Abstract
As one of the three proteinogenic aromatic amino acids, l-phenylalanine is widely applied in the food, chemical and pharmaceutical industries, especially in production of the low-calorie sweetener aspartame. Microbial production of l-phenylalanine has become attractive as it possesses the advantages of environmental friendliness, low cost, and feedstock renewability. With the progress of metabolic engineering, systems biology and synthetic biology, production of l-phenylalanine from glucose in Escherichia coli with relatively high titer has been achieved by improving the intracellular levels of precursors, alleviating transcriptional repression and feedback inhibition of key enzymes, increasing the export of l-phenylalanine, engineering of global regulators, and overexpression of rate-limiting enzymes. In this review, successful metabolic engineering strategies for increasing l-phenylalanine accumulation from glucose in E. coli are described. In addition, perspectives for further improvement of production of l-phenylalanine are discussed.
Keywords: Escherichia coli, Metabolic engineering, l-Phenylalanine, Glucose
Introduction
As one of the three proteinogenic aromatic amino acids, l-phenylalanine is widely used in food additives, animal feed, infusion fluids, or as a building block for drug synthesis. As it can be used in the biosynthesis of the low-calorie sweetener aspartame, demand for l-phenylalanine has increased worldwide (Sprenger 2007b). Because of its commercial importance and wide application, increasing attention has been paid to l-phenylalanine production. Chemical synthesis uses nonrenewable toxic materials and generates racemic mixtures of d- and l-phenylalanine, which is undesirable (Otrokhov et al. 2013). Accordingly, direct microbial fermentation from low cost raw materials has become more favorable than other strategies (Rodriguez et al. 2014). Escherichia coli, a model organism with the advantages of a clear genetic background, simple genetic manipulation and fast growth in cheap media, is widely used for the production of l-phenylalanine and other aromatic compounds (Gu et al. 2012, 2016; Liu et al. 2015). However, in wild strains of E. coli, titer of l-phenylalanine is limited by strong and complex regulation of the biosynthesis pathway.
Previous l-phenylalanine producers were mainly obtained by classical mutagenesis and screening procedures. To obtain l-phenylalanine producers, structural analogues fluorophenylalanine or chlorophenylalanine was firstly supplemented into the medium. And then, mutant strains with resistant against the antimetabolites will exhibit restored growth and can easily be selected (De Boer and Dijkhuizen 1990). Through DNA sequencing, it was found that the mutant strains usually possessed altered allosteric binding sites of key enzymes (Ikeda 2006). However, unexpected mutations will be inevitably generated in the process of classical mutagenesis, which may affect further improvement of the mutant strain (Dong et al. 2011). Accordingly, rational engineering technologies have been used to achieve deletion, overexpression, and genomic integration of target genes to improve l-phenylalanine production in E. coli strains (Table 1).
