Abstract
A series of novel structurally simple analogues based on nitidine was designed and synthesized in search of potent anticancer agents. The antitumor activity against human cancer cell lines (HepG2, A549, NCI-H460, and CNE1) was performed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay in vitro. The results showed that some of them had good anticancer activities, especially derivatives with a [(dimethylamino)ethyl]amino side chain in the C-6 position. Planar conjugated compounds 15a, 15b, and 15c, with IC50 values of 1.20 μM, 1.87 μM, and 1.19 μM against CNE1 cells, respectively, were more active than nitidine chloride. Compound 15b and compound 15c with IC50 values of 1.19 μM and 1.37 μM against HepG2 cells and A549 cells demonstrated superior activities to nitidine. Besides, compound 5e which had a phenanthridinone core displayed extraordinary cytotoxicity against all test cells, particularly against CNE1 cells with the IC50 value of 1.13 μM.
Keywords: nitidine, phenanthridine, phenanthridinone, antitumor activity
1. Introduction
Nowadays, cancer is a major public health issue in most of countries with high mortality rates [1,2,3]. Therefore, it is necessary and urgent to develop novel antitumor drugs with higher activity but lower toxicity. Natural products and their related derivatives have been proved to be important candidates in the discovery and development of bioactive drugs owing to the lower mammalian toxicity [4]. Quaternary benzo[c]phenanthridines are alkaloids with extensive bioactivities [5,6,7,8,9]. Among them, natural occurring nitidine, fagaronine, chelerythrine, and sanguinarine exhibit good antitumor activity. The synthesis of their derivatives is of great interest because of the lower activity in vivo owing to the instability of the iminium salt moiety in the structure [10,11,12]. As a synthetic derivative of benzo[c]phenanthridine, NK 314 displays high antitumor activity not only in vitro but also in vivo and can be regarded as a promising anticancer drug which inspired chemist to develop more structurally modified or structurally simple alternatives [13,14,15,16].
Nitidine, as a best-known member of benzo[c]phenanthridine alkaloids, has received much attention because of its broad range of biological activities, including anti-inflammatory, anti-malarial, antibacterial, anti-HIV, and especially antitumor [17,18,19,20,21,22,23,24,25,26,27,28,29]. Despite the excellent activity against the cancer cells, little progress has been made, attributed to the unsatisfactory activity in vivo and complicated total synthetic route. As shown in the structure, phenanthridine is the common but significant N-heterocyclic core existing in benzo[c]phenanthridine and some drugs [30,31,32]. Thus, structurally simple phenanthridine alternatives may display similar antitumor activity. Considering the necessity of 8,9-dimethoxy groups and steric interaction on the skeletons for the biological effects from the QSAR model based on benzo[c]phenanthridine [33], a class of analogues based on nitidine were designed and synthesized. The antitumor activity was evaluated against human cell lines of HepG2, A549, NCI-H460, and CNE1 in vitro.
2. Results and Discussion
2.1. Synthesis
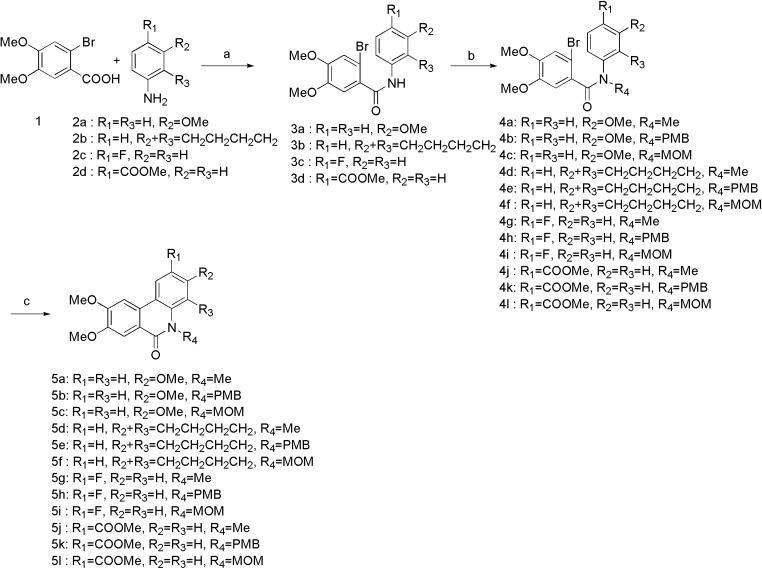
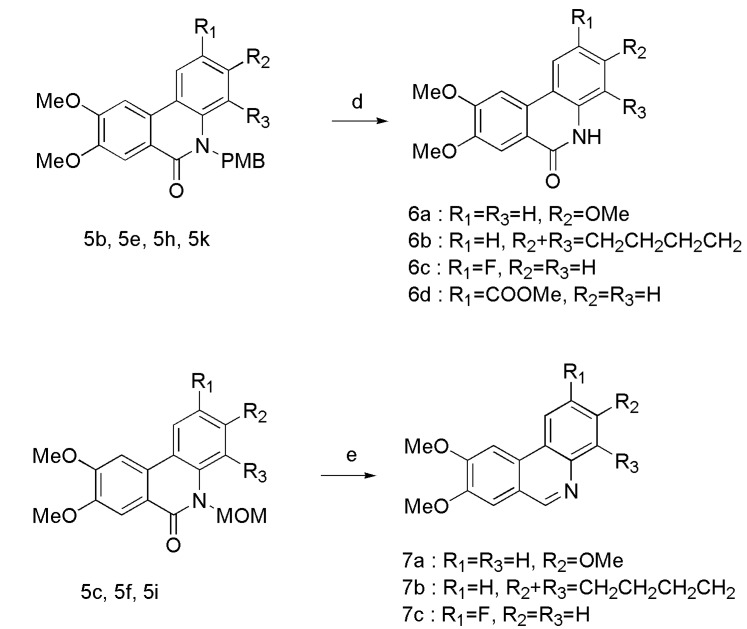
The analogues were synthesized according to the procedures in Scheme 1, Scheme 2 and Scheme 3. Firstly, the 2-bromo-4,5-dimethoxybenzoic acid was converted to acid chloride in situ by refluxing in SOCl2. After evaporation of SOCl2 under reduced pressure, the acid chloride was directly reacted with various commercially available anilines (2a–d) in dry DCM. Different groups were introduced to obtain corresponding N-protected compounds 4a–l. Intramolecular Heck coupling in the presence of Pd(oAc)2/P(o-tol)3/K2CO3/DMF gave the phenanthridinone derivatives 5a–l in good yield. N-PMB protected benzamides 5b, 5e, 5h, and 5k were treated with TFA to afford the phenanthridinone derivatives 6a-6d, while N-MOM protected benzamides 5c, 5f, and 5i were treated with LiAlH4 to provide planar phenanthridine derivatives 7a–c.
Scheme 1.
Reagents and conditions: (a) SOCl2, iPrNEt, DCM, reflux, 78–91%; (b) NaH, DMF, PMBCl or MOMCl, rt, 92–98%; (c) Pd(oAc), P(o-tol)3, K2CO3, DMF, 155 °C, 78–90%; (d) TFA, 75 °C, 54–78%; (e) LiAlH4, dry THF, 0 °C-rt, 45–55%.
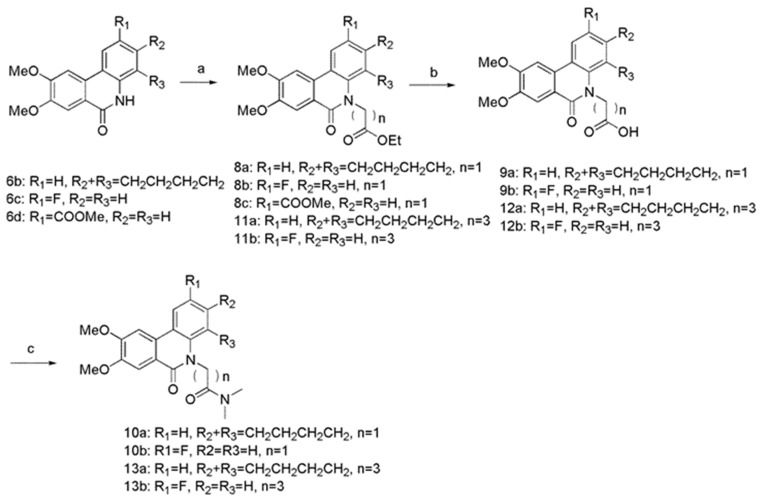
Scheme 2.
Reagents and conditions: (a) ethyl bromoacetate or ethyl 4-bromobutyrate, Cs2CO3, TBAI, DMF, 90 °C, 76–95%; (b) MeOH, H2O, 10N NaOH, rt, 93–98%; (c) triethylamine, THF, CH2Cl2, isobutyl chloroformate, dimethylamine, 0 °C-rt, 82–85%.
