Abstract
Variants in PTCH2 have been described to be associated with Nevoid Basal Cell Carcinoma Syndrome (NBCCS). We report a family with a healthy female who is homozygous for a frameshift variant, c.269delG, p.(Gly90Alafs*4), in PTCH2 and her heterozygous daughter. The variant predicts a frameshift and a premature stop codon. A summary of reported heterozygous individuals with germline PTCH2 variants along with the existence of a healthy homozygous individual question whether variants in PTCH2 are associated with NBCCS.
Subject terms: Cancer genomics, Risk factors
The Patched 2 (PTCH2) gene and its close homolog, PTCH1, both encode transmembrane receptors of the patched gene family in the sonic hedgehog pathway1. Variants in the PTCH1 gene have been found to cause nevoid basal cell carcinoma syndrome (NBCCS), also known as Gorlin syndrome, with typical clinical manifestations of multiple basal cell carcinomas, odontogenic keratocysts of the jaw, palmar and plantar pits, intracranial ectopic calcification, facial dysmorphism (macrocephaly, cleft lip/palate), and eye anomalies (cataract, pigmentary changes of the retinal epithelium and developmental defects)2,3. According to the NBCCS criteria of Evans et al., at least two major and one minor criteria or one major and three minor criteria must be fulfilled for a patient to be diagnosed with NBCCS2,3. Autosomal dominantly inherited NBCCS is transmitted with complete penetrance and variable expressivity4,5. Large epidemiological studies of NBCCS report different ethnicity-dependent penetrances of NBCCS: the most significant is the lower prevalence of basal cell carcinoma in Japanese NBCCS patients over 20 years of age (51.4%) compared to American NBCCS patients (91%), Australian NBCCS patients (85%), and British NBCCS patients (73%)2,6–8. It has been suggested that PTCH2 variants can also cause NBCCS, albeit with a milder phenotype9.
PTCH1 plays an important role in the sonic hedgehog pathway, which is a regulator of patterning and development in the embryo and adult. In brief, the pathway comprises the sonic hedgehog ligand (Shh), PTCH1/PTCH2 transmembrane receptors and the G protein-coupled receptor Smoothened (Smo). The best-studied member of the patched gene family, the PTCH1 receptor, suppresses the release of Smo, but when Shh binds to the PTCH1 receptor, this inhibition is alleviated, resulting in Smo activation and translocation from the plasma membrane to the primary cilium. While PTCH1 is the primary Shh receptor, PTCH2 has a minor compensatory role in Shh signal transduction, although its function is yet to be fully understood10. Deficient PTCH1 and PTCH2 receptors decrease the inhibition of Smo, which leads to increased activity of transcription factors11.
We here present a healthy female homozygous for a PTCH2 frameshift variant, and we therefore question the association between PTCH2 variants and NBCCS.
The clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. Both patients gave oral and written informed consent for publication. Since the investigations were performed as part of a diagnostic workup, we did not apply for ethics approval.
Patient 1: An 18-year-old woman of Middle-Eastern descent with consanguineous parents (1st cousins once removed) was diagnosed with severe hypermobile Ehlers-Danlos syndrome (EDS), congenital scoliosis, epilepsy, and asthma at the age of 2 years (Fig. 1a). During the following years, as a likely consequence of EDS, she underwent three umbilical hernia operations. At 14 years old, she was diagnosed with sleep apnea and began using autoCPAP as treatment during the nights. Additionally, she suffered from joint instability, which resulted in nearly chronic subluxations of the bilateral carpometacarpal joints. She was using orthotics and a walker to maintain balance and stability while walking. Furthermore, the patient was treated for chronic rhinosinusitis, weakened mastication, and difficulty in swallowing. Lastly, the patient had also been affected by migraine and fluctuations of eye accommodation and refraction. To our knowledge, she did not have any of the symptoms included in the diagnostic criteria for NBCCS. Whole-exome sequencing was performed on blood from the patient.
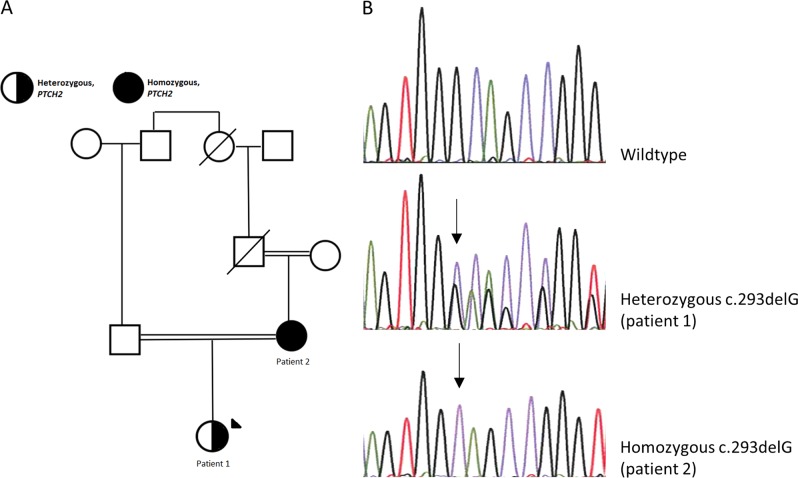
Fig. 1. Pedigree and electropherogram of the family with a PTCH2 variant.
a Pedigree of the family with a PTCH2 variant. b Electropherogram of the PTCH2 c.269delG variant showing wild-type, heterozygous (patient 1) and homozygous (patient 2) individuals
Patient 2: The 39-year-old mother of patient 1 had been diagnosed with hypermobility syndrome at the age of 34 years. Furthermore, she had been affected with recurrent respiratory infections with hemoptysis for several years in her early thirties. She reported no NBCSS manifestations and was further examined for three major NBCCS criteria. Skin examination by a dermatologist revealed no basal cell carcinomas or palmar or plantar pits, and a dental CT scan showed no cysts of the jaw. We refrained from exposing the patient to unnecessary radiation, and hence she was not examined for the fourth major criterion, calcification of the falx cerebri. She did not have first-degree relatives affected with NBCCS, which is the fifth major criterion. To our knowledge, she did not fulfill any of the minor criteria for NBCCS.
Variant analysis of whole-exome sequencing data showed no pathogenic variants explaining the severe Ehlers Danlos syndrome in patient 1. However, as an incidental finding, a heterozygous variant, c.269delG, p.(Gly90Alafs*4), was identified in exon 3 of PTCH2, leading to a frameshift and a premature stop codon. Sanger sequencing of DNA from her mother showed homozygosity for the c.269delG, p.(Gly90Alafs*4) variant (Fig. 1b).
To our knowledge, the PTCH2 variant has not been described previously, and it is not reported in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) or the Genome Aggregation Database (gnomAD)(http://gnomad.broadinstitute.org), which comprises more than 245,000 alleles in this region.
Patient 2 with the homozygous PTCH2 variant had worn traditional ethnic clothing covering the arms, legs, and hair from a young age, and she had darkly pigmented skin. Only her face and hands were exposed to ultraviolet radiation, and hence the risk of developing basal cell carcinomas could be considered low. However, ~80% of reported basal cell carcinomas are located in the face, head, and neck, and her risk of basal cell carcinoma is therefore not negligible12.
It is notable that patient 1 is affected with scoliosis, as 40% of NBCCS cases are reported to have scoliosis13, but this is not significant as scoliosis can occur as part of many syndromic diseases.
Previously, three papers have described eight individuals, including six belonging to one family, with heterozygous PTCH2 variants (Table 1). Six individuals had palmar or plantar pits, three individuals had keratocysts of the jaw, two had bifid ribs, and one individual had multiple basal cell carcinomas. A newborn had embryonal rhabdomyosarcoma14,15. None of the 10 PTCH2 individuals listed in Table 1 fulfilled the diagnostic criteria for NBCCS proposed by Evans et al.3, whereas using the diagnostic criteria proposed by Kimonis et al., 7 of the 10 individuals with PTCH2 variants would be diagnosed with NBCCS, as these criteria demand only that two major criteria be fulfilled7.
Table 1.