Table 1.
Comparison of l-phenylalanine production in different recombinant strains of E. coli
| Strain | Relevant characteristics | Titer (g/L) | Conversion rate (mol/mol) | Productivity (g/L/h) | Culture methods | References |
|---|---|---|---|---|---|---|
| WSH-Z06 (pAP-B03) | E. coli K-12 with l-tyrosine auxotrophic | 35.38 | 26% | N | 3-L fed-batch fermentation | Haiyan et al. (2010) |
| HD-A2 | Multiple random mutagenesis of E. coli W3110 and overexpressing aroFWT, pheAFBR and aroA | 62.47 | 25.74% | N | 5-L fed-batch fermentation | Ding et al. (2016) |
| BR-42 (pAP-B03) | Multiple random mutagenesis of E. coli WSH-Z06 and overexpressing aroFWT and pheAFBR | 57.63 | 26.4% | 1.153 | 3-L fed-batch fermentation | Zhou et al. (2011) |
| Xllp21 | W3110-derived l-tyrosine auxotrophic with overexpressed aroFWT, aroD, and pheAFBR, inactivated tyrR, and genomic integrated galP and glk | 72.9 | 28.3% | N | 5-L fed-batch fermentation | Liu et al. (2018) |
| W3110 (pNpheABK15) | W3110 with overexpressed pheAFBR, ydiB, aroK and aroG15 | 23.8 | 16.8% | 0.073 | 15-L fed-batch fermentation | Liu et al. (2013) |
| pR15BABKG | WSH-Z06△crr with overexpressed pheA, aroG15, ydiB, aroK, tyrB and yddG | 47 | 27.5% | N | 5-L fed-batch fermentation | Liu et al. (2013) |
| W3110-4 (pF20) | W3110 (△pheA△tyrA △aroF) with overexpressed aroFFBR and pheAFBR | 32 | N | N | 300-L fed-batch fermentation | Gerigk et al. (2002b) |
| E. coli aroF-wt | w3110 Fnr+ (△pheA△tyrA △aroF)/pJF119EH-aroFWT-pheAFBR-aroLWT | 35 | N | N | 20-L fed-batch fermentation | Gerigk et al. (2002a) |
N not reported
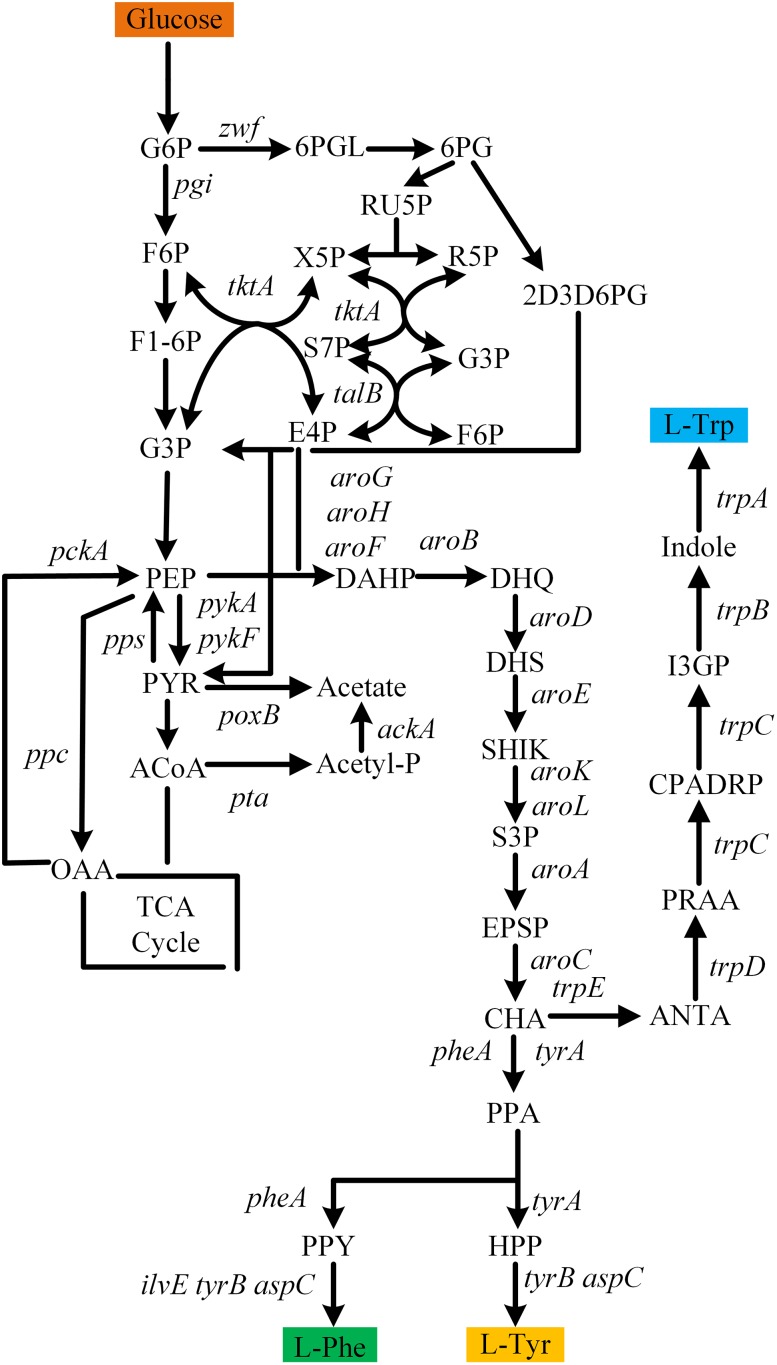
In E. coli, two precursors of the l-phenylalanine biosynthesis pathway are phosphoenolpyruvate (PEP) and erythrose 4-phosphate (E4P). As the three aromatic amino acids share the same biosynthesis pathway from 3-deoxy-d-arabino-heptulosonate 7-phosphate (DAHP) to chorismate, the overall l-phenylalanine biosynthesis pathway is often divided into the common pathway and the specific l-phenylalanine pathway that branches at the point of chorismate. As shown in Fig. 1, three DAHP synthase isoenzymes-AroF, AroG and AroH-are responsible for the first and rate limited step in the l-phenylalanine biosynthesis pathway (Umbarger 1978). AroG contributes about 80% of the overall DAHP activity, while the contributions of AroF and AroH to the total DAHP activity are only about 15% and 5%, respectively (Herrmann and Weaver 1999). Another rate-limiting enzyme in the l-phenylalanine pathway is chorismate mutase-prephenate dehydrogenase (CM-PDT), encoded by pheA, which is feedback inhibited by allosteric binding of l-phenylalanine. Notably, the last step in the formation of l-phenylalanine is a transamination reaction of phenylpyruvate with glutamate as the amino donor, and this step can be catalyzed by TyrB, AspC and IlvE.
Fig. 1.