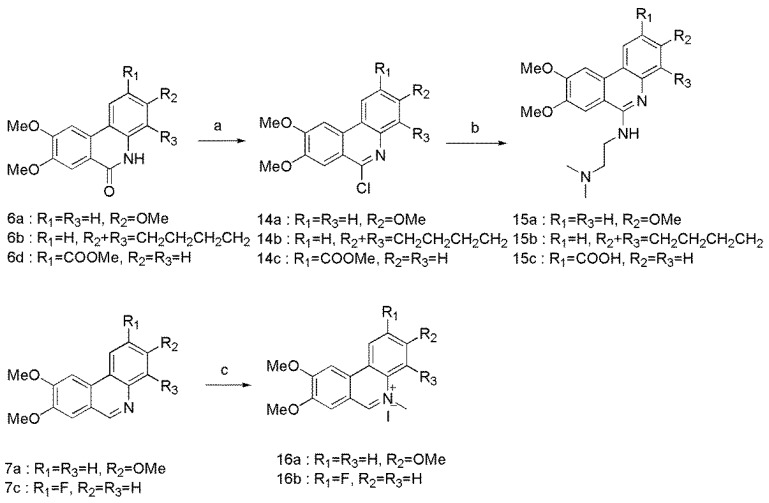
Scheme 3.
Reagents and conditions: (a) POCl3, 105 °C, 83–85%; (b) Me2N(CH2)2NH2, reflux, 59–78%. (c) MeI, toluene, 80 °C, 75–84%.
The route to the desired N-5 substituted derivatives was illustrated in Scheme 2. We prepared N-5 carboxyl substituted derivatives 9a, 9b, 12a, and 12b by initially forming compounds 8a, 8b, 11a, and 11b, followed by hydrolysis of the ester moiety. Treatment of carboxyl derivatives with dimethylamine provided 10a, 10b, 13a, and 13b.
The general synthetic methodology for the synthesis of C-6 substituted derivatives was outlined in Scheme 3. 6a, 6b, and 6d were treated with POCl3 to afford the C-6 chlorinated analogues of 14a–c, followed by substitution reaction with N,N-dimethylethylenediamine to provide derivatives 15a–c. Compounds 7a and 7c underwent N-methylation with iodomethane to form phenanthridinium salts 16a and 16b.
2.2. Evaluation of Antitumor Activity
The antitumor activities of the derivatives were evaluated against the human cancer cell lines including HepG2, A549, NCI-H460, and CNE1 in vitro by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Nitidine chloride was also presented as the comparative criterion. The results were summarized in Table 1. Derivatives which did not demonstrate obvious activity were not presented in the table.
Table 1.
Antitumor activity of some compounds.
| Compounds | IC50 (μM) | |||
|---|---|---|---|---|
| HepG2 | A549 | NCI-H460 | CNE1 | |
| 5a | >50 | 36.20 ± 1.52 | / | 26.74 ± 3.54 |
| 5b | >50 | 44.57 ± 3.02 | 31.03 ± 6.91 | 14.75 ± 1.96 |
| 5c | >50 | 34.56 ± 3.28 | / | 11.25 ± 3.59 |
| 5e | 9.07 ± 1.14 | 2.77 ± 0.11 | 12.59 ± 3.57 | 1.13 ± 0.17 |
| 5h | / | / | 26.13 ± 4.35 | / |
| 5k | / | >50 | 48.01 ± 0.21 | >50 |
| 5l | 25.01 ± 4.85 | / | >50 | 42.446 ± 1.95 |
| 6a | 44.49 ± 2.91 | / | / | >50 |
| 6b | >50 | / | 48.79 ± 1.92 | >50 |
| 7b | >50 | >50 | / | 4.38 ± 1.66 |
| 7c | 33.67 ± 2.51 | 45.84 ± 1.79 | 34.69 ± 3.42 | 8.57 ± 1.21 |
| 8c | 16.79 ± 5.33 | / | >50 | 37.98 ± 4.91 |
| 9a | 26.96 ± 3.82 | 41.45 ± 2.05 | / | 34.50 ± 2.31 |
| 11a | / | 44.00 ± 2.90 | / | 23.84 ± 4.57 |
| 14c | >50 | >50 | >50 | 38.52 ± 1.50 |
| 15a | 1.68 ± 0.10 | 1.94 ± 0.09 | 11.38 ± 0.53 | 1.20 ± 0.14 |
| 15b | 1.19 ± 0.09 | 2.11 ± 0.24 | 8.98 ± 0.58 | 1.87 ± 0.18 |
| 15c | 2.15 ± 0.17 | 1.37 ± 0.15 | 5.24 ± 0.51 | 1.19 ± 0.18 |
| 16a | 25.41 ± 5.15 | 18.69 ± 3.34 | 28.09 ± 4.20 | 8.79 ± 1.66 |
| 16b | >50 | 33.01 ± 6.61 | 21.48 ± 3.96 | 25.43 ± 1.99 |
| Nitidine chloride | 1.40 ± 0.16 | 1.88 ± 0.24 | 2.35 ± 0.35 | 1.85 ± 0.08 |
It was found that most of target compounds showed moderate to high antitumor activity, with the IC50 value range from 1.13 μM to 48.79 μM. Compounds 5e, 15a, 15b, 15c, and 16a exhibited good inhibition against all of the test cell line.
For HepG2 cells, compounds 5e, 15a, 15b, and 15c displayed high activity with the IC50 values of 9.07 μM, 1.68 μM, 1.19 μM, and 2.15 μM, respectively. Among them, compound 15b had the best inhibition and was superior to nitidine chloride. Similar results for A549 cell and NCI-H460 cell were gave, and compound 15c had the highest activity with IC50 of 1.37 μM against A549 cell and 5.24 μM against NCI-H460 cell, which were better than or close to nitidine chloride.
For CNE1 cells, N-5 substituted phenanthridinone derivatives 5a, 5b, 5c, and 5e showed moderate to high activity than phenanthridinones 6a–d, revealing that the introduction of substituent in the N-5 position could significantly improve the antitumor activity. In particular, compound 5e exhibited the highest cytotoxicity against CNE1 cell with the IC50 value of 1.13 μM and was 1.6-fold more active than nitidine chloride (IC50: 1.85 μM). However, derivatives including 8–13 with a long chain in N-5 position did not demonstrate obvious activity. In addition, all of the planar derivatives (except 7a and 14a–c) exhibited high cytotoxicity with the IC50 values range from 1.19 μM to 15.17 μM.
It should be noted that planar conjugated structure, especially with substituent in the C-6 position, enhanced the antitumor activity. The moiety of ammonium salt was less important than the benzo[c]phenanthridine alkaloids. Moreover, short chain in the N-5 position of the phenanthridinone derivatives improved the antitumor activity, while the presence of long alkyl chain may decrease the activity.
3. Materials and Methods
3.1. General Information
All commercial reagents and solvents were used as received without further purification unless otherwise indicated. Synthetic compounds 3a–d and 4a–l were directly used for reaction without further purification and characterization. Melting points were recorded on an SGW X-4 microscope melting point apparatus (Shanghai Tech Instrument Co., Ltd., Shanghai, China). Infrared spectra (IR) were performed on NICOLET iS10 sepectrometer (Shimazu Co., Ltd., Kyoto, Japan). NMR spectra were recorded on a Bruker Avance 500MHz spectrometer (Bruker Co., Ltd., Zurich, Switzerland) at room temperature with tetramethylsilane (TMS) as an internal standard and CDCl3 or DMSO-d6 as solvents. Mass spectra (MS) were obtained by LCMS-IT-TOF spectrometer (Shimadzu Co., Ltd., Kyoto, Japan) or TSQ Quantum Ultra (Thermo Scientific Co., Ltd., Madison, WI, USA). Elemental analysis for C, H, O, and N were carried out with Elementar VarioMICRO Cube analyzer (Elementar, Frankfurt, Germany).
3.2. General Procedure for the Synthesis of Compounds 5a–l
2-bromo-4,5-dimethoxy-N-(3-methoxyphenyl)-N-methylbenzamide (4a) (2.00 g, 5.26 mmol), Pd(oAc)2 (0.118 g, 0.526 mmol), P(o-tol)3 (0.32 g, 1.052 mmol), K2CO3 (2.90 g, 21.0 mmol), and dry DMF (20 mL) were mixed under nitrogen atmosphere, and the mixture was stirred at 100 °C overnight. After cooled to room temperature, the reaction mixture was extracted with CH2Cl2. The organic phase was washed with H2O and brine, and then dried over anhydrous Na2SO4. After being concentrated under reduced pressure, the residue was purified on silica gel column chromatography (PE/EA, v/v = 1:1) to obtain compound 5a. Same method was used to provide compounds 5b–l from 4b–l, respectively. Derivatives 5b, 5c, 5e, 5f, 5h, 5i, 5k, and 5l were used without purification and further characterization.
3,8,9-trimethoxy-5-methyl-5H-phenanthridin-6-one (5a) White solid, yield 87%, m.p. 191.0–193.0 °C. FTIR (KBr, cm−1) 3441, 3132, 1643, 1608, 1510, 1463, 1402, 1313, 1262, 1230, 1146, 1032, 872, 821, 775, 726. 1H-NMR (CDCl3) δ 8.06 (d, J = 8.6 Hz, 1H), 7.89 (s, 1H), 7.48 (s, 1H), 6.91–6.87 (m, 2H), 4.08 (s, 3H), 4.02 (s, 3H), 3.94 (s, 3H), 3.78 (s, 3H). 13C-NMR (CDCl3) δ 160.3, 153.5, 149.2, 139.2, 128.8, 128.4, 124.1, 113.0, 109.3, 109.0, 105.5, 102.2, 100.4, 56.3, 56.2, 55.7, 30.1. ESI-MS m/z: 338.34 ([M + K]+). Anal. Calcd for C17H17NO4: C 68.21; H 5.72; N 4.68; O 21.68. Found: C 67.92; H 5.58; N 4.55; O 21.40 (See Supplementary Materials).
