Abstract
Bioassay directed isolation of secondary metabolites from the rhizomes of Zingiber montanum (Fam. Zingiberaceae) led to the isolation of mono-, sesqui-, and di-terpenes. The compounds were characterized as (E)-8(17),12-labdadiene-15,16-dial (1), zerumbol (2), zerumbone (3), buddledone A (4), furanodienone (5), germacrone (6), borneol (7), and camphor (8) by analysing one-dimensional (1D) (1H and 13C) and two-dimensional (2D) (COSY, HSQC, HMBC, and NOESY) NMR data and mass spectra. Among these terpenes, compounds 1 and 2 revealed potential antibacterial activity (minimum inhibitory concentrations (MIC) values 32–128 µg/mL; 0.145–0.291 mM)) against a series of clinical isolates of multi-drug resistant (MDR) and Methicillin resistant Staphylococcus aureus (MRSA).
Keywords: antimicrobial resistance; multi-drug resistant (MDR); methicillin resistant Staphylococcus aureus (MRSA); Zingiber monatnum; terpenes; (E)-8(17),12-labdadiene-15,16-dial; zerumbol
1. Introduction
Antimicrobial resistance has increasingly become a major public health issue that currently claims 700,000 lives every year. It is predicted that if no action is taken, there will be approximately 10 million deaths each year globally by 2050, which will be more than the predicted number of deaths by cancer [1] and will cause a cumulative 100 trillion USD of economic output due to the rise of drug-resistant infections [1]. The morbidity due to resistant infection has doubled since 2007, which equals the burden of HIV, influenza, and tuberculosis [1]. As antibiotic resistance occurs naturally, its mishandling and misuse in humans and animals accelerate the process of development of resistant infection [2]. Infectious diseases, like pneumonia, tuberculosis, gonorrhoea, and salmonellosis are becoming difficult to treat because the antibiotics that are used to treat them are becoming less effective [2]. The gram-positive bacterium Staphylococcus aureus relates to an extensive range of infection of skin and soft tissue, pneumonia, endocarditis, sepsis and bacteremia [3] that causes nosocomial infection (resistant to methicillin and vancomycin). Therefore, it is no doubt important to discover new antibiotics to act against multi-drug resistant (MDR) and Methicillin resistant Staphylococcus aureus (MRSA).
Since the accidental discovery of penicillin from Penicillium notatum, a huge number of antibiotics have been developed from microbes. However, the development of resistance of existing antibiotics to pathogenic microorganisms necessitates the development of new antibiotics from natural sources, including plants, microbes, and marine resources. Medicinal plants have been under-exploited for antimicrobial drug discovery, although plants are considered as leads for the development of several medicines. Some key examples of plant derived medicines include cardioactive digoxin from Digitalis lanata [4], anticancer vincristine and vinblastine from Catharanthus roseus [5], analgesic morphine from Papaver somniferum [6], antimalarial artemisinin from Artemisia annua [7], and antiinflamatory salicin from the bark of the willow tree Salix alba L. [8]. There are also significant reports of medicinal plants being used as systemic and topical antimicrobial agents in Ayurvedic [9] and Traditional Chinese Medicine [10], as well as in western herbal medicine [11] due to their self-protection strategy to counter bacteria and fungi in their own environment. Hyperforin isolated from the medicinal plant Hypericum perforatum exhibited antimicrobial activity with minimum inhibitory concentrations (MIC) value of 0.1 mg/L against methicillin-resistant Staphylococcus aureus (MRSA) and penicillin-resistant variants [12]. A series of new acylphloroglucinol isolated from Hypericum olympicum showed highly promising antibacterial activity (MICs 0.50–1 µg/mL) against a series of clinical isolates of MRSA strains [13].
Zingiber montanum (Fam. Zingiberaceae), an herbaceous plant that produces a clump of leaves from large rhizomes, is indigenous to Bangladesh, India, Malaysia, Thailand, Indonesia, and Sri Lanka [14]. Traditionally, it has been used for the treatment of asthma, sprains, muscular pain, inflammation, wounds, and as a mosquito repellent, a carminative, and an antidysentery agent [15,16]. Z. montanum has been reported to exhibit antioxidant [17], radioprotective [17,18], antiulcer [19], and anti-inflammatory [20] properties. In regard to the phytochemical investigation on Z. montanum, a number of monoterpene and sesquiterpene hydrocarbons have recently been reported using gas chromatography-flame ionization detection and gas chromatography-mass spectrometry [21]. The antibacterial, antifungal, allelopathic, and acetylcholinesterase inhibitory activities of these terpenes have also been reported [21]. As part of our research into anti-infective secondary metabolites from Bangladesh medicinal plants, the authors report the bioassay directed isolation and identification of a total of eight terpenes from Z. montanum and also their antibacterial activity against a panel of clinical isolates of multi-drug resitant (MDR) and methicillin resistance Staphycococcus aureus (MRSA) strains.
2. Results and Discussion
The n-hexane, CHCl3, and MeOH extracts from the rhizomes of Z. montanum were initially screened for antibacterial activity (Table 1) against clinical isolates of MRSA strains. Whilst MeOH extract did not exhibit any activity at a concentration of 512 µg/mL, both n-hexane and CHCl3 extracts showed activity against the MRSA strains tested with MICs of 64–256 µg/mL. Vacuum liquid chromatography (VLC) fractionation on active crude extracts, followed by further purification using column chromatography over Sephadex LH20, solid phase extraction (SPE), and/or preparative TLC led to the isolation of seven terpenes (2–8) from n-hexane extract and two terpenes (1 and 8) from the CHCl3 extract. Among these compounds, 1 and 2 exhibited promising antibacterial activity against MRSA strains with MICs of 64–128 µg/mL (0.145–0.291 mM).
Table 1.
Antibacterial activity of crude extracts against standard, multi-drug resistant (MDR) and methicillin-resistant strains of Staphylococcus aureus in µg/mL.
| Extracts/Antibiotic | MICs in µg/mL | ||||
|---|---|---|---|---|---|
| SA1199B | XU212 | EMRSA15 | RN4229 | ATCC25941 | |
| n-Hexane | 128 | 128 | 256 | 256 | 128 |
| Chloroform | 64 | 128 | 128 | 128 | 256 |
| Methanol | >512 | >512 | >512 | >512 | >512 |
| Norfloxacin | 32 | 64 | 16 | 8 | 16 |
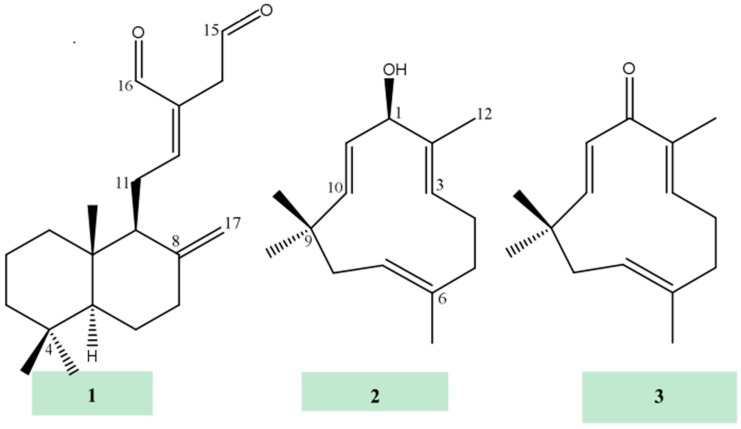
Compound 1 was isolated as colourless amorphous powder from the CHCl3 extract of Z. montanum. The molecular formula of 1 was established as C20H30O2 from the [M + H]+ at m/z 303.23122 (calculated for C20H31O2 at 303.23240) in the high resolution of mass spectrometry. The 1H-NMR (600 MHz, CDCl3, Table 2) spectrum showed the presence of two sets of aldehyde protons (δH 9.40 and 9.63), one olefinic proton resonating at δH 6.76 (J = 6.6 Hz), exomethylene protons at 4.36 and 4.86, three sets of methyl protons, and a number of peaks for methine and methylene protons. The 13C-NMR spectrum showed the presence of a total of 20 carbons including two aldehyde carbons (193.8 and 197.5), an exomethylene (108.1), an olefinic methine (135.0), two aliphatic methines, four quaternary carbons, three methyl carbons, and seven methylene carbons. In HMBC, two sets of methyl protons at δH 0.82 (δC 22.1 from HSQC) and δH 0.89 (δC 33.7 from HSQC) showed a common 2J connectivity to a carbon at 33.7 (C-4) and 3J connection with methylene carbon at δC 42.0 (C-3; δH 1.41 and 1.18 from HSQC) and methine carbon at δC 55.8 (C-5; δH 1.13 from HSQC). H-5 revealed 3J HMBC correation to methylene carbons at 42.0 (C-3), 38.1 (C-7; δH 2.02 and 2.42 from HSQC), methine carbon at 56.6 (C-9; δH 1.90 from HMQC), and methyl carbon at 14.6 (C-20; δH 0.72 from HSQC). H-9 exhibited HMBC interactions to C-10 (by 2J), C-11 (by 2J), C-12 (160.4 by 3J; δH 6.76 from HSQC), C-17 (108.1 by 3J; δH 4.36 and 4.86 from HSQC), and C-20 (by 3J). H-12 showed 3J HMBC connectivity to C-9, methylene carbon at 39.6 (C-14; δH 3.41 and 3.46 from HSQC), and aldehydic carbon at 193.8 (C-16; δH 9.40 from HSQC). The other aldehydic proton at H 9.63 (H-15; C 197.5 from HSQC) showed 3J HMBC correation to a quaternary carbon at 135.0 (C-13). Accordingly, structure of 1 was confirmed as (E)-8(17),12-labdadiene-15,16-dial [22]. The NMR spectra of compound 1 are available in Supplementary Material. This compound has previously reported from Alpinia chinensis [22] and Curcuma heyneana [23]. This is the first report of its isolation from the genus Zingiber.
Table 2.
1H- (600 MHz), 13C- (150 MHz) NMR and HMBC (600 MHz) data of 1 in CDCl3.
| Position | 1H | 13C | HMBC | |
|---|---|---|---|---|
| 2 J | 3 J | |||
| 1 | 1.06, m, 1H; 1.68, m, 1H | 39.4 | - | C-9 |
| 2 | 1.50, m, 1H; 1.57, m, 1H | 19.5 | C-3 | C-4 |
| 3 | 1.18, m, 1H; 1.41, m, 1H | 42 | - | C-18, C19 |
| 4 | - | 33.6 | - | - |
| 5 | 1.13, m, 1H | 55.8 | C6 | C3, C7, C9, C19, C20 |
| 6 | 1.34, m, 1H; 1.75, m, 1H | 24.4 | C5, C7 | C10 |
| 7 | 2.02, m, 1H; 2.42, m, 1H | 38.1 | C8 | C5, C17 |
| 8 | - | 148.4 | - | - |
| 9 | 1.90, m, 1H | 56.6 | C10, C11 | C12, C17, C20 |
| 10 | - | 39.8 | - | - |
| 11 | 2.31, m, 1H; 2.49, m, 1H | 24.8 | C9 | C8, C13 |
| 12 | 6.76, t, J = 6.6 Hz, 1H | 160.4 | - | C9, C14, C16 |
| 13 | - | 135 | - | - |
| 14 | 3.41, d, J = 16.8 Hz, 1H | 39.6 | C13 | C12, C16 |
| 3.46, d, J = 16.7 Hz, 1H | ||||
| 15 | 9.63, t, J = 14.4 Hz, 1H | 197.5 | C14 | C13 |
| 16 | 9.40, s, 1H | 193.8 | C13 | C12, C14 |
| 17 | 4.36, s, 1H; 4.86, s, 1H | 108.1 | C8 | C7, C9 |
| 18 | 0.88, s, 3H | 33.7 | - | C3, C5, C19 |
| 19 | 0.82, s, 3H | 22.1 | - | C3, C5, C18 |
| 20 | 0.72, s, 3H | 14.6 | C10 | C5, C9 |
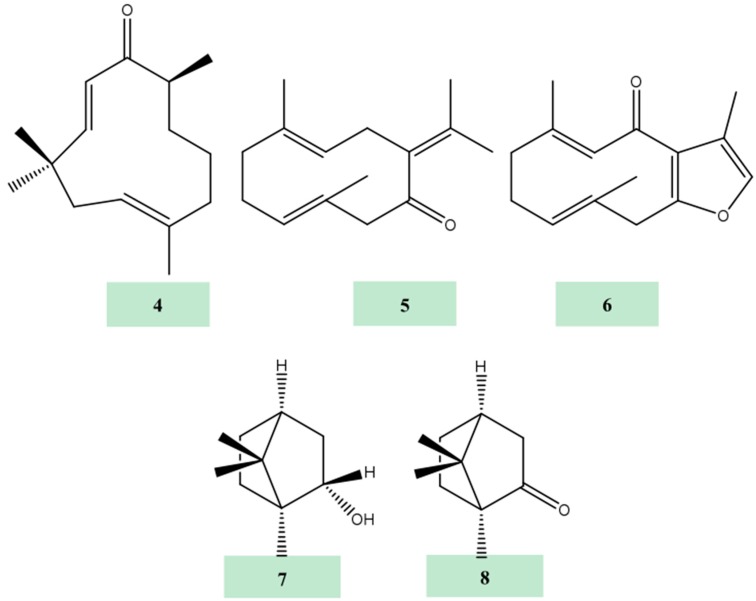
Compound 2 was isolated colourless oil from the n-hexane extract of Z. montanum. The IR spectrum revealed the presence of a hydroxyl group (3300 cm−1) and double bond (1610 cm−1). The high resolution of mass spectroscopy showed the [M + H]+ at m/z 221.18956 (calculated for C15H25O, at 221.19054), which confirmed the molecular formula of 2 as C15H24O. The 1H-NMR spectrum (CDCl3, 600 MHz, Table 3) of 2 showed the presence of four methyl singlets resonating at δH 1.04, 1.06, 1.43, 1.65, four sets of olefinic protons at 4.82 (dd, J = 10.2, 4.4 Hz), 5.20 (d, J = 7.5 Hz), 5.23 (d, J = 16.2 Hz), and 5.56 (dd, J = 16.2, 7.5 Hz), an oxymethine proton at 4.63 as doublet (J = 7.5 Hz), and also couple of methylene protons peaks between 1.87–2.35 Hz. The 13C-NMR spectrum (150 MHz, CDCl3) revealed the presence of a total of 15 carbons, including an oxymethine carbon at 78.8. The DEPT135 identified four methyl, three methylene, one oxymethine, four olefinic methine, and the remaining three as quaternary carbons. Among the later three quaternary carbons, one at 37.3 is aliphatic and remaining two are connected double bonds. The complete structure of this compound was established by two-dimensional (2D) NMR spectra, predominantly by HSQC and HMBC. In the 1H-1H COSY spectrum, the trans double bonded protons showed expected interaction between them. In the HMBC experiment, the trans double bonded proton at 5.23 (δC 139.5 from HSQC) and methyl protons at 1.65 (H-12; δC 12.8 from HSQC) revealed a common 3J interaction to an oxymethine carbon at 78.8 (C-1). Olefinic protons at δH 4.82 (H-7; δC 125.0 ppm from HSQC) and 5.20 (H-3; δC 124.8 ppm from HSQC) and methyl protons at 1.43 (H-13; δC 15.3 ppm from HSQC) showed 3J correlation to a methylene carbon at 39.5 (C-5; δH 2.35 ppm from HSQC). Protons at 2.20 and 2.24 (H-4; δC 24.4 from HSQC) exhibited 3J correlations to quaternary carbons at 142.2 (C-2) and 133.2 (C-6). Two sets of methyl protons at 1.04 (H-14; δC 24.9) and 1.06 (H-15; δC 29.9) revealed common 2J correlation to a quaternary carbon at 37.3 (C-9) and 3J interaction to methine carbon at 139.5 (C-10, δH 5.23) and methylene at 42.4 (C-8, δH 1.87, 2.32). H-10 also revealed 3J interaction to both methyl group carbons at 24.9 (C-14) and 29.9 (C-15). The COSY experiment exhibited usual interaction (H-10 to H-11; H-4 to both H-3 and H-5; H-7 to H-8). Accordingly, compound 2 was identified as (2Z,6Z,10E)-2,6,9,9-tetramethylcycloundeca-2,6,10-trien-1-ol, commonly known as zerumbol (2) [24]. The NMR spectra of compound 2 are available in Supplementary Material. Compounds 3–8 were identified as zerumbone (3) [23,25], buddledone A (4) [26], germacrone (5) [23,27], furanodienone (6) [28], borneol (7) [29], and camphor (8) [29]. Among these compounds, zerumbol (2), buddledone A (4), germacrone (5), and furanodienone (6) have been reported first time from the genus Zingiber, while zerumbone (3) was reported from Z. zerumbet [30]. Chemical structures of compounds 1–8 are incorporated in Figure 1.
Table 3.
1H- (600 MHz) and 13C- (150 MHz) NMR and HMBC (500 MHz) data of 2 in CDCl3.
| Position | 1H | 13C | HMBC | |
|---|---|---|---|---|
| 2 J | 3 J | |||
| 1 | 4.63, d, J = 7.5 Hz, 1H | 78.8 | - | C3, C10, C12 |
| 2 | - | 142.2 | - | - |
| 3 | 5.20, d, J = 7.5 Hz, 1H | 124.8 | - | C1, C12 |
| 4 | 2.20, m, 1H; 2.24, m, 1H | 24.4 | C3 | C6 |
| 5 | 2.35, m, 2H | 39.5 | - | C3, C13 |
| 6 | - | 133.2 | - | - |
| 7 | 4.82, dd, J = 10.2, 4.4 Hz, 1H | 125 | - | C5, C9 |
| 8 | 1.87, m, 1H; 2.32, m, 1H | 42.4 | C7 | C6, C10 |
| 9 | - | 37.3 | - | - |
| 10 | 5.23, d, J = 16.2 Hz, 1H | 139.5 | - | C1, C14, C15 |
| 11 | 5.56, dd, J = 16.2, 7.5 Hz, 1H | 131.2 | C1 | C9, C13 |
| 12 | 1.65, s, 3H | 12.8 | C2 | C1, C3 |
| 13 | 1.43, s, 3H | 15.3 | C6 | C5, C7 |
| 14 | 1.04, s, 3H | 24.9 | C9 | C8, C10, C15 |
| 15 | 1.06, s, 3H | 29.9 | C9 | C8, C10, C14 |
Figure 1.
Chemical structures of terpenes isolated from Z. montanum.
Compounds 1–8 were assessed for their antibacterial activities against multi-drug resistant and methicillin resistant Staphylococcus aureus, notably SA1199B, XU212, RN4229, EMRSA15, MRSA27819, and MRSA340702. The minimum inhibitory concentrations (MICs) of these compounds are presented in Table 4. Norfloxacin was used as positive control for the comparison of antibacterial potencial of these compounds. Among these compounds, 1 and 2 displayed the highest activities with MICs in the range of 32–128 µg/mL (0.145–0.291 mM) against the test organisms. Compound 1 is a labdane diterpene with exomethylene at C-8, an olefine at C-12, and two aldehyde groups at C-16 and 17. The presence of these groups and unsaturations could account for significant antibacterial activity against MRSA strains. Although compounds 2 and 3 are structurally very similar, they differ in activity. We suggest that the presence of a hydroxyl group instead of carbonyl group at C-1 might make compound 2 more active than compound 3. The antibacterial activity of compounds 3–8 were above 128 µg/mL (0.557–0.842 mM), the highest concentrations at which the compounds were tested. Monoterpene and sesquiterpenes from Zingiber were reported with antibacterial activity against Staphylococcus aureus (MTCC 96) and Staphylococcus epidermidis (MTCC 435) [21].
Table 4.
Minimum inhibitory concentrations (MICs) (in mM) of compounds (1–8) against standard, multi-drug resistant (MDR) and methicillin-resistant strains of Staphylococcus aureus.
| Compound | SA1199B | XU212 | ATCC25941 | RN4220 | EMRSA15 | MRSA27819 | MRSA340702 |
|---|---|---|---|---|---|---|---|
| 1 | 0.212 | 0.424 | 0.212 | 0.212 | 0.212 | 0.424 | 0.424 |
| 2 | 0.291 | 0.582 | 0.582 | 0.582 | 0.145-0.291 | 0.582 | >0.582 |
| 3 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 |
| 4 | >0.582 | >0.582 | >0.582 | >0.582 | >0.582 | >0.582 | >0.582 |
| 5 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 |
| 6 | >0.557 | >0.557 | >0.557 | >0.557 | >0.557 | >0.557 | >0.557 |
| 7 | >0.831 | >0.831 | >0.831 | >0.831 | >0.831 | >0.831 | >0.831 |
| 8 | >0.842 | >0.842 | >0.842 | >0.842 | >0.842 | >0.842 | >0.842 |
| Norfloxacin | 0.100 | 0.200 | 0.050 | 0.025 | 0.050 | 0.100 | 0.401 |
3. Material and Methods
3.1. General
Analytical solvents, such as n-hexane, chloroform, ethyl acetate, acetone, and methanol were purchased from Fisher Scientific, Loughborough, UK. Dimethyl sulfoxide, sodium chloride, norfloxacin and 3-[4,5-dimethylthiazol-2-yl]-2,5-iphenyltetrazolium bromide (MTT) used during antibacterial assay were purchased from Sigma Aldrich (Dorset, UK). Silica gel 60H used for vacuum liquid chromatography was purchased from Merck Millipore, UK. Sephadex LH 20 used for gel filtration chromatography was purchased from GE healthcare, Uppasala, Sweden. Prepacked silica column (normal phase) used for solid phase extraction (SPE) was purchased from Phenomenex, Cheshire, UK. Analytical and preparative TLC carried out on 0.2 mm silica gel 60 F254 was purchased from Merck, Darmstadt, Germany. Spots on the TLC plates were visualized under short UV (254 nm) and long UV (366 nm), and also by spraying them with 1% vanillin in concentrated H2SO4 followed by heating at 100 °C for 3–6 min. The NMR spectroscopy was performed with Bruker AMX 600 NMR spectrometer, Coventry, UK (600 MHz for 1H, and 150 MHz for 13C) in the Department of Chemistry at University College London (London, UK). High Resolution Mass Spectrometry (HRMS) was performed in Liverpool John Moores University (Liverpool, UK). IR spectroscopy was recorded on Agilent FT-IR (Cary 630, Stockport, UK).
3.2. Plant Material
The rhizomes of Zingiber montanum were collected from Bangladesh National Botanical Garden, Dhaka, Bangladesh in September 2016. The plant was identified by the Bangladesh National Herbarium, Mirpur, Dhaka, Bangladesh, where a voucher specimen (DACB 43550) of this collection was deposited.
3.3. Extraction and Isolation of Compounds
The rhizomes of Z. montanum were sun dried for 2–3 days, followed by drying in the oven at a temperature of 30–35 °C for 30 min prior to grinding. Subsequently, the plant materials were ground into fine powders using a grinder. The ground plant material (242 g) was Soxhlet extracted with solvents of increasing polarity: n-hexane, chloroform, and methanol (approximately 700 mL, 10–15 cycles each). Each of the extracts was concentrated using rotary evaporator under reduced pressure at a maximum temperature of 40 °C to yield 9.38 g, 10.22 g, and 23.0 g of n-hexane, chloroform, and methanol extracts, respectively. The antibacterial screening was performed on these crude extracts against clinical isolates of MRSA strains. Hexane (MICs 128–256 µg/mL) and chloroform (MICs 64–256 µg/mL) extracts appeared to be active and they were further fractionated by vacuum liquid chromatography (VLC). A portion of n-hexane (6.5 g) or chloroform (7.6 g) were adsorbed into silica gel (70–230 mesh) and loaded into VLC column, which was uniformly packed with VLC grade silica gel (60H), followed by eluting with stepwise gradient of mobile phase initially with mixture of n-hexane and ethyl acetate and then with EtOAc and MeOH mixtures. The eluted fractions (200 mL each) were evaporated using rotary evaporator and analysed by TLC. Based on TLC results, the similar fractions were bulked together for further purifications by solid phase extraction (SPE), column chromatography over Sephadex LH20, and/or preparative TLC. The basic principle of SPE is similar to VLC, but SPE was used in smaller scale fractionation or further purification of compounds from the VLC fractions or pooled fractions from Sephadex LH20 column chromatography. For column chromatography over Sephadex LH20, the glass column was packed with the slurry of Sephadex LH-20, which was soaked in the solvent (50% chloroform in n-hexane or 100% chloroform) half an hour prior to the packing of the column. The sample was dissolved in a small amount of appropriate solvent and then applied onto the top of the adsorbent. The column was eluted with 50–75% chloroform in n-hexane, followed by 100% chloroform and then CHCl3 + MeOH mixtures of increasing polarity. During preparative TLC, the sample was applied uniformly as band in the sample application zone (2 cm above from the bottom edge of TLC plate) on commercially available TLC aluminium plates (pre-coated silica gel 60 PF254). The TLC plates were developed with appropriate mobile phase up to the upper edge of plates. In addition, the multiple development technique was also adapted for a better accomplishment of separation of compounds of very similar polarity.
VLC fraction eluted with 5–10% of EtOAc in n-hexane of n-hexane extract was subjected to column chromatography over Sephadex LH20. PTLC (mobile phase 15% EtOAc in hexane) on Sephadex column eluted with 50% CHCl3 in n-hexane yielded compounds 2 (3 mg) and 3 (10 mg), whereas compound 4 (9 mg) was obtained from sephadex column eluted with 100% chloroform. SPE on the VLC fraction eluted with 15% of EtOAc in n-hexane of n-hexane extract provided six sub-fractions. Preparative TLC (mobile phase 4% EtOAc in hexane plus two drops glacial acetic acid) on SPE sub-fraction eluted with 4% EtOAc in hexane yielded compounds 5 (7 mg) and 6 (4 mg), whilst preparative TLC (mobile phase 4% EtOAc in hexane plus two drops glacial acetic acid) on SPE sub-fraction eluted with 4% EtOAc in hexane led to the isolation of compounds 6 (5 mg) and 7 (7 mg). Similarly, VLC fraction of chloroform extract was subjected to SPE and preparative TLC for the purification of compounds. SPE on VLC fraction eluted with 15% EtOAc in n-hexane followed PTLC (mobile phase 4% EtOAc in hexane) on SPE sub-fraction eluted with 4% EtOAc in hexane yielded 8 (4 mg), while compound 1 (6.5 mg) was isolated from the VLC fraction eluted with 25% EtOAc in n-hexane, followed by PTLC (mobile phase 15% EtOAc in hexane) on SPE sub-fraction eluted with 10% EtOAc in hexane.
3.4. Antibacterial Assay against Clinical Isolates of Multi-Drug Resistant and Methicillin Resistant Staphylococcus Aureus
The antibacterial activity of crude extracts and the isolated compounds were tested against clinical isolates of MRSA strains by microtitre assay using 96 well plates to determine the minimum inhibitory concentrations (MICs). Mueller–Hinton broth (MHB) used in this study was purchased from Oxoid, Hamshire, UK and prepared as instructed by the supplier; however, MHB was adjusted to contain 20 mg/L and 10 mg/L of Ca2+ and Mg2+, respectively. The clinical isolates of S. aureus strains used in this study included ATCC25923, SA1199B, RN4220, XU212, EMRSA15, MRSA340702, and MRSA274829. A standard laboratory strain, ATCC25923, was also used in this study, which is sensitive to antibiotics, like tetracycline [31]. SA1199B over-expresses the NorA MDR efflux pump [32], RN4220 possesses the MsrA macrolide efflux protein [33], XU212 is a Kuwaiti hospital isolate that is an MRSA strain possessing the TetK tetracycline efflux pump [31], whilst the EMRSA15 strain [34] is epidemic in the UK. All S. aureus strains were subcultured on nutrient agar (Oxoid) and incubated for approximately 24 h at 37 °C prior to MIC determination. All of the bacterial strains were prepared in 9 g/L saline water with an inoculum density of 5 × 105 colony forming unit (cfu/mL) by comparison with the 0.5 MacFarland turbidity standard.
The stock solution of control positive (Norfloxacin) was prepared by dissolving the antibiotic (2.0 mg) in DMSO (244 µL) and diluting 16 fold with MHB to obtain the desired concentration of the antibiotic stock solution (512 µg/mL). Similarly, stock solutions of crude extract (2048 µg/mL) and isolated compounds (256–512 µg/mL) were prepared by dissolving in required amount of DMSO, followed by 16 fold dilution with MHB.
During the experiment, using a multi-channel pipette an aliquot of 100 µL of MHB was dispensed into each well of 96-well plate except those in the last column. Then 100 µL of stock solution of crude extract or isolated compounds and antibiotic was added in duplicate to the wells of the first column of 96-well plate (total content 200 µL), followed by mixing the content thoroughly and transferring 100 µL of this content to the wells of the second column of 96-well plate using a multi-channel pipette. This two-fold serial dilution process was continued to the 10th well, followed by the addition of the final 100 µL solution to the empty wells of 12th column of 96 well-plate. The inoculum (100 µL) of each bacterium at a density of 5 × 105 cfu/mL was added to all wells, except those in the final (12th) column. The contents of the wells in the 11th and 12th columns represented growth control (bacteria, but no antibiotic, extract, or compounds) and sterility control (antibiotic, extract, or compounds but no bacteria), respectively. Every assay was performed in duplicate. The plates were incubated for 18 h at 37 °C. For the measurement of MIC, 20 µL of a 5 mg/mL methanolic solution of 3-[4,5-dimethylthiazol-2-yl]-2,5-iphenyltetrazolium bromide (MTT; Sigma) was added to each of the wells, followed by incubation for 20–30 min at 37 °C. Bacterial growth was indicated by a colour change from yellow (colour of MTT) to dark blue. The MIC was recorded as the lowest concentration at which no growth (yellow color) was observed [13]. If no growth was observed at any of the concentrations tested, the assay was repeated starting from a stock solution of lower concentration. If growth was observed at all of the concentrations tested, the assay was repeated, starting with a stock solution of higher concentration.
4. Conclusions
In this study, the crude extracts of the rhizomes of Z. montanum and compounds that were isolated from active extracts were assessed against a panel of clinical isolates of multi-drug resistant (MDR) and methicillin resistant Staphylococcus aureus (MRSA), including SA1199B, XU212, RM4221, EMRSA15, MRSA27819, and MRSA340702. Bioassay directed isolation using a range of chromatographic techniques, including vacuum liquid chromatography (VLC), solid phase extraction (SPE), column chromatography over Sephadex LH20, and preparative thin layer chromatography (PTLC) led to the identification of two monoterpenes, borneol (7) and camphor (8), and five sesquiterpnes, zerumbol (2), zerumbone (3), buddledone A (4), furanodienone (5), germacrone (6), and a diterpene, (E)-8(17),12-labdadiene-15,16-dial (1). Among these terpenes, compounds 1 and 2 displayed significant activity with MICs of 32–128 µg/mL (0.145–0.291 mM) against the clinical isolates of MRSA strains tested. Such activity encourages the authors to carry out bioassay guided phytochemical investigation on related members of Zingiberacae family for the identification of lead anti-Staphylococcal compounds.
Acknowledgments
Holly Siddique is grateful to the School of Health, Sport and Bioscience at the University of East London for the award of PhD studentship. The authors sincerely thank to Simon Gibbons at the UCL School of Pharmacy for providing the MRSA strains for antibacterial assay and Satyajit D Sarker at Liverpool John Moores University for providing mass spectrometry data of compounds.
Supplementary Materials
NMR and mass spectra of active compounds together with results of antibacterial activity using 96 well plates are available in supplementary materials.
Author Contributions
H.S. as part of her PhD project collected and extracted the plant, isolated and identified the compounds, carried out antibacterial assay and prepared the initial draft manuscript. M.M.R. and B.P. supervised the various aspects of the project, helped with the interpretation of data and contributed towards the preparation of the manuscript. M.M.R. as the Director of Study and Corresponding Author also prepared and submitted the final version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–8 are available from the authors.
References
- 1.O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance. [(accessed on 15 February 2018)];2016 Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- 2.World Health Organization (WHO) Antibiotic Resistance. [(accessed on 18 November 2018)];2018 Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance.
- 3.Faridi A., Kareshk A.T., Fatahi-Bafghi M., Ziasistani M., Ghahraman M.R.K., Seyyed-Yousefi S.Z., Shakeri N., Kalantar-Neyestanaki D. Detection of methicillin-resistant Staphylococcus aureus (MRSA) in clinical samples of patients with external ocular infection. Iran. J. Microbiol. 2018;10:215–219. [PMC free article] [PubMed] [Google Scholar]
- 4.Hollman A. Digoxin comes from Digitalis lanata. Br. Med. J. 1996;312:912. doi: 10.1136/bmj.312.7035.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alam M.M., Naeem M., Khan M.M.A., Uddin M. Catharanthus Roseus. Springer; Cham, Switzerland: 2017. Vincristine and Vinblastine Anticancer Catharanthus Alkaloids: Pharmacological Applications and Strategies for Yield Improvement; pp. 277–307. [Google Scholar]
- 6.Patrick G. An Introduction to Medicinal Chemistry. 5th ed. Oxford University Press; Oxford, UK: 2013. The Opioid Analgesic; p. 632. [Google Scholar]
- 7.Butler A., Hensman T. Drugs for the fever. Educ. Chem. 2000;37:151. [Google Scholar]
- 8.Mahdi J.G. Medicinal potential of willow: A chemical perspective of aspirin discovery. J. Saudi Chem. Soc. 2010;14:317–322. doi: 10.1016/j.jscs.2010.04.010. [DOI] [Google Scholar]
- 9.Gautam R., Saklani A., Jachak S.M. Indian medicinal plants as a source of antimycobacterial agents. J. Ethnopharmacol. 2007;110:200–234. doi: 10.1016/j.jep.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z., Yu P., Zhang G., Xu L., Wang D., Wang L., Zeng X., Wang Y. Design, synthesis and antibacterial activity of novel andrographolide derivatives. Bioorg. Med. Chem. 2010;18:4269–4274. doi: 10.1016/j.bmc.2010.04.094. [DOI] [PubMed] [Google Scholar]
- 11.Oluwatuyi M., Kaatz G.W., Gibbons S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry. 2004;65:3249–3254. doi: 10.1016/j.phytochem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Schempp C.M., Pelz K., Wittmer A., Schöpf E., Simon J.C. Antibacterial activity of hyperforin from St John’s wort against multi-resistant Staphylococcus aureus and Gram-positive bacteria. Lancet. 1999;353:2129. doi: 10.1016/S0140-6736(99)00214-7. [DOI] [PubMed] [Google Scholar]
- 13.Shiu W.K.P., Rahman M.M., Curry J., Stapleton P.D., Zloh M., Malkinson J.P., Gibbons S. Antibacterial acylphloroglucinols from Hypericum olympicum. J. Nat. Prod. 2012;75:336–343. doi: 10.1021/np2003319. [DOI] [PubMed] [Google Scholar]
- 14.Khare C.P. Indian Medicinal Plants: An Illustrated Dictionary. Springer; Berlin/Heidelberg, Germany: 2007. p. 733. [Google Scholar]
- 15.Farnsworth N.R., Bunyapraphatsara N. Thai Medicinal Plants: Recommended for Primary Health Care System. Medicinal Plants Information Center; Bangkok, Thailand: 1992. [Google Scholar]
- 16.Singh C.B., Manglembi N., Swapana N., Chanu S.B. Ethnobotany, phytochemistry and pharmacology of Zingiber cassumunar Roxb. (Zingiberaceae) J. Pharmacogn. Phytochem. 2015;4:1–6. [Google Scholar]
- 17.Sharma G.J., Thokchom D.S. Antioxidant and radioprotective properties of Zingiber montanum (J. König) A. Dietr. Planta Med. 2011;77:127. doi: 10.1055/s-0031-1273656. [DOI] [Google Scholar]
- 18.Thokchom D.S., Sharma T.D., Sharma G.J. Radioprotective effect of rhizome extract of Zingiber montanum in Rattus norvegicus. Radiat. Environ. Biophys. 2012;51:311–318. doi: 10.1007/s00411-012-0425-x. [DOI] [PubMed] [Google Scholar]
- 19.Al-Amin M., Sultana G.N., Hossain C.F. Antiulcer principle from Zingiber montanum. J. Ethnopharmacol. 2012;141:57–60. doi: 10.1016/j.jep.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 20.Masuda T., Jitoe A., Mabry M.J. Isolation and structure determination of cassumunarins A, B and C: New anti-inflammatory antioxidants from a tropical ginger, Zingiber cassumunar. J. Am. Oil Chem. Soc. 1995;72:1053–1057. doi: 10.1007/BF02660721. [DOI] [Google Scholar]
- 21.Verma R.S., Joshi N., Padalia R.C., Singh V.R., Goswami P., Verma S.K., Iqbal H., Chanda D., Verma R.K., Darokar M.P., et al. Chemical composition and antibacterial, antifungal, allelopathic and acetylcholinesterase inhibitory activities of cassumunar-ginger. J. Sci. Food Agric. 2018;98:321–327. doi: 10.1002/jsfa.8474. [DOI] [PubMed] [Google Scholar]
- 22.Sy K.L., Brown D.G. Labdane diterpenoids from Alpinia chinensis. J. Nat. Prod. 1997;60:904–908. doi: 10.1021/np970243d. [DOI] [Google Scholar]
- 23.Firman K., Kinoshita T., Itai A., Sankawa U. Terpenoids from Curcuma Heyneana. Phytochemistry. 1988;27:3887–3891. doi: 10.1016/0031-9422(88)83038-3. [DOI] [Google Scholar]
- 24.Takashi K., Nagao R., Masuda T., Hill R.K., Morita M., Takatani M., Sawada S., Okamoto T. The chemistry of Zerumbone IV: Asymmetric synthesis of Zerumbol. J. Mol. Catal. B Enzym. 2002;17:75–79. [Google Scholar]
- 25.Nathaniel C., Elaine-Lee Y.L., Yee B.C., How C.W., Yim H.S., Rasadee A., Ng H.S. Zerumbone-loaded nanostructured lipid carrier induces apoptosis in human colorectal adenocarcinoma (Caco-2) cell line. Nanosci. Nanotechnol. Lett. 2016;8:294–302. doi: 10.1166/nnl.2016.2136. [DOI] [Google Scholar]
- 26.Cai Z., Yongpruksa N., Harmata M. Total synthesis of the terpenoid buddledone A: 11-membered ring-closing metathesis. Org. Lett. 2012;14:1661–1663. doi: 10.1021/ol300400x. [DOI] [PubMed] [Google Scholar]
- 27.Simova S.D., Bozhkova N.V., Orahovats A.S. 1H and 13C NMR studies of some germacrones and isogermacrones. Org. Magn. Reson. 1984;22:431–433. doi: 10.1002/mrc.1270220707. [DOI] [Google Scholar]
- 28.Brieskorn C.H., Noble P. Furanosesquiterpenes from the essential oil of myrrh. Phytochemistry. 1983;22:1207–1211. doi: 10.1016/0031-9422(83)80223-4. [DOI] [Google Scholar]
- 29.Uchio Y. Constituents of the essential oil of Chrysanthemum japonense. Nojigiku alcohol and its acetate. Bull. Chem. Soc. Jpn. 1978;51:2342–2346. doi: 10.1246/bcsj.51.2342. [DOI] [Google Scholar]
- 30.Da Silva T.M., Pinheiro C.D., Orlandi P.P., Pinheiro C.C., Sontes G.S. Zerumbone from Zingiber zerumbet (L.) smith: A potential prophylactic and therapeutic agent against the cariogenic bacterium Streptococcus mutans. BMC Complement. Altern. Med. 2018;18:301. doi: 10.1186/s12906-018-2360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibbons S., Udo E.E. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother. Res. 2000;14:139–140. doi: 10.1002/(SICI)1099-1573(200003)14:2<139::AID-PTR608>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Kaatz G.W., Seo S.M., Ruble C.A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1993;37:1086–1094. doi: 10.1128/AAC.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross J.L., Farrell A.M., Eady E.A., Cove J.H., Cunliffe W.J.J. Characterisation and molecular cloning of the novel macrolide-streptogramin B resistance determinant from Staphylococcus epidermidis. Antimicrob. Agents Chemother. 1989;24:851–862. doi: 10.1093/jac/24.6.851. [DOI] [PubMed] [Google Scholar]
- 34.Richardson J.F., Reith S. Characterization of a strain of methicillin-resistant Staphylococcus aureus (EMRSA-15) by conventional and molecular methods. J. Hosp. Infect. 1993;25:45–52. doi: 10.1016/0195-6701(93)90007-M. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.