Abstract
Background: To assess the efficacy of health coaching (HC) delivered through videoconferencing (VC) to favorably change physical activity (PA), weight, and metabolic markers in adults with high body mass index (BMI).
Materials and Methods: Thirty adults (BMI ≥30 kg/m2) were randomly assigned to one of three groups: VC, in-person (IP), or control group (CG). Participants received wireless watches and weight scales to sync with their personal smartphones; recorded data were wirelessly uploaded to a secure database. Participants assigned to VC and IP received individualized HC by a multidisciplinary team (registered dietitian, exercise physiologist, and medical doctor) based on data uploaded over the 12-week intervention. Steps/day and weight loss were analyzed through analyses of covariance.
Results: Within- and between-group changes in weight (kg), glucose, insulin, hemoglobin A1c (HbA1c), and Homeostasis Model Assessment estimate of insulin resistance (HOMA-IR) were analyzed through analyses of variance. Weight loss was greater (p < 0.05) for VC (8.23 ± 4.5 kg; 7.7%) than IP (3.2 ± 2.6 kg; 3.4%) and CG (2.9 ± 3.9 kg; 3.3%), respectively. Steps/day were significantly higher in VC than IP at week 4 and VC was significantly higher than the CG at weeks 6, 8, 9, and 11 (p ≤ 0.05). No within- or between-group differences were found for glucose, insulin, or HbA1C. HOMA-IR decreased for VC only (p ≤ 0.05).
Conclusions: Our innovative, multidisciplinary, telemedicine HC delivered through VC led to more favorable changes in weight loss, PA (steps/day), and HOMA-IR than IP or no HC. VC may be an economical approach to improve health and promote behavior change in obese adults. Clinical Trial Registration Number: ClinicalTrials.gov identifier NCT03278951.
Keywords: m-Health, telehealth, telemedicine, home health monitoring
Introduction
Obesity is a global public health issue. Currently, 66% of adults in the United States are overweight (≥25 kg/m2) or obese (≥30 kg/m2) by body mass index (BMI).1,2 Body composition characterized by high fat mass increases the risk of type II diabetes mellitus (T2DM), hypertension, stroke, and cancer.2 The 2009 economic burden of obesity was $147 billion for the United States.3 Lifestyle changes, including diet and exercise, can help reduce obesity and associated healthcare costs.4,5
Current evidence suggests that health coaching (HC) sessions for lifestyle modification improve program compliance, weight loss, and chronic disease-related health outcomes.6–9 HC has been performed by: nurses,6,10 health counselors,4,11 diabetes educators,12 and primary care providers.12–14 Most HC interventions have been group based and delivered by healthcare professionals through telephone,4,10,11 web-based chatting,6,12 or in-person (IP), and web-based features.13,14 Despite the potentially positive impact of group-based HC by videoconferencing (VC) on weight loss and metabolic health,15–18 use of individualized VC session interventions is scarce.19 Evidence suggests that multidisciplinary teams of healthcare professionals may enhance program adherence and medical monitoring; however, traveling to receive care is often a barrier.5,19,20
This study was designed to determine the impacts of didactically similar HC interventions delivered by VC or IP visits on body weight, blood glucose, hemoglobin A1c (HbA1c), and Homeostasis Model Assessment estimate of insulin resistance (HOMA-IR) in adults with BMI ≥30 kg/m2.
Materials and Methods
Subject Population
Thirty adults (BMI ≥30 kg/m2; Table 1) participated in this study, which was approved by the University of New Mexico (UNM) Institutional Review Board.
Table 1.
