Abstract
Three previously undescribed iridoids, cornusfurals A–C, were isolated from the ethanolic extracts of fruits of Cornus officinalis. Their structures were elucidated by spectroscopic methods, including one-dimensional and two-dimensional nuclear magnetic resonance, ultraviolet spectroscopy, infrared spectroscopy, and mass spectrometry. The neuroprotective activity was evaluated by measuring corticosterone-induced damage in PC12 cells. The results showed that cornusfural B decreased corticosterone-induced PC12 cell damage compared with that in model cells.
Keywords: Cornus officinalis, iridoid, neuroprotective activity
1. Introduction
Cornus officinalis (Cornus) is a shrub distributed in Eastern Asia. The fruits of C. officinalis, named “shanzhuyu”, have traditionally been used for nourishing the liver and kidneys for thousands of years [1]. Extracts or constituents of C. officinalis have been reported to possess neuroprotective, antidiabetic, anti-inflammatory, antioxidant, and cardiovascular effects [2,3,4,5,6]. Phytochemical investigation of the fruits of C. officinalis has demonstrated the presence of iridoid glycosides, flavonoids, terpenoids, and polyphenols [7,8,9,10,11]. Among these, iridoids were found to be the most important constituents and are considered to be active components of the extracts.
Depression is a common mental disorder characterized by persistent depression, which imposes a great burden on patients. Despite the high incidence of depression, the pathogenesis of this disease has not been fully elucidated [12]. The identification of antidepressant drugs from natural products is an important step in the development of novel therapeutics. PC12 cells, derived from the pheochromocytoma of the adrenal medulla in rats, are widely used in studies because of typical neuron characteristics [13]. Corticosterone-induced PC12 neuronal damage is useful as an in vitro experimental model for depression studies [14]. Loganin, the main iridoid glycoside from C. officinalis, has been reported to have antidepressive activity in recent studies [15,16]. Moreover, investigations of iridoid analogs from C. officinalis have revealed a number of biologically and structurally interesting compounds. In our previous pharmacology studies, we found that the macroporous resin 40% ethanol elution fraction of the ethanol extract of C. officinalis exhibited potent neuroprotective activity, and four new iridoid glycosides were isolated [17]. According to the HPLC spectroscopic characteristics, there are still many similar constituents which were suspected to have potential activities in this fraction. Thus, the 40% ethanol elution fraction was further evaluated in this study. Herein, the new iridoids were isolated, and their biological activities were discussed.
2. Results and Discussion
2.1. Characterization
The crude extract of the fruits of C. officinalis was divided into five fractions by macroporous resin column chromatography. The generated 40% ethanol elution fraction was further isolated by the combination of silica gel column chromatography, low-pressure liquid chromatography, Sephadex LH-20 chromatography, and HPLC, generating three new compounds (Figure 1).
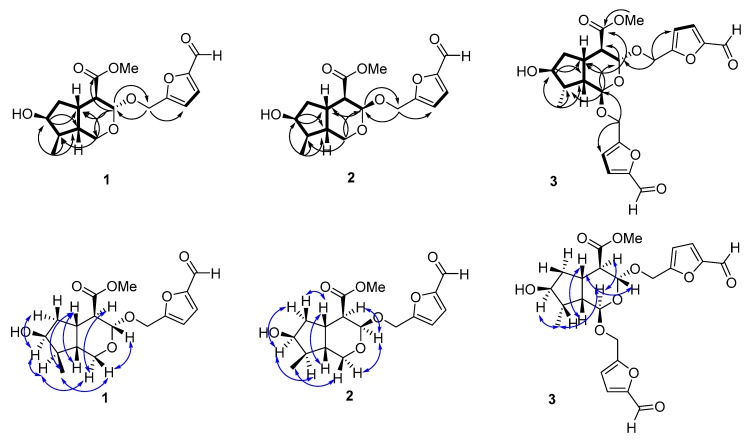
Figure 1.
The structures of compounds 1–3.
