntary eccentric repetitions performed on an isokinetic dynamometer | 1. Control group (n=13);2. Vibration group (n=13) | LV applied to the biceps brachii and treatment arm using a vibratory device (MyoVolt, Christchurch) after exercise for 15 minutes at 120 Hz | Electromyography, VAS, PPT, CK, ROM, normalized isometric strength, concentric strength | Before and immediately and 24, 48, and 72 hours after exercise |
| Kim et al.41 | 2017 | RCT | 30 Healthy male adults | Weight-bearing arm using weight equivalent to 60% of one repetition maximum slowly lowered at the same pace and lift with assistance | 1. Control group (n=10);2. Vibration before exercise group (n=10);3. Vibration after exercise group (n=10) | LV applied to the middle of biceps muscle using an AT-1000 system during a relaxed state before or after exercise for 5 minutes, 60 Hz | PPT, CK, LDH | Before and 24, 48, and 72 hours after exercise |
| Aminian-Far et al.43 | 2011 | RCT | 32 Untrained volunteers (22 women and 10 men) | Exercise performed on the dominant-limb knee extensors against the lever arm of the isokinetic dynamometer | 1. Vibration group (n=15);2. Control group (n=17) | WBV applied using a Power Plate Pro 5 before eccentric exercise at 35 Hz, 5 mm for 60 s | Tight circumference, PPT, VAS, maximal isometric torque, PIT, CK | Before and 1, 2, 3, 4, 7, and 14 days after exercise |
| Rhea et al.39 | 2009 | RCT | 16 Adult men | Exercise including resistance training and repeated sprint exercise | 1. Static stretch group (n=8);2. Static stretch with WBV group (n=8) | WBV applied using an iTonic platform immediately after exercise and again later the same day. Vibration performed after exercise for 30 s, 50 Hz, 2 mm amplitude with the gastrocnemius, hamstring and quadriceps muscles on the platform | VAS | Before and 12, 24, 48, and 72 hours after exercise |
RCT: randomized controlled trial; VAS: visual analogue scale; WBV: whole‐body vibration; LV: local vibration; CK: creatine kinase, PIT: peak isometric torque; LDH: lactate dehydrogenase; ROM: range of motion; PPT: pressure pain threshold; RM: repetition maximum.
Risk of bias
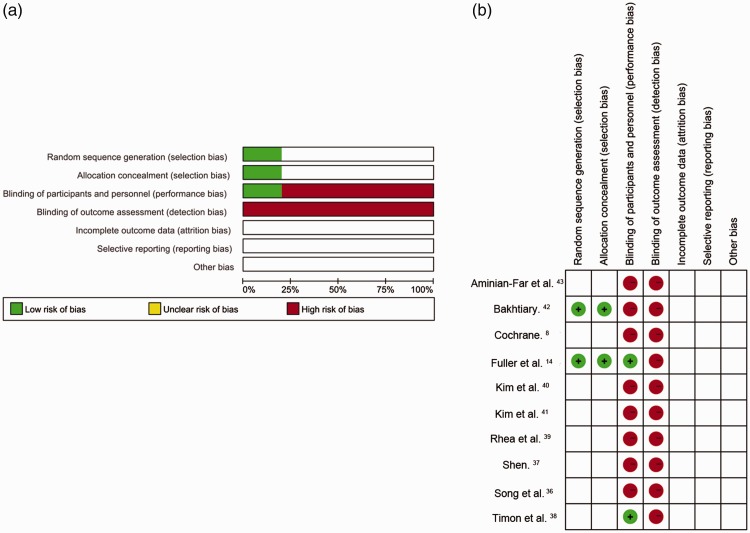
Two independent reviewers assessed the risk of bias, according to allocation, blinding, incomplete outcome bias, selective reporting bias, and other bias. As shown in Figure 2a and 2b, two studies described the generation of the random sequences and allocation concealment, and were evaluated as having a low risk of bias.14,42 The other studies mentioned ‘random’ assignment but did not provide any detailed description of the random sequence generation and were therefore judged to have an unclear risk of bias. Two studies used single blinding,14,38 while the others did not mention any blinding of participants or researchers. However, given that the participants were likely to have felt the vibration during the intervention, we considered that the importance of blinding in a physiotherapy RCT was less important than in a drug trial.44 None of the included studies reported blinding in the outcome recorders.
