Summary
The aim of the study was to examine whether a rat model of liver cirrhosis induced by carbon tetrachloride (CCl4) is a suitable model of muscle wasting and alterations in amino acid metabolism in cirrhotic humans. Rats were treated by intragastric gavage of CCl4 or vehicle for 45 days. Blood plasma and different muscle types—tibialis anterior (mostly white fibres), soleus (red muscle) and extensor digitorum longus (white muscle) ‐ were analysed at the end of the study. Characteristic biomarkers of impaired hepatic function were found in the plasma of cirrhotic animals. The weights and protein contents of all muscles of CCl4‐treated animals were lower when compared with controls. Increased concentrations of glutamine (GLN) and aromatic amino acids (phenylalanine and tyrosine) and decreased concentrations of branched‐chain amino acids (BCAA), glutamate (GLU), alanine and aspartate were found in plasma and muscles. In the soleus muscle, GLN increased more and GLU and BCAA decreased less than in the extensor digitorum and tibialis muscles. Increased chymotrypsin‐like activity (indicating enhanced proteolysis) and decreased α‐ketoglutarate and ATP levels were found in muscles of cirrhotic animals. ATP concentration also decreased in blood plasma. It is concluded that a rat model of CCl4‐induced cirrhosis is a valid model for the investigation of hepatic cachexia that exhibits alterations in line with a theory of role of ammonia in pathogenesis of BCAA depletion, citric cycle and mitochondria dysfunction, and muscle wasting in cirrhotic subjects. The findings indicate more effective ammonia detoxification to GLN in red than in white muscles.
Keywords: ammonia detoxification, cachexia, glutamine, liver cirrhosis
1. INTRODUCTION
Muscle wasting is a serious complication of liver cirrhosis that increases the risk of encephalopathy and significantly worsens the quality of life and prognosis of the illness.1, 2, 3, 4 A number of studies have demonstrated poor responsiveness of hepatic cachexia to the anabolic signals and that conventional nutritional strategies are insufficient to stop the loss of muscle mass.5, 6, 7
The pathogenesis of hepatic cachexia is unclear. Undoubtedly, maldigestion and malabsorption of food, the inhibitory effect of ammonia on protein synthesis and compromised gut barrier function resulting in bacterial infections all play a role.8, 9, 10 Several studies have shown that ammonia increases catabolism of branched‐chain amino acids (BCAAs; valine, leucine and isoleucine) in muscles and decreases their level in the blood.11, 12, 13, 14 It has been postulated that enhanced ammonia detoxification to glutamine (GLN) may increase the drain of α‐ketoglutarate (α‐KG) from the tricarboxylic acid (TCA) cycle (cataplerosis) resulting in mitochondrial dysfunction and decreased ATP production.15, 38
To understanding the pathogenesis of muscle wasting and to enable preclinical testing of selected agents, clinically relevant models of hepatic cachexia are essential. The most common method for experimentally inducing liver cirrhosis in rats is to use multiple doses of carbon tetrachloride (CCl4), which produces most of the features of abnormal hepatic function observed in humans including portal‐systemic shunts, ascites, hyperammonaemia, hypoalbuminaemia, low BCAA and high aromatic amino acid (phenylalanine and tyrosine) levels in the blood.8, 17, 18 The morphological characteristic of the cirrhotic liver usually is described as micronodular, and most of the deductions regarding the histogenesis of cirrhosis have been based on this model.19 Less frequently other hepatotoxins are used (eg, thioacetamide, dimethylnitrosamine and galactosamine). A surgical alternative to develop liver cirrhosis is the ligation of the common bile duct, which has been shown to be a relevant model to study muscle mass loss in cirrhosis.20 Unfortunately, relationships between the degree of the loss of muscle mass and amino acid metabolism in muscles are not well characterized in any of the cirrhosis models.
The aim of the present study was to examine whether muscle wasting and supposed alterations due to hyperammonaemia develop in the rat model of liver cirrhosis induced by the chronic administration of CCl4. The main attention was given to alterations in the tibialis anterior muscle (TIB) composed mostly by white fibres and small portion of red fibres. As a number of articles have demonstrated that white (fast‐twitch, glycolytic) fibres are more sensitive to catabolic signals when compared with red (slow‐twitch) fibres,21, 22 selected measurements were also performed in soleus (SOL, red muscle) and extensor digitorum longus (EDL, white muscle) muscles.
2. MATERIALS AND METHODS
2.1. Animals and materials
Male Wistar rats (10 weeks old, body weight 200‐220 g) obtained from Charles River (Sulzfeld, Germany) were housed in standardized cages in quarters with controlled temperature and a 12‐hour light‐dark cycle. The rats were maintained on an ST‐1 (Velas, CR) standard laboratory diet and provided drinking water ad libitum. The chemicals were obtained from Sigma Chemical (St. Louis, MO, USA), Lachema (Brno, CR), Waters (Milford, MA, USA), Biomol (Hamburg, Germany) and Merck (Darmstadt, Germany).
2.2. Ethical approval
The Animal Care and Use Committee of Charles University, Faculty of Medicine in Hradec Kralove, (licence no. 144879/2011‐MZE‐17214) specifically approved this study on November 1, 2016 (identification code MSMT‐33747/2016‐4). All experimental procedures complied with the National Institutes of Health guidelines.
2.3. Experimental design
At the beginning of the study the animals were randomly assigned to an experimental group and a control group. Liver cirrhosis was induced by intragastric administration of CCl4 dissolved in olive oil (1:1) in dose of 2 mL CCl4/kg, three times a week, for 45 days.9, 17, 18 The control animals underwent vehicle administration only. At the end of the study, the overnight fasted animals were euthanized by exsanguination from the abdominal aorta after they had been administered ether anaesthesia. The SOL, EDL and TIB of both legs were quickly removed and weighed, and small pieces (approximately 0.1 g) of the tissues were frozen in liquid nitrogen. The left leg muscles were used for measurement of amino acid and protein content, and the right leg muscles were used for other analyses. Blood was collected in heparinized tubes and immediately centrifuged for 15 minutes at 2200 g using a refrigerated centrifuge; blood plasma was transferred into a clean polypropylene tube.
2.4. Amino acid concentrations in blood plasma and tissues
Amino acid concentrations were determined in the supernatants of deproteinized blood plasma and tissue samples using high‐performance liquid chromatography (HPLC; Alliance 2695, Waters) after derivatization with 6‐aminoquinolyl‐N‐hydroxysuccinimidyl carbamate with norleucine as an internal standard. The results are expressed as μmol/L of blood plasma or nmol/g wet muscle.
2.5. Branched‐chain keto acid concentrations
The reversed‐phase HPLC combined with o‐phenylenediamine derivatization was used for the determination of branched‐chain keto acid (BCKA) in samples of blood plasma. The samples were pH‐adjusted with 6 M sodium acetate (pH 6.0) prior to the chromatographic analysis. The keto acids were separated on LichroCart 125 × 4 mm, Purospher Star RP‐18 (5 μm) endcapped analytical column (Merck Millipore) using the mobile phase consisting of methanol and water at a flow rate of 0.8 mL/min in a gradient mode. The quinoxalinol derivatives of BCKAs were detected using fluorescence with emission and excitation at 410 nm and 350 nm, respectively, and were quantified by the internal standard method. The results are expressed as μmol/L of blood plasma.
2.6. Chymotrypsin‐like activity of proteasome and cathepsin B and L activities
The Chymotrypsin‐like activity (CHTLA) of the proteasome and cathepsin B and L activities were determined using the fluorogenic substrates Suc‐LLVY‐MCA23 and Z‐FA‐MCA,24 respectively, as previously described in detail.21 The fluorescence of the samples was measured at the excitation wavelength of 340 nm and the emission wavelength of 440 nm (Tecan Infinite™ 200, Tecan Austria GmbH, Salzburg, Austria). A standard curve was established for 7‐amino‐4‐methylcoumarin (AMC), which allowed expression of the enzyme activities in nmol of AMC/g protein/h.
2.7. Adenine nucleotides
ATP, ADP and AMP levels were measured in blood plasma and TIB using HPLC. The reversed‐phase HPLC (Alliance 2695, Waters) combined with ultraviolet (UV) detection was used for the determination of nucleotide concentrations. The HPLC conditions were as follows: LichroCart 250 × 4 mm, Purospher Star RP‐18 (5 μm) endcapped analytical column (Merck Millipore) using the mobile phase consisting of methanol and buffer (50 mmol/L potassium phosphate buffer, pH = 6) at a flow rate 0.4 mL/min in a gradient mode. Peaks were detected at 254 nm and quantified by the external standard method. The results are expressed as μmol/L of blood plasma or μmol/g of wet muscle.
2.8. TCA cycle intermediates
Tricarboxylic acid cycle components, including α‐KG, citrate, fumarate, cis‐aconitate and succinate, were quantified in blood plasma and TIB by HPLC (Alliance 2695, Waters) equipped with a C18 YMC‐Triart analytical column in isocratic mode with 20 mmol/L potassium phosphate buffer (pH 2.9). The wavelength for detection was set at 210 nm. The results are expressed as μmol/g of wet muscle.
2.9. Other techniques
The plasma levels of glucose, albumin, urea, creatinine, bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), creatine phosphokinase (CPK) and ammonia were measured using commercial tests (Boehringer, Mannheim, Germany), Elitech (Sées, France), Lachema (Brno, CR) and Sigma Chemical.
2.10. Statistical analyses
The results are expressed as means ± standard error of the means (SEM). The Mann‐Whitney U test was used to determine the effects of CCl4 treatment. NCSS 2001 statistical software (Kaysville, UT, USA) was used for analyses. The differences were considered significant at P < 0.05.
3. RESULTS
3.1. Alterations in blood biochemistry
Significantly lower blood plasma concentrations of glucose, albumin, urea and creatinine and significantly higher concentrations of bilirubin, ALT, AST, ALP, CPK and ammonia were observed in CCl4‐treated animals (Table 1).
Table 1.
Blood biochemistry
| Control (n = 10) | Cirrhosis (n = 13) | |
|---|---|---|
| Glucose (mmol/L) | 8.71 ± 0.38 | 4.69 ± 0.27a |
| Albumin (g/L) | 41.1 ± 0.3 | 27.4 ± 1.9a |
| Urea (mmol/L) | 6.90 ± 0.37 | 5.60 ± 0.73a |
| Creatinine (μmol/L) | 31 ± 1 | 26 ± 2a |
| Bilirubin (μmol/L) | 2.9 ± 0.1 | 24.8 ± 6.7a |
| ALT (μkat/L) | 0.57 ± 0.06 | 6.58 ± 0.99a |
| AST (μkat/L) | 1.27 ± 0.05 | 14.28 ± 2.16a |
| ALP (μkat/L) | 1.34 ± 0.11 | 5.66 ± 0.70a |
| CPK (μkat/L) | 2.80 ± 0.30 | 4.60 ± 0.30a |
| Ammonia (μmol/L) | 31 ± 3 | 111 ± 12a |
Means ± SEM.
P ˂ 0.05 (Mann‐Whitney U test).
3.2. Alterations in body weight and food intake
There was a significantly lower increase in the gain of body weight in animals treated by CCl4 (Figure 1). At the end of the study, the body weight reached 495 ± 12 g in controls and 420 ± 13 g in CCl4‐treated animals. The intake of food was lower in CCl4‐treated animals in the initial phase of the study. Starting on the 4th day of the study, the differences in daily food intake were not significant.
Figure 1.
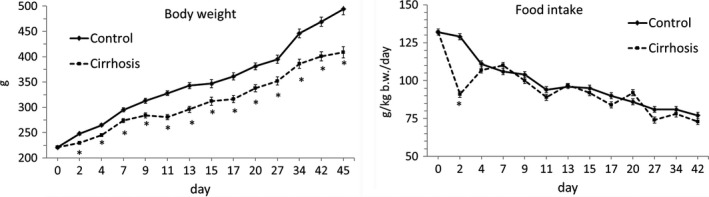
Alterations in body weight and food intake. Means ± SEM. *P ˂ 0.05 (Mann‐Whitney U test)
3.3. Alterations in weight and protein content of muscles
The weights and protein contents of all muscles obtained from the CCl4‐treated animals were lower than muscles of controls. The relative protein content (g/kg body weight) was lower only in the TIB. The differences in protein concentration among muscles were insignificant (Table 2).
Table 2.
Weights and protein content of muscles
| Control (n = 10) | Cirrhosis (n = 13) | |
|---|---|---|
| TIB | ||
| Weight | ||
| g | 0.84 ± 0.02 | 0.68 ± 0.03a |
| g/kg b.w. | 1.71 ± 0.05 | 1.61 ± 0.04 |
| Protein | ||
| Concentration (mg/g) | 174 ± 3 | 159 ± 5 |
| Content (g) | 146 ± 5 | 107 ± 5a |
| Content (g/kg b.w.) | 297 ± 10 | 255 ± 9a |
| SOL | ||
| Weight | ||
| g | 0.23 ± 0.01 | 0.18 ± 0.01a |
| g/kg b.w. | 0.47 ± 0.01 | 0.43 ± 0.01a |
| Protein | ||
| Concentration (mg/g) | 180 ± 9 | 176 ± 4 |
| Content (g) | 41.9 ± 2.0 | 31.7 ± 1.2a |
| Content (g/kg b.w.) | 85.3 ± 5.1 | 75.6 ± 2.3 |
| EDL | ||
| Weight | ||
| g | 0.21 ± 0.00 | 0.16 ± 0.01a |
| g/kg b.w. | 0.42 ± 0.01 | 0.39 ± 0.01 (0.06) |
| Protein | ||
| Concentration (mg/g) | 164 ± 4 | 173 ± 6 |
| Content (mg) | 34.4 ± 1.4 | 28.3 ± 1.3a |
| Content (g/kg b.w.) | 69.9 ± 3.2 | 67.4 ± 2.8 |
Means ± SEM.
P ˂ 0.05 (Mann‐Whitney U test).
3.4. Alterations in protein breakdown
Chronic administration of CCl4 enhanced CHTLA in TIB. The effect on cathepsin B and L activities was insignificant (Figure 2).
Figure 2.
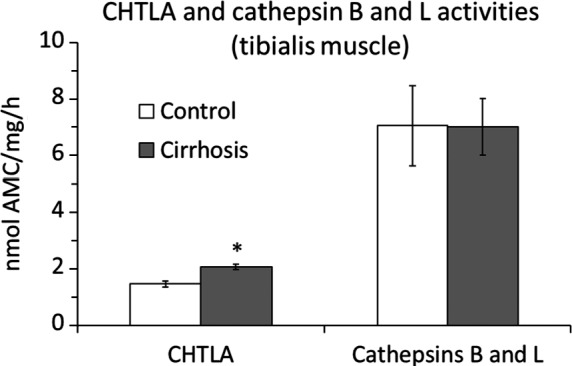
Alterations in protein breakdown. Means ± SEM. *P ˂ 0.05 (Mann‐Whitney U test)
3.5. Amino acid and BCKA concentrations in blood plasma
The blood plasma concentrations of GLN and aromatic amino acids (PHE and TYR) increased whereas the BCAA, glutamate (GLU), alanine (ALA) and ASP decreased in cirrhotic rats (Figure 3). The BCKA levels were altered heterogeneously. A significant decrease was found in KIC (ketoleucine) and KMV (ketoisoleucine), whereas KIV (ketovaline) concentration was unaltered.
Figure 3.
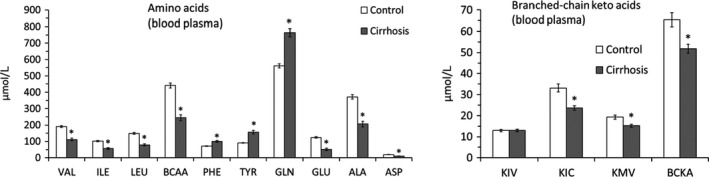
Alterations in amino acid and branched‐chain keto acid concentrations in blood plasma. Means ± SE. *P ˂ 0.05 (Mann‐Whitney U test)
3.6. Amino acid concentrations in muscles
Amino acid concentrations in the muscles of cirrhotic animals exhibited a similar pattern to those in blood plasma (Table 3). GLN, PHE and TYR concentrations increased whereas BCAAs, GLU, ALA and ASP decreased in all types of examined muscles. In SOL, GLN increased more and GLU and BCAA decreased less than in EDL and TIB.
Table 3.
Amino acid concentrations in muscles
| TIB | SOL | EDL | ||||
|---|---|---|---|---|---|---|
| Control (n = 10) | Cirrhosis (n = 13) | Control (n = 9) | Cirrhosis (n = 13) | Control (n = 10) | Cirrhosis (n = 13) | |
| VAL | 218 ± 13 | 130 ± 9a | 134 ± 7 | 91 ± 7a | 173 ± 5 | 107 ± 8a |
| ILE | 131 ± 12 | 75 ± 7a | 69 ± 3 | 52 ± 5a | 92 ± 3 | 54 ± 5a |
| LEU | 207 ± 15 | 126 ± 12a | 104 ± 5 | 70 ± 6a | 123 ± 6 | 72 ± 5a |
| Σ BCAA | 556 ± 40 | 331 ± 27a | 308 ± 15 | 214 ± 16a | 389 ± 14 | 232 ± 17a |
| PHE | 121 ± 14 | 139 ± 8a | 64 ± 2 | 111 ± 5a | 114 ± 2 | 133 ± 4a |
| TYR | 181 ± 12 | 263 ± 15a | 103 ± 5 | 209 ± 20a | 129 ± 2 | 220 ± 12a |
| GLN | 3240 ± 168 | 3876 ± 175a | 5109 ± 234 | 8013 ± 281a | 4156 ± 132 | 5575 ± 253a |
| GLU | 1412 ± 136 | 621 ± 136a | 3467 ± 82 | 2192 ± 227a | 2639 ± 104 | 820 ± 148a |
| ALA | 2228 ± 73 | 964 ± 81a | 1930 ± 70 | 944 ± 92a | 1882 ± 59 | 680 ± 59a |
| ASP | 350 ± 39 | 176 ± 23a | 2718 ± 113 | 883 ± 180a | 519 ± 47 | 163 ± 12a |
Means ± SEM in nmol/g of muscle.
P ˂ 0.05 (Mann‐Whitney U test).
3.7. Alterations in TCA cycle intermediates and adenine nucleotides
We found a marked decrease in αKG in TIB of the CCl4‐treated animals. Cis‐aconitate was also significantly decreased, whereas succinate increased. The citrate concentration was unaltered (Figure 4).
Figure 4.
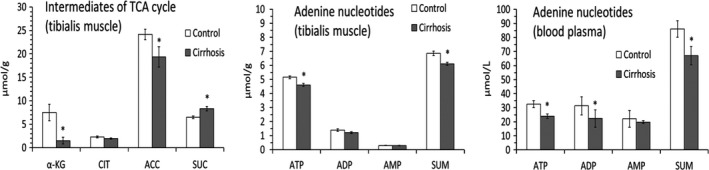
Alterations in TCA cycle intermediates and adenine nucleotides. Means ± SEM. *P ˂ 0.05 (Mann‐Whitney U test)
A significant decrease in ATP and total level of adenine nucleotides was found in TIB of cirrhotic rats. In blood plasma, decreased concentrations of ATP, ADP and total level of adenine nucleotides were found.
4. DISCUSSION
The data collectively show that CCl4‐induced cirrhosis in rats is a valid experimental model for the investigation of pathogenesis and therapy of hepatic cachexia. Alterations observed in blood plasma and muscles of cirrhotic rats are similar to those observed in cirrhotic patients. Of special importance are hyperammonaemia, hypoalbuminaemia, low BCAA and high aromatic amino acids, as well as decreased urea and glucose levels. Decreased blood plasma concentrations of BCAA and increased concentrations of aromatic amino acids in blood plasma found in cirrhotic rats are characteristic for subjects with liver cirrhosis,25, 26 but not for acute liver injury.27 The BCAA deficiency and their positive effects on protein metabolism in muscles provide a clear rationale to recommend their supplementation in patients with cirrhosis. In fact, the lack of success has been reported in a number of clinical trials, and some adverse effects of enhanced BCAA consumption have been suggested.28, 29
Decreased weight and protein content of muscles are in line with reports showing a decrease in the cross‐sectional area of muscles and muscle fibres in CCl4‐treated mice30 and rats.31 Enhanced CHTLA in the muscles of cirrhotic rats indicates that enhanced protein degradation plays a role in the loss of muscle tissue. The finding is in agreement with the report of upregulation of the proinflammatory cytokines and increased expression of NF‐κB and MuRF‐1 in cirrhotic mice.30 Indirect markers of muscle wasting in CCl‐4 treated animals are decreased creatinine concentration and increased CPK activity in the blood.
4.1. Alterations in amino acid concentrations in muscles related to ammonia detoxification to GLN
Alterations in amino acid concentrations in muscles of CCl4 treated animals are in line with a well‐defined role of muscles in ammonia detoxification to GLN (Figure 5). Of special interest should be the increased concentrations of GLN and decreased concentrations of BCAA, GLU and ALA. The alterations indicate enhanced synthesis of GLN from GLU and the shift of GLU metabolism from its use as a donor of nitrogen to pyruvate for synthesis of ALA to GLN synthesis. The BCAAs decrease due to their use as donors of nitrogen to α‐KG for synthesis of GLU, which is a direct substrate for ammonia detoxification to GLN.12, 13
Figure 5.
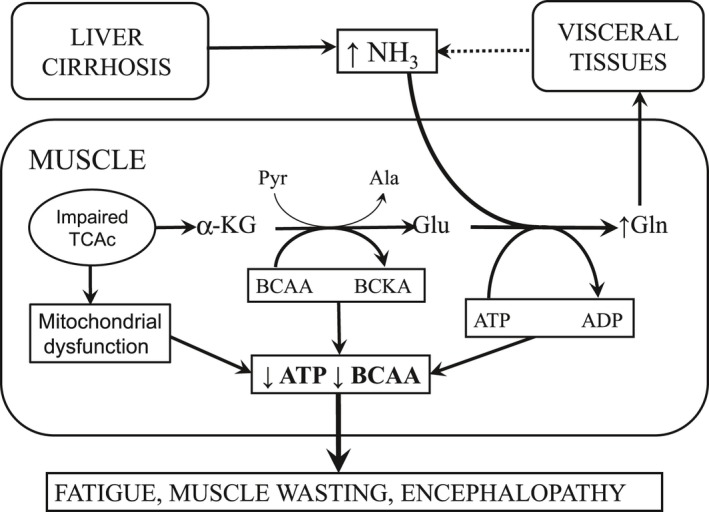
Metabolic reactions involved in ammonia detoxification to GLN in muscles resulting in enhanced BCAA catabolism, cataplerosis of α‐KG, mitochondrial dysfunction and ATP depletion. In addition, GLN synthesis from GLU catalysed by glutamine synthetase is the ATP consuming reaction. Clinical manifestations are fatigue and muscle wasting
Unfortunately, the number of studies which refer to amino acid changes in the muscles due to cirrhosis is limited and most of the data are older. A marked decrease in the BCAA in muscles in patients with cirrhosis was reported by Iob et al.32 Plauth et al.33 reported decreased levels of VAL and ILE, and unaltered concentration of LEU, in quadriceps femoris muscle. A significant decrease in VAL and unaltered levels of ILE and LEU in patients with stable liver cirrhosis were found by Montanari et al.34
4.2. Alterations in BCKA concentrations
An observed decrease in the BCKA in the blood plasma of CCl4‐treated animals is in line with reports of decreased BCKA levels in cirrhotic rats and patients with liver cirrhosis.35, 36 These findings support the suggestion that hyperammonaemia decreases BCAA not only by the enhanced use of their nitrogen for the synthesis of GLU, but also activates their oxidation. Enhanced oxidation of the BCAA in hyperammonaemic conditions has been reported in a number of studies.11, 12, 14, 18 Activation of the BCKA dehydrogenase, which regulates irreversible oxidation of BCKAs, has been shown in the cirrhotic liver of rats with chronic liver failure.35
The unaltered concentration of KIV (ketovaline) might be explained by impaired gluconeogenesis from VAL in the liver (VAL is glucogenic and unlike LEU and ILE is not catabolized to acetyl‐CoA). The finding is in agreement with the report of delayed clearance of VAL and KIV from blood in cirrhotic patients and supports the suggestion that people with cirrhosis have a diminished tolerance for VAL.36
4.3. Alterations in intermediates of TCA cycle and adenine nucleotides
The decrease in α‐KG in the muscles of rats with cirrhosis supports the theory that enhanced ammonia detoxification to GLN causes cataplerotic efflux of α‐KG from the TCA cycle and impairs the energy status of muscles (Figure 5). Hyperammonaemia decreased mitochondrial respiration, NAD+/NADH ratio, TCA cycle intermediates and ATP content in C2C12 myotubes.15, 38 The cause of the reduction of α‐KG but no other intermediates of the TCA cycle could be compensatory anaplerotic reactions including metabolites of ILE and VAL that can enter the TCA cycle via succinyl CoA. This speculation is supported by increased succinate concentration in the TIB of cirrhotic rats.
Decreased ATP levels in blood plasma and muscles of CCl4‐treated animals indicate impaired energy status, which may play a role in fatigue and muscle wasting. The data reported in the present study indicate a role of cataplerosis induced by ammonia detoxification to GLN. ATP consumption in synthesis of GLN from GLU also may have a role. ATP and the total level of adenine nucleotides were markedly reduced in skeletal muscle biopsy specimens of patients with liver cirrhosis.37, 39 A decrease of 25%‐39% in blood ATP levels was reported in patients with alcoholic hepatitis and both alcoholic and non‐alcoholic liver cirrhosis.16
4.4. Differences between red and white muscles
A number of articles have demonstrated that white muscles (fast‐twitch, glycolytic) are more sensitive to various signals when compared with red (slow‐twitch) muscles utilizing glycogen and fat for energy.21, 22 In the present study, the differences in the effect of cirrhosis on the weight loss and protein content in various muscle types were insignificant. However, marked differences were manifested in GLN, GLU and BCAA levels. In SOL, the increase in GLN concentration was higher and the decrease in GLU and BCAA lower when compared with EDL. These finding indicate higher rates of ammonia detoxification to GLN in SOL (red muscle) than in EDL (white muscle). Higher protein turnover and higher BCAA aminotransferase activity in red compared to white muscles might play a role in this metabolic difference.21, 40
5. CONCLUSIONS
We conclude that the rat model of liver cirrhosis induced by CCl4 exhibits (i) muscle mass loss and multiple metabolic similarities with liver cirrhosis in humans, and (ii) alterations in GLN, ALA, BCAA, TCA cycle and ATP concentrations in blood plasma and muscles, which are in line with the theory of a role of ammonia in the pathogenesis of hepatic cachexia and BCAA depletion in patients with cirrhosis. The findings also indicate higher rates of ammonia detoxification to GLN in red (slow‐twitch) than in white (fast‐twitch) muscles.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
FUNDING SOURCE STATEMENT
This work was supported by the programme PROGRES Q40/02.
AUTHOR CONTRIBUTIONS
The authors meet ICMJE authorship criteria, and nobody who qualifies for authorship has been excluded. M.H. provided conception and design of the study, interpreted results of experiments and prepared the manuscript. M.V. performed the experiments, analysed data and edited the manuscript.
ACKNOWLEDGEMENTS
The authors wish to thank R. Fingrova and D. Jezkova for their technical assistance.
Holeček M, Vodeničarovová M. Muscle wasting and branched‐chain amino acid, alpha‐ketoglutarate and ATP depletion in a rat model of liver cirrhosis. Int. J. Exp. Path. 2018;99:274–281. 10.1111/iep.12299
REFERENCES
- 1. Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanai T, Shiraki M, Ohnishi S, et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res. 2016;46:743‐751. [DOI] [PubMed] [Google Scholar]
- 3. Nardelli S, Lattanzi B, Torrisi S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol. 2017;15:934‐936. [DOI] [PubMed] [Google Scholar]
- 4. Peng S, Plank LD, McCall JL, et al. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257‐1266. [DOI] [PubMed] [Google Scholar]
- 5. Ng EH, Lowry SF. Nutritional support and cancer cachexia. Hematol Oncol Clin North Am. 1991;5:161‐194. [PubMed] [Google Scholar]
- 6. Shaw JHF, Wolfe RR. An integrated analysis of glucose, fat, and protein metabolism in severely traumatized patients: Studies in the basal state and the response to total parenteral nutrition. Ann Surg. 1989;209:63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Streate SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987;27:262‐266. [DOI] [PubMed] [Google Scholar]
- 8. Blondé‐Cynober F, Plassart F, Rey C, et al. Assessment of the carbon tetrachloride‐induced cirrhosis model for studies of nitrogen metabolism in chronic liver disease. Ann Nutr Metab. 1994;38:238‐248. [DOI] [PubMed] [Google Scholar]
- 9. Holecek M, Skopec F, Sprongl L. Protein metabolism in cirrhotic rats: effect of dietary restriction. Ann Nutr Metab. 1995;39:346‐354. [DOI] [PubMed] [Google Scholar]
- 10. Schott K, Poetter U, Neuhoff V. Ammonia inhibits protein synthesis in slices from young rat brain. J Neurochem. 1984;42:644‐646. [DOI] [PubMed] [Google Scholar]
- 11. Holecek M, Sprongl L, Tichy M. Effect of hyperammonemia on leucine and protein metabolism in rats. Metabolism Clin Exp. 2000;49:1330‐1334. [DOI] [PubMed] [Google Scholar]
- 12. Holecek M, Kandar R, Sispera L, et al. Acute hyperammonemia activates branched‐chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids. 2011;40:575‐584. [DOI] [PubMed] [Google Scholar]
- 13. Holeček M, Vodeničarovová M. Effects of branched‐chain amino acids on muscles under hyperammonemic conditions. J Physiol Biochem. 2018;74:523‐530. [DOI] [PubMed] [Google Scholar]
- 14. Leweling H, Breitkreutz R, Behne F, et al. Hyperammonemia‐induced depletion of glutamate and branched‐chain amino acids in muscle and plasma. J Hepatol. 1996;25:756‐762. [DOI] [PubMed] [Google Scholar]
- 15. Davuluri G, Allawy A, Thapaliya S, et al. Hyperammonaemia‐induced skeletal muscle mitochondrial dysfunction results in cataplerosis and oxidative stress. J Physiol. 2016a;594:7341‐7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagenmakers AJ, Coakley JH, Edwards RH. Metabolism of branched‐chain amino acids and ammonia during exercise: clues from McArdle‘s disease. Int J Sports Med. 1990;11(Suppl. 2):S101‐S113. [DOI] [PubMed] [Google Scholar]
- 17. Holecek M, Mraz J, Tilser I. Plasma amino acids in four models of experimental liver injury in rats. Amino Acids. 1996a;10:229‐241. [DOI] [PubMed] [Google Scholar]
- 18. Holecek M, Tilser I, Skopec F, et al. Leucine metabolism in rats with cirrhosis. J Hepatol. 1996b;24:209‐216. [DOI] [PubMed] [Google Scholar]
- 19. Rubin E, Popper H. The evolution of human cirrhosis deduced from observations in experimental animals. Medicine (Baltimore). 1967;46:163‐183. [DOI] [PubMed] [Google Scholar]
- 20. Bosoi CR, Oliveira MM, Ochoa‐Sanchez R, et al. The bile duct ligated rat: a relevant model to study muscle mass loss in cirrhosis. Metab Brain Dis. 2017;32:513‐518. [DOI] [PubMed] [Google Scholar]
- 21. Holeček M, Mičuda S. Amino acid concentrations and protein metabolism of two types of rat skeletal muscle in postprandial state and after brief starvation. Physiol Res. 2017;66:959‐967. [DOI] [PubMed] [Google Scholar]
- 22. Kadlcikova J, Holecek M, Safranek R, et al. Effects of proteasome inhibitors MG132, ZL3VS and AdaAhx3L3VS on protein metabolism in septic rats. Int J Exp Pathol. 2004;85:365‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gomes‐Marcondes MC, Tisdale MJ. Induction of protein catabolism and the ubiquitin‐proteasome pathway by mild oxidative stress. Cancer Lett. 2002;180:69‐74. [DOI] [PubMed] [Google Scholar]
- 24. Koohmaraie M, Kretchmar DH. Comparisons of four methods for quantification of lysosomal cysteine proteinase activities. J Anim Sci. 1990;68:2362‐2370. [DOI] [PubMed] [Google Scholar]
- 25. Campollo O, Sprengers D, Dam G, et al. Protein tolerance to standard and high protein meals in patients with liver cirrhosis. World J. Hepatol. 2017;9:667‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dam G, Sørensen M, Buhl M, et al. Muscle metabolism and whole blood amino acid profile in patients with liver disease. Scand J Clin Lab Invest. 2015;75:674‐680. [PubMed] [Google Scholar]
- 27. Holecek M, Skalská H, Mráz J. Plasma amino acid levels after carbon tetrachloride induced acute liver damage. A dose‐response and time‐response study in rats. Amino Acids. 1999;16:1‐11. [DOI] [PubMed] [Google Scholar]
- 28. Holeček M. Branched‐chain amino acid supplementation in treatment of liver cirrhosis: updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition. 2017;41:80‐85. [DOI] [PubMed] [Google Scholar]
- 29. Plauth M, Schütz T. Branched‐chain amino acids in liver disease: new aspects of long known phenomena. Curr Opin Clin Nutr Metab Care. 2011;14:61‐66. [DOI] [PubMed] [Google Scholar]
- 30. Giusto M, Barberi L, Di Sario F, et al. Skeletal muscle myopenia in mice model of bile duct ligation and carbon tetrachloride‐induced liver cirrhosis. Physiol Rep. 2017;5(7):e13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weber FL, Macechko PT, Kelson SR, et al. Increased muscle protein catabolism caused by carbon tetrachloride hepatic injury in rats. Gastroenterology. 1992;102:1700‐1706. [DOI] [PubMed] [Google Scholar]
- 32. Iob V, Coon WW, Sloan M. Free amino acids in liver, plasma, and muscle of patients with cirrhosis of the liver. J Surg Res. 1967;7:41‐43. [DOI] [PubMed] [Google Scholar]
- 33. Plauth M, Egberts EH, Abele R, et al. Characteristic pattern of free amino acids in plasma and skeletal muscle in stable hepatic cirrhosis. Hepatogastroenterology. 1990;37:135‐139. [PubMed] [Google Scholar]
- 34. Montanari A, Simoni I, Vallisa D, et al. Free amino acids in plasma and skeletal muscle of patients with liver cirrhosis. Hepatology. 1988;8:1034‐1039. [DOI] [PubMed] [Google Scholar]
- 35. Honda T, Fukuda Y, Nakano I, et al. Effects of liver failure on branched‐chain alpha‐keto acid dehydrogenase complex in rat liver and muscle: comparison between acute and chronic liver failure. J Hepatol. 2004;40:439‐445. [DOI] [PubMed] [Google Scholar]
- 36. Schauder P, Schröder K, Herbertz L, et al. Evidence for valine intolerance in patients with cirrhosis. Hepatology. 1984;4:667‐670. [DOI] [PubMed] [Google Scholar]
- 37. Möller P, Bergström J, Fürst P, et al. Muscle biopsy studies in patients with moderate liver cirrhosis with special reference to energy‐rich phosphagens and electrolytes. Scand J Gastroenterol. 1984;19:267‐272. [PubMed] [Google Scholar]
- 38. Davuluri G, Krokowski D, Guan BJ, et al. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l‐leucine in cirrhosis. J Hepatol. 2016b;65:929‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hernández‐Muñoz R, Glender W, Díaz‐Muñoz M, et al. Alterations of ATP levels and of energy parameters in the blood of alcoholic and nonalcoholic patients with liver damage. Alcohol Clin Exp Res. 1991;15:500‐503. [DOI] [PubMed] [Google Scholar]
- 40. Yang Q, Birkhahn RH. Branched‐chain transaminase and keto acid dehydrogenase activities in burned rats: evidence for a differential adaptation according to sex. Nutrition. 1997;13:640‐645. [DOI] [PubMed] [Google Scholar]