Cornusfural A (1) was obtained as an amorphous white solid. The molecular formula C17H22O7 was deduced from the quasimolecular ion peak at m/z 361.1247 [M + Na]+ (calcd 361.1257) in the high-resolution electrospray ionization mass spectrometry (HRESIMS) with an unsaturation of seven. The IR spectrum displayed the presence of hydroxyl (3257 cm−1) and carbonyl (1727, 1672 cm−1) groups. The 1H-NMR data of 1 (Table 1) indicated the presence of nine methine protons, including two oxygenated methines at δH 4.69 (1H, d, J = 8.4 Hz) and 4.09 (1H, m); two olefinic methines at δH 6.57 (1H, d, J = 3.5 Hz) and 7.34 (1H, d, J = 3.5 Hz); three aliphatic methines at δH 2.36 (1H, m), 1.80 (1H, m), and 1.84 (1H, m); one aldehyde at δH 9.52 (1H, s); three methylenes at δH 3.75 (1H, dd, J = 5.0, 12.1 Hz), 3.82 (1H, dd, J = 5.0, 12.1 Hz), 1.75 (1H, m), 1.79 (1H, m), 4.62 (1H, d, J = 13.6 Hz), and 4.71 (1H, d, J = 13.6 Hz); one methoxy at δH 3.61 (3H, s); and one methyl at δH 0.95 (3H, d, J = 6.6 Hz). 13C-NMR data gave 17 carbons, including one methyl (δC 12.4), two oxygenated methylenes (δC 64.9, 62.7), two oxygenated methines (δC 101.6, 75.5), two carbonyl carbons (δC 174.8, 179.5), and four olefinic carbons (δC 159.5, 154.2, 124.4, 112.7), as detailed in Table 1.
Table 1.
1H-NMR and 13C-NMR spectroscopic data of compounds 1–3a.
| No. | Compound 1 | Compound 2 | Compound 3 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1α | 3.75 (dd, J = 5.0, 12.1) | 64.9 | 3.99 (dd, J = 3.9, 12.0) | 58.9 | 4.95 (d, J = 2.9) | 101.3 |
| 1β | 3.82 (dd, J = 5.0, 12.1) | 3.54 (dd, J = 1.4, 12.0) | ||||
| 3 | 4.69 (d, J = 8.4) | 101.6 | 5.04 (d, J = 3.8) | 97.3 | 5.07 (d, J = 8.6) | 97.7 |
| 4 | 2.26 (dd, J = 8.4, 12.1) | 52.5 | 2.48 (dd, J = 3.8, 11.9) | 49.2 | 2.30 (dd, J = 8.6, 12.3) | 51.7 |
| 5 | 2.36 (m) | 39.1 | 2.65 (m) | 32.5 | 2.51 (m) | 37.4 |
| 6α | 1.79 (m) | 40.4 | 1.83 (m) | 42.5 | 1.69 (m) | 40.2 |
| 6β | 1.75(m) | 1.77 (m) | 1.81 (m) | |||
| 7 | 4.09 (m) | 75.5 | 4.08 (m) | 74.6 | 4.08 (m) | 74.9 |
| 8 | 1.80 (m) | 40.7 | 1.90 (m) | 39.7 | 1.84 (m) | 40.9 |
| 9 | 1.84 (m) | 43.5 | 1.68 (m) | 42.9 | 1.88 (m) | 47.8 |
| 10 | 0.95 (d, J = 6.6) | 12.4 | 0.97 (d, J = 6.8) | 12.2 | 0.97 (d, J = 6.3) | 12.7 |
| 11 | - | 174.8 | - | 173.0 | - | 174.4 |
| 12 | 3.61 (s) | 52.3 | 3.56 (s) | 52.2 | 3.62 (s) | 52.5 |
| 1′α | 4.62 (d, J = 13.6) | 62.7 | 4.53 (d, J = 13.7) | 61.9 | 4.70 (d, J = 13.3) | 63.1 |
| 1′β | 4.71 (d, J = 13.6) | 4.64 (d, J = 13.7) | 4.78 (d, J = 13.3) | |||
| 2′ | - | 159.5 | - | 159.3 | - | 159.5 |
| 3′ | 6.57 (d, J = 3.5) | 112.7 | 6.58 (d, J = 3.6) | 113.1 | 6.67 (d, J = 3.6) | 113.0 |
| 4′ | 7.34 (d, J = 3.5) | 124.4 | 7.35 (d, J = 3.6) | 124.4 | 7.36 (d, J = 3.6) | 124.4 |
| 5′ | - | 154.2 | - | 154.3 | - | 154.3 |
| 6′ | 9.52 (s) | 179.5 | 9.53 (s) | 179.4 | 9.54 (s) | 179.5 |
| 1″α | 4.69 (d, J = 13.4) | 62.8 | ||||
| 1″β | 4.81 (d, J = 13.4) | |||||
| 2″ | - | 159.2 | ||||
| 3″ | 6.63 (d, J = 3.6) | 112.9 | ||||
| 4″ | 7.35 (d, J = 3.6) | 124.4 | ||||
| 5″ | - | 154.3 | ||||
| 6″ | 9.52 (s) | 179.5 |
a 1H-NMR data (δ) were measured in methanol-d4 at 500 MHz and 13C-NMR data (δ) were measured in methanol-d4 at 125 MHz for compounds 1-3. Coupling constants (J) in Hz are given in parentheses. The assignments were based on 1H-1H COSY, HSQC, HMBC and NOESY experiments.
In combination with analysis of the 1H-1H COSY spectrum (Figure 2), the NMR data showed that there was a four-spin system involving C3-C4-C5-C6-C7-C8-C9-C1, C5-C9, C8-C10, and C3′-C4′ in 1. In the HMBC spectrum, the correlations of H-1/C-3, C5, and C-8; H-3/C-1, C-5 and C-11, H-7/C-5, and C-9; and H-10/C-7, C-8, and C-9 determined the cyclopentane iridoid carbon skeleton. In addition, a set of a 5-hydroxymethyl furfural moiety, which was proven by its characteristic signals at δH 4.62 (1H, d, J = 13.6 Hz), 4.71 (1H, d, J = 13.6 Hz), 6.57 (1H, d, J = 3.5 Hz), 7.34 (1H, d, J = 3.5 Hz), and 9.52 (1H, s), was present in 1. The location of the 5-hydroxymethyl furfural group was established by the key cross-peaks from H-1′ to C-3 and from H-3 to C-1′ in the HMBC spectrum.
Figure 2.
The HMBC ( ), 1H-1H COSY (
), 1H-1H COSY ( ) and NOESY (
) and NOESY ( ) spectra of compounds.
) spectra of compounds.
The relative configuration of 1 was deduced as depicted by the NOESY spectrum and coupling constants. The J3,4 = 8.4 Hz and J4,5 = 12.1 Hz suggested that the methoxycarbonyl group was β-equatorial [18,19], whereas the position of the furfural unit was α-equatorial. In the NOESY spectrum, correlations of H-3/H-1β/H-10/H-5/H-9 and H-4/H-1α/H-8/H-7/H-6α indicated that H-3, H-5, and H-9 were of the β-configuration, whereas the H-4, H-7, and H-8 were of the α-configuration. The absolute configuration of 1 was substantiated based on biogenetic grounds in that nearly all iridoids found in nature have a configuration of 5S and 9R, and by analogy to the known compounds that were found to have closely comparable NMR data and similar optical rotation values [18]. The ECD spectrum of 1 exhibited negative Cotton effects at 234 and 272 nm arising from 5-hydroxymethylfurfural (Supplementary Materials). Thus, the configurations of H-3/4/5/7/8/9 should be 3S/4R/5S/7S/8R/9R. Therefore, the structure of 1 was established as cornusfural A.
Compound 2 was found to have the same formula (C17H22O7) as 1 with the HRESIMS ion at m/z 361.1247 [M + Na]+ (calcd for 361.1257, C17H22O7Na) and required seven degrees of unsaturation. Moreover, this compound exhibited similar IR, UV, and NMR data as 1, suggesting their structural resemblance. However, the upfield shifts of C-1 (δC 58.9), C-3 (δC 97.3), C-4 (δC 49.2), and C-5 (δC 32.5) and the coupling constants J3,4 = 3.8 Hz and J4,5 = 11.9 Hz showed that the iridoid skeleton of 2 was an epimer of 1, which was confirmed by the NOESY correlations of H-3 with H-4 and H-1α. Thus, the spectroscopic data of 2 indicated that this compound was an epimer of 1 and that the configuration of H-3 was α-oriented. Compared to compound 1, the absolute configuration of 2 was (3R, 4R, 5S, 7S, 8R, and 9R). Hence, the structure of 2 (cornusfural B) was established as shown.
Compound 3 was obtained as a white powder. The molecular weight was determined by HRESIMS, which showed an [M + Na]+ ion at m/z 485.1407 (calcd for 485.1418, C32H38O15Na), indicating 11 degrees of unsaturation. The IR spectrum showed absorption bands due to hydroxy (3256 cm−1) and carbonyl (1727 and 1669 cm−1) groups. The 1H-NMR spectrum of compound 3 showed that there was a doublet methyl group δH 0.97 (3H, d, J = 6.3 Hz); three pairs of geminal coupled methylene groups δH 1.67–1.72 (1H, m, H-6α), 1.80–1.82 (1H, m, H-6β), 4.70 (1H, d, J = 13.3Hz, H-1′α), 4.78 (1H, d, J = 13.3Hz, H-1′β), 4.69 (1H, d, J = 13.4 Hz, H-1′′α), and 4.81 (1H, d, J = 13.4 Hz, H-1′′β); four methine protons δH 2.30 (1H, dd, J = 8.6, 12.3 Hz, H-4), 2.49–2.53 (1H, m, H-5), 1.84 (1H, m, H-8), and 1.88 (1H, o, H-9); an oxygenated methine proton δH 4.08–4.09 (1H, m, H-7); two acetal protons δH 4.95 (1H, d, J = 2.9 Hz, H-1) and 5.07 (1H, d, J = 8.6 Hz, H-3); four olefinic protons δH 6.67 (1H, d, J = 3.6 Hz, H-3′), 7.36 (1H, d, J = 3.6 Hz, H-4′), 6.63 (1H, d, J = 3.6 Hz, H-3′′), and 7.35 (1H, d, J = 3.6 Hz, H-4′′); and two aldehyde groups δH 9.54 (1H, s, H-6′) and 9.52 (1H, s, H-6′′). There were 23 carbons found in the 13C-NMR spectrum, and there were three carbonyl groups, that is, δC 174.4 (C-11), 179.5 (C-6′), and 179.5 (C-6′′). The 1H-1H COSY spectra found correlations of H-3/H-4/H-5, H-5/H-6/H-7/H-8, H-8/H-9/H-10, and H-5/H-9. In the HMBC spectrum, the correlations of H-1 to C-3/C-5/C-8, H-3 to C-5/C-11, H-7 to C-5/C-6/C-8/C-9, and H-10 to C-7/C-8/C-9 indicated that compound 3 was an iridoid-type compound. According to the spectra and correlations, there were two additional 5-hydroxymethylfurfural groups connecting to the iridoid skeleton. The HMBC correlations from H-1′ to C-3 and from H-1′′ to C-1 revealed that the 5-hydroxymethylfurfural moieties were present at C-1 and C-3, respectively.
The relative stereochemistry of 3 was determined by the NOESY spectrum and coupling constants. The large coupling constant J4,5 = 11.4 Hz between H-4 and H-5 indicated that H-4 was located in the α-oriented configuration, and J3,4 = 8.6 Hz indicated that H-3 was β-oriented. In the NOESY spectrum, correlations between H-3 and H-5/H-9 indicated that H-5 and H-9 were both β-oriented. Moreover, NOESY correlations of H-4 with H-1 and of H-10 with H-1 and H-7 confirmed that H-1, H-10, and H-7 were all α-oriented. According to the biogenetic grounds in which the absolute configurations of H-5 and H-9 were 5S and 9R, respectively, and because the negative Cotton effects were at 234 and 281 nm, it can be said the compound 3 had an absolute configuration of (1R, 3R, 4R, 5S, 7S, 8S, and 9R). Analysis of the HSQC, 1H-1H COSY, and HMBC spectra led to the complete assignments of the proton and carbon signals in compound 3. Therefore, compound 3 was characterized as cornusfural C.
2.2. Neuroprotective Effects of Compounds 1–3
Corticosterone-induced PC12 neuronal damage is useful as an in vitro experimental model for depression studies [14]. The neuroprotective effects of compounds 1–3 were assessed (Table 2). Compound 2 exhibited neuroprotective activity compared with the model (complete medium with 500 μM corticosterone).
Table 2.
Neuroprotective effects of compounds 1–3 at a concentration of 10−5 M (means ± SD, n = 6).
| Sample | Viability (%) |
|---|---|
| Control | 100.00 ± 1.21 |
| Model | 53.54 ± 1.82 ### |
| 1 | 57.42 ± 2.74 |
| 2 | 68.23 ± 2.26 *** |
| 3 | 59.46 ± 3.62 |
###p < 0.01 vs. control, *** p < 0.05 vs. model.
2.3. Discussion
As the results showed, only compound 2 exhibited neuroprotective activity in in vitro experiments. The structural analysis of these new compounds showed that compounds 1 and 3 had the same configuration of 3α, while compound 2 had a 3β configuration. The current data showed that the neuroprotective activity of these iridoid derivatives might be associated with the stereochemistry of C-3, and the 3β-substituents might be the active groups. However, the mechanism of the effect of C-3 configuration needs further confirmation by studying the structure–activity relationships of more similar compounds. Although there are many reports on the neuroprotective activities of iridoids from C. officinalis, the structure–activity relationship has not been reported [20,21,22]. Morroniside, the main active component of C. officinalis, was even evaluated in the form of a mixture in the neuroprotective study. Therefore, more efforts are suggested to explore the structure–activity relationships of iridoids from C. officinalis.
3. Materials and Methods
3.1. Plant Material
The fruits of C. officinalis were purchased from Tong-Ren-Tang Company in Beijing, People’s Republic of China, and were authenticated by Professor Wen Wang, Xuanwu Hospital of Capital Medical University. Voucher specimen number 20090305 was deposited at the Beijing Union University, Beijing Key Laboratory of Bioactive Substances, and Functional Foods, Beijing, China.
3.2. General Experimental Procedures
The HRESIMS data were generated on a Thermo QE Spectrometer (Thermo Scientific Inc., Waltham, MA, USA). The specific rotation data were obtained with a JASCO P-2000 polarimeter (JASCO Inc., Tokyo, Japan). IR spectra were recorded as KBr disks on a Nicolet Impact 400 FT-IR Spectrophotometer (Nicolet Instrument Inc., Madison, WI, USA). The circular dichroism spectra and UV data were recorded on a JASCO J-1005 circular dichroism spectrometer (JASCO Corporation, Tokyo, Japan). The one- and two-dimensional NMR spectra were recorded in CD3OD with TMS as the internal standard on Varian 500 MHz and Bruker AV500-III spectrometers (Bruker Corporation, Billerica, MA, USA). A Waters 2996 series was coupled with an RP-C18 column (Sunfire, 250 mm × 19 mm i.d.; Alltech Associates, Inc., Bannockburn, IL, USA) and a Waters 2998 dual-wavelength absorbance detector (Waters Corporation, Milford, MA, USA). Column chromatography was performed with silica gel (160–200 mesh; Qingdao Marin Chemical Inc., Qingdao, China) and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden). Thin-layer chromatography (TLC) was used with glass precoated silica gel GF254 plates. Spots were visualized under UV light or by spraying with 8% H2SO4 in 95% EtOH followed by heating.
3.3. Cell lines, Chemicals, and Biochemicals
PC12 cells (adrenal gland; pheochromocytoma) were purchased from the Shanghai Institute of Biochemistry and Cell Biology, CAS (Shanghai, China). DMSO, corticosterone, and MTT were obtained from Sigma (St. Louis, MO, USA). The methanol used for HPLC isolation, which was of HPLC grade, was purchased from Fisher (Waltham, MA, USA). The solvents used to open the column isolation (Silica gel and Sephadex LH-20 gel column) in the study, such as chloroform, acetone, and methanol, were of ACS grade (Beijing, China).
3.4. Extraction and Isolation
Air-dried fruits (10 kg) of C. officinalis were exhaustively extracted with 50% aqueous solution (100 L × 3, 1 h) at reflux. The ethanolic extracts were concentrated under reduced pressure to dryness. The residue was suspended in H2O and applied to a Diaion HP-20 column chromatography (Mitsubishi Chemical Corporation, Nagasaki, Japan) by a stepwise gradient of EtOH/H2O (0:100, 20:80, 40:60, 70:30, and 95:5, v/v) to yield five fractions (fractions A–E). The separation of fraction B (EtOH:H2O = 40:60, 500 g) was carried out on a silica gel column eluted with CHCl3/MeOH (15:1 to 8:1, v/v) to afford four major fractions (B1–B4) based on TLC analysis. The separation of fraction B1 (73 g) was carried out on a silica gel column eluted with CHCl3/MeOH (50:1 to 3:1) to provide four subfractions B1-1–B1-4. Fraction B1-2 (42.2 g) was subjected to silica gel column chromatography, eluted with CHCl3/MeCOMe (10:1 to 2:1, v/v), to give three subfractions B1-2-1–B1-2-3. Fraction B1-2-1 (10.1 g) was separated using a Sephadex LH-20 column eluted with CHCl3/MeOH (2:1) as the mobile phase to give three subfractions B1-2-1-1–B1-2-1-3. Fraction B1-2-1-2 (3.4 g) was further fractionated over a Sephadex LH-20 column eluted with petroleum ether/CHCl3/MeOH (5:5:1) as the mobile phase, and the subfractions were purified by preparative HPLC using 60% MeOH/H2O (18 mL/min) to yield compounds 1 (18 mg) and 2 (11 mg). Fraction B1-2-3 was subjected to CombiFlash HCN silica gel column chromatography by a gradient elution with petroleum ether/acetone (20:1, 10:1, 8:1, 6:1, 3:1, 1:1) to yield five fractions B1-2-3-1–B1-2-3-5. Fraction B-1-2-3-3 was purified by preparative HPLC using 55% MeOH/H2O (18 mL/min) as the mobile phase to yield compound 3 (9 mg).
3.5. Compounds Characterization Data
Cornusfural A (1): White amorphous powder, [α −121.2 (c 0.06, CH3OH); UV (MeOH) λmax (log ε): 230 (4.01) nm, 280 (4.21) nm; CD (MeOH) 234 (Δε −1.49), 272 (Δε −6.31) nm; IR νmax 3257, 2959, 2872, 1727, 1672, 1521, 1457 cm−1; 1H-NMR (methanol-d4, 500 MHz) and 13C-NMR (methanol-d4, 125 MHz) spectral data see Table 1; (+)-HRESIMS m/z 361.1247 [M + Na]+ calcd for 361.1257 C17H22O7 Na).
Cornusfural B (2): White amorphous powder, [α +120 (c 0.06, CH3OH); UV (MeOH) λmax (log ε): 230 (3.85) nm, 280 (4.11) nm; CD (MeOH) 234 (Δε +4.11), 281 (Δε +13.5) nm; IR νmax 3431, 2954, 2877, 1736, 1675, 1521, 1436 cm−1; 1H-NMR (methanol-d4, 500 MHz) and 13C-NMR (methanol-d4, 125 MHz) spectral data see Table 1; (+)-HRESIMS m/z 361.1247 [M + Na]+ (calcd for 361.1257, C17H22O7Na).
Cornusfural C (3): White amorphous powder, [α −44.2 (c 0.03, CH3OH); UV (MeOH) λmax (log ε): 232 (3.80) nm, 280 (4.02) nm; CD (MeOH) 234 (Δε −3.11), 281 (Δε −13.9) nm; IR νmax 3256, 2959, 2926, 1727, 1669, 1523, 1458 cm−1; 1H-NMR (methanol-d4, 500 MHz) and 13C-NMR (methanol-d4, 125 MHz) spectral data see Table 1; (+)-HRESIMS m/z 485.1407 [M + Na]+ (calcd for 485.1418, C32H38O15Na).
3.6. Neuroprotection Bioassays
PC12 cells were cultured in RPMI 1640 medium supplemented with 1% streptomycin, 5% horse serum, and 5% fetal bovine serum. The cell suspensions were seeded in 96-well culture plates (2 × 104 cells/mL) and cultured for 24 h. Then, the medium was replaced with different fresh media, including the control (complete medium), the model (complete medium with 500 μM corticosterone), and the sample (the test compounds at a concentration of 10 μM), and the cells were cultured for 24 h. Next, 10 μL MTT (5 mg/mL) was added to each well. After incubation for 2 h, the medium was removed, and 100 μL DMSO was added to dissolve the formazan crystals generated by the reaction. The optical density was then measured on a microplate reader (Molecular Devices, SFO, USA) at 570 nm. Cell viability was indicated as a percentage of the control.
4. Conclusions
Three new iridoids, that is, cornusfural A (1), cornusfural B (2), and cornusfural C (3), containing a furan ring, were isolated from the fruits of C. officinalis, and the neuroprotective effects of these compounds were evaluated. Compound 2 showed neuroprotective activities. The neuroprotective activities of iridoid glycosides have been evaluated through a variety of in vitro and in vivo studies [20,21,22]; however, iridoid aglycones isolated from C. officinalis have rarely been studied. Importantly, we identified one new iridoid aglycone exhibiting neuroprotective effects, thereby providing a potential new neuroprotective agent for further antidepressants research. According to the literature, iridoid glycosides are the main component in this plant, but the quality of iridoid aglycones are less. If the iridoid aglycones have significant biological activities, a large number of aglycones can be obtained through the hydrolysis of iridoid glycosides, which could be used for further animal experiments. This indicated that Cornus officinalis is a good resource of bioactive compounds and functional food.
Supplementary Materials
The supplementary materials are available online.
Author Contributions
X.-y.S. supervised the study. L.-l.J. and X.W. wrote and revised the paper. L.-l.J., X.-j.Z., J.J., and B.Z. performed the isolation of the compounds. X.W. and J.-j.L. analyzed the data and performed the biological assays.
Funding
The authors gratefully acknowledge the grants from the Key projects of the Beijing Natural Sciences Foundation and Beijing Municipal Education Committee (No. KZ201811417049); the Strengthening University by Talents Program of Beijing Union University (Grant No. BPHR2018AZ01); and the Scientific Research Common Program of Beijing Municipal Commission of Education (Grant No. KM201811417008).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 2 are available from the authors.
References
- 1.Chinese Pharmacopoeia Commission . Pharmacopoeia of the People’s Republic of China. Volume 1. China Medical Science Press; Beijing, China: 2015. pp. 27–28. [Google Scholar]
- 2.Wang W., Xu J.D., Li L., Wang P.C., Ji X.M., Ai H.X., Zhang L. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res. Bull. 2010;83:196–201. doi: 10.1016/j.brainresbull.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Kang D.G., Choi D.H., Lee J.K., Lee Y.J., Moon M.K., Yang S.N., Kwon T.O., Kwon J.W., Kim J.S., Lee H.S. Endothelial NO/cGMP-dependent vascular relaxation of cornuside isolated from the fruits of Cornus officinalis. Planta Med. 2007;73:1436–1440. doi: 10.1055/s-2007-990243. [DOI] [PubMed] [Google Scholar]
- 4.He K., Song S.H., Zou Z.Y., Feng D., Wang Y.Z., Li X.G., Ye X.L. The hypoglycemic and synergistic effect of loganin, morroniside, and ursolic acid isolated from the fruits of Cornus officinalis. Phytother. Res. 2016;30:283–291. doi: 10.1002/ptr.5529. [DOI] [PubMed] [Google Scholar]
- 5.An Y.A., Hwang J.Y., Lee J.S., Kim Y.C. Cornus officinalis methanol extract upregulates melanogenesis in melan-a cells. Eur. Toxicol. Res. 2015;31:165–172. doi: 10.5487/TR.2015.31.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huwang K.A., Huang Y.J., Song J. Antioxidant activities and oxidative stress inhibitory effects of ethanol extracts from Cornus officnalis on raw 264.7 cells. BMC Complementary Altern. Med. 2016;16:1–9. doi: 10.1186/s12906-016-1172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma W., Wang K.J., Cheng C.S., Yan G.Q., Lu W.L., Ge J.F. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J. Ethnopharmacol. 2014;153:840–845. doi: 10.1016/j.jep.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Ye X.S., He J., Cheng Y.C., Zhang L., Qiao H.Y., Pan X.G., Zhang J., Liu S.N., Zhang W.K., Xu J.K. Cornusides A-O, Bioactive iridoid glucoside dimers from the fruits of Cornus officinalis. J. Nat. Prod. 2017;80:3103–3111. doi: 10.1021/acs.jnatprod.6b01127. [DOI] [PubMed] [Google Scholar]
- 9.Lee S.H., Tanaka T., Nonaka G.I., Nishioka I. Sedoheptulose digallate from Cornus officnalis. Phytochemistry. 1989;28:3469–3472. doi: 10.1016/0031-9422(89)80366-8. [DOI] [Google Scholar]
- 10.Lee J., Jang D.S., Kim N.H., Lee Y.M., Kim J., Kim J.S. Galloyl glucoses from the seeds of Cornus officinalis with inbibitory activity against protein glycation, aldose reductase, and cataractogenesis ex vivo. Biol. Pharm. Bull. 2011;34:443–446. doi: 10.1248/bpb.34.443. [DOI] [PubMed] [Google Scholar]
- 11.Xie X.Y., Wang R., Shi Y.P. Chemical constituents from the fruits of Cornus officinalis. Biochem. Syst. Ecol. 2012;45:120–123. doi: 10.1016/j.bse.2012.07.025. [DOI] [Google Scholar]
- 12.Ledford H.D. If depression were cancer. Nature. 2014;515:183–185. doi: 10.1038/515182a. [DOI] [PubMed] [Google Scholar]
- 13.Mao Q.Q., Xian Y.F., Ip S.P., Tsai S.H., Che C.T. Protective effects of peony glycosides against corticosterone-induced cell death in PC12 cells through antioxidant action. Cell. Mol. Neurobiol. 2011;133:1121–1125. doi: 10.1016/j.jep.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 14.Li Y.F., Gong Z.H., Cao J.B., Wang H.L., Luo Z.P., Li J. Antidepressant-like effect of agmatine and its possible mechanism. Eur. J. Pharm. 2003;469:81–88. doi: 10.1016/S0014-2999(03)01735-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W.K., Xu J.K., He J., Qiao H.Y., Ye X.S. Application of Loganin in Manufacture of Medicine or Health Product for Preventing and Treating Depression, Anxiety and Other Mental Disorder Diseases. 106176789 A 20161207. CN Patent. 2016 Dec 7;
- 16.Rajabi M., Mohaddes G., Farajdokht F., Nayebi Rad S., Mesgari M., Babri S. Impact of loganin on pro-inflammatory cytokines and depression-and anxiety-like bhaviors in male diabetic rats. Physiol. Int. 2018;105:199–209. doi: 10.1556/2060.105.2018.1.8. [DOI] [PubMed] [Google Scholar]
- 17.Wang X., Liu C.H., Li J.J., Zhang B., Ji L.L., Shang X.Y. Iridoid glycosides from the fruits of Cornus officinalis. J. Asian Nat. Prod. Res. 2018;20:934–942. doi: 10.1080/10286020.2018.1497609. [DOI] [PubMed] [Google Scholar]
- 18.Hu J., Mao X., Shi X.D., Li H. Chemical constituents of the barks of Litsea rubescens. Chem. Nat. Comp. 2017;53:694–697. doi: 10.1007/s10600-017-2093-1. [DOI] [Google Scholar]
- 19.Kocsis A., Szabo L.F. New bis-iridoids from Dipsacs laciniatus. J. Nat. Prod. 1993;56:1486–1499. doi: 10.1021/np50099a007. [DOI] [Google Scholar]
- 20.Jeong E.J., Kim T.B., Yang H.J., Kang S.Y., Kim S.Y., Sung S.H., Kim Y.C. Neuroprotective iridoid glycosides from Cornus officinalis fruits against glutamate-induced toxicity in HT22 hippocampal cells. Phytomedicine. 2012;19:317–321. doi: 10.1016/j.phymed.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 21.Zhao L.H., Ding Y.X., Zhang L., Li L. Cornel iridoid glycoside improve memory and promotes neuronal survival in fimbria-fornix transected rats. Eur. J. Pharm. 2010;647:68–74. doi: 10.1016/j.ejphar.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Wang W., Sun F.L., An Y., Ai H.X., Zhang L., Huang W.T., Li L. Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur. J. Pharm. 2009;613:19–23. doi: 10.1016/j.ejphar.2009.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.