Figure 2.
Risk-of-bias graph (a) and summary (b)
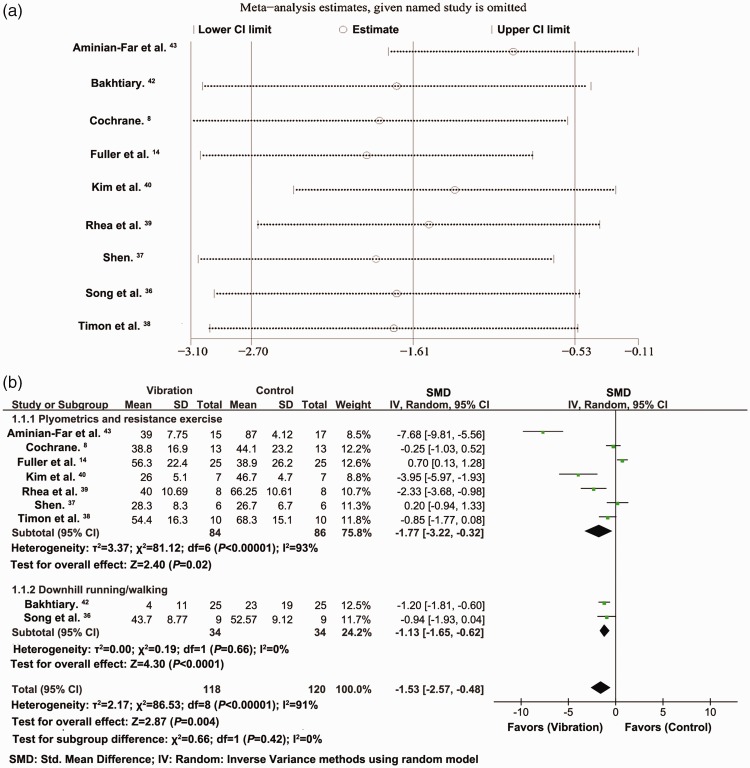
Effect of vibration on VAS rating at 24 hours
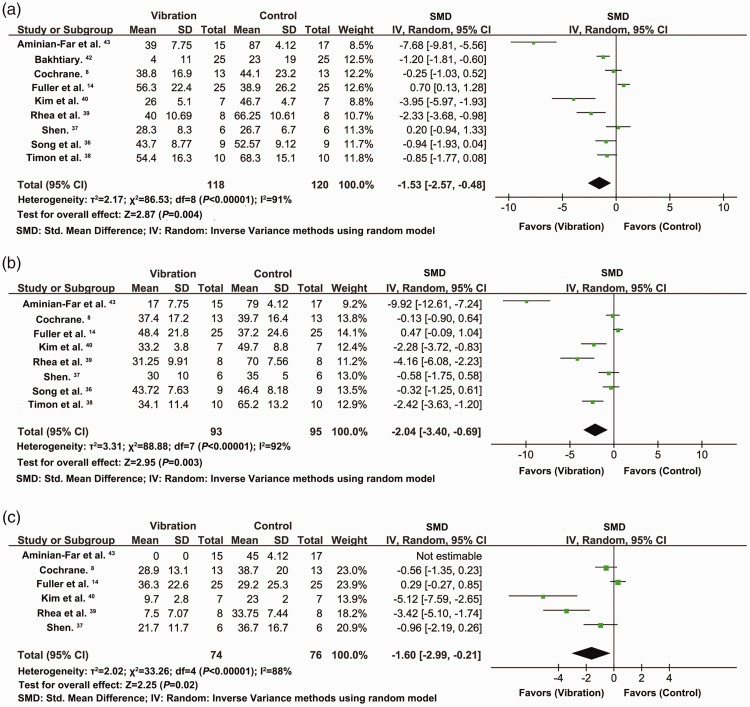
Analysis of nine studies with 238 participants indicated that the VAS scores at 24 hours after exercise decreased significantly in participants who received the vibration intervention compared with the control group (SMD = −1.53, 95% CI = −2.57 to −0.48, P = 0.004, I2 = 91%) (Figure 3a). The VAS scores were derived from the right leg in Bakhtiary et al.42 and from the flexion data in Aminian-Far et al.43 VAS analysis for the right leg and extension showed SMD = −1.28, 95% CI = −2.21 to −0.35, P = 0.007, I2 = 89%, analysis for the left leg and flexion showed SMD = −1.50, 95% CI = −2.53 to −0.47, P = 0.004, I2 = 91%, and VAS analysis for the left leg and extension showed SMD =−1.26, 95% CI = −2.18 to −0.34, P =0.008, I2 = 89%.
Figure 3.
Effects of vibration on VAS at 24 (a), 48 (b), and 72 hours (c) after exercise
Effect of vibration on VAS rating at 48 hours
Analysis of eight studies with 188 participants demonstrated that the VAS scores at 48 hours after exercise also decreased significantly after vibration intervention, compared with the control group (SMD = −2.04, 95% CI = −3.40 to −0.69, P = 0.003, I2 = 92%). The VAS scores were derived from data in flexion in Aminian-Far et al.43) (Figure 3b). The results for VAS in extension were SMD = −2.03, 95% CI = −3.39 to −0.68, P =0.003, I2 = 92%.
Effect of vibration on VAS rating at 72 hours
Analysis of six studies with 150 participants showed significant improvement in VAS scores at 72 hours after exercise following vibration intervention compared with the control group (SMD = −1.60, 95% CI = −2.99 to −0.21, P = 0.02, I2 = 88%) (Figure 3c).
Effect of vibration on CK levels at 24 hours
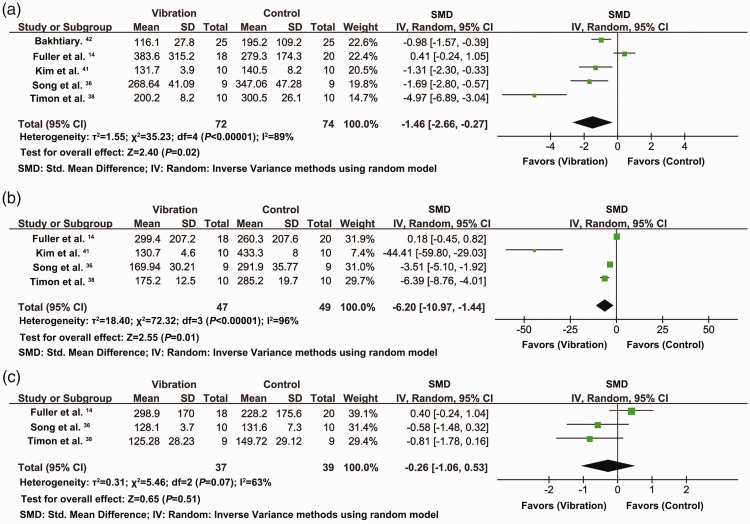
Analysis of five studies with 146 participants indicated that CK levels decreased significantly at 24 hours following vibration intervention compared with the control groups (SMD = −1.46, 95% CI = −2.66 to −0.27, P = 0.02, I2 = 89%) (Figure 4a). The CK data in Kim et al.41 were derived from pre-exercise data, and when the post-exercise data were adopted, the results were SMD = −1.29, 95% CI = −2.45 to −0.14, P = 0.03, I2 = 88%.
Figure 4.
Effects of vibration on CK levels at 24 (a), 48 (b), and 72 hours (c) after exercise
Effect of vibration on CK levels at 48 hours
Analysis of four studies with 96 participants showed that CK levels decreased significantly 48 hours after vibration intervention, compared with the control group (SMD = −6.20, 95% CI = −10.90 to −1.44, P = 0.01, I2 = 96%) (Figure 4b). The CK data in Kim et al.41 were derived from pre-exercise data, and when the post-exercise data were adopted, the results were SMD = −6.10, 95% CI = −10.89 to −1.30, P = 0.01, I2 = 96%.
Effect of vibration on CK levels at 72 hours
Analysis of three studies with 76 participants indicated that there was no significant difference in CK levels at 72 hours after vibration intervention compared with the control groups (Figure 4c). The CK data in Kim et al.41 were derived from pre-exercise data, but the result remained non-significant when the post-exercise data were adopted.
Subgroup analysis and sensitivity analysis
The above analyses demonstrated high heterogeneity (I2 > 50%). We therefore conducted sensitivity analysis to investigate the influence of each study. The total effect rating in terms of the primary outcome (VAS 24 hours) was stable when the included RCTs were removed one at a time (I2 > 50%) (Figure 5a). Meta-regression requires a minimum of 10 included studies, but the VAS 24 hours data was only based on nine studies and the planned meta-regression analysis was therefore not performed.
Figure 5.
Sensitivity analysis (a) and subgroup analysis according to method of inducing DOMS (b) in relation to VAS 24 hours
We further explored the source of the heterogeneity by subgroup analyses based on the primary outcome of VAS 24 hours, to detect potential clinical, statistical, and methodological heterogeneities. Subgroup analysis showed that the method of inducing DOMS, including downhill running/walking, and plyometrics and resistance training, contributed to the heterogeneity in VAS 24 hours (Figure 5b). Other subgroup analyses indicated that sex, region of study, type of vibration (WBV or LV), vibration before or after exercise, frequency of vibration, amplitude of vibration, duration of each vibration session, total vibration duration, blinding, and concealment, did not contribute to the heterogeneity in VAS 24 hours.
Publication bias
Funnel plots require a minimum of 10 studies, but the primary outcome of VAS 24 hours was only measured in nine studies and publication bias could therefore not be assessed by this method or using Egger’s test.
Discussion
The use of vibration to prevent and treat DOMS is growing in popularity in gyms and sports stadiums; however, direct evidence of its efficacy is still lacking. We searched four medicine, two physiotherapy and sports, and three Chinese databases and identified a total of 10 RCTs that investigated this issue.
The VAS is the direct pain index used by subjects to report DOMS, and is frequently assessed in clinical investigations of pain in patients with muscle pain and osteoarthritis, due to its convenience and reliability. The results of the current meta-analysis indicated that vibration reduced muscle pain at 24, 48, and 72 hours. Interestingly, the SMD of VAS at 48 hours following exercise was −2.04, which was greater than the changes at 24 (−1.53) and 72 hours (−1.60), suggesting that vibration treatment can achieve peak pain relief at 48 hours. An increase in CK levels commonly represents muscle fiber damage, during which CK is released into the lymphatic system and consequently into the serum. CK blood levels thus commonly represent a key marker of muscle damage and injury.18 According to this meta-analysis, vibration alleviated muscle damage and inflammation at 24 and 48 hours, consistent with the changes in VAS. The CK SMD at 48 hours was −6.20, which was greater than the change at 24 hours (−1.46), supporting the idea that vibration had the greatest benefit in terms of relieving pain and down-regulating CK levels at 48 hours after exercise. To the best of our knowledge, the current study represents the first meta-analysis to investigate the efficacy of vibration for DOMS, based on more credible quantitative results compared with an earlier descriptive systematic review.19
Some of the studies included in this meta-analysis increased the risk of heterogeneity. Subgroup analysis indicated that the method of inducing DOMS, including downhill running/walking, and plyometrics and resistance training (Table 1),42 contributed to the heterogeneity in VAS at 24 hours. The I2 values for VAS and CK at 24 hours in the downhill running/walking subgroup were 0% and 18%, respectively. The mechanism responsible for DOMS is currently unclear. Asmussen proposed that lengthening (eccentric) but not shortening (concentric) muscle contraction was the primary factor causing DOMS.45 Plyometrics and resistance exercise frequently use eccentric exercise to induce DOMS, resulting in pain, fatigue, and increased CK levels, while downhill running or walking can also elicit DOMS.46,47 However, a recent report suggested that the hamstrings did not perform an absolute eccentric muscle action during the swing phase, especially in running.48 Furthermore, the plyometrics and resistance exercise methods that induced DOMS differed among the studies included in the exercise subgroup (Table 1), which may be responsible for the high heterogeneity in this subgroup. Further clinical studies should thus be conducted with consistent methods of inducing DOMS.
The current study had some limitations. First, the number of included studies was relatively small. However, DOMS is usually only elicited by excessive sports participation and thus commonly occurs in athletes and fitness enthusiasts, but not in other individuals. Furthermore, the doctors in the included trials were not blinded, or were only single blinded, but this was likely because the rehabilitation process (i.e., vibration) would be evident to the participants, in contrast to the situation in trials of internal medication. The relatively small sample size and the lack of blinding meant that the quality of the evidence in this meta-analysis was relatively poor, and further large-scale, blinded RCTs are needed in the future.
Additionally, the pressure pain threshold (PPT) is frequently used as an index for rating the intensity of muscle pain,49 but differences in the units used to measure PPT in the current literature meant that it could not be used in the current analysis. For example, PPT was recorded in N,8 Kpa,15 or kg/cm,40 and varied in numerical values at different ranges and locations.41–43 Furthermore, the range of motion was explored in different joints, including the knee in two studies15,36 and the elbow in one.8 Furthermore, other strength, movement, and electromyography indexes were lacking or differed among studies in the present literature. Further large-scale RCTs should thus include consistent measurement indexes.
Conclusion
In conclusion, we demonstrated that vibration intervention could alleviate DOMS and reduce serum CK levels, based on a meta-analysis of 10 RCTs including 258 participants. Vibration may therefore be a beneficial and useful physiotherapy for alleviating DOMS. However, the quality of the existing evidence is relatively poor, and future large-scale, blinded RCTs using unified units and consistent methods of inducing DOMS are needed to clarify the role of vibration in patients with DOMS.
Acknowledgements
The authors would like to thank Darryl J. Cochrane (School of Sports and Exercise, Massey University, New Zealand) for kindly providing raw data for his report in 2017.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the National Natural Science Fund of China (81774443).
References
- 1.Meamarbashi A. Herbs and natural supplements in the prevention and treatment of delayed-onset muscle soreness. Avicenna J Phytomed 2017; 7: 16–26. [PMC free article] [PubMed] [Google Scholar]
- 2.Koh HW, Cho SH, Kim CY, et al. Effects of vibratory stimulations on maximal voluntary isometric contraction from delayed onset muscle soreness. J Phys Ther Sci 2013; 25: 1093–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulick DT, Kimura IF. Delayed onset muscle soreness: what is it and how do we treat it? J Sport Rehabil 1996; 5: 234–243. [Google Scholar]
- 4.Yu JY, Jeong JG, Lee BH. Evaluation of muscle damage using ultrasound imaging. J Phys Ther Sci 2015; 27: 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler AA, Jacobson BH. Effect of whole-body vibration on delayed onset muscular soreness, flexibility, and power. J Strength Cond Res 2013; 27: 2527–2532. [DOI] [PubMed] [Google Scholar]
- 6.Dupuy O, Douzi W, Theurot D, et al. An evidence-based approach for choosing post-exercise recovery techniques to reduce markers of muscle damage, soreness, fatigue, and inflammation: a systematic review with meta-analysis. Front Physiol 2018; 9: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung K, Hume P, Maxwell L. Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med 2003; 33: 145–164. [DOI] [PubMed] [Google Scholar]
- 8.Cochrane DJ. Effectiveness of using wearable vibration therapy to alleviate muscle soreness. Eur J Appl Physiol 2017; 117: 501–509. [DOI] [PubMed] [Google Scholar]
- 9.Rittweger J, Mutschelknauss M, Felsenberg D. Acute changes in neuromuscular excitability after exhaustive whole body vibration exercise as compared to exhaustion by squatting exercise. Clin Physiol Funct Imaging 2003; 23: 81–86. [DOI] [PubMed] [Google Scholar]
- 10.Sands WA, McNeal JR, Stone MH, et al. Flexibility enhancement with vibration: acute and long-term. Med Sci Sports Exerc 2006; 38: 720–725. [DOI] [PubMed] [Google Scholar]
- 11.Verschueren SMP, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res 2004; 19: 352–359. [DOI] [PubMed] [Google Scholar]
- 12.Costantino C, Gimigliano R, Olvirri S, et al. Whole body vibration in sport: a critical review. J Sports Med Phys Fitness 2014; 54: 757–764. [PubMed] [Google Scholar]
- 13.Lee CL, Chu IH, Lyu BJ, et al. Comparison of vibration rolling, nonvibration rolling, and static stretching as a warm-up exercise on flexibility, joint proprioception, muscle strength, and balance in young adults. J Sports Sci 2018; 36: 2575–2582. [DOI] [PubMed] [Google Scholar]
- 14.Fuller JT, Thomson RL, Howe PRC, et al. Vibration therapy is no more effective than the standard practice of massage and stretching for promoting recovery from muscle damage after eccentric exercise. Clin J Sport Med 2015; 25: 332–337. [DOI] [PubMed] [Google Scholar]
- 15.Dabbs NC, Black CD, Garner J. Whole-body vibration while squatting and delayed-onset muscle soreness in women. J Athl Train 2015; 50: 1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammadi H, Sahebazamani M. Influence of vibration on some of functional markers of delayed onset muscle soreness. Int J Appl Exerc Physiol 2012; 1 http://www.ijaep.com/index.php/IJAE/article/view/59 [Google Scholar]
- 17.Broadbent S, Rousseau JJ, Thorp RM, et al. Vibration therapy reduces plasma IL6 and muscle soreness after downhill running. Br J Sports Med 2010; 44: 888–894. [DOI] [PubMed] [Google Scholar]
- 18.Kosar AC, Candow DG, Putland JT. Potential beneficial effects of whole-body vibration for muscle recovery after exercise. J Strength Cond Res 2012; 26: 2907–2911. [DOI] [PubMed] [Google Scholar]
- 19.Veqar Z, Imtiyaz S. Vibration therapy in management of delayed onset muscle soreness (DOMS). J Clin Diagn Res 2014; 8: LE01–LE04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weerakkody NS, Percival P, Hickey MW, et al. Effects of local pressure and vibration on muscle pain from eccentric exercise and hypertonic saline. Pain 2003; 105: 425–435. [DOI] [PubMed] [Google Scholar]
- 21.Lau WY, Nosaka K. Effect of vibration treatment on symptoms associated with eccentric exercise-induced muscle damage. Am J Phys Med Rehabil 2011; 90: 648–657. [DOI] [PubMed] [Google Scholar]
- 22.Custer L, Peer KS, Miller L. The effects of local vibration on balance, power, and self-reported pain after exercise. J Sport Rehabil 2017; 26: 193–201. [DOI] [PubMed] [Google Scholar]
- 23.Barnes MJ, Perry BG, Mündel T, et al. The effects of vibration therapy on muscle force loss following eccentrically induced muscle damage. Eur J Appl Physiol 2012; 112: 1189–1194. [DOI] [PubMed] [Google Scholar]
- 24.Manimmanakorn N, Ross JJ, Manimmanakorn A, et al. Effect of whole-body vibration therapy on performance recovery. Int J Sports Physiol Perform 2015; 10: 388–395. [DOI] [PubMed] [Google Scholar]
- 25.Hazell TJ, Olver TD, Hamilton CD, et al. Addition of synchronous whole-body vibration to body mass resistive exercise causes little or no effects on muscle damage and inflammation. J Strength Cond Res 2014; 28: 53–60. [DOI] [PubMed] [Google Scholar]
- 26.Ayles S, Graven-Nielsen T, Gibson W. Vibration-induced afferent activity augments delayed onset muscle allodynia. J Pain 2011; 12: 884–891. [DOI] [PubMed] [Google Scholar]
- 27.Gojanovic B, Feihl F, Liaudet L, et al. Whole body vibration training elevates creatine kinase levels in sedentary subjects. Swiss Med Wkly 2011; 141: w13222. [DOI] [PubMed] [Google Scholar]
- 28.Imtiyaz S, Veqar Z, Shareef MY. To compare the effect of vibration therapy and massage in prevention of delayed onset muscle soreness (DOMS). J Clin Diagn Res 2014; 8: 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Hoyo M, Carrasco L, Da Silva-Grigoletto ME, et al. Impact of an acute bout of vibration on muscle contractile properties, creatine kinase and lactate dehydrogenase response. Eur J Sport Sci 2013; 13: 666–673. [DOI] [PubMed] [Google Scholar]
- 30.Marin PJ, Zarzuela R, Zarzosa F, et al. Whole-body vibration as a method of recovery for soccer players. Eur J Sport Sci 2012; 12: 2–8. [Google Scholar]