8,9-dimethoxy-5-methyl-2,3,4,5-tetrahydro-1H-benzo[c]phenanthridin-6-one (5d) White solid, yield 90%, m.p. 167.0–169.0 °C. FTIR (KBr, cm−1) 3441, 3132, 2931, 1640, 1604, 1516, 1493, 1456, 1419, 1374, 1312, 1271, 1234, 1212, 1153, 1028, 907, 768. 1H-NMR (CDCl3) δ 7.85–7.87 (m, 2H), 7.51 (s, 1H), 7.06 (d, J = 8.2 Hz, 1H), 4.06 (s, 3H), 4.02 (s, 3H), 3.74 (s, 3H), 3.60 (s, 1H), 3.33 (s, 1H), 2.95 (t, J = 6.3 Hz, 4H), 1.86 (m, 2H), 1.68 (m, 2H). 13C-NMR (CDCl3) δ 153.4, 149.5, 139.7, 139.2, 129.1, 127.7, 124.7, 119.6, 118.6, 108.8, 102.7, 56.3, 56.2, 39.7, 30.3, 29.6, 23.4, 22.3. ESI-MS: m/z: 324.16 ([M + H]+). Anal. Calcd for C20H21NO3: C 74.28; H 6.55; N 4.33; O 14.84. Found: C 74.03; H 6.68; N 4.24; O 14.92.
2-fluoro-8,9-dimethoxy-5-methyl-5H-phenanthridin-6-one (5g) White solid, yield 87%, m.p. 215.0–216.0 °C. FTIR (KBr, cm−1) 3433, 3134, 1640, 1594, 1515, 1465, 1403, 1320, 1277, 1187, 1027, 856, 808, 780, 616. 1H-NMR (CDCl3) δ 7.94 (s, 1H), 7.82–7.79 (dd, J = 2.7, 6.9 Hz, 1H), 7.47 (s, 1H), 7.38–7.35 (dd, J = 4.4, 4.7 Hz, 1H), 7.25-7.21 (m, 1H), 4.09 (s, 3H), 4.04 (s, 3H), 3.81 (s, 3H). 13C-NMR (CDCl3) δ 160.8, 158.5 (d, J = 241.1 Hz), 153.3, 150.3, 134.0, 127.3, 120.5 (d, J = 7.7 Hz), 120.0, 116.5 (d, J = 9.0 Hz), 115.8 (d, J = 23.0 Hz), 109.2, 108.5 (d, J = 23.5 Hz), 102.7, 56.3, 56.2, 30.2. ESI-MS m/z: 288.12 ([M + H]+). Anal. Calcd for C16H14FNO3: C 66.89; H 4.91; N 4.88; O 16.71. Found: C 66.61; H 4.73; N 4.55; O 16.45.
8,9-dimethoxy-5-methyl-6-oxo-5,6-dihydro-phenanthridine-2-carboxylic acid methyl ester (5j) White solid, yield 84%, m.p. 217.6–218.6 °C. FTIR (KBr, cm−1) 3435, 3134, 1711, 1648, 1613, 1516, 1402, 1314, 1267, 1112, 1032. 1H-NMR (CDCl3) δ 8.84 (d, J = 1.9 Hz, 1H), 8.16-8.14 (dd, J = 1.9, 1.9 Hz, 1H), 7.93 (s, 1H), 7.67 (s, 1H), 7.44 (d, J = 8.9 Hz, 1H), 4.14 (s, 3H), 4.05 (s, 3H), 4.00 (s, 3H), 3.84 (s, 3H). 13C-NMR (CDCl3) δ 166.9, 161.4, 153.8, 150.5, 140.9, 129.6, 128.0, 124.8, 124.0, 119.8, 119.1, 115.1, 109.3, 102.9, 56.5, 56.4, 52.4, 30.4. ESI-MS m/z: 318.12 ([M + H]+). Anal. Calcd for C18H17NO5: C 66.05; H 5.23; N 4.28; O 24.44. Found: C 66.21; H 5.14; N 3.96; O 24.16.
3.3. General Procedure for the Synthesis of Compounds 6a–d
TFA (6 mL) was slowly added to the flask with 5b (1.70 g, 4.193 mmol) under nitrogen atmosphere at 75 °C. The mixture was stirred overnight. After cooling, the reaction mixture was quenched with ethyl acetate and water, and then extracted with ethyl acetate. The organic phase was washed with H2O, aqueous NaHCO3 and brine, and dried over anhydrous Na2SO4. The crude product was purified on silica gel column chromatography (DCM/EA, v/v = 1:1) to obtain compound 6a. The same method was used for 6b–d from reactants 5e, 5h, and 5k.
3,8,9-trimethoxy-5H-phenanthridin-6-one (6a) White solid, yield 69%, m.p. 267.4–269.1 °C. FTIR (KBr, cm−1) 3440, 3160, 1662, 1612, 1503, 1404, 1330, 1258, 1210, 1176, 1098, 1044, 877, 840, 802. 1H-NMR (CDCl3) δ 9.80 (br, 1H), 7.99 (d, J = 8.9 Hz, 1H), 7.51 (s, 1H), 6.89 (d, J = 8.9 Hz, 1H), 6.74 (s, 1H), 4.09 (s, 3H), 4.04 (s, 3H), 3.91 (s, 3H). 13C-NMR (DMSO-d6) δ 160.8, 159.7, 153.4, 148.7, 137.5, 129.5, 124.7, 117.9, 111.4, 109.9, 107.9, 103.6, 99.3, 56.1, 55.6, 55.3. ESI-MS m/z: 286.12 ([M + H]+). Anal. Calcd for C16H15NO4: C 67.36; H 5.30; N 4.91; O 22.43. Found: C 67.07; H 5.38; N 4.76; O 22.24.
8,9-dimethoxy-2,3,4,5-tetrahydro-1H-benzo[c]phenanthridin-6-one (6b) White solid, yield 76%, m.p. 253.0–255.0 °C. FTIR (KBr, cm−1) 3439, 3132, 1651, 1610, 1496, 1398, 1234, 1129, 1079, 836. 1H-NMR (CDCl3) δ 8.56 (br, 1H), 7.87-7.86 (m, 2H), 7.59 (s, 1H), 7.03 (d, J = 8.0 Hz, 1H), 4.09 (s, 3H), 4.04 (s, 3H), 2.88 (t, J = 6.1 Hz, 2H), 2.77 (t, J = 6.4 Hz, 2H), 2.01-1.96 (m, 1H), 1.87-1.83 (m, 2H). 13C-NMR (CDCl3) δ 166.7, 153.9, 149.7, 138.4, 1335, 132.1, 124.0, 121.9, 119.6, 116.0, 108.6, 103.0, 56.4, 56.3, 30.1, 23.6, 22.8, 22.5. ESI-MS m/z: 310.15 ([M + H]+). Anal. Calcd for C19H19NO3: C 73.77; H 6.19; N 4.53; O 15.52. Found: C 73.43; H 6.02; N 4.36; O 15.38.
2-fluoro-8,9-dimethoxy-5H-phenanthridin-6-one (6c) White solid; yield 54%, m.p. 192.1–193.0 °C. FTIR (KBr, cm−1) 3438, 3142, 1643, 1600, 1535, 1510, 1402, 1262, 1213, 1029, 824. 1H-NMR (CDCl3) δ 7.94 (br, 1H), 7.63–7.61 (m, 2H), 7.33 (s, 1H), 7.10–7.05 (m, 2H), 3.93 (s, 3H), 3.92 (s, 1H). 13C-NMR (CDCl3) δ 164.9, 160.0 (d, J = 243.7), 151.4, 148.8, 133.8, 129.0, 122.0, 122.0, 116.0, 116.0, 115.9, 113.4, 110.0, 56.5, 56.4. ESI-MS m/z: 274.35 ([M + H]+). Anal. Calcd for C15H12FNO3: C 65.93; H 4.43; N 5.13; O 17.57. Found: C 65.27; H 4.30; N 5.06; O 17.42.
8,9-dimethoxy-6-oxo-5,6-dihydro-phenanthridine-2-carboxylic acid methyl ester (6d) White solid, yield 78%, m.p. 296.5–299.0 °C. FTIR (KBr, cm−1) 3438, 3156, 1709, 1663, 1617, 1514, 1402, 1267, 1092, 1035. 1H-NMR (DMSO-d6) δ 11.93 (s, 1H), 8.84–8.82 (m, 1H), 8.00–7.97 (m, 1H), 7.84 (d, J = 3.0 Hz, 1H), 7.70 (s, 1H), 7.39 (d, J = 9.0 Hz, 1H), 4.06 (s, 3H), 3.91 (s, 3H), 3.90 (s, 3H). 13C-NMR (DMSO-d6) δ 166.1, 161.0, 153.9, 150.3, 140.0, 129.6, 128.8, 125.1, 124.0, 119.8, 117.8, 116.7, 108.3, 104.6, 56.7, 56.1, 52.6. ESI-MS m/z: 314.11 ([M + H]+). Anal. Calcd for C17H15NO5: C 65.17; H 4.83; N 4.47; O 25.53. Found: C 65.62; H 4.91; N 4.19; O 25.17.