The l-phenylalanine biosynthesis pathway in Escherichia coli
Regulation of l-phenylalanine biosynthesis in E. coli
In E. coli, TyrR and TrpR are the main transcriptional regulators of genes in the l-phenylalanine biosynthesis pathway (Wallace and Pittard 1969). Transcription of aroF, aroL, and tyrB is repressed by TyrR (Pittard and Davidson 1991; Wilson et al. 1994). TrpR can repress the transcription of aroH, aroL, and also its own expression (Gunsalus and Yanofsky 1980). To increase the accumulation of l-phenylalanine, tyrR and trpR are often inactivated simultaneously or individually.
In addition, l-phenylalanine production is also limited by feedback inhibition of enzymes in E. coli such as DAHP synthases and CM-PDT. The three DAHP synthase isozymes are feedback inhibited by l-phenylalanine, l-tyrosine and l-tryptophan, respectively, but CM-PDT is only sensitive to the concentration of l-phenylalanine (Ikeda 2006). In addition, shikimate dehydrogenase, encoded by aroE, is feedback inhibited by shikimate. This is the only enzyme in the aromatic amino acid biosynthesis pathways of E. coli that is subject to feedback inhibition by an intermediate rather than an end-product (Sprenger 2007a).
In E. coli, the expression of pheA (encoding CM-PDT) is also regulated by an attenuation mechanism. When high concentrations of l-phenylalanine are present in the medium, the ribosomes can successfully translate the leader peptide sequence by enough charged tRNAPhe, in turn preventing the formation of the second stem and loop and allowing formation of the third structure. As a result, transcription by RNA polymerase will be terminated. It was reported that 75% of the transcription of the pheA leader region was stalled when rich medium was applied for the cultivation of E. coli (Gavini and Davidson 1991).
Metabolic engineering for l-phenylalanine production in E. coli
Improving the intracellular levels of precursors of l-phenylalanine
To increase the intracellular concentration of E4P, transketolase (encoded by tktA) and transaldolase (encoded by talB) are often selected as engineering targets (Zhao and Winkler 1994). In addition, it was reported that inactivation of phosphoglucose isomerase was also advantageous for the supply of E4P by blocking glycolysis (Mascarenhas et al. 1991). By using glycerol instead of glucose as carbon source, the carbon flow directed into the pentose phosphate pathway can be obviously increased (Khamduang et al. 2009; Thongchuang et al. 2012).
When one glucose molecule is assimilated into E. coli cells by the phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS), one molecule of PEP will be consumed (Gosset 2005; Postma and Lengeler 1985). It was reported that about 50% of the intracellular PEP is consumed by the PTS system, while only 1.5% of PEP is directed into aromatic amino acid biosynthesis (Flores et al. 2002; Sprenger 2007b). Accordingly, the PTS is often selected as the first engineering target to increase the PEP level. However, the growth of mutant strains with deficiency of PTS components is often impaired due to limitations on the transport of glucose. To solve this problem, adaptive evolution processes can be employed to improve the growth of PTS-defect strains on glucose, and versatile mutant strains with recovered growth on glucose were obtained, such as PB12 (Flores et al. 1996). It was found that this strain directs more PEP into the aromatic synthesis pathway than other strains (Flores et al. 2002), and it can be used to produce various aromatic compounds by further genetic modifications (Carmona et al. 2015; Escalante et al. 2010). Another optional strategy is replacement of the PTS by the glucose facilitator and glucokinase from Zymomonas mobilis, or the galactose permease and glucokinase from E. coli (Balderas-Hernandez et al. 2009; Yi et al. 2002).
Apart from engineering the PTS, decreasing the carbon flux from PEP into the tricarboxylic acid cycle was also necessary to further increase the PEP pool, including inactivation of pyruvate kinase PykF (Meza et al. 2012; Sabido et al. 2014) and phosphoenolpyruvate carboxylase Ppc, and improving the expression of PEP synthetase PpsA to recycle pyruvate to PEP (Patnaik and Liao 1994; Yi et al. 2002).
Engineering of global regulators
CsrA is a regulatory protein of carbohydrate metabolism and can also regulate the intracellular PEP titer. It was reported that the titer of PEP could be increased by deletion of csrA or increasing the expression of negative regulatory RNA of csrA encoded by csrB (Tatarko and Romeo 2001; Yakandawala et al. 2008). Engineering of csrB and/or csrA combined with tktA overexpression was more effective than traditional strategies in enhancing l-phenylalanine production (Yakandawala et al. 2008). In addition, Fis, a nucleoid-associated protein, was found to be abundant during the exponential phase and decreased obviously in the stationary phase. Perhaps Fis is needed for transcription of growth related genes, and overexpression of Fis can put the host into a more metabolically active state (Tyagi et al. 2017). To test this hypothesis, the fis gene was overexpressed in recombinant strain WF123456 and the l-phenylalanine titer was indeed increased 1.2-fold compared with the control (Tyagi et al. 2017). In addition, Ojima et al. found that excess expression of yggG, encoding a stress-responsive gene, could repress the secretion of acetate, which may be of benefit for production of l-phenylalanine in E. coli (Ojima et al. 2009). Accordingly, they introduced yggG into an l-phenylalanine producer (E. coli AJ12741) with feedback-resistant AroG; the recombinant strain could produce 6.4 g/L l-phenylalanine, 73% higher than the original strain in batch fermentation.