- 31.Cormie P, Deane RS, Triplett NT, et al. Acute effects of whole-body vibration on muscle activity, strength, and power. J Strength Cond Res 2006; 20: 257. [DOI] [PubMed] [Google Scholar]
- 32.Dabbs NC, Black CD, Garner JC. Effects of whole body vibration on muscle contractile properties in exercise induced muscle damaged females. J Electromyogr Kinesiol 2016; 30: 119–125. [DOI] [PubMed] [Google Scholar]
- 33.Orr R, O’Keefe K, Selvadurai H, et al. The effect of whole body vibration exposure on muscle function in cystic fibrosis patients-A pilot efficacy trial. J Sci Med Sport 2010; 13: e102–e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pournot H, Tindel J, Testa R, et al. The acute effect of local vibration as a recovery modality from exercise-induced increased muscle stiffness. J Sports Sci Med 2016; 15: 142–147. [PMC free article] [PubMed] [Google Scholar]
- 35.Abd T. The effects of vibration on delayed onset of muscle soreness before eccentric exercise in non-dominant biceps muscle. Physiotherapy 2015; 101: e28. [Google Scholar]
- 36.Song FM, Liu BX. Effect of whole body vibration intervention and static stretching on delayed onset muscle soreness after eccentric exercise (In Chinese). Journal of Shandong Sport University 2017; 33: 74–79. [Google Scholar]
- 37.Shen YH. Comparison of the effects of vibration training and static traction on the relief of delayed onset muscle soreness (In Chinese). Journal of Military Physical Education and Sports 2017; 36: 48–51. [Google Scholar]
- 38.Timon R, Tejero J, Brazo-Sayavera J, et al. Effects of whole-body vibration after eccentric exercise on muscle soreness and muscle strength recovery. J Phys Ther Sci 2016; 28: 1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhea MR, Bunker D, Marin PJ, et al. Effect of iTonic whole-body vibration on delayed-onset muscle soreness among untrained individuals. J Strength Cond Res 2009; 23: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 40.Kim YS, Park HS, Song EM, et al. Effects of whole-body vibration on DOMS and comparable study with ultrasound therapy. https://pdfs.semanticscholar.org/92d0/5b93cc5aa12ee0bb7cb24924dc19c27e29b6.pdf (2011, accessed date month year)
- 41.Kim JY, Kang DH, Lee JH, et al. The effects of pre-exercise vibration stimulation on the exercise-induced muscle damage. J Phys Ther Sci 2017; 29: 119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakhtiary AH, Safavi-Farokhi Z, Aminian-Far A. Influence of vibration on delayed onset of muscle soreness following eccentric exercise. Br J Sports Med 2007; 41: 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aminian-Far A, Hadian MR, Olyaei G, et al. Whole-body vibration and the prevention and treatment of delayed-onset muscle soreness. J Athl Train 2011; 46: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo J, Li L, Gong Y, et al. Massage alleviates delayed onset muscle soreness after strenuous exercise: a systematic review and meta-analysis. Front Physiol 2017; 8: 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asmussen E. Observations on experimental muscular soreness. Acta Rheumatol Scand 1956; 2: 109–116. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama A, Aoi W, Takami M, et al. Effect of downhill walking on next-day muscle damage and glucose metabolism in healthy young subjects. J Physiol Sci Epub ahead of print 20 April 2018. doi: 10.1007/s12576-018-0614-8. [DOI] [PMC free article] [PubMed]
- 47.Ely MR, Romero SA, Sieck DC, et al. A single dose of histamine-receptor antagonists prior to downhill running alters markers of muscle damage and delayed onset muscle soreness. J Appl Physiol 2016; 122: 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hooren BV, Bosch F. Is there really an eccentric action of the hamstrings during the swing phase of high-speed running? Part II: implications for exercise. J Sport Sci 2016; 35: 2313–2321. [DOI] [PubMed] [Google Scholar]
- 49.Fleckenstein J, Niederer D, Auerbach K, et al. No effect of acupuncture in the relief of delayed-onset muscle soreness: results of a randomized controlled trial. Clin J Sport Med 2016; 26: 471–477. [DOI] [PubMed] [Google Scholar]