3.4. General Procedure for the Synthesis of Compounds 7a–c
LiAlH4 (325 mg, 8.56 mmol) was added to the solution of 5c (940 mg, 2.85 mmol) in dry THF (30 mL) under nitrogen atmosphere at 0 °C, and then the mixture was stirred for 4 h at room temperature. The progress of the reaction was monitored by TLC. The reaction mixture was cooled to room temperature, followed by extraction with DCM. The organic phase was washed with H2O and brine, dried over anhydrous Na2SO4, and then concentrated under reduced pressure. The crude product was purified on silica gel column chromatography (PE/EA, v/v = 1:2) to obtain compound 7a. The same method was used for 7b and 7c from compounds 5f and 5i.
3,8,9-trimethoxyphenanthridine (7a) White solid, yield 55%, m.p. 163.0–165.0 °C. FTIR (KBr, cm−1) 3435, 3141, 1619, 1504, 1398, 1274, 1213, 1162, 1034, 840, 808. 1H-NMR (CDCl3) δ 9.13 (s, 1H), 8.36 (d, J = 8.0 Hz, 1H), 7.81 (s, 1H), 7.63 (s, 1H), 7.35 (s, 1H), 7.33–7.30 (m, 1H), 4.15 (s, 3H), 4.07 (s, 3H), 4.00 (s, 3H). 13C-NMR (CDCl3) δ 159.6, 153.3, 152.1, 149.5, 145.6, 128.8, 123.0, 121.0, 118.2, 118.1, 109.7, 107.9, 101.5, 56.3, 56.2, 55.7. ESI-MS m/z: 270.12 ([M + H]+). Anal. Calcd for C16H15NO3: C 71.36; H 5.61; N 5.20; O 17.82. Found: C 70.90; H 5.43; N 5.15; O 17.55.
8,9-dimethoxy-1,2,3,4-tetrahydrobenzo[c]phenanthridine (7b) White solid, yield 45%, m.p. 178.6–179.8 °C. FTIR (KBr, cm−1) 3437, 3140, 2930, 1612, 1502, 1405, 1269, 1201, 1157, 1027, 846. 1H-NMR (CDCl3) δ 9.14 (s, 1H), 8.18 (d, J = 8.7 Hz, 1H), 7.82 (s, 1H), 7.35 (d, J = 8.7 Hz, 1H), 7.31 (s, 1H), 4.12 (s, 3H), 4.06 (s, 3H), 3.40 (t, J = 5.3 Hz, 2H), 2.97 (t, J = 6.0 Hz, 2H), 1.99–1.96 (m, 2H), 1.93–1.90 (m, 2H). 13C-NMR (CDCl3) δ 152.9, 150.2, 149.7, 142.5, 136.9, 135.6, 128.8, 128.5, 121.5, 121.4, 118.8, 107.7, 101.8, 56.2, 56.2, 30.3, 25.8, 23.3, 23.1. ESI-MS m/z: 294.15 ([M + H]+). Anal. Calcd for C19H19NO2: C 77.79; H 6.53; N 4.77; O 10.91. Found: C 77.34; H 6.38; N 4.52; O 10.94.
2-fluoro-8,9-dimethoxyphenanthridine (7c) White solid, yield 47%, m.p. 178.0–179.0 °C. FTIR (KBr, cm−1) 3436, 3134, 1617, 1511, 1400, 1270, 1195, 1151, 1098, 1028, 848. 1H-NMR (CDCl3) δ 9.11 (s, 1H), 8.16–8.05 (m, 1H), 8.04–8.02 (m, 1H), 7.74 (s, 1H), 7.45–7.41 (m, 1H), 7.37 (s, 1H), 4.15 (s, 3H), 4.08 (s, 3H). 13C-NMR (CDCl3) δ 161.3 (d, J = 247.4), 153.3, 150.9 (d, J = 2.0), 150.7, 140.6, 132.2 (d, J = 9.0), 127.9 (d, J = 4.4), 125.3, 121.9, 117.0 (d, J = 24.4), 108.0, 106.6 (d, J = 23.2), 102.1, 56.4, 56.3, ESI-MS m/z: 258.11 ([M + H]+). Anal. Calcd for C15H12FNO2: C 70.03; H 4.70; N 5.44; O 12.44. Found: C 69.61; H 5.01; N 5.22; O 12.77.
3.5. General Procedure for the Synthesis of Compounds 8a–c and 11a–b
To the solution of 6b (216 mg, 0.70 mmol) in DMF (5 mL), Cs2CO3 (456.83 mg, 1.40 mmol), TBAI (38.84 mg, 0.105 mmol), and ethyl bromoacetate (526.83 mg, 3.15 mmol) were added, and the mixture was stirred for 1 h at 90 °C. After cooled to room temperature, the mixture was extracted with ethyl acetate. The organic phase was washed with H2O and brine, dried over anhydrous Na2SO4, and then concentrated under reduced pressure. The residue was purified on silica gel column chromatography (PE/EA, v/v = 4:1) to obtain compound 8a. Same method was used for compounds 8b, 8c from 6c and 6d. Ethyl 4-bromobutyrate was used instead of ethyl bromoacetate to 11a, 11b from 6b and 6c.
(8,9-dimethoxy-6-oxo-1,3,4,6-tetrahydro-2H-benzo[c]phenanthridin-5-yl)-acetic acid ethyl ester (8a) White solid, yield 87.%, m.p. 152.3–153.7 °C. FTIR (KBr, cm−1) 3440, 3132, 2927, 1753, 1599, 1525, 1401, 1333, 1266, 1203, 1175, 1125, 1087, 1042, 860, 767. 1H-NMR (CDCl3) δ 8.05 (d, J = 8.2 Hz, 1H), 7.77 (s, 1H), 7.73 (s, 1H), 7.19 (d, J = 8.2 Hz, 1H), 5.10 (s, 2H), 4.25 (q, J = 7.1 Hz, 2H), 4.11 (s, 3H), 4.06 (s, 3H), 3.16 (t, J = 6.1 Hz, 2H), 2.92 (t, J = 5.9 Hz, 2H), 1.93–1.86 (m, 4H), 1.28 (t, J = 6.3 Hz, 3H). 13C-NMR (CDCl3) δ 169.7, 155.8, 152.8, 149.4, 140.5, 137.1, 134.1, 131.3, 126.0, 120.1, 118.6, 113.2, 104.8, 102.4, 63.1, 61.0, 56.2, 56.2, 30.2, 25.2, 23.4, 23.3, 14.4. ESI-MS m/z: 396.17 ([M + H]+). Anal. Calcd for C23H25NO5: C 69.86; H 6.37; N 3.54; O 20.23. Found: C 69.71; H 6.20; N 3.42; O 20.89.
(2-fluoro-8,9-dimethoxy-6-oxo-6H-phenanthridin-5-yl)-acetic acid ethyl ester (8b) White solid, yield 95%, m.p. 152.3–153.7 °C. FTIR (KBr, cm−1) 3439, 3128, 1744, 1659, 1600, 1509, 1399, 1258, 1208, 1024, 845. 1H-NMR (CDCl3) δ 7.28–7.25 (m, 1H), 6.90–6.87 (m, 2H), 6.84 (s, 1H), 6.65 (s, 1H), 4.55 (s, 2H), 4.25 (q, J = 7.2 Hz, 2H), 3.80 (s, 3H), 3.71 (s, 3H), 1.31 (t, J = 7.1 Hz, 3H). 13C-NMR (CDCl3) δ 169.1, 169.0, 161.6 (d, J = 240 Hz), 150.0, 148.0, 138.7, 138.6, 129.4, 129.4, 116.1, 116.0, 115.4, 112.0, 110.6, 61.7, 56.2, 56.2, 51.5, 29.8, 14.3. ESI-MS m/z: 360.09 ([M + H]+). Anal. Calcd for C19H18FNO5: C 63.50; H 5.05; N 3.90; O 22.26. Found: C 63.04; H 4.85; N 3.62; O 21.10.
5-ethoxycarbonylmethyl-8,9-dimethoxy-6-oxo-5,6-dihydro-phenanthridine-2-carboxylic acid methyl ester (8c) White solid, yield 87%, m.p. 211.9–214.0 °C. FTIR (KBr, cm−1) 3432, 3134, 1740, 1711, 1648, 1614, 1517, 1400, 1322, 1270, 1215, 1118, 1046, 1017, 835, 769. 1H-NMR (CDCl3) δ 8.85 (s, 1H), 8.10 (d, J = 8.9 Hz, 1H), 7.91 (s, 1H), 7.67 (s, 1H), 7.17 (d, J = 8.9 Hz, 1H), 5.21 (s, 2H), 4.25 (q, J = 7.2 Hz, 2H), 4.14 (s, 3H), 4.04 (s, 3H), 3.99 (s, 3H), 1.27 (t, J = 7.2 Hz, 4H). 13C-NMR (CDCl3) δ 168.3, 166.7, 154.2, 150.5, 140.1, 129.7, 128.4, 125.2, 124.4, 119.3, 119.2, 114.6, 109.4, 103.1, 62.0, 56.6, 56.4, 52.4, 44.7, 14.3. ESI-MS m/z: 400.13 ([M + H]+). Anal. Calcd for C21H21NO7: C 63.15; H 5.30; N 3.51; O 28.04. Found: C 63.27; H 5.41; N 3.49; O 27.86.