Overcoming transcriptional repression, feedback inhibition and attenuation of key enzymes
As DAHP synthase controls the amount of carbon flow into the l-phenylalanine biosynthesis pathway (Ogino et al. 1982), the removal of feedback inhibition of DAHP synthase isoenzymes may be helpful to increase l-phenylalanine yield. With the progress of structural analysis, the crucial amino acid residues of DAHP synthase isoenzymes have been identified. As a result, various feedback-resistant enzymes, such as AroG(Leu76Val), AroF(Pro148Leu), and AroF(Gln152Ile) have been obtained (Ger et al. 1994; Kikuchi et al. 1997), and some of them are summarized in Table 2.
Table 2.
Summary of deregulated key enzymes in l-phenylalanine biosynthesis pathway of E. coli
| Enzyme | Mutant sites | References |
|---|---|---|
| AroG | D146 N, M147I | Ding et al. (2014) |
| AroG | S180F | Ger et al. (1994) |
| AroG | L76V | Kikuchi et al. (1997) |
| AroH | P18L, V147M, G149D, G149C, A177T | Ray et al. (1988) |
| AroF | Deletion of I11 | Zhao et al. (2011) |
| AroF | P148L | Weaver and Herrmann (1990) |
| AroF | N8K | Jossek et al. (2001) |
| PheA | Deletion of T304 and Q306 | Nelms et al. (1992) |
| PheA | Deletion of 301–386 amino acids residues | Zhang et al. (1998) |
| PheA | N5S, L8P, D54N, L55M, S235A | Baez-Viveros et al. (2004) |
Interestingly, it was found that wild-type aroF could achieve even higher final l-phenylalanine titers (34 g/L) than the aroFFBR-containing strain (28 g/L) due to the higher activity of wild-type AroF (Gerigk et al. 2002a). In 2004, another group also constructed a recombinant E. coli strain containing aroFWT and pheAFBR inserted in an IPTG-inducible plasmid for l-phenylalanine production (Takors 2004). By overexpression of pheAFBR and aroFWT in l-tyrosine auxotrophic E. coli strain WSH-Z06, the l-phenylalanine titer reached 35.38 g/L in a 3-L fermentor, 2.81-fold higher than that of the parental strain (Haiyan et al. 2010). These examples imply that wild-type DAHP synthases can also be employed for l-phenylalanine production.
In E. coli, ydiB, encoding bifunctional enzyme shikimate dehydrogenase/quinate dehydrogenase, can transform 3-dehydroshikimate to shikimate like AroE. It was reported that ydiB-overexpressing strains could achieve a higher l-phenylalanine titer than aroE (Lutke-Eversloh and Stephanopoulos 2008), indicating this strategy could overcome the feedback regulation of aroE. In addition, aroL was reported as the preferred shikimate kinase for increasing l-phenylalanine yield because of its lower Km. However, the activity of AroL is partly inhibited at high substrate concentration, while AroK works well in the same conditions (Oldiges et al. 2004). Accordingly, overexpression of aroK in E. coli will produce more l-phenylalanine than overexpression of aroL.
For CM-PDT, three mutants with substitution or an in-frame deletion located within codons 304–310 of the pheA gene were obtained. The mutated enzymes exhibited both high enzymatic activity and almost complete resistance to feedback inhibition when 200 mM l-phenylalanine was present (Nelms et al. 1992). In addition, PheA containing residues 1–285 and residues 1–300 retained full mutase and dehydratase activity and showed no feedback inhibition (Zhang et al. 1998). In 2004, through two protein-evolutionary cycles of CM-PDT, two mutant genes, pheAev1 and pheAev2, were obtained. Recombinant strain PB12 with overexpressed tktA, aroGFBR and pheAev2 could produce 0.33 g l-phenylalanine per gram of glucose, corresponding to 60% of the maximum theoretical yield (0.55 g/g) (Baez-Viveros et al. 2004).
Other engineering targets for l-phenylalanine production
The dissolved oxygen (DO) in culture is considered an important factor for bacterial growth and l-phenylalanine productivity. To increase the DO level, co-expression of a hemoglobin gene from Vitreoscilla with aroF and pheAFBR was performed in E. coli CICC10245, which led to 21.9% more biomass and 16.6% more l-phenylalanine production, while only approximately 5% more glucose was consumed (Wu et al. 2018).
Another strategy to relieve the feedback inhibition of key enzymes is reduction of intracellular accumulation of l-phenylalanine. In E. coli, the protein YddG is responsible for the export of aromatic amino acids. It was reported that E. coli strains overexpressing yddG accumulated less l-phenylalanine within the cell and exported l-phenylalanine threefold faster than the control (Doroshenko et al. 2007).