Subject Characteristics
| CONTROL GROUP (N = 10), MEAN ± SD | IN-PERSON GROUP (N = 10), MEAN ± SD | VIDEOCONFERENCING GROUP (N = 10), MEAN ± SD | ||||
|---|---|---|---|---|---|---|
| VARIABLES | BASELINE | 12 WEEKS | BASELINE | 12 WEEKS | BASELINE | 12 WEEKS |
| Age (years) | 44.5 ± 12.1 | — | 42.2 ± 10.2 | — | 43.0 ± 10.7 | — |
| Body weight (kg) | 95.9 ± 16.4 | 92.9 ± 18.3 | 101.5 ± 21.5 | 98.2 ± 22.7 | 112.8 ± 25.8 | 104.7 ± 27.1 |
| Weight loss (kg) | — | 2.9 ± 3.9 | — | 3.2 ± 4.5 | — | 8.23 ± 4.5a |
| Weight loss (%) | — | 3.3 ± 4.2 | — | 3.4 ± 2.6 | — | 7.7 ± 4.9 |
| No. of subjects that met 5% of WL, n | — | 3 | — | 1 | — | 6 |
| No. of subjects that met 3% of WL, n | — | 5 | — | 6 | — | 9 |
| Height (cm) | 167.9 ± 8.2 | — | 168.5 ± 9.4 | — | 171.5 ± 9.8 | — |
| BMI (kg/m2) | 34.5 ± 5.3 | 33.2 ± 6.2 | 35.3 ± 5.2 | 34.4 ± 5.3 | 38.6 ± 9.8 | 35.8 ± 10.1 |
| Glucose (ng/mL) | 5.4 ± 0.43 | 5.5 ± 0.4 | 5.2 ± 0.9 | 5.5 ± 1.2 | 5.4 ± 0.37 | 5.1 ± 0.40 |
| HOMA-IR | 2.0 ± 1.7 | 1.8 ± 2.1 | 2.1 ± 1.5 | 2.0 ± .72 | 3.2 ± 3.1 | 1.5 ± 0.9b |
| HbA1c (%) | 5.6 ± 0.20 | 5.5 ± 0.16 | 5.7 ± 1.4 | 5.8 ± 0.96 | 5.6 ± 0.20 | 5.5 ± 0.21 |
| Insulin (mg/dL) | 8.4 ± 7.2 | 7.1 ± 7.5 | 7.5 ± 3.3 | 8.1 ± 1.7 | 13.3 ± 12.5 | 6.8 ± 4.7 |
| Steps/day | 4,324.3 ± 2,000.7 | 5,002.4 ± 2,640.3 | 3,641.7 ± 1,167 | 6,236.2 ± 2,393.4 | 3,755.1 ± 1,610.2 | 7,054.6 ± 2,068.7a |
Indicates a significant difference from IP and CG (p ≤ 0.05).
Indicates a within-group significant difference for the VC group (p ≤ 0.05).
BMI, body mass index; CG, control group; HbA1c, hemoglobin A1c; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; IP, in-person; n, sample that obtained respective percent weight loss; SD, standard deviation; VC, videoconferencing; WL, weight loss.
Inclusion criteria required that participants were English speaking, nondiabetic, ambulatory, <396 pounds in weight, leading a sedentary lifestyle (<7,000 steps/day)21 as confirmed by the run-in period, not regularly engaging in moderate-intensity activities, owners of an iPhone or Android smartphone, and able to travel for scheduled appointments. Individuals were excluded if they used medications or dietary supplements that could affect body composition; had T2DM; lost >3 kg body weight or dramatically changed physical activity (PA) patterns within the past 6 months; have or had cancer, eating disorders, uncontrolled blood pressure, or neurological or psychological disorders; or had undergone obesity-related surgery (i.e., gastric bypass, etc.). This 12-week intervention utilized a randomized, repeated measures, quasi-experimental design.
Baseline and Postintervention Sessions
Following phone screening, eligible participants were scheduled for an individualized orientation and baseline visit. Baseline and post-testing sessions took place at UNM's Exercise Physiology Lab (Lab). At the Lab, participants were consented and completed a health history questionnaire and the International Physical Activity Questionnaire (IPAQ) short version.22 Barefoot standing height (cm) was measured using a wall-mounted stadiometer (SECA®, Chino, CA), while nude weight (kg) was measured on a calibrated digital scale (MedWeigh® MS-3900; Itin Scale Company, Brooklyn, NY) and reported to a research team member. Height and weight were measured in duplicate, then averaged and used to calculate BMI. Similar procedures were performed at postintervention follow-up visits.
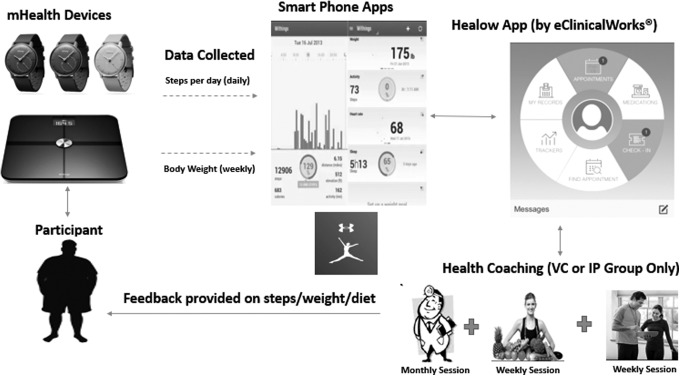
During the baseline orientation, participants were issued and familiarized with the Withings® Body Analyzer weight scale and the Withings Activite Steel step-tracking accelerometer watch (Withings, Inc., Cambridge, MA). The Withings app housed the step and weight data from the accelerometer and scale integrated wirelessly to each participant's smartphone. The Healow app (eClinicalWorks®; eCW, Westborough, MA) transferred data wirelessly from the Withings app dashboard to a secure electronic medical records (EMR) online database (eClinicalWorks). This EMR was only accessible by the research team and participants (Fig. 1). All participants had 24 h/7 day access to eClinicalWorks technical support and research team. Each participant had a unique username and password for the Healow app, which each participant created during the orientation session. eClinicalWorks developed a comprehensive information security policy for the Healow app that was based on the Health Insurance Portability and Accountability Act (HIPAA) Security and Privacy Rules and supported required as dictated by the HITECH Act.
Fig. 1.
Mobile health device and telemedicine database framework. VC, videoconferencing; IP, in-person.
All participants were instructed to enter daily food and beverage intake into MyFitnessPal. The prescribed daily caloric target followed American Heart Association (AHA) dietary recommendations23 for the VC and IP groups.
VC participants were familiarized with the VC aspects of the Healow app. VC and IP participants were familiarized with online curriculum manuals created by a team of health professionals at inHealth Medical Services, Inc., as previously described.19
Run-In Period
During the 1-week run-in period, participants were instructed to wear the accelerometer watch on their nondominant wrist for at least 24 h/day while awake24 and to weigh nude on the Withings scale at least once/week after overnight fasting and voiding. After the run-in-period, participants were randomized to VC, IP or control group (CG) by the website Random Lists.*
Experimental Procedures
Following standardized procedures similar to a commercially available weight loss program from inHealth Medical Services, Inc., every week, participants weighed themselves in the morning after voiding. Body weight from the Withings scale was uploaded weekly; accelerometer step counts were uploaded daily. Data were transmitted wirelessly by participants and transferred from the Withings app dashboard to the EMR online database (eClinicalWorks), which was only accessible by the research team and participants; participants could see their real-time data through the Withings app and Healow app.
For preintervention and postintervention assessments, participants reported to the Lab in the morning after a 12-h fast with water ad libitum. Venous samples were acquired from a prominent antecubital vein for serum (glucose and insulin) and whole blood (HbA1c) and sent to (Quest® Laboratory, Albuquerque, NM) for processing. A HOMA-IR value was calculated as follows: HOMA-IR = fasting insulin × fasting glucose/22.5.25
Intervention
Following the run-in, participants were instructed to continue m-health device data uploads.
Participants in CG received m-health devices, no HC sessions, or team member feedback on steps per day from the Withings watch or calories entered into MyFitnessPal. Throughout the intervention, those assigned to the VC and IP groups received didactically similar HC educational materials19 and feedback delivered by the same registered dietitian and exercise physiologist in accordance with group assignment. During the dietitian sessions, daily and weekly caloric intakes retrieved from the MyFitnessPal app were reviewed, discussed, and adjustments made as needed. During the HC sessions with the exercise physiologist, discussions included current exercise routine, goal setting, and PA progression (i.e., more steps/day, more minutes/day). The project's medical doctor oversaw all dietary and exercise recommendations. PA recommendations followed the American College of Sports Medicine guidelines of ≥30 min of moderate-to-vigorous-intensity PA 5 days per week for a targeted minimum of 150 min/week.26
Statistical Analyses
Separate within- and between-group one-way analyses of variance (ANOVAs) for baseline body weight, steps per day, fasting blood glucose, insulin, HbA1c, and HOMA-IR were applied. Separate mixed-model group × time (pre-post) analyses of covariance were conducted for steps/day and body weight. Covariates were average run-in steps/day and baseline body weight (kg). Group-specific steps/day were summed and averaged by week to identify pre-post between-group differences by one-way ANOVAs. Separate one-way between-group ANOVAs were applied to identify postintervention differences of HbA1c, glucose, insulin, and HOMA-IR. Post hoc analyses using Bonferroni corrections for multiple comparisons were performed when significant main effects or differences were found. Graph time points (weeks) were calculated as adjusted least mean square (LMS) and standard error (SE) to examine the treatment effect on body weight loss and steps/day over time. All data were analyzed using R.27 Statistical significance was defined as p ≤ 0.05.
Results
Ten participants were randomized into each of the VC, IP, and CG groups, with no participant attrition. Baseline and post-testing values are shown in Table 1. No significant baseline differences were found between groups for steps/day, body weight, blood glucose, HbA1c, insulin, or HOMA-IR (p > 0.05).
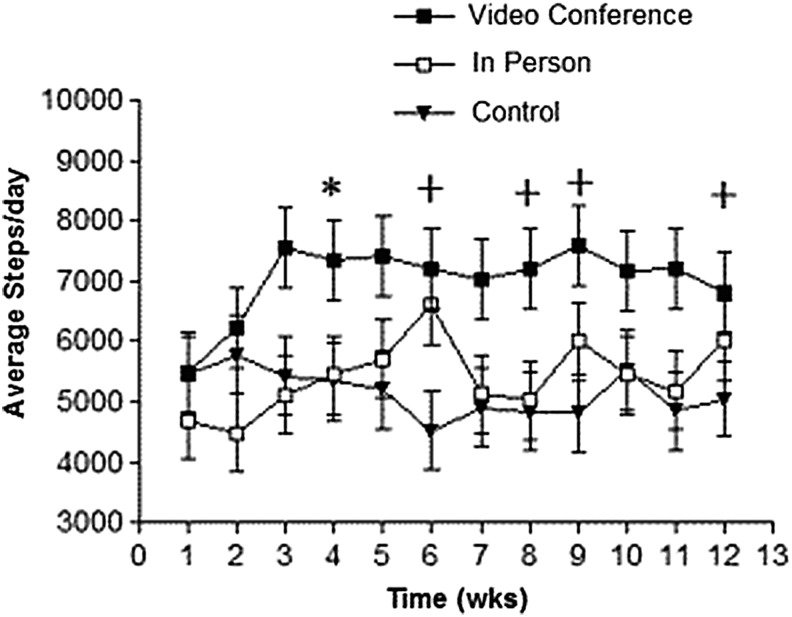
There was no main effect of time for steps/day [F(11, 394.28) =1.36; p = 0.18]. There was a main effect by group for VC [F(2, 30.1) = 3.75; p = 0.03] with steps/day (averaged by week) increasing from baseline to week 12 (Table 1). There was a significant interaction by time and group for steps/day with VC being higher than IP by 1,063 steps/day at week 4, and VC being higher than CG at week 6 by 2,107 steps/day, week 8 by 1,883 steps/day, week 9 by 2,318 steps/day, and week 11 by 1,961 steps/day [F(22, 394.22) = 1.62; p = 0.03] (Fig. 2; data presented as LMS ± SE).
Fig. 2.
Comparison of daily step average per day by group (n = 10 for each group). *Significant difference between VC and IP groups; +significant difference between VC and control group; p < 0.05. Each time point (weeks) is presented as adjusted LMS and SE. LMS, least mean square; SE, standard error.
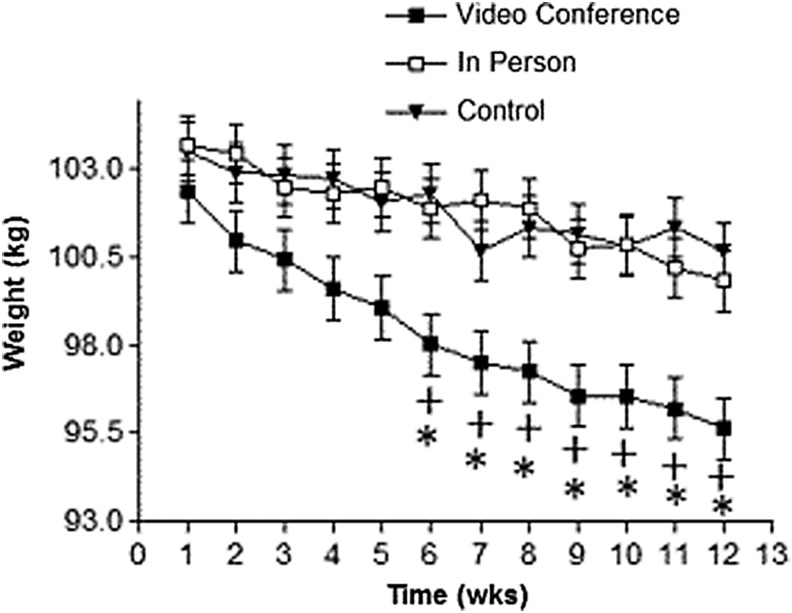
For body weight loss, there was a main effect by time [F(11, 297.1), = 20.4 p = 0.001] and by group for VC [F(2, 33.3) = 7.71; p = 0.01]. There was a significant time and group interaction for weight loss for VC compared to IP and CG for weeks 6–12 [F(22, 297.1) = 1.88; p = 0.01] (Fig. 3; data presented as LMS ± SE). There was a significant (p < 0.001) difference for postinvention weight loss between VC (8.23 kg) compared to IP (3.2 kg) and CG (2.9 kg) (Table 1).
Fig. 3.
Comparison of weekly body weight in kg by group (n = 10 for each group). *Significant difference between VC and IP group; +significant difference between VC and control group; p < 0.05. Each time point (weeks) is presented as adjusted LMS and SE.
There were no within- or between-group differences for blood glucose, insulin, or HbA1c, nor any group by time interactions for HbA1c. The 1.7 U decrease in VC's HOMA-IR by week 12 was significant (p = 0.05).
Discussion
Our multidisciplinary team delivery of one-on-one HC through VC was more effective than IP delivery for increasing body weight loss and PA. Since our VC and IP groups received didactically similar HC interventions and CG received none from the team, we hypothesized that the VC and IP groups would see similar changes over time for body weight loss, PA, fasting blood glucose, HbA1c, and HOMA-IR and that CG results would differ significantly from those of the intervention groups. However, we only found significantly greater improvements for VC in body weight lost, steps/day, and HOMA-IR compared to IP and CG. No other between-group differences were found.
Steps/Day
Baseline step counts for all groups were below 7,000 per day, defining participants as sedentary.21 The VC group averaged significantly higher steps/day over the time course of the study. This is somewhat similar to Vadheim et al.'s27 findings of nonsignificant differences between their VC and onsite HC groups for self-reported PA (min/week) following a 16-week body weight loss program. Unlike the subjective self-reporting of PA by Vadheim's participants, ours recorded steps/day through accelerometry.
VC had significantly higher steps/day than CG at weeks 6, 8, 9, and 12 (Fig. 3). VC also had higher attendance at HC sessions than CG. This supports previous comments4,12,28 about a direct relationship between increasing efficacy in PA interventions and frequency of contact with health coaches. However, time spent traveling to scheduled appointments may have competed with the available time IP participants had for exercising.
Body Weight Loss
The VC group achieved a significantly greater weight loss from baseline to postintervention than the other groups (Table 1). Azar et al.15 investigated the efficacy of VC for a diabetes prevention program (DPP) with a weight loss focus. Like this study, they reported significant weight loss differences between their VC (3.6 kg) and control (0.4 kg) groups.17 A 12-week group-based VC pilot study19 for men with BMIs >28 kg/m2 emulated the LEARN® behavioral program with a dietitian and certified athletic trainer leading weekly nutrition and behavioral exercise sessions, respectively; all sessions were delivered by VC. Weight loss was significantly greater compared to the controls. Our results are also consistent with Alencar et al.19 who performed 12 weeks of VC HC weekly with a registered dietitian, and monthly HC with a medical doctor; they reported significant differences for body weight loss of (7.34 ± 5.2 kg) for VC and (1.2 ± 3.9 kg) for CG, respectively.
In contrast, our results differ from those of Vadheim et al.27 who found that DPP participants assigned to IP (n = 13) and VC (n = 14) achieved similar weight losses (6.5 ± 3.1 and 6.7 ± 3.1 kg, respectively). We unexpectedly found significant differences between our IP and VC groups. Absences from IP HC sessions may be one reason for the weight loss differences between our groups. Our VC members had perfect attendance for all HC sessions; those in our IP group attended, on average, 80% of the sessions. Average HC attendance of both groups was 77.5% in the Vadheim study.27 IP group-based programs are considered a gold standard in behavioral treatment of individuals with BMIs ≥30 kg/m2; however, attendance at IP counseling group sessions is known to drop over time.10,15 As previously mentioned, inconsistent attendance at IP meetings may induce lapses toward goals. Attendance drops may be due to challenges in scheduling and travel, and future research is needed to address these challenges.
A possible explanation for the similar amount of weight lost for the IP and CG groups may be attributed to the proprietary feedback (notifications) from the MyFitnessPal application. While this is plausible, a deeper investigation of CG data indicated 4 of the group's 10 participants failed to upload their weight data for 10 of the 12 weeks. This deviation from instructions given at baseline precluded the majority of CG benefiting from the automated feedback about weight change from the previous weigh-in, macronutrient content of self-reported food intake, and a caloric restriction target. Even though we found no statistically significant changes between IP and CG for weight loss, it is important to mention that the positive outcome in weight loss for CG may be attributed to the double-digit weight loss (in kg) of three participants in that group.
Glycemic Control
We found no between-group differences for blood insulin, glucose, HbA1c, and HOMA-IR resulting from our 12-week intervention. There was, however, a significant decrease (1.7 U) for HOMA-IR in our VC group. Our glycemic control results are similar to those of Laitinen et al.16 who recruited diabetics with BMIs ≥30 kg/m2 for a group-based nutritional counseling intervention. They found significant fasting blood glucose differences between their VC and IP groups. Conversely, Luley et al.18 randomly assigned participants (BMI ≥30 kg/m2) into a telemedicine (wireless scales+accelerometers) or a control (no wireless devices) group. Fasting blood glucose and HbA1c decreased significantly in the telemedicine group, whereas neither of our intervention groups significantly decreased these variables. Of importance, although, are the participant medical history differences (diabetic status) and study duration (3 vs. 6 months); we recruited nondiabetic adults for a 3-month intervention.
A 3-month intervention appears too short for documenting pre-to-post intervention changes in HbA1c. Possible explanations for our participants showing no significant changes in glucose- and insulin-related variables might include insufficient weekly step count goals that complemented our aggressive dietary restrictions. As per the Look AHEAD study,29 intensive dietary and exercise alterations are needed to trigger significant changes in HbA1c in diabetics. Since none of our participants were diabetic at baseline, an even more aggressive exercise program may have been needed to invoke change. We did not observe any differences between groups for glycemic control, although weight loss ≥5% of body weight is known to reduce insulin levels and improve glycemic control.30 However, the significant improvement in HOMA-IR within our VC group (7.7% body weight loss) suggests that our intervention resulted in improved insulin sensitivity. Even though our between-group changes in insulin and glucose were not statistically significant, the within-group decrease in HOMA-IR for VC may reduce the likelihood that these nondiabetic individuals will develop diabetes.30,31 Furthermore, the slight decreases in HOMA-IR for IP and CG may be clinically significant with longer study durations.
Limitations
The short length of this study may have precluded attainment of significant diet- and exercise-induced changes for all variables of interest. Nonetheless, we report that individual sessions of HC delivered IP or through videoconference can unequivocally contribute to significant weight loss. We found no peer-reviewed literature validating the Withings accelerometer against a criterion method; therefore, we assumed there was similar step tracking capabilities across the three groups. We also did not have access to raw accelerometry data to determine our participants' frequency, intensity, and duration of activity bouts. Consequently, we were unable to objectively confirm if activity bouts were periodically in the moderate-to-vigorous-intensity ranges. Knowing the frequency, intensity, and duration of activity bouts would have allowed for a more specific recommendation regarding daily step goals.
The same multidisciplinary team members delivered HC to the intervention groups. Baseline and postintervention assessments were conducted by the same research team member. Consequently, there was no blinding of team members to group assignment or during statistical analyses, although IP and VC interventions were didactically similar. Individual participant motivation to change, or lack thereof, may have contributed to differences in weight loss and related outcomes, but was not measured. Using the Transtheoretical Model Stages of Change questionnaire (Jossey-Bass, Inc., Hoboken, NJ) at baseline and follow-up to identify participant readiness for change would enrich the understanding of our HC efficacy.16,32,33 Last, although fully powered, our sample size was small, limiting the extent of additional analyses. Therefore, our outcomes can be generalized only to adults who are 35–45 years of age, nondiabetic, and have baseline BMIs ≥30 kg/m2.
Strengths
To our knowledge, this is the first study to employ a multidisciplinary team approach to individualized HC by VC versus IP. In addition, the data transmitted from our m-health devices (steps/day and nude weight/week) provided objective measures instead of the subjective self-report data captured in previous group-based VC interventions.18–20 The remote tracking of body weight and PA by intervention group members and research team personnel likely motivated participants and provided health coaches with up-to-date data critical for individualizing conferencing sessions.
Suggestions for Future Research
Future studies evaluating the cost-effectiveness of a telemedicine intervention like ours would be beneficial. Additional comparisons (i.e., group vs. individualized; diabetic vs. nondiabetic) of our VC HC approach to investigate the impact on weight loss and other health outcomes (e.g., lipid profile, glycemic control, and inflammatory markers) are also warranted. Periodic postintervention follow-ups (i.e., at 6 weeks, 6 months, and 1 year postintervention) may provide insights into factors contributing to long-term health behavior changes. Measuring participants' motivation to change behavior before HC interventions could provide insight into further tailoring HC to each individual.
Conclusions
Our innovative, multidisciplinary, telemedicine HC delivered through VC led to favorable changes in weight loss, PA, and HOMA-IR that surpassed changes when HC was delivered in person.
Acknowledgments
We thank all our participants; our gratitude is also extended to inHealth Medical Services, Inc., for project-related content, modules, and technical support. We also want to thank eClinicalWorks for their platform services and Healow application. In addition, we thank Stuart Pett, MD, who donated his time and medical expertise. Research reported in this publication was supported by the National Institute of General Medical Sciences under Award number 8UL1GM118979-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding from inHealth Medical Services, Inc., was for laboratory blood markers only and labs were conducted and analyzed by an outside lab.
Footnotes
Disclosure Statement
Dr. Michelle Alencar owns stock inHealth Medical Services, Inc. inHealth Medical Services, Inc., provided project-related content, including subject education content, modules, and technology support only. All other authors declare no conflict of interests.
References
- 1. Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis 2014;56:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CDC. What causes overweight and obesity? National Institutes of Health and the US Department of Health & Human Services, 2015 [Google Scholar]
- 3. Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–w831 [DOI] [PubMed] [Google Scholar]
- 4. Huber JM, Shapiro JS, Wieland ML, Croghan IT, Douglas KSV, Schroeder DR, et al. . Telecoaching plus a portion control plate for weight care management: A randomized trial. Trials 2015;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HS, Cho JH, Yoon KH. New directions in chronic disease management. Endocrinol Metab (Seoul) 2015;30:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett GG, Herring SJ, Puleo E, Stein EK, Emmons KM, Gillman MW. Web-based weight loss in primary care: A randomized controlled trial. Obesity (Silver Spring) 2010;18:308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kivela K, Elo S, Kyngas H, Kaariainen M. The effects of health coaching on adult patients with chronic diseases: A systematic review. Patient Educ Couns 2014;97:147–157 [DOI] [PubMed] [Google Scholar]
- 8. Leveille SG, Huang A, Tsai SB, Allen M, Weingart SN, Iezzoni LI. Health coaching via an Internet portal for primary care patients with chronic conditions: A randomized controlled trial. Med Care 2009;47:41–47 [DOI] [PubMed] [Google Scholar]
- 9. Olsen JM, Nesbitt BJ. Health coaching to improve healthy lifestyle behaviors: An integrative review. Am J Health Promot 2010;25:e1–e12 [DOI] [PubMed] [Google Scholar]
- 10. Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, et al. . Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med 2011;365:1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Odnoletkova I, Goderis G, Nobels F, Aertgeerts B, Annemans L, Ramaekers D. Nurse-led telecoaching of people with type 2 diabetes in primary care: Rationale, design and baseline data of a randomized controlled trial. BMC Fam Pract 2014;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hersey JC, Khavjou O, Strange LB, Atkinson RL, Blair SN, Campbell S, et al. . The efficacy and cost-effectiveness of a community weight management intervention: A randomized controlled trial of the health weight management demonstration. Prev Med 2012;54:42–49 [DOI] [PubMed] [Google Scholar]
- 13. Bennett JA, Perrin NA, Hanson G, Bennett D, Gaynor W, Flaherty-Robb M, et al. . Healthy aging demonstration project: Nurse coaching for behavior change in older adults. Res Nurs Health 2005;28:187–197 [DOI] [PubMed] [Google Scholar]
- 14. Lisspers J, Hofman-Bang C, Nordlander R, Ryden L, Sundin O, Ohman A, et al. . Multifactorial evaluation of a program for lifestyle behavior change in rehabilitation and secondary prevention of coronary artery disease. Scand Cardiovasc J 1999;33:9–16 [DOI] [PubMed] [Google Scholar]
- 15. Azar KM, Aurora M, Wang EJ, Muzaffar A, Pressman A, Palaniappan LP. Virtual small groups for weight management: An innovative delivery mechanism for evidence-based lifestyle interventions among obese men. Transl Behav Med 2015;5:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laitinen J, Korkiakangas E, Alahuhta M, Keinanen-Kiukaanniemi S, Rajala U, Timonen O, et al. . Feasibility of videoconferencing in lifestyle group counselling. Int J Circumpolar Health 2010;69:500–511 [DOI] [PubMed] [Google Scholar]
- 17. Liou TH, Chen CH, Hsu CY, Chou P, Chiu HW. A pilot study of videoconferencing for an Internet-based weight loss programme for obese adults in Taiwan. J Telemed Telecare 2006;12:370–373 [DOI] [PubMed] [Google Scholar]
- 18. Luley C, Blaik A, Reschke K, Klose S, Westphal S. Weight loss in obese patients with type 2 diabetes: Effects of telemonitoring plus a diet combination—The Active Body Control (ABC) Program. Diabetes Res Clin Pract 2011;91:286–292 [DOI] [PubMed] [Google Scholar]
- 19. Alencar MK, Johnson K, Mullur R, Gray V, Gutierrez E, Korosteleva O. The efficacy of a telemedicine-based weight loss program with video conference health coaching support. J Telemed Telecare 2017. [Epub ahead of print]; DOI: 10.1177/1357633X17745471 [DOI] [PubMed] [Google Scholar]
- 20. Ganguli A, Clewell J, Shillington AC. The impact of patient support programs on adherence, clinical, humanistic, and economic patient outcomes: A targeted systematic review. Patient Prefer Adherence 2016;10:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tudor-Locke C. Resources for data interpretation and reporting. In: Shephard RJ, Tudor-Locke C, eds. The objective monitoring of physical activity: Contributions of accelerometry to epidemiology, exercise science and rehabilitation. Cham, Switzerland: Springer International Publishing, 2016:133–158 [Google Scholar]
- 22. Scholes S, Bridges S, Fat LN, Mindell JS. Comparison of the physical activity and sedentary behaviour assessment questionnaire and the short-form International Physical Activity Questionnaire: An analysis of health survey for England data. PLoS One 2016;11:e0151647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jensen M, Ryan D, Apovian C, Ard J, Comuzzie A, Donato K, et al. . Obesity Society: 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129(Suppl 2):S102–S138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller GD, Jakicic JM, Rejeski WJ, Whit‐Glover MC, Lang W, Walkup MP, et al. . Effect of varying accelerometry criteria on physical activity: The look ahead study. Obesity (Silver Spring) 2013;21:32–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Araki-Sasaki R, et al. . Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care 2001;24:362–365 [DOI] [PubMed] [Google Scholar]
- 26. American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. Philadelphia, PA: Lippincott Williams & Wilkins, 2013 [DOI] [PubMed] [Google Scholar]
- 27. Vadheim LM, McPherson C, Kassner DR, Vanderwood KK, Hall TO, Butcher MK, et al. . Adapted diabetes prevention program lifestyle intervention can be effectively delivered through telehealth. Diabetes Educ 2010;36:651–656 [DOI] [PubMed] [Google Scholar]
- 28. Lin JS, O'connor E, Evans CV, Senger CA, Rowland MG, Groom HC. Behavioral counseling to promote a healthy lifestyle in persons with cardiovascular risk factors: A systematic review for the US Preventive Services Task Force healthy lifestyle counseling in persons with cardiovascular risk factors. Ann Intern Med 2014;161:568–578 [DOI] [PubMed] [Google Scholar]
- 29. Espeland M. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the look AHEAD trial. Diabetes Care 2007;30:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Blair SN, Church TS. Effects of clinically significant weight loss with exercise training on insulin resistance and cardiometabolic adaptations. Obesity (Silver Spring) 2016;24:812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diabetes Prevention Program Research Group. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hussain AA, Claussen B, Ramachandran A, Williams R. Prevention of type 2 diabetes: A review. Diabetes Res Clin Pract 2007;76:317–326 [DOI] [PubMed] [Google Scholar]
- 33. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psychol 1992;47:1102. [DOI] [PubMed] [Google Scholar]