Summary of PTCH2 literature reports
| Reference | Present report (patient 2) | Present report (patient 1) | Taeubner (16) | Fujii9 | Fan14 | Fan | Fan | Fan | Fan | Fan |
|---|---|---|---|---|---|---|---|---|---|---|
| PTCH2 variant | c.269delG (homozygous) | c.269delG (heterozygous) | c.1864C>T (rs11573586) (heterozygous)a | c.1172_1173delCT (rs777588680) (heterozygous) | c.2157G>A (rs121434397) (heterozygous) | c.2157G>A (heterozygous) | c.2157G>A (heterozygous) | c.2157G>A (heterozygous) | c.2157G>A (heterozygous) | c.2157G>A (heterozygous) |
| Variant type | Frameshift | Frameshift | Missense (p.(His622Tyr)) | Frameshift (p.(Ser(391Ter)) | Missense (p.(Arg719Gln)) | Missense | Missense | Missense | Missense | Missense |
| In silico prediction of pathogenicity | ||||||||||
| SIFT | Deleterious (0.01) | Tolerated (0.19) | ||||||||
| MutationTaster | Probably damaging (0.978) | Probably damaging (0.999) | ||||||||
| Polyphen2 | Benign (0.10) | Benign (0.33) | ||||||||
| CADD score | 1.9 | 3.6 | ||||||||
| Allele frequency in gnomAD | – | – | 2683/276,572 alleles (26 homozygous) | 65/277,190 alleles (1 homozygous) | 8/276,184 alleles (0 homozygous) | |||||
| Sex | F | F | M | F | M | F | F | F | M | F |
| Age (years) | 39 | 18 | 0 | 13 | 45 | 57 | 42 | 40 | 17 | 25 |
| Ethnicity | Middle Eastern | Middle Eastern | Turkish | Japanese | Chinese | Chinese | Chinese | Chinese | Chinese | Chinese |
| Major NBCCS criteria | ||||||||||
| Basal cell carcinoma | No | – | No | No | No | Yes (multiple) | No | No | No | No |
| Keratocysts of the jaw | No | – | No | Yes | No | No | No | No | No | Yes |
| Palmar or plantar pits | No | – | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Ectopic calcification | – | – | No | No | No | No | No | No | No | No |
| First-degree relative with NBCCS | No | No | No | No | No | No | No | No | No | No |
| Number of major criteria fulfilled | 0 | 0 | – | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Number of minor criteria fulfilled | 0 | 0 | – | 1 (bifid ribs) | 0 | 0 | 0 | 0 | 1 (bifid ribs) | 0 |
| Other | No | No | Embryonal rhabdomyosarcoma | No | No | No | No | No | Yes | No |
| NBCCS (Evans criteria) | No | No | – | No | No | No | No | No | No | No |
| NBCCS (Kimonis criteria)b | No | No | – | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
aThis individual also carries a PTCH1 variant.
bAs opposed to the criteria of Evans et al., Kimonis et al. define bifid ribs as a major criterion and require only two major criteria or one major and two minor criteria for a patient to be diagnosed with NBCCS.
The reported PTCH2 variants (Table 1) included one 2-bp deletion resulting in a frameshift and two missense variants. One of the missense variants, c.1864C>T, p.(His622Tyr), was found together with a PTCH1 variant in a newborn with rhabdomyosarcoma, for whom no follow-up was reported. This PTCH2 missense variant is very frequent in gnomAD, with 26 homozygous individuals reported, and in silico programs give a low probability of the variant being pathogenic. The other missense variant (c.2156G>A, p.(Arg719Gln)) was reported in six members of one family, of whom none had NBCCS according to the Evans criteria but all fulfilled the Kimonis diagnostic criteria. The variant was reported in eight individuals in gnomAD, and in silico predictions do not support its pathogenicity. The frameshift variant, c.1172_1173delCT, was found in 65 alleles, including one homozygous individual.
A variety of PTCH1 variants cause NBCCS, including missense, nonsense, frameshift, and splice site variants. The gnomAD database reports 27 different loss of function (LOF) variants in PTCH1 (transcript size 8057 bp) and 85 LOF variants in PTCH2 (transcript size 4298 bp), of which one was reported as homozygous (/mentary table S1). Assuming the variants are not on the same alleles, these LOF variants are found in 59 individuals in PTCH1 and in 480 individuals in PTCH2; hence, LOF variants seem to be well-tolerated in PTCH2.
Analyses of knockout (KO) mice revealed that PTCH1 −/− mutants were lethal at embryonic day 9.5 due to hyperactivation of Shh signaling. By contrast, PTCH2 −/− mutants were viable, fertile and apparently normal; however, alopecia, epidermal hyperplasia, dermal hyperplasia, hair follicle loss, and ulceration were frequently observed with progressing age in PTCH2 −/− mutant male mice, whereas PTCH2 −/− mutant female mice did not show any abnormalities. Neither male nor female PTCH2 double KO mice developed basal cell carcinomas10,16
Homozygosity for a frameshift variant in the PTCH2 gene does not seem to have caused any NBCCS manifestations in the patient reported here, and KO mice do not display any NBCCS manifestations. Furthermore, none of the previously reported PTCH2 individuals have been diagnosed with NBCCS according to one of the proposed sets of diagnostic criteria3. Finally, loss of function variants in PTCH2 are frequent in population studies.
These observations question whether variants in PTCH2 are associated with NBCCS; if, however, it is the case, the penetrance could be considered very low. Further studies of larger cohorts of individuals with PTCH2 variants should clarify this.
Supplementary information
Acknowledgements
We thank the family for their participation. The study was supported by grant number A180-A11311-17-S7 from the Danish Cancer Society.
HGV database
The relevant data from this Data Report are hosted at the Human Genome Variation Database at 10.6084/m9.figshare.hgv.2534
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information is available for this paper at 10.1038/s41439-019-0041-2.
References
- 1.Smyth I, et al. Isolation and characterization of human patched 2 (PTCH2), a putative tumour suppressor gene inbasal cell carcinoma and medulloblastoma on chromosome 1p32. Hum. Mol. Genet. 1999;8:291–7. doi: 10.1093/hmg/8.2.291. [DOI] [PubMed] [Google Scholar]
- 2.Evans DG, et al. Complications of the naevoid basal cell carcinoma syndrome: results of a population based study. J. Med. Genet. 1993;30:460–4. doi: 10.1136/jmg.30.6.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans D. G., Farndon P. A. Nevoid Basal Cell Carcinoma Syndrome. (eds Adam M. P., Ardinger H. H., Pagon R. A., Wallace S. E., Bean L. J. H., Stephens K.) (GeneReviews((R)), Seattle (WA), 1993). [PubMed]
- 4.Bare JW, Lebo RV, Epstein EH., Jr. Loss of heterozygosity at chromosome 1q22 in basal cell carcinomas and exclusion of the basal cell nevus syndrome gene from this site. Cancer Res. 1992;52:1494–8. [PubMed] [Google Scholar]
- 5.Bialer MG, Gailani MR, McLaughlin JA, Petrikovsky B, Bale AE. Prenatal diagnosis of Gorlin syndrome. Lancet. 1994;344:477. doi: 10.1016/S0140-6736(94)91810-4. [DOI] [PubMed] [Google Scholar]
- 6.Endo M, et al. Nationwide survey of nevoid basal cell carcinoma syndrome in Japan revealing the low frequency of basal cell carcinoma. Am. J. Med. Genet. A. 2012;158A:351–7. doi: 10.1002/ajmg.a.34421. [DOI] [PubMed] [Google Scholar]
- 7.Kimonis VE, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am. J. Med. Genet. 1997;69:299–308. doi: 10.1002/(SICI)1096-8628(19970331)69:3<299::AID-AJMG16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 8.Shanley S, et al. Nevoid basal cell carcinoma syndrome: review of 118 affected individuals. Am. J. Med. Genet. 1994;50:282–90. doi: 10.1002/ajmg.1320500312. [DOI] [PubMed] [Google Scholar]
- 9.Fujii K, Ohashi H, Suzuki M, Hatsuse H, Shiohama T, Uchikawa H, et al. Frameshift mutation in the PTCH2 gene can cause nevoid basal cell carcinoma syndrome. Fam. Cancer. 2013;12:611–4. doi: 10.1007/s10689-013-9623-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhulyn O, Nieuwenhuis E, Liu YC, Angers S, Hui CC. Ptch2 shares overlapping functions with Ptch1 in Smo regulation and limb development. Dev. Biol. 2015;397:191–202. doi: 10.1016/j.ydbio.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers. 2016;8:e22. doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong CS, Strange RC, Lear JT. Basal cell carcinoma. BMJ. 2003;327:794–8. doi: 10.1136/bmj.327.7418.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo Muzio L, et al. Nevoid basal cell carcinoma syndrome. Clinical findings in 37 Italian affected individuals. Clin. Genet. 1999;55:34–40. doi: 10.1034/j.1399-0004.1999.550106.x. [DOI] [PubMed] [Google Scholar]
- 14.Fan Z, et al. A missense mutation in PTCH2 underlies dominantly inherited NBCCS in a Chinese family. J. Med. Genet. 2008;45:303–8. doi: 10.1136/jmg.2007.055343. [DOI] [PubMed] [Google Scholar]
- 15.Taeubner J, et al. Congenital embryonal rhabdomyosarcoma caused by heterozygous concomitant PTCH1 and PTCH2 germline mutations. Eur. J. Hum. Genet. 2018;26:137–42. doi: 10.1038/s41431-017-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieuwenhuis E, et al. Mice with a targeted mutation of patched2 are viable but develop alopecia and epidermal hyperplasia. Mol. Cell. Biol. 2006;26:6609–22. doi: 10.1128/MCB.00295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The relevant data from this Data Report are hosted at the Human Genome Variation Database at 10.6084/m9.figshare.hgv.2534