In brief, to construct an l-phenylalanine producer from wild E. coli strains, the following engineering targets can be considered: repressor proteins TyrR and TrpR; feedback regulation of aroG, aroE and pheA; the intracellular levels of PEP and E4P; the l-phenylalanine transport system YddG; and the global regulators CsrA, CsrB, Fis and YggG.
Conclusions and perspectives
With the disadvantages of being labor-intensive, time-consuming, and producing undefined mutations, classical mutagenesis and screening have been replaced by rational metabolic engineering strategies in the construction of l-phenylalanine producing strains (Dong et al. 2011). However, only a few recombinant strains achieve a relatively satisfactory titer of l-phenylalanine. Further improvements can be expected by investigating novel engineering targets. An insight into intracellular flux distribution during l-phenylalanine production was obtained by flux variability analysis. According to the results, malic enzyme knockout mutants were constructed and exhibited well process performances in l-phenylalanine production (Michael et al. 2014).
As synthetic biology develops, more and more tools can be applied to the generation of large diversified libraries and high-throughput screening processes. In 2016, a mtr promoter-based biosensor was constructed and employed in FACS high-throughput screening of an E. coli MG1655 mutant library (Mahr et al. 2016). The best mutant could produce 4.3-fold l-phenylalanine levels compared with the wild-type strain. This suggests that a combination strategy can be implemented to obtain an improved l-phenylalanine producer. Firstly, random mutation and high-throughput screening can be carried out to obtain a base strain with deregulated feedback inhibition and transcriptional repression, and then whole genome sequencing implemented to verify which genes are affected. Next, defined metabolic engineering strategies focusing on traditional targets can be performed. Third, unwanted mutations generated in the random mutagenesis can be removed. By combining random mutation, rational engineering, and high-throughput screening methods, a recombinant strain with higher l-phenylalanine production may be generated.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31600066, 31870105, 31741007), the Shandong Provincial Natural Science Foundation (ZR2016CB20, ZR2016CL02), and the State Key Laboratory of Microbial Technology Open Projects Fund (M2016-10).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Baez-Viveros JL, Osuna J, Hernandez-Chavez G, Soberon X, Bolivar F, Gosset G. Metabolic engineering and protein directed evolution increase the yield of l-phenylalanine synthesized from glucose in Escherichia coli. Biotechnol Bioeng. 2004;87:516–524. doi: 10.1002/bit.20159. [DOI] [PubMed] [Google Scholar]
- Balderas-Hernandez VE, Sabido-Ramos A, Silva P, Cabrera-Valladares N. Metabolic engineering for improving anthranilate synthesis from glucose in Escherichia coli. Microb Cell Fact. 2009;8:19. doi: 10.1186/1475-2859-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer LD, Dijkhuizen L. Microbial and enzymatic processes for l-phenylalanine production. Adv Biochem Eng Biotechnol. 1990;41:1–27. [Google Scholar]
- Carmona SB, Moreno F, Bolívar F, Gosset G, Escalante A. Inactivation of the PTS as a strategy to engineer the production of aromatic metabolites in Escherichia coli. J Mol Microbiol Biotechnol. 2015;25:195–208. doi: 10.1159/000380854. [DOI] [PubMed] [Google Scholar]
- Ding R, Liu L, Chen X, Cui Z, Zhang A, Ren D, Zhang L. Introduction of two mutations into AroG increases phenylalanine production in Escherichia coli. Biotechnol Lett. 2014;36:2103–2108. doi: 10.1007/s10529-014-1584-4. [DOI] [PubMed] [Google Scholar]
- Ding D, Liu Y, Xu Y, Ping Z, Li H, Zhang D, Sun J. Improving the production of l-phenylalanine by identifying key enzymes through multi-enzyme reaction system in vitro. Sci Rep. 2016;6:32208. doi: 10.1038/srep32208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Quinn PJ, Wang X. Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for the production of l-threonine. Biotechnol Adv. 2011;29:11–23. doi: 10.1016/j.biotechadv.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Doroshenko V, Airich L, Vitushkina M, Kolokolova A, Livshits V, Mashko S. YddG from Escherichia coli promotes export of aromatic amino acids. FEMS Microbiol Lett. 2007;275:312–318. doi: 10.1111/j.1574-6968.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- Escalante A, Calderon R, Valdivia A, Anda RD, Hernández G, Ramirez OT. Metabolic engineering for the production of shikimic acid in an evolved Escherichia coli strain lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system. Microb Cell Fact. 2010;9:21. doi: 10.1186/1475-2859-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores N, Xiao J, Berry A, Bolivar F, Valle F. Pathway engineering for the production of aromatic compounds in Escherichia coli. Nat Biotechnol. 1996;14:620–623. doi: 10.1038/nbt0596-620. [DOI] [PubMed] [Google Scholar]
- Flores S, Gosset G, Flores N, de Graaf AA, Bolivar F. Analysis of carbon metabolism in Escherichia coli strains with an inactive phosphotransferase system by (13)C labeling and NMR spectroscopy. Metab Eng. 2002;4:124–137. doi: 10.1006/mben.2001.0209. [DOI] [PubMed] [Google Scholar]
- Gavini N, Davidson BE. Regulation of pheA expression by the pheR product in Escherichia coli is mediated through attenuation of transcription. J Biol Chem. 1991;266:7750–7753. [PubMed] [Google Scholar]
- Ger YM, Chen SL, Chiang HJ, Shiuan D. A single Ser-180 mutation desensitizes feedback inhibition of the phenylalanine-sensitive 3-deoxy-d-arabino-heptulosonate 7-phosphate (DAHP) synthetase in Escherichia coli. J Biochem. 1994;116:986–990. doi: 10.1093/oxfordjournals.jbchem.a124657. [DOI] [PubMed] [Google Scholar]
- Gerigk M, Bujnicki R, Ganpo-Nkwenkwa E, Bongaerts J, Sprenger G, Takors R. Process control for enhanced l-phenylalanine production using different recombinant Escherichia coli strains. Biotechnol Bioeng. 2002;80:746–754. doi: 10.1002/bit.10428. [DOI] [PubMed] [Google Scholar]
- Gerigk M, Maass D, Kreutzer A, Sprenger G, Bongaerts J, Wubbolts M, Takors R. Enhanced pilot-scale fed-batch l-phenylalanine production with recombinant Escherichia coli by fully integrated reactive extraction. Bioprocess Biosyst Eng. 2002;25:43–52. doi: 10.1007/s00449-002-0280-2. [DOI] [PubMed] [Google Scholar]
- Gosset G. Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate: sugar phosphotransferase system. Microb Cell Fact. 2005;4:14. doi: 10.1186/1475-2859-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P, Yang F, Kang J, Wang Q, Qi Q. One-step of tryptophan attenuator inactivation and promoter swapping to improve the production of l-tryptophan in Escherichia coli. Microb Cell Fact. 2012;11:30. doi: 10.1186/1475-2859-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P, Su T, Wang Q, Liang Q, Qi Q. Tunable switch mediated shikimate biosynthesis in an engineered non-auxotrophic Escherichia coli. Sci Rep. 2016;6:29745. doi: 10.1038/srep29745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus RP, Yanofsky C. Nucleotide sequence and expression of Escherichia coli trpR, the structural gene for the trp aporepressor. Proc Natl Acad Sci USA. 1980;77:7117. doi: 10.1073/pnas.77.12.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiyan Z, Xianyan L, Tianwen W, Guocheng D, Jian C. Enhanced l-phenylalanine biosynthesis by co-expression of pheA(fbr) and aroF(wt) Bioresour Technol. 2010;101:4151–4156. doi: 10.1016/j.biortech.2010.01.043. [DOI] [PubMed] [Google Scholar]
- Herrmann KM, Weaver LM. The Shikimate pathway annual review of plant physiology and plant. Mol Biol. 1999;50:473–503. doi: 10.1146/annurev.arplant.50.1.473. [DOI] [PubMed] [Google Scholar]
- Ikeda M. Towards bacterial strains overproducing l-tryptophan and other aromatics by metabolic engineering. Appl Microbiol Biotechnol. 2006;69:615–626. doi: 10.1007/s00253-005-0252-y. [DOI] [PubMed] [Google Scholar]
- Jossek R, Bongaerts J, Sprenger GA. Characterization of a new feedback-resistant 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase AroF of Escherichia coli. FEMS Microbiol Lett. 2001;202:145–148. doi: 10.1111/j.1574-6968.2001.tb10795.x. [DOI] [PubMed] [Google Scholar]
- Khamduang M, Packdibamrung K, Chutmanop J, Chisti Y, Srinophakun P. Production of l-phenylalanine from glycerol by a recombinant Escherichia coli. J Ind Microbiol Biotechnol. 2009;36:1267–1274. doi: 10.1007/s10295-009-0606-z. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Tsujimoto K, Kurahashi O. Mutational analysis of the feedback sites of phenylalanine-sensitive 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase of Escherichia coli. Appl Environ Microbiol. 1997;63:761–762. doi: 10.1128/aem.63.2.761-762.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SP, Xiao MR, Zhang L, Xu J, Ding ZY, Gu ZH, Shi GY. Production of l-phenylalanine from glucose by metabolic engineering of wild type Escherichia coli W3110. Process Biochem. 2013;48:413–419. doi: 10.1016/j.procbio.2013.02.016. [DOI] [Google Scholar]