4-(8,9-dimethoxy-6-oxo-1,3,4,6-tetrahydro-2H-benzo[c]phenanthridin-5-yl)-butyric acid ethyl ester (11a) White solid, yield 76%, m.p. 104.8–106.8 °C. FTIR (KBr, cm−1) 3434, 3137, 2921, 1728, 1595, 1528, 1403, 1322, 1267, 1228, 1176, 1035, 859, 766. 1H-NMR (CDCl3) δ 8.05 (d, J = 8.4 Hz, 1H), 7.78 (s, 1H), 7.64 (s, 1H), 7.18 (d, J = 8.4 Hz, 1H), 4.70 (t, J = 6.2 Hz, 2H), 4.14–4.10 (m, 5H), 4.06 (s, 3H), 3.25 (t, J = 6.1 Hz, 2H), 2.93 (t, J = 6.1 Hz, 2H), 2.58 (t, J = 7.4 Hz, 2H), 2.33–2.27 (m, 2H), 1.95–1.87 (m, 4H), 1.22 (t, J = 7.6 Hz, 3H). 13C-NMR (CDCl3) δ 173.6, 156.9, 152.6, 149.3, 141.1, 137.0, 134.1, 131.0, 125.6, 119.7, 118.5, 113.8, 104.7, 102.4, 65.0, 60.5, 56.2, 56.2, 31.7, 30.3, 25.4, 24.8, 23.5, 23.3, 14.3. ESI-MS m/z: 424.20 ([M + H]+). Anal. Calcd for C25H29NO5: C 70.90; H 6.90; N 3.31; O 18.89. Found: C 70.82; H 6.95; N 3.38; O 18.77.
4-(2-fluoro-8,9-dimethoxy-6-oxo-6H-phenanthridin-5-yl)-butyric acid ethyl ester (11b) Yellow oil, yield 79.%. FTIR (KBr, cm−1) 3437, 3120, 2934, 1729, 1643, 1598, 1508, 1444, 1405, 1323, 1259, 1210, 1184, 1092, 1023, 848, 787. 1H-NMR (CDCl3) δ 7.15–7.12 (m, 2H), 6.91–6.87 (m, 1H), 6.81 (s, 1H), 6.54 (s, 1H), 4.12 (q, J = 7.1 Hz, 2H), 3.94 (t, J = 6.7 Hz, 1H), 3.78 (s, 3H), 3.71 (s, 3H), 2.45 (t, J = 6.8 Hz, 1H), 2.00–1.94 (m, 2H), 1.24 (t, J = 7.1 Hz, 3H). 13C-NMR (CDCl3) δ 173.1, 168.7, 161.4 (d, J = 248.0), 149.7, 148.0, 138.0, 138.0, 129.5, 129.4, 116.2, 116.0, 115.3, 111.7, 110.5, 60.6, 56.2, 56.2, 489, 31.7, 23.1, 14.3. ESI-MS m/z: 388.12 ([M + H]+). Anal. Calcd for C21H22FNO5: C 65.11; H 5.72; N 3.62; O 20.65. Found: C 64.94; H 5.63; N 3.65; O 20.32.
3.6. General Procedure for the Synthesis of Compounds 9a–b and 12a–b
To the solution of 8a (107 mg, 0.27 mmol) in MeOH (27 mL) was added the solution of 10N NaOH (18 mL). The reaction mixture was refluxed for 16 h at room temperature. And then 2 M HCl was added to adjust pH~2. The aqueous layer was extracted with ethyl acetate. The combined organic phase was washed with H2O and brine, dried over anhydrous Na2SO4, and then concentrated under reduced pressure. The residue was purified on silica gel column chromatography (DCM/MeOH, v/v = 15:1) to obtain compound 9a. The same method was used for 9b, 12a, and 12b from reactants 8b, 11a, and 11b.
(8,9-dimethoxy-6-oxo-1,3,4,6-tetrahydro-2H-benzo[c]phenanthridin-5-yl)-acetic acid (9a) White solid, yield 93%, m.p. 181.3–182.3 °C. FTIR (KBr, cm−1) 3130, 2932, 1724, 1599, 1402, 1330, 1261, 1211, 1173, 1127, 1036. 1H-NMR (CDCl3) δ 8.09 (d, J = 8.4 Hz, 1H), 7.80 (s, 1H), 7.68 (s, 1H), 7.27 (d, J = 8.4, 1H), 5.18 (s, 2H), 4.13 (s, 3H), 4.06 (s, 3H), 3.17 (t, J = 6.0 Hz, 2H), 2.94 (t, J = 6.0 Hz, 2H), 1.95–1.86 (m, 4H). 13C-NMR (CDCl3) δ 157.1, 153.4, 149.7, 137.8, 133.6, 131.7, 126.7, 120.3, 118.7, 112.9, 104.5, 102.4, 65.4, 56.3, 56.3, 30.3, 29.9, 25.2, 23.2, 23.1. ESI-MS m/z: 368.14 ([M + H]+). Anal. Calcd for C21H21NO5: C 68.65; H 5.76; N 3.81; O 21.77. Found: C 68.50; H 5.34; N 3.73; O 21.52.
(2-fluoro-8,9-dimethoxy-6-oxo-6H-phenanthridin-5-yl)-acetic acid (9b) White solid, yield 96%, m.p. 107.0–108.8 °C. FTIR (KBr, cm−1) 3439, 3132, 1638, 1509, 1400, 1258, 1212, 1014, 844. 1H-NMR (CDCl3) δ 7.25 (m, 1H), 6.90–6.88 (m, 2H), 6.84 (s, 1H), 6.65 (s, 1H), 4.61 (s, 2H), 3.80 (s, 3H), 3.72 (s, 3H). 13C-NMR (CDCl3) δ 169.7, 162.5, 160.5, 150.0, 148.0, 138.6, 138.6, 129.3, 129.2, 116.1, 115.9, 115.2, 112.1, 110.4, 56.3, 56.1, 29.8. ESI-MS m/z: 332.12 ([M + H]+). Anal. Calcd for C17H14FNO5: C 61.63; H 4.26; N 4.23; O 24.15. Found: C 61.42; H 4.55; N 4.05; O 23.80.
4-(8,9-dimethoxy-6-oxo-1,3,4,6-tetrahydro-2H-benzo[c]phenanthridin-5-yl)-butyric acid (12a) White solid, yield 95%, m.p. 209.0–210.0 °C. FTIR (KBr, cm−1) 3443, 3130, 2931, 1703, 1594, 1503, 1401, 1325, 1270, 1208, 1040, 1001, 864, 774. 1H-NMR (DMSO-d6) δ 8.33 (d, J = 8.4 Hz, 1H), 8.01 (s, 1H), 7.58 (s, 1H), 7.19 (d, J = 8.4 Hz, 1H), 4.59 (t, J = 7.1 Hz, 2H), 4.03 (s, 3H), 3.93 (s, 3H), 3.16 (t, J = 7.7 Hz, 2H), 2.88 (t, J = 4.8 Hz, 2H), 2.48 (t, J = 6.7 Hz, 2H), 2.16–2.11 (m, 2H), 1.87-1.81 (m, 4H). 13C-NMR (DMSO-d6) δ 174.7, 156.8, 153.2, 149.6, 140.7, 136.8, 133.2, 130.8, 125.8, 119.9, 119.7, 113.1, 104.5, 103.5, 65.3, 56.4, 56.1, 31.3, 29.9, 25.2, 24.6, 23.3, 23.2. ESI-MS m/z: 396.18 ([M + H]+). Anal. Calcd for C23H25NO5: C 69.86; H 6.37; N 3.54; O 20.23. Found: C 69.88; H 6.46; N 3.29; O 20.51.
4-(2-fluoro-8,9-dimethoxy-6-oxo-6H-phenanthridin-5-yl)-butyric acid (12b) White solid, yield 98. %, m.p. 47.5–49.6 °C. FTIR (KBr, cm−1) 3440, 3132, 1644, 1509, 1400, 1257, 1213, 1024, 841. 1H-NMR (CDCl3) δ 7.14 (dd, J = 8.7, 4.8 Hz, 2H), 6.91 (t, J = 8.4 Hz, 1H), 6.82 (s, 1H), 6.53 (s, 1H), 3.97 (d, J = 6.3 Hz, 2H), 3.79 (s, 3H), 3.70 (s, 3H), 2.54 (t, J = 7.3 Hz, 2H), 2.00–1.95 (m, 2H). 13C-NMR (CDCl3) δ 176.7, 169.2, 161.5 (d, J = 251.0 Hz), 149.9, 148.1, 137.8, 130.2, 129.4 (d, J = 8.7 Hz), 116.2 (d, J = 22 Hz), 115.3, 111.6, 110.6, 100.1, 56.3, 56.2, 48.8, 31.3, 23.0. ESI-MS m/z: 360.13 ([M + H]+). Anal. Calcd for C19H18FNO5: C 63.50; H 5.05; N 3.90; O 22.26. Found: C 63.24; H 4.92; N 3.68; O 22.01.
3.7. General Procedure for the Synthesis of Compounds 10a–b and 13a–b
Compound 9a (36.8 mg, 0.1 mmol) was dissolved in THF (10 mL) and CH2Cl2 (4 mL) at 0 °C. Triethylamine (20 μL) and isobutyl chloroformate (18 μL) were added (18 μL), and the mixture was stirred for 20 min. Then dimethylamine (0.5 mL) was added at 0 °C and the mixture was stirred for 30 min at room temperature. After evaporation of solvent under reduced pressure, the mixture was extracted with ethyl acetate. The combined organic phase was washed with H2O and brine, dried over anhydrous Na2SO4, and then concentrated under reduced pressure. The crude product was purified on silica gel column chromatography (DCM/MeOH, v/v = 30:1) to obtain compound 10a. The same method was used for 10b, 13a, and 13b from 9b, 12a, and 12b.
2-(8,9-dmethoxy-6-oxo-1,3,4,6-tetrahydro-2H-benzo[c]phenanthridin-5-yl)-N,N-dimethyl-acetamide (10a) White solid, yield 85%, m.p. 196.0–197.8 °C. FTIR (KBr, cm−1) 3435, 3134, 2931, 1667, 1601, 1502, 1400, 1324, 1269, 1215, 1175, 1122, 1035, 769. 1H-NMR (CDCl3) δ 8.05 (d, J = 8.4 Hz, 1H), 7.78 (s,1H), 7.77 (s, 1H), 7.19 (d, J = 8.4 Hz, 1H), 5.27 (s, 2H), 4.11 (s, 3H), 4.06 (s, 3H), 3.19 (s, 3H), 3.18 (t, J = 6.5 Hz, 2H), 3.04 (s, 3H), 2.92 (t, J = 6.0 Hz, 2H), 1.94–1.85 (m, 4H). 13C-NMR (CDCl3) δ 168.6, 156.0, 152.8, 149.4, 140.7, 137.0, 133.9, 131.3, 125.9, 120.1, 118.6, 113.4, 104.8, 102.4, 63.4, 56.3, 56.2, 36.6, 35.8, 30.2, 25.2, 23.5, 23.2. ESI-MS m/z: 395.19 ([M + H]+). Anal. Calcd for C23H26N2O5: C 70.03; H 6.64; N 7.10; O 16.22. Found: C 69.71; H 6.55; N 6.96; O 16.19.
2-(2-fluoro-8,9-dimethoxy-6-oxo-6H-phenanthridin-5-yl)-N,N-dimethyl-acetamide (10b) White solid; yield 83%, m.p. 57.0–59.0 °C. FTIR (KBr, cm−1) 3442, 3123, 2934, 1652, 1600, 1508, 1399, 1325, 1257, 1212, 1155, 1032, 927, 847, 790. 1H-NMR (CDCl3) δ 7.37–7.34 (m, 2H), 6.87–6.82 (m, 2H), 6.75 (s, 1H), 4.63 (s, 2H), 3.79 (s, 3H), 3.71 (s, 3H), 3.07 (s, 3H), 3.02 (s, 3H). 13C-NMR (CDCl3) δ 169.1, 167.4, 149.9, 148.1, 130.0, 129.6, 129.6, 115.9, 115.7, 115.2, 114.4, 112.3, 110.4, 108.4, 56.3, 56.2, 51.5, 36.6, 36.1. ESI-MS m/z: 361.17 ([M + H]+). Anal. Calcd for C19H19FN2O4: C 63.68; H 5.34; N 7.82; O 17.86. Found: C 63.27; H 5.09; N 7.72; O 17.48.
4-(8,9-dimethoxy-6-oxo-1,3,4,6-tetrahydro-2H-benzo[c]phenanthridin-5-yl)-N,N-dimethyl-butyramide (13a) White solid, yield 84.%, m.p. 249.2–251.5 °C. FTIR (KBr, cm−1) 3436, 3135, 2924, 1643, 1597, 1501, 1403, 1320, 1264, 1229, 1205, 1174, 1034, 996, 868, 776. 1H-NMR (CDCl3) δ 8.05 (d, J = 8.3 Hz, 1H), 7.78 (s, 1H), 7.65 (s, 1H), 7.18 (d, J = 8.3 Hz, 1H), 4.70 (t, J = 6.2 Hz, 2H), 4.11 (s, 3H), 4.05 (s, 3H), 3.25 (t, J = 5.9 Hz, 2H), 2.99 (s, 3H), 2.96 (s, 3H), 2.58 (t, J = 7.6 Hz, 2H), 2.58 (t, J = 7.5 Hz, 2H), 2.35-2.29 (m, 2H), 1.94-1.88 (m, 4H). 13C-NMR (CDCl3) δ 172.7, 156.9, 152.5, 149.2, 141.2, 136.9, 134.1, 131.0, 125.5, 119.6, 118.5, 113.8, 104.6, 102.4, 66.3, 56.2, 56.1, 37.3, 35.6, 30.3, 30.2, 25.4, 25.0, 23.4, 23.3. ESI-MS m/z: 423.22 ([M + H]+). Anal. Calcd for C25H30N2O4: C 71.07; H 7.16; N 6.63; O 15.15. Found: C 70.82; H 7.22; N 6.37; O 15.31.
4-(2-fluoro-8,9-dimethoxy-6-oxo-6H-phenanthridin-5-yl)-N,N-dimethyl-butyramide (13b) White solid, yield 82.%, m.p. 48.9–50.5 °C. FTIR (KBr, cm−1) 3437, 3132, 1646, 1508, 1400, 1258, 1211, 1024, 846. 1H-NMR (CDCl3) δ 7.18–7.05 (m, 1H), 6.88 (t, J = 8.5 Hz, 1H), 6.81 (s, 1H), 6.54 (s, 1H), 3.95 (t, J = 6.9 Hz, 2H), 3.78 (s, 3H), 3.69 (s, 3H), 3.02(s, 3H), 2.94 (s, 3H), 2.49 (t, J = 7.3 Hz, 2H), 2.02-1.97 (m, 2H). 13C-NMR (CDCl3) δ 172.3, 168.8, 149.7, 148.0, 138.2, 130.6, 129.6, 129.5, 116.2, 116.0, 115.3, 111.8, 110.5, 100.1, 56.2, 49.3, 37.4, 35.6, 30.8, 23.4. ESI-MS m/z: 387.16 ([M + H]+). Anal. Calcd for C21H23FN2O4: C 65.27; H 6.00; N 7.25; O 16.56. Found: C 64.92; H 5.97; N 6.97; O 16.37.
3.8. General Procedure for the Synthesis of Compounds 14a–c
POCl3 (0.64 mL) was added to compound 6a (100 mg, 0.35 mmol) in flask. Then the reaction mixture was stirred for 2 h at 105 °C. After cooled to room temperature, the mixture was poured carefully into a beaker filled with ice water. Concentrated ammonia water was added untill pH > 7. The precipitation was washed with water and purified on silica gel column chromatography (PE/DCM, v/v = 1:1) to obtain compound 14a. The same method was used for 14b and 14c from compounds 6b and 6d.
6-chloro-3,8,9-trimethoxyphenanthridine (14a) White solid, yield 83%, m.p. 174.1–175.6 °C. FTIR (KBr, cm−1) 3443, 3133, 1617, 1579, 1503, 1400, 1313, 1234, 1213, 1155, 1113, 1038, 912, 845. 1H-NMR (CDCl3) δ 8.29 (d, J = 9.1 Hz, 1H), 7.77 (s, 1H), 7.72 (s, 1H), 7.47 (d, J = 2.6 Hz, 1H), 7.29 (d, J = 2.6 Hz), 4.14 (s, 3H), 4.09 (s, 3H), 3.96 (s, 3H). 13C-NMR (CDCl3) δ 160.1, 153.8, 150.4, 149.8, 144.5, 130.9, 123.0, 119.0, 118.5, 118.0, 109.0, 107.1, 101.8, 56.4, 56.3, 55.8. ESI-MS m/z: 304.08 ([M + H]+). Anal. Calcd for C16H14ClNO3: C 63.27; H 4.65; N 4.61; O 15.80. Found: C 63.41; H 4.80; N 4.39; O 16.26.
6-chloro-8,9-dimethoxy-1,2,3,4-tetrahydrobenzo[c]phenanthridine (14b) White solid, yield 85%, m.p. 201.3–202.3 °C. FTIR (KBr, cm−1) 3434, 3133, 2928, 1613, 1578, 1523, 1501, 1465, 1402, 1299, 1249, 1206, 1160, 1081, 1043, 953, 840. 1H-NMR (CDCl3) δ 8.13 (d, J = 8.4 Hz, 1H), 7.83 (s, 1H), 7.72 (s, 1H), 7.34 (d, J = 8.4 Hz, 1H), 4.14 (s, 3H), 4.09 (s, 3H), 3.35 (t, J = 6.1 Hz, 2H), 2.96 (t, J = 6.0 Hz, 2H), 1.96-1.89 (m, 4H)). 13C-NMR (CDCl3) δ 153.2, 149.0, 148.5, 141.9, 137.6, 135.3, 130.9, 128.7, 121.5, 119.6, 118.6, 106.9, 102.2, 56.3, 56.3, 30.2, 25.3, 23.1, 23.1. ESI-MS m/z: 328.11 ([M + H]+). Anal. Calcd for C19H18ClNO2: C 69.62; H 5.53; N 4.27; O 9.76. Found: C 69.62; H 5.48; N 4.17; O 9.45.
9-chloro-6,7-dimethoxy-phenanthrene-3-carboxylic acid methyl ester (14c) White solid, yield 83.%, m.p. 194.9–196.6 °C. FTIR (KBr, cm−1) 3444, 3135, 1716, 1615, 1514, 1400, 1254, 1163, 1101, 1037, 849. 1H-NMR (CDCl3) δ 9.08 (d, J = 1.4 Hz, 1H), 8.26 (dd, J = 8.6, 1.7 Hz, 1H), 8.06 (d, J = 8.6 Hz, 1H), 7.91 (s, 1H), 7.74 (s, 1H), 4.19 (s, 3H), 4.10 (s, 3H), 4.03 (s, 3H). 13C-NMR (CDCl3) δ 167.0, 154.1, 152.4, 150.9, 145.5, 130.6, 129.6, 128.5, 128.3, 124.6, 123.4, 120.4, 107.3, 102.5, 56.7, 56.4, 52.7. ESI-MS m/z: 332.07 ([M + H]+). Anal. Calcd for C17H14ClNO4: C 61.55; H 4.25; N 4.22; O 19.29. Found: C 61.76; H 4.72; N 4.02; O 19.77.
3.9. General Procedure for the Synthesis of Compounds 15a–c
To compound 14a (60 mg, 0.20 mmol) was added N,N-dimethylaminoethylamine (696.5 mg, 7.90 mmol) under nitrogen atmosphere, and the reaction mixture was stirred at 105 °C for 6 h. After cooled to room temperature, the solvent was removed under reduced pressure and extracted with CH2Cl2. The organic phase was washed with 5% NaOH and H2O, dried over anhydrous Na2SO4, and then concentrated. The crude product was purified on silica gel column chromatography (DCM/MeOH, v/v = 5:1) to obtain compound 15a. The same method was use for 15b and 15c from 14b and 14c.
N,N-dimethyl-N’-(3,8,9-trimethoxy-phenanthridin-6-yl)-ethane-1,2-diamine (15a) Yellow solid, yield 59.%, m.p. 126.0–128.0 °C. FTIR (KBr, cm−1) 3414, 3138, 1617, 1592, 1400, 1306, 1209, 1171, 1039, 841, 801. 1H-NMR (CDCl3) δ 8.08 (d, J = 8.8 Hz, 1H), 7.67 (d, J = 2.4 Hz, 1H), 7.34 (s, 1H), 7.16 (s, 1H), 6.94 (dd, J = 8.8, 2.3 Hz, 1H), 6.41 (br, 1H), 4.07 (s, 3H), 4.05 (s, 3H), 3.93 (s, 3H), 3.81 (t, J = 6.2 Hz, 2H), 2.74 (t, J = 6.2 Hz, 2H), 2.37 (s, 6H). 13C-NMR (CDCl3) δ 160.2, 153.7, 153.6, 149.8, 129.4, 122.3, 114.4, 112.8, 112.1, 106.5, 104.8, 102.3, 59.4, 57.7, 56.2, 55.6, 45.9, 44.6, 39.8. ESI-MS m/z: 356.19 ([M + H]+). Anal. Calcd for C20H25N3O3: C 67.58; H 7.09; N 11.82; O 13.50. Found: C 67.92; H 7.15; N 11.70; O 13.26.
N’-(8,9-dimethoxy-1,2,3,4-tetrahydro-benzo[c]phenanthridin-6-yl)-N,N-dimethyl-ethane-1,2-diamine (15b) Yellow solid, yield 78.%, m.p. 121.6–123.0 °C. FTIR (KBr, cm−1) 3430, 3134, 2926, 1593, 1530, 1488, 1401, 1262, 1253, 1207, 1037, 837, 784. 1H-NMR (CDCl3) δ 7.98 (d, J = 8.3 Hz, 1H), 7.77 (s, 1H), 7.42 (s, 1H), 7.06 (d, J = 8.3 Hz, 1H), 6.40 (br, 1H), 4.09 (s, 3H), 4.08 (s, 3H), 3.88 (t, J = 5.6 Hz, 2H), 3.21 (t, J = 6.2 Hz, 2H), 2.91 (t, J = 6.2 Hz, 2H), 2.84 (t, J = 6.7 Hz, 2H), 2.42 (s, 6H), 1.96–1.85 (m, 4H). 13C-NMR (CDCl3) δ 152.0, 151.9, 149.1, 141.8, 136.8, 132.5, 129.8, 123.9, 118.5, 117.9, 113.1, 103.8, 103.0, 58.7, 56.5, 56.0, 45.4, 39.2, 30.3, 25.4, 23.6, 23.4. ESI-MS m/z: 380.22 ([M + H]+). Anal. Calcd for C23H29N3O2: C 72.79; H 7.70; N 11.07; O 8.43. Found: C 72.24; H 7.85; N 10.83; O 8.91.
6-(2-dimethylamino-ethylamino)-8,9-dimethoxy-phenanthridine-2-carboxylic acid (15c) Yellow solid, yield 69%, m.p. 192.4–193.5 °C. FTIR (KBr, cm−1) 3451, 3142, 1687, 1619, 1590, 1511, 1398, 1262, 1209, 1115, 1033, 843. 1H-NMR (CDCl3) δ 8.95 (s, 1H), 8.13-8.11 (m, 1H), 7.89 (s, 1H), 7.73 (d, J = 8.3, Hz, 1H), 7.23 (s, 1H), 6.24 (br, 1H), 4.14 (s, 3H), 4.06 (s, 3H), 3.83-3.79 (m, 2H), 2.70 (t, J = 5.3 Hz, 2H), 2.34 (s, 6H). 13C-NMR (CDCl3) δ 167.4, 154.4, 152.4, 149.9, 148.1, 129.4, 128.4, 126.8, 124.4, 123.6, 120.1, 113.8, 103.4, 103.3, 58.2, 56.4, 56.4, 45.4, 38.9, 29.8. ESI-MS m/z: 384.17([M + H]+). Anal. Calcd for C21H25N3O4: C 65.78; H 6.57; N 10.96; O 16.69. Found: C 65.59; H 6.71; N 10.95; O 16.45.
3.10. General Procedure for the Synthesis of Compounds 16a–b
CH3I (158.2 mg, 1.11 mmol) was added to the suspension of compound 7a (100 mg, 0.37 mmol) in dry toluene, and the mixture was stirred for 16 h at 80 °C. After cooled to room temperature, the solution was filtrated and washed with toluene and ether to obtain compound 16a. The same method was used for 16b from compound 7c.
3,8,9-trimethoxy-5-methyl-phenanthridinium iodide (16a) Yellow solid, yield 84%, m.p. 245.2–247.2 °C. FTIR (KBr, cm−1) 3423, 3131, 1624, 1501, 1402, 1294, 1232, 1172, 1033, 836. 1H-NMR (DMSO-d6) δ 9.81 (s, 1H), 9.09 (d, J = 9.2 Hz, 1H), 8.30 (s, 1H), 7.82 (s, 1H), 7.71–7.66 (m, 2H), 4.55 (s, 3H), 4.19 (s, 3H), 4.09 (s, 3H), 4.00 (s, 3H). 13C-NMR (DMSO-d6) δ 161.8, 158.8, 151.5, 151.0, 135.9, 132.9, 127.0, 120.0, 119.3, 118.5, 109.9, 103.2, 101.0, 57.7, 56.9, 56.7, 45.8. ESI-MS m/z: 284.14 ([M + H]+). Anal. Calcd for C17H18NO3: C 49.65; H 4.41; N 3.41; O 11.67. Found: C 49.52; H 4.32; N 3.49; O 12.17.
2-fluoro-8,9-dimethoxy-5-methyl-phenanthridinium iodide (16b) Yellow solid, yield 75%, m.p. 246.7–247.7 °C.FTIR (KBr, cm−1) 3438, 3120, 3016, 1606, 1516, 1474, 1438, 1400, 1279, 1205, 1131, 1036, 988, 876. 1H-NMR (DMSO-d6) δ 9.89 (s, 1H), 9.08 (d, J = 10.3 Hz, 1H), 8.55–8.52 (m, 1H), 8.40 (s, 1H), 8.03–7.99 (m, 1H), 7.92 (s, 1H), 4.59 (s, 3H), 4.20 (s, 3H), 4.03 (s, 3H). 13C-NMR (DMSO-d6) δ 162.1 (d, J = 249.6 Hz), 158.6, 152.0, 151.9, 131.7 (d, J = 3.8 Hz), 130.0, 127.2 (d, J = 9.9 Hz), 123.3 (d, J = 9.6 Hz), 120.3 (d, J = 25.1 Hz), 119.6, 110.7, 110.4 (d, J = 25.0 Hz), 104.7, 58.0, 56.8, 46.1. ESI-MS m/z: 272.11 ([M + H]+). Anal. Calcd for C16H15FINO2: C 48.14; H 3.79; N 3.51; O 8.02. Found: C48.30; H 3.72; N 3.62; O 8.43.
3.11. Cytotoxicity Assay In Vitro
All cell lines used in this study, including HepG2, A549, NCI-H460, and CNE1, were purchased from the cell bank of Chinese Academy of Sciences (Shanghai, China) and cultured at 37 °C in a humidified atmosphere containing 5% CO2. HepG2, A549 and CNE1 cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% (v/v) FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. NCI-H460 cell line was cultured in ATCC Modified 1640 Medium supplemented with 10% (v/v) FBS, 100 U/mL penicillin and 100 μg/mL streptomycin.
The antitumor activity of new compounds was evaluated by MTT assay in vitro. 5 × 103 cells in 100 μL medium were plated to each well of a 96-well flat-bottom microtiter plate and cultured overnight. Then the cells were treated with serial diluted candidate compounds. The control was treated with equivalent DMSO. After 48 h, 10 μL MTT stock (5 mg/mL) was added to each well and incubated for 2 h. Then, the supernatants were discarded and 100 μL DMSO was added to each well. After 10 minutes’ shaking, the optical density at the wavelengths of 490 nm (OD490) was measured on a SPARK microplate reader (Tecan, Männedorf, Switzerland). All samples were repeated 3 times and each time tested in triplicate. The cell inhibition rate of each sample was calculated by the following formula. The IC50 values of the compounds were calculated using SPSS 17.0 software.
| Inhibition (%) = [1 − (ODsample − ODblank)/(ODcontrol − ODblank)] × 100%. | (1) |
4. Conclusions
In conclusion, we have synthesized series of structurally simple phenanthridine analogues based on nitidine and evaluated their antitumor activities against human cancer cell lines including HepG2, A549, NCI-H460, and CNE1 cells. Most of the derivatives exhibited moderate to high activity, especially compounds 15a, 15b, and 15c. It was found that the C-6 modified structure could greatly increase the antitumor activity, and the structure of ammonium salt was not necessary to the antitumor activity in the test compounds. The result motivated us to investigate more C-6 substituted derivatives and their structure–activity relationships to discover potent antitumor drugs with high activity and excellent selectivity.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 21602038), Fundamental Research Fund of Guangxi Institute of Botany (16002) and the Director of Capital Program of Guangxi Key Laboratory of Functional Phytochemicals Research and Utilization (ZRJJ2016-2).
Supplementary Materials
The following are available online. FTIR, 1H-NMR, 13C-NMR and ESI-MS spectra of compounds.
Author Contributions
S.-Q.Q. carried out the experiment. J.-R.S. conceptualized and designed the experiment route, and wrote the paper. L.-C.L. participated in the discussion of antitumor activity. H.L. and D.-P.L. supervised the work.
Funding
This research was funded by the National Natural Science Foundation of China (No. 21602038), Fundamental Research Fund of Guangxi Institute of Botany (16002) and the Director of Capital Program of Guangxi Key Laboratory of Functional Phytochemicals Research and Utilization (ZRJJ2016-2).
Conflicts of Interest
The authors declare no conflict interest.
Footnotes
Sample Availability: Samples of the compounds 4–16 are available from the authors.
References
- 1.Wei F., Wu Y., Tang L., Xiong F., Guo C., Li X., Zhou M., Xiang B., Li X., Li G., et al. Trend analysis of cancer incidence and mortality in China. Sci. China Life Sci. 2017;60:1271–1275. doi: 10.1007/s11427-017-9172-6. [DOI] [PubMed] [Google Scholar]
- 2.Chen J.-G., Chen H.-Z., Zhu J., Yang Y.-L., Zhang Y.-H., Huang P.-X., Chen Y.-S., Zhu C.-Y., Yang L.-P., Shen K., et al. Cancer survival in patients from a hospital-based cancer registry, China. J. Cancer. 2018;9:851–860. doi: 10.7150/jca.23039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce A., Sharp L., Hanly P., Barchuk A., Bray F., de Camargo Cancela M., Gupta P., Meheus F., Qiao Y.L., Sitas F., et al. Productivity losses due to premature mortality from cancer in Brazil, Russia, India, China, and South Africa (BRICS) A population-based comparison. Cancer Epidemiol. 2018;53:27–34. doi: 10.1016/j.canep.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Moga M.A., Dimienescu O.G., Arvatescu C.A., Ifteni P., Ples L. Anticancer Activity of Toxins from Bee and Snake Venom-An Overview on Ovarian Cancer. Molecules. 2018;23:692. doi: 10.3390/molecules23030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krane B.D., Fagbule M.O., Shamma M. The Benzophenanthridine Alkaloids. J. Nat. Prod. 1984;47:1–43. doi: 10.1021/np50031a001. [DOI] [Google Scholar]
- 6.Nyangulu J.M., Hargreaves S.L., Sharples S.L., Mackay S.P., Waigh R.D., Duval O., Mberu E.K., Watkins W.M. Antimalarial benzo[c]phenanthridines. Bioorg. Med. Chem. Lett. 2005;15:2007–2010. doi: 10.1016/j.bmcl.2005.02.074. [DOI] [PubMed] [Google Scholar]
- 7.Hatae N., Fujita E., Shigenobu S., Shimoyama S., Ishihara Y., Kurata Y., Choshi T., Nishiyama T., Okada C., Hibino S. Antiproliferative activity of O4-benzo[c]phenanthridine alkaloids against HCT-116 and HL-60 tumor cells. Bioorg. Med. Chem. Lett. 2015;25:2749–2752. doi: 10.1016/j.bmcl.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Cordell G.A., Farnsworth N.R. A review of selected potential anticancer plant principles. Heterocycles. 1976;4:393–427. [Google Scholar]
- 9.Nakanishi T., Suzuki M., Mashiba A., Ishikawa K., Yokotsuka T. Synthesis of NK109, an anticancer benzo[c]phenanthridine alkaloid. J. Org. Chem. 1998;63:4235–4239. doi: 10.1021/jo9718758. [DOI] [Google Scholar]
- 10.Dostál J., Slavík J. Some aspects of the chemistry of quaternary benzo[c]phenanthridine alkaloids. Stud. Nat. Prod. Chem. 2002;27:155–184. [Google Scholar]
- 11.Matkar S.S., Wrischnik L.A., Hellmann-Blumberg U. Production of hydrogen peroxide and redox cycling can explain how sanguinarine and chelerythrine induce rapid apoptosis. Arch. Biochem. Biophys. 2008;477:43–52. doi: 10.1016/j.abb.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Kosina P., Vacek J., Papouskova B., Stiborova M., Styskala J., Cankar P., Vrublova E., Vostalova J., Simanek V., Ulrichova J. Identification of benzo[c]phenanthridine metabolites in human hepatocytes by liquid chromatography with electrospray ion-trap and quadrupole time-of-flight mass spectrometry. J. Chromatogr. B. 2011;879:1077–1085. doi: 10.1016/j.jchromb.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Guo L., Liu X., Nishikawa K., Plunkett W. Inhibition of topoisomerase IIα and G2 cell cycle arrest by NK314, a novel benzo[c]phenanthridine currently in clinical trials. Mol. Cancer Ther. 2007;6:1501–1508. doi: 10.1158/1535-7163.MCT-06-0780. [DOI] [PubMed] [Google Scholar]
- 14.Parhi A., Kelley C., Kaul M., Pilch D.S., LaVoie E.J. Antibacterial activity of substituted 5-methylbenzo[c]phenanthridinium derivatives. Bioorg. Med. Chem. Lett. 2012;22:7080–7083. doi: 10.1016/j.bmcl.2012.09.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao F.-J., Yang R., Lv C., Ma Q., Lei M., Geng H.-L., Zhou L. Pseudocyanides of sanguinarine and chelerythrine and their series of structurally simple analogues as new anticancer lead compounds: Cytotoxic activity, structure–activity relationship and apoptosis induction. Eur. J. Phar. Sci. 2015;67:45–54. doi: 10.1016/j.ejps.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Lynch M.A., Duval O., Sukhanova A., Devy J., MacKay S.P., Waigh R.D., Nabiev I. Synthesis, Biological Activity and Comparative Analysis of DNA Binding Affinities and Human DNA Topoisomerase I Inhibitory Activities of Novel 12-Alkoxy-benzo[c]phenanthridinium Salts. Bioorg. Med. Chem. Lett. 2011;11:2643–2646. doi: 10.1016/S0960-894X(01)00520-0. [DOI] [PubMed] [Google Scholar]
- 17.Arthur H.R., Hui W.H., Ng Y.L. An examination of the rutaceae of Hong Kong. Part II. The alkaloids, nitidine and oxynitidine, from Zanthoxylum nitidum. J. Chem. Soc. 1959:1840–1845. doi: 10.1039/jr9590001840. [DOI] [Google Scholar]
- 18.Wang Z., Jiang W., Zhang Z., Qian M., Du B. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-κB pathway in RAW 264.7 cells. J. Ethnopharmacol. 2012;144:145–150. doi: 10.1016/j.jep.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 19.Bouquet J., Rