Abstract
Background:
Cervical cancer caused by the human papillomavirus (HPV) is endemic in East Africa. Recent, dramatic, increases in the incidence of oropharyngeal cancer in the United States and Europe are linked to the same high risk HPV genotypes responsible for cervical cancer. Currently, there is extremely limited data regarding the role of HPV in head and neck cancers in Africa. Evidence of HPV as anetiologic agent in head and neck cancers in Africa would have important prevention and treatment implications.
Methods:
A retrospective single institution review of oral tongue and oropharyngeal squamous cell carcinomas diagnosed between 2005 and 2013 was performed. Individual case data for 51 patients with biopsy proven squamous cell carcinoma (SCC) from the oropharynx (n = 22) and oral tongue (n = 29) were identified. Formalin fixed, paraffin embedded biopsy samples were obtained and evaluated for p16 by immunohistochemistry and HPV genotype 16 specific oncogenes, E6 and E7, by PCR.
Results:
All of the positive controls, but none of the oropharyngeal samples stained positively for p16. Two of the oral tongue samples stained positive for p16. None of the oropharyngeal or oral tongue cases demonstrated PCR products for HPV-16 E6 or E7.
Conclusions:
Though Mozambique has extremely high levels of HPV positive cervical cancer this study demonstrates an absence of HPV positive oropharyngeal or oral tongue squamous cell carcinoma within biopsy samples from a single referral hospital in Maputo, the capital of Mozambique.
Keywords: Africa; Africa, Eastern; Carcinoma; Squamous cell; Head and neck neoplasms; Human papillomavirus 16; Mozambique; Oropharyngeal neoplasms; Paraffin embedding; Polymerase chain reaction; Immunohistochemistry
1. Introduction
Head and neck cancers are the sixth most common cancer worldwide [1]. In the developed world, declining trends in smoking have been associated with a declining incidence of head and neck squamous cell carcinoma (HNSCC). A notable exception is squamous cell carcinomas of the oropharynx (OP). This trend has been noted worldwide with incident cases of OPSCC increasing from 18% to 31% of total HNSCCs [2]. Sweden and the US, for example, have noted a seven fold increase in OPSCC over the past 30 years [3,4] and an overall increase in oral tongue, base of tongue and tonsillar SCC [5]. The data for this trend in Africa is limited to one study from South Africa that showed, through a review of their pathology based cancer registry, an increase in oropharyngeal cancers among “coloured” South Africans from 1992 to 2001 [6]. Interestingly, the trend in incidence of OPSCC within the developed world is accompanied by a distinct change in patient characteristics. These cases are arising in younger, non-smoking individuals [7]. This eventually led to the discovery of the relationship of these cancers with the human papillomavirus (HPV), specifically high risk genotype 16. Though the presence of HPV DNA has been noted in oral and OP mucosal tumors since the 1980s and cervical cancer research has found HPV 16 and 18 to be tumorigenic, causation with OPSCC was not clearly identified until 2000 [7–9].
That year Gillison et al. described HPV related OPSCC as a distinct oncologic entity, both molecularly and pathologically. In a cohort of 253HNSCC samples they found 62 to be HPV positive and, of those, 90% were HPV 16. Thirty four of the 62HPV positive tumors were from the OP and 32 of those arose from the lingual or palantine tonsil. Molecular and pathologic analysis of these HPV positive tumors demonstrated decreased risk of p53 mutation, a non-keratizining/basaloid morphology and southern blot evidence of viral DNA integration. And, the HPV positive tumors demonstrated a 15 month survival benefit over HPV negative tumors [7]. D’souza further defined the relationship between HPV-16 and OPSCC by showing of 100 patients newly diagnosed with OPSCC, 72% had HPV 16 DNA in the tumor. And, considering the risk of HPV infection with sexual practices, they identified both number of sexual and oral sex partners as risk factors of OPSCC [8]. The increased survival benefit was further supported by Lindquist who showed a disease specific survival at five years of 81% for HPV positive versus 36% for HPV negative tonsillar SCC [10].
Though these advances bode well for those with OPSCC in the developed world, data regarding HPV status of patients in the developing world, specifically Africa, are sparse. One systematic review looked at worldwide HPV types in HNSCC and included data from 60 studies in over 20 countries, but only one study included data from Africa. Though the review demonstrated a 35.6% HPV positivity rate for OPSCC, conclusions concerning Africa cannot be drawn since the one study including an African country had a total of three OPSCC cases from one country, Sudan [11]. Ndiaye, in 2013, evaluated the presence of HPV in 117HNSCC from all head and neck subsites in Senegal. They identified five OPSCC cases, none of which were positive for HPV by PCR or IHC for p16 [12]. Another study from Ghana found 15 of 78HNSCC tumors HPV positive (86.7% HPV-16), but eight of the 15 were from the larynx and only two were from the pharynx [13]. In contrast, Paquette et al., in 2013, evaluated OPSCC samples from 55 black South Africans from 2005 to 2010 and found 49.1% of these cancers were driven by HPV [14]. Other studies from Africa, specifically Nigeria and Egypt, merely examine types of head and neck cancers, their locations and some demographic data without mention of histology or HPV status [15,16]. This leaves a large knowledge gap regarding HPV related HNSCC in Africa and, specifically, sub-Saharan Africa versus the developed world.
Despite the lack of research in HNSCC in Africa, there has been a significant amount research regarding cervical cancer in sub-Saharan Africa [17,18], including Mozambique. East Africa has one of the highest rates of cervical cancer in the world accounting for 14.3% of all cancers, male and female [1]. In Mozambique, one study demonstrated 100% of cervical cancers were HPV related and 47% were HPV 16 and 31.3% were HPV 18 [19]. Another study found 70 of 72 cervical cancers in Mozambique positive for HPV with HPV-16 and 18 accounting for 69% of the tumors [20].
The high rates of HPV positive cervical cancer in East Africa and epidemiological data ranking head and neck cancers as the sixth most common in the region would suggest HPV may be playing a role in HNSCC. This study aims to evaluate the presence of HPV related oropharyngeal and oral tongue SCCs in Mozambique and, to our knowledge, this is the first such study to do so in East Africa.
2. Materials and methods
Approval for the study was obtained from both the Yale University Human Investigation Committee (HIC Protocol #: 1301011341) and the Eduardo Mondlane University’s Scientific and Bioethics Committee. Informed consent was not required and the review was performed in compliance with the Health Insurance Portability and Accountability Act. Study samples were identified through a retrospective, computerized database search of all pathologic specimens received by the Department of Pathology at the Hospital Central de Maputo (HCM) from 2005 to 2013. Specifically, location, SNOMED topography and morphology and pathologic diagnosis were used to identify squamous cell carcinoma samples of the oropharynx (tonsil, base of tongue, soft palate and posterior pharyngeal wall) and oral tongue. The pathology reports were then reviewed to confirm adequate detail regarding location and pathology. Each sample was then given a de-identified study number. Demographic data was obtained but was limited to what was provided on the pathology report and included age, sex, race, profession, symptoms, location of biopsy, histology and diagnosis. Due to the decentralized nature of record keeping at HCM, further information regarding treatments, outcomes or other patient specific co-morbidities (including smoking or HIV status) was unavailable.
The paraffin blocks of the samples meeting criteria were then recovered as possible. Each sample was then cut, stained with H&E and reviewed by the chief of pathology in Maputo (CC) to confirm the diagnosis of SCC. Samples that met criteria for inclusion were then re-cut and underwent immunohistochemistry (IHC) for p16 (mouse anti-human, SC-56330, Santa Cruz Biotechnology, Dallas, TX) using a protocol derived from the Yale University School of Medicine and the Immunocruz mouse ABC staining system (SC-2017, Santa Cruz Biotechnology, Dallas, TX). To control for variability in staining, each IHC run included an identical positive cervical cancer control. Stained samples were then reviewed for positivity by the chief of pathology at HCM (CC). Pictures of all stained samples were taken with a photographic microscope in Maputo (Leica) and were reviewed electronically by an experienced head and neck pathologist at Yale (MP).
To determine the presence of HPV-16 genomic sequences, PCR was conducted on DNA extracted from each sample. Briefly, DNA was extracted from the formalin fixed paraffin embedded samples utilizing the QIAamp DNA FFPE Tissue Kit and Deparaffinization Solution (Qiagen, Venlo, the Netherlands) according to manufacturer protocol. The number of 4 μm paraffin slices used per sample depended on the size of the specimen sample in the block to help insure adequate DNA extraction. For instance, if the specimen was a small biopsy with a few nest of tumor cells 7–10 slices were used as compared to 2–3 for a large tissue sample. PCR was then performed using primers for HPV-16 E6 (Forward: ATG CAC CAA AAG AGA ACT G Reverse: TTA CAG CTG GGT TTC TCT AC) and E7 (Forward: ATG CAT GGA GAT ACA CCT AC Reverse: TTATGG TTT CTG AGA ACA GAT GG) annealing at 47 °C. Products were run on a 0.8% agarose gel with ethidium bromide according to standard protocols. Each set of PCR reactions was performed with a positive control using DNA extracted from SCC090, a SCC cell line naturally transfected with HPV-16. All the above work was performed within the departments of Pathology and Parasitology at the Hospital Central de Maputo and Eduardo Mondlane University in Maputo, Mozambique.
3. Results
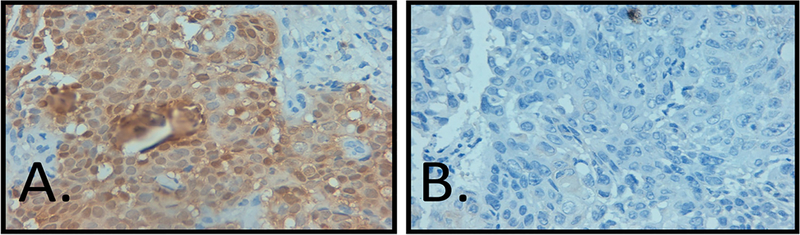
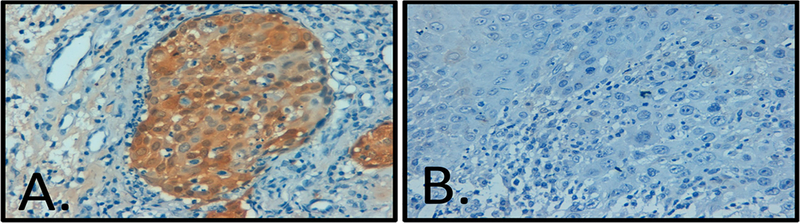
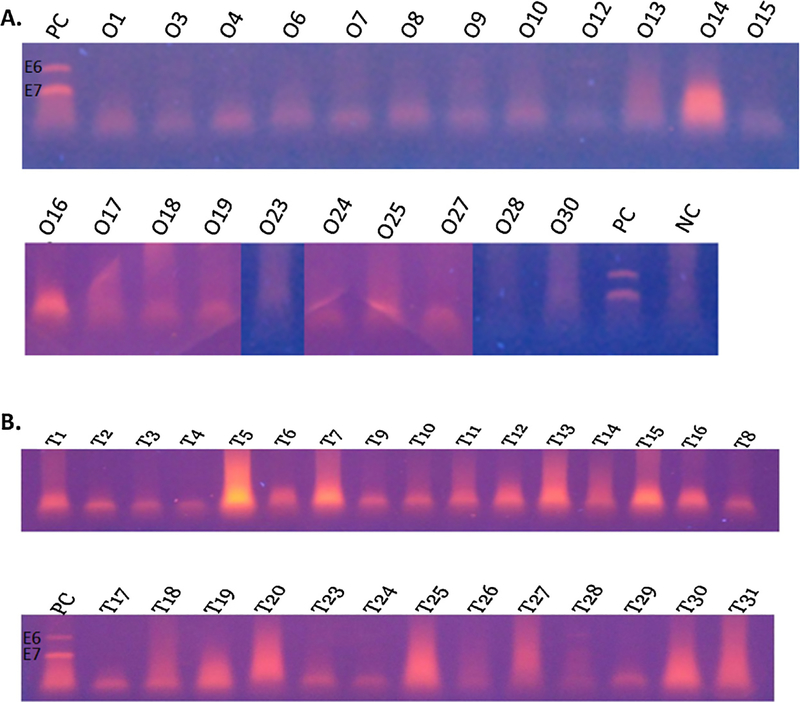
A total of 61 samples were identified through the database search. Of these, ten samples were excluded from the study due to incorrect diagnosis reported on the pathology report found on rereview or inability to locate the paraffin block (eight OP and two OT). This resulted in 22 oropharyngeal and 29 oral tongue tumors available to study. Oropharyngeal and oral tongue tumors were 55.2% and 32.2% female, respectively, with one specimen in each group lacking gender data. The average age for patients with OP and OT tumors was 59.3 and 51.8 years, respectively (Table 1a and b). The most common subsite noted for OPSCC was the tonsil, followed by base of tongue, though the majority (n = 15) were denoted as just oropharynx (Table 1a). The oropharyngeal tumors demonstrated pathology ranging from poorly to well differentiated squamous cell carcinoma with 82% (n = 18) showing keratinization, though none had a basaloid morphology. The oral tongue tumors also demonstrated pathology ranging from poorly to well differentiated squamous cell carcinoma with 72% (n = 21) demonstrating keratinization and, again, no basaloid morphology. Immunohistochemistry for p16 was found to be negative for all oropharyngeal samples (Fig. 1). Two oral tongue samples were positive for p16 (Fig. 2). DNAwas extracted as described above. PCR for all samples, OP and OT, were supposed negative for the presence of HPV 16 E6 and E7 as compared to the control DNA (Fig. 3).
Table 1.
Demographic information and sample data for oropharyngeal (A) and oral tongue (B) samples. In one sample sex data was not obtaineda, Excluded from experimentationb BOT—base of tongue, NOS—not otherwise specified, SCC—squamous cell carcinoma, IHC—immunohistochemistry, PCR— polymerization chain reaction.
| A | |
| Oropharynx (n = 30) | |
| Sex% (n)a | |
| Male | 44.8 (13) |
| Female | 55.2 (16) |
| Avg. Age (Std dev) | 59.3 (14.2) |
| Location% (n) | |
| Tonsil | 23.3 (7) |
| BOT | 16.7 (5) |
| Palate | 6.7 (2) |
| Pharyngeal Wall | 3.3 (1) |
| Oropharynx NOS | 50 (15) |
| Pathology% (n) | |
| SCC | 73.3 (22) |
| SCC—Keratinizing | 81.8 (18) |
| Other (Not SCC)b | 26.7 (8) |
| p16 IHC% (n) | |
| Positive | 0 (0) |
| PCR HPV-16 E6/ E7 % (n) | |
| Positive | 0 (0) |
| B | |
| Oral Tongue (n = 31) | |
| Sex% (n)a | |
| Male | 67.8 (21) |
| Female | 32.2 (10) |
| Avg. Age (Std dev) | 51.8 (15.8) |
| Pathology% (n) | |
| SCC | 93.5 (29) |
| SCC—Keratinizing | 72.4 (21) |
| Other (Not SCC)b | 6.5 (2) |
| p16 IHC% (n) | |
| Positive | 6.9 (2) |
| PCR HPV-16 E6/E7 % (n) | |
| Positive | 0 (0) |
one sex missing.
Excluded from experimentation.
Fig. 1.
(A) Representative cervical cancer positive control with overexpressed p16 by IHC at 40× and (B) No expression seen in a representative OP squamous cell carcinoma (40×).
Fig. 2.
Immunohistochemistry for p16 in oral tongue squamous cell carcinoma: (A) focally positive tumor and (B) p16 negative tumor (both at 40×).
Fig. 3.
PCR products from DNA extracted from FFPE samples for HPV-16 specific E6 and E7 (A) oropharyngeal SCC (B) oral tongue SCC. Missing numbers represent samples that did not meet criteria for inclusion in the study (egg: not SCC or unable to obtain specimen). PC—positive control with two bands representing E6 (upper band) and E7, NC—negative control.
4. Discussion
We conducted a single institution retrospective review of the presence of HPV related OP/OT SCC in Mozambique. In stark contrast to the United States and Western Europe and despite high rates of HPV −16 driven cervical cancer, HPV related SCC of the oropharynx and the oral tongue was not identified in the examined cohorts.
Though this is a pilot study, we believe the data does provide an accurate snap shot of the HPV status of OP/OT SCC in Mozambique. Mozambique is a country of roughly 26 million people, but, despite the size, there are only three academic pathology departments, with the one in Maputo (where the study was conducted) being the largest and the referral center for the country. This was reflected in the study by a number of biopsies originating outside Maputo Province. In addition, the majority of the otolaryngologists serving the country practice in Maputo, limiting the opportunity for tonsillectomy or biopsy outside the capital. Based on this we feel the samples represent a cross-section of the Mozambican population with access to otolaryngology or other medical services.
A notable limitation of the project is sample size. With most studies in the US and elsewhere providing data on hundreds of patients, our study was limited to 51, but this may be partially explained through the natural history of OPSCC and medical resources within Mozambique. Though improving significantly, the current life expectancy in Mozambique is 52 years [21]. A recent study from Germany evaluating 275 patients with tensile cancer demonstrated a median age of 56 and 59 for p16 positive and negative tumors, respectively [22]. An evaluation of the burden of potentially HPV associated OP and OC cancers in the US showed only 3% of tonsil tumors present before 40 years of age [23]. More locally, a study from South Africa demonstrated an average age of 57.5 years in their evaluation of 55 OPSCC samples [14]. Our data’s average age coincided with both of these studies (59y for OP and 52y for OT), but a significant portion of people in Mozambique, may not live long enough to develop this cancer. Secondly, the report from Germany noted 80% of the p16 positive tumors presented as T1 or T2 tumors [22]. In discussions with otolaryngologists in Maputo, many patients who do present to the hospital with head and neck cancers do so at such a late stage that biopsy is often not performed and patients are generally palliated with a tracheosotomy. And, as with many developing countries, the lack of prompt and widely accessible medical care, especially cancer care, is limited, particularly by economics. GDP per capita in Mozambique is $565 and is often difficult for families to provide basic necessities, making medical care often unobtainable [24].
Another potential limitation was the use of p16 as a surrogate marker for HPV transcriptional activity. Identification of p16 via IHC in tumor samples has been shown to be a reliable marker of HPV infection in OPSCC with only a small percentage showing either false positive or false negative rates for HPV status. Lewis, after reviewing the available literature suggests “it is widely acknowledged that p16 overexpression is a very sensitive marker for transcriptionally-active HPV” [25]. The p16 protein has also been shown to independently correlate with better survival of HNSCC patients and, at this point, there is only inconclusive data that p16 must be tested with an HPV specific test in OPSCC [25]. Though other cancer types do overexpress p16 (e.g., sinonasal undifferentiated carcinoma and some lung cancers) the overexpression of p16 taken with anatomic location and pathologic evaluation should sufficiently support a diagnosis of HPV related OPSCC [25]. Interestingly, two of our oral tongue samples did overexpress p16. Akin to the OP, p16 positive OTSCC were once thought to be HPV related, however recent data failed to correlate overexpression of p16 in the oral tongue with HPV. Poling et al. found nine of 78 non-smoking oral tongue cancers were positive for p16 on IHC, but HPV E6/E7 mRNA transcripts were detected in only one [26].
OPSCC has been associated with other high risk HPV genotypes including 18, 31, and 33 and only evaluating our samples for HPV-16 may be considered a limitation. However, HPV-16 has been demonstrated as the causative agent in over 90% of cases in the developed world [3]. One caveat is Paquette et al’s study from South Africa. They found 72% of their HPV related oropharyngeal cancers were HPV-16 positive (alone or co-infection), but 11.8% of their samples were HPV-31 positive alone [14]. Nevertheless, with the majority of cervical cancers in Mozambique positive for HPV 16, the worldwide prevalence of the HPV-16 genotype in OPSCC and the strength of the p16 IHC test, we felt confident only testing for HPV 16 would capture the genotype of the vast majority of any positive tumors.
Despite the small sample size of this study, the prevalence of HPV within Mozambican society as demonstrated by the ubiquity of cervical cancer would suggest at least a portion of OP tumors would be HPV positive. One potential explanation this was not seen may be cultural practices. D’souza demonstrated a significant increased risk of HPV related OPSCC with greater than six oral sexual partners with an odds ratio of 3.4 [8] highlighting the role oral sex plays in the transmission of HPV. The increasing incidence of OPSCC may be following evolving sexual trends as approximately 65–66% of 15–24 year olds in the US have participated in oral sex [27]. No data was readily available on the rates of oral sex in sub-Saharan Africa, but a recent study by Davidson et al. has provided a limited glimpse. In 2014, Davidson evaluated the HPV status of 125 male factory workers in South Africa and supplemented this with a survey regarding sexual practices. They found a 5.6% prevalence rate of HPV infection with only two showing high risk genotype 16 or 68. Notably, the survey concluded oral sex was an uncommon practice for the majority of the study participants, but both men with high risk HPV participated in oral sex [28]. Though this study is limited and within a specific population in neighboring South Africa, it does suggest a much more limited practice of oral sex in the region and may help explain the lack of HPV positive tumors in this study.
5. Conclusions
This is the first study evaluating the presence of HPV related OPSCC and OTSCC in East Africa and, surprisingly, it was not identified. This finding is likely a multifactorial phenomenon with cultural practices, health/cancer care access and economics all playing important roles.
Acknowledgements
Wendell Yarbrough, MD and lab, Emilia Noormahomed, MD, Eliane Carmo dos Santos Moneiro, MD, Bea Carbone, Neil Gordon, MD, Chip Schooley, MD and Stephen Bickler, MD. The work of Carla Carrilho was supported by the grant number R24TW008908 from the Fogarty International Center.
Abbreviations:
- HPV
human papillomavirus
- SCC
squamous cell carcinoma
- HNSCC
head and neck squamous cell carcinoma
- OP
oropharynx/oropharyngeal
- OT
oral tongue
- HCM
Hospital Central de Maputo
- EMU
Eduardo Mondlane University
- IHC
immunohistochemistry
- PCR
polymerase chain reaction
Footnotes
Conflicts of interest
None.
References
- [1].Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008, Int. J. Cancer 127 (12) (2010) 2893–2917 (accessed 15.12.15) [DOI] [PubMed] [Google Scholar]
- [2].Marur S, D’Souza G, Westra WH, Forastiere AA, HPV-associated head and neck cancer: a virus-related cancer epidemic, Lancet Oncol. 11 (August (8)) (2010) 781–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ramqvist T, Dalianis T, Oropharyngeal cancer epidemic and human papillomavirus, Emerg. Infect. Dis 16 (11) (2010) 1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hammarstedt L, Dahlstrand H, Lindquist D, Onelov L, Ryott M, Luo J, et al. , The incidence of tonsillar cancer in Sweden is increasing, Acta oto- laryngologica 127 (9) (2007) 988–992 [DOI] [PubMed] [Google Scholar]
- [5].Shiboski CH, Schmidt BL, Jordan RC, Tongue and tonsil carcinoma: increasing trends in the U. S. population ages 20–44 years, Cancer 1103 (9) (2005) 1843–1849 [DOI] [PubMed] [Google Scholar]
- [6].Ayo-Yusuf OA, Lalloo R, Johnson NW, Trends and ethnic disparities in oral and oro-pharyngeal cancers in South Africa, 1992–2001, SADJ 68 (May (4)) (2013) 168–173 [PubMed] [Google Scholar]
- [7].Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. , Evidence for a causal association between human papillomavirus and a subset ofhead and neck cancers,J. Natl. Cancer Inst 9 (2000) 709–720 [DOI] [PubMed] [Google Scholar]
- [8].D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. , Case-control study of human papillomavirus and oropharyngeal cancer, New Engl. J. Med 19 (2007) 1944–1956 [DOI] [PubMed] [Google Scholar]
- [9].Kreimer AR, Clifford GM, Boyle P, Franceschi S, Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review, Cancer Epidemiol. Biomarkers Prev. 14 (2) (2005) 467–475 [DOI] [PubMed] [Google Scholar]
- [10].Lindquist D, Romanitan M, Hammarstedt L, Nasman A, Dahlstrand H, Lindholm J, et al. , Human papillomavirus is a favourable prognostic factor in tonsillar cancerand its oncogenicrole is supported bythe expressionofE6 and E7, Mol. Oncol 1 (December (3)) (2007) 350–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, et al. , Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study,J. Natl. Cancer Inst 23 (2003) 1772–1783 [DOI] [PubMed] [Google Scholar]
- [12].Ndiaye C, Alemany L, Diop Y, Ndiaye N, Dieme MJ, Tous S, et al. , The role of human papillomavirus in head and neck cancer in Senegal, Infectious Agents Cancer 8 (1) (2013) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kaba G, Dzudzor B, Gyasi RK, Asmah RH, Brown CA, Kudzi W, et al. , Human papillomavirus genotypes in a subset of head and neck squamous cell carcinoma, West Afr. J. Med 33 (April-June (2)) (2014) 121–124 [PubMed] [Google Scholar]
- [14].Paquette C, Evans MF, Meer SS, Rajendran V, Adamson CS, Cooper K, Evidence that alpha-9 human papillomavirus infections are a major etiologic factor for oropharyngeal carcinoma in black South Africans, Head Neck Pathol. 7 (December (4))(2013) 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Attar E, Dey S, Hablas A, Seifeldin IA, Ramadan M, Rozek LS, et al. , Head and neck cancer in a developing country: a population-based perspective across 8 years, Oral Oncol. 46 (August (8)) (2010) 591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].da Lilly-Tariah OB, Somefun AO, Adeyemo WL, Current evidence on the burden of head and neck cancers in Nigeria, Head Neck Oncol. 1 (2009) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].De Vuyst H, Alemany L, Lacey C, et al. , The burden of human papillomavirus infections and related diseases in sub-saharan Africa, Vaccine 31 (05) (2013) F32–F46, doi: 10.1016/j.vaccine.2012.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Firnhaber C, Mayisela N, Mao L, et al. , Validation of Cervical Cancer Screening Methods in HIV Positive Women from Johannesburg South Africa. G. Samimi, ed. PLoS ONE. 2013. 8 (1) e53494. doi: 10.1371/journal.pone.0053494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Castellsague X, Klaustermeier J, Carrilho C, Albero G, Sacarlal J, Quint W, et al. , Vaccine-related HPV genotypes in women with and without cervical cancer in Mozambique: burden and potential for prevention, Int. J. Cancer 8 (2008) 1901–1904 [DOI] [PubMed] [Google Scholar]
- [20].Naucler P, Da Costa FM, Ljungberg O, Bugalho A, Dillner J, Human papillomavirus genotypes in cervical cancers in Mozambique, J. Gen. Virol 85 (Pt 8) (2004) 2189–2190 [DOI] [PubMed] [Google Scholar]
- [21].World Health Organization [Internet]: Geneva: WHO; c2014. Mozambique country information; [about 1 screen]. Available from: http://www.who.int/countries/moz/en/ [Google Scholar]
- [22].Psychogios G, Alexiou C, Agaimy A, Brunner K, Koch M, Mantsopoulos K, et al. , Epidemiology and survival of HPV-related tonsillar carcinoma, Cancer Med. 3 (June (3)) (2014) 652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ryerson AB, Peters ES, Coughlin SS, Chen VW, Gillison ML, Reichman ME, et al. , Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003, Cancer 113 (Suppl. 10) (2008) 2901–2909 (accessed 15.11.15) [DOI] [PubMed] [Google Scholar]
- [24].The World Bank [Internet]: Washington DC: The World Bank Group; c2014. GDP per capita: [about 6 screens]. Available from: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD [Google Scholar]
- [25].Lewis JS Jr., p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma, Head Neck Pathol. 6 (Suppl. 1) (2012) S75–S82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Poling JS, Ma XJ, Bui S, Luo Y, Li R, Koch WM, et al. , Human papillomavirus (HPV) status of non-tobacco related squamous cell carcinomas of the lateral tongue, Oral Oncol. 50 (4) (2014) 306–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Copen CE, Chandra A, Martinez G, Prevalence and timing of oral sex with opposite-sex partners among females and males aged 15–24 years: United States, 2007–2010. National Health Statistics Reports. 2012. August 16 (56):1–14. [PubMed] [Google Scholar]
- [28].Davidson CL, Richter KL, Van der Linde M, Coetsee J, Boy SC, Prevalence of oral and oropharyngeal human papillomavirus in a sample of South African men: a pilot study, S. Afr. Med.J 104 (May (5)) (2014) 358–361 [DOI] [PubMed] [Google Scholar]