- Liu SP, Zhang L, Mao J, Ding ZY, Shi GY. Metabolic engineering of Escherichia coli for the production of phenylpyruvate derivatives. Metab Eng. 2015;32:55–65. doi: 10.1016/j.ymben.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xu Y, Ding D, Wen J, Zhu B, Zhang D. Genetic engineering of Escherichia coli to improve l-phenylalanine production. BMC Biotechnol. 2018;18:5. doi: 10.1186/s12896-018-0418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutke-Eversloh T, Stephanopoulos G. Combinatorial pathway analysis for improved l-tyrosine production in Escherichia coli: identification of enzymatic bottlenecks by systematic gene overexpression. Metab Eng. 2008;10:69–77. doi: 10.1016/j.ymben.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Mahr R, Boeselager RFV, Wiechert J, Frunzke J. Screening of an Escherichia coli promoter library for a phenylalanine biosensor. Appl Microbiol Biotechnol. 2016;100:6739–6753. doi: 10.1007/s00253-016-7575-8. [DOI] [PubMed] [Google Scholar]
- Mascarenhas D, Ashworth DJ, Chen CS. Deletion of pgi alters tryptophan biosynthesis in a genetically engineered strain of Escherichia coli. Appl Environ Microbiol. 1991;57:2995–2999. doi: 10.1128/aem.57.10.2995-2999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza E, Becker J, Bolivar F, Gosset G, Wittmann C. Consequences of phosphoenolpyruvate: sugar phosphotransferase system and pyruvate kinase isozymes inactivation in central carbon metabolism flux distribution in Escherichia coli. Microb Cell Fact. 2012;11:127. doi: 10.1186/1475-2859-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael W, Julia TN, Christoph A, Sprenger GA, Dirk WB. Improvement of constraint-based flux estimation during l-phenylalanine production with Escherichia coli using targeted knock-out mutants. Biotechnol Bioeng. 2014;111:1406–1416. doi: 10.1002/bit.25195. [DOI] [PubMed] [Google Scholar]
- Nelms J, Edwards RM, Warwick J, Fotheringham I. Novel mutations in the pheA gene of Escherichia coli K-12 which result in highly feedback inhibition-resistant variants of chorismate mutase/prephenate dehydratase. Appl Environ Microbiol. 1992;58:2592–2598. doi: 10.1128/aem.58.8.2592-2598.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T, Garner C, Markley JL, Herrmann KM. Biosynthesis of aromatic compounds: 13C NMR spectroscopy of whole Escherichia coli cells. Proc Natl Acad Sci USA. 1982;79:5828–5832. doi: 10.1073/pnas.79.19.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojima Y, Komaki M, Nishioka M, Iwatani S, Tsujimoto N, Taya M. Introduction of a stress-responsive gene, yggG, enhances the yield of l-phenylalanine with decreased acetic acid production in a recombinant Escherichia coli. Biotechnol Lett. 2009;31:525. doi: 10.1007/s10529-008-9906-z. [DOI] [PubMed] [Google Scholar]
- Oldiges M, Kunze M, Degenring D, Sprenger GA, Takors R. Stimulation, monitoring, and analysis of pathway dynamics by metabolic profiling in the aromatic amino acid pathway. Biotechnol Prog. 2004;20:1623–1633. doi: 10.1021/bp0498746. [DOI] [PubMed] [Google Scholar]
- Otrokhov GV, Morozova OV, Vasil’, eva IS, Shumakovich GP, Zaitseva EA, Khlupova ME, Yaropolov AI. Biocatalytic synthesis of conducting polymers and prospects for its application. Biochemistry. 2013;78:1539–1553. doi: 10.1134/S0006297913130117. [DOI] [PubMed] [Google Scholar]
- Patnaik R, Liao JC. Engineering of Escherichia coli central metabolism for aromatic metabolite production with near theoretical yield. Appl Environ Microbiol. 1994;60:3903–3908. doi: 10.1128/aem.60.11.3903-3908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard AJ, Davidson BE. TyrR protein of Escherichia coli and its role as repressor and activator. Mol Microbiol. 1991;5:1585–1592. doi: 10.1111/j.1365-2958.1991.tb01904.x. [DOI] [PubMed] [Google Scholar]
- Postma PW, Lengeler JW. Phosphoenolpyruvate: carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985;49:232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JM, Yanofsky C, Bauerle R. Mutational analysis of the catalytic and feedback sites of the tryptophan-sensitive 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase of Escherichia coli. J Bacteriol. 1988;170:5500–5506. doi: 10.1128/jb.170.12.5500-5506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Martinez JA, Flores N, Escalante A, Gosset G, Bolivar F. Engineering Escherichia coli to overproduce aromatic amino acids derived compounds. Microb Cell Fact. 2014;13:126. doi: 10.1186/s12934-014-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabido A, Sigala JC, Hernandez-Chavez G, Flores N, Gosset G, Bolivar F. Physiological and transcriptional characterization of Escherichia coli strains lacking interconversion of phosphoenolpyruvate and pyruvate when glucose and acetate are coutilized. Biotechnol Bioeng. 2014;111:1150–1160. doi: 10.1002/bit.25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger G. Aromatic amino acid biosynthesis-pathways, regulation and metabolic engineering. Heidelberg: Springer; 2007. pp. 93–127. [Google Scholar]
- Sprenger GA. From scratch to value: engineering Escherichia coli wild type cells to the production of l-phenylalanine and other fine chemicals derived from chorismate. Appl Microbiol Biotechnol. 2007;75:739–749. doi: 10.1007/s00253-007-0931-y. [DOI] [PubMed] [Google Scholar]
- Takors R. Model-based analysis and optimization of an ISPR approach using reactive extraction for pilot-scale l-phenylalanine production. Biotechnol Prog. 2004;20:57–64. doi: 10.1021/bp0257473. [DOI] [PubMed] [Google Scholar]
- Tatarko M, Romeo T. Disruption of a global regulatory gene to enhance central carbon flux into phenylalanine biosynthesis in Escherichia coli. Curr Microbiol. 2001;43:26–32. doi: 10.1007/s002840010255. [DOI] [PubMed] [Google Scholar]
- Thongchuang M, Pongsawasdi P, Chisti Y, Packdibamrung K. Design of a recombinant Escherichia coli for producing l-phenylalanine from glycerol. World J Microbiol Biotechnol. 2012;28:2937–2943. doi: 10.1007/s11274-012-1104-4. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Saini D, Guleria R, Mukherjee KJ. Designing an Escherichia coli strain for phenylalanine overproduction by metabolic engineering. Mol Biotechnol. 2017;59:1–11. doi: 10.1007/s12033-017-9999-5. [DOI] [PubMed] [Google Scholar]
- Umbarger HE. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Wallace BJ, Pittard J. Regulator gene controlling enzymes concerned in tyrosine biosynthesis in Escherichia coli. J Bacteriol. 1969;97:1234–1241. doi: 10.1128/jb.97.3.1234-1241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Herrmann KM. Cloning of an aroF allele encoding a tyrosine-insensitive 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase. J Bacteriol. 1990;172:6581–6584. doi: 10.1128/jb.172.11.6581-6584.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TJ, Maroudas P, Howlett GJ, Davidson BE. Ligand-induced self-association of the Escherichia coli regulatory protein TyrR. J Mol Biol. 1994;238:309–318. doi: 10.1006/jmbi.1994.1294. [DOI] [PubMed] [Google Scholar]
- Wu WB, Guo XL, Zhang ML, Huang QG, Qi F, Huang JZ. Enhancement of l-phenylalanine production in Escherichia coli by heterologous expression of Vitreoscilla hemoglobin. Biotechnol Appl Biochem. 2018;65:476–483. doi: 10.1002/bab.1605. [DOI] [PubMed] [Google Scholar]
- Yakandawala N, Romeo T, Friesen AD, Madhyastha S. Metabolic engineering of Escherichia coli to enhance phenylalanine production. Appl Microbiol Biotechnol. 2008;78:283–291. doi: 10.1007/s00253-007-1307-z. [DOI] [PubMed] [Google Scholar]
- Yi J, Li K, Draths KM, Frost JW. Modulation of phosphoenolpyruvate synthase expression increases shikimate pathway product yields in E. coli. Biotechnol Prog. 2002;18:1141–1148. doi: 10.1021/bp020101w. [DOI] [PubMed] [Google Scholar]
- Zhang S, Pohnert G, Kongsaeree P, Wilson DB, Clardy J, Ganem B. Chorismate mutase-prephenate dehydratase from Escherichia coli. Study of catalytic and regulatory domains using genetically engineered proteins. J Biol Chem. 1998;273:6248–6253. doi: 10.1074/jbc.273.11.6248. [DOI] [PubMed] [Google Scholar]
- Zhao G, Winkler ME. An Escherichia coli K-12 tktA tktB mutant deficient in transketolase activity requires pyridoxine (vitamin B6) as well as the aromatic amino acids and vitamins for growth. J Bacteriol. 1994;176:6134–6138. doi: 10.1128/jb.176.19.6134-6138.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZJ, Zou C, Zhu YX, Dai J, Chen S, Wu D, Wu J, Chen J. Development of l-tryptophan production strains by defined genetic modification in Escherichia coli. J Ind Microbiol Biotechnol. 2011;38:1921–1929. doi: 10.1007/s10295-011-0978-8. [DOI] [PubMed] [Google Scholar]
- Zhou H, Liao X, Liu L, Wang T, Du G, Chen J. Enhanced l-phenylalanine production by recombinant Escherichia coli BR-42 (pAP-B03) resistant to bacteriophage BP-1 via a two-stage feeding approach. J Ind Microbiol Biotechnol. 2011;38:1219. doi: 10.1007/s10295-010-0900-9. [DOI] [PubMed] [Google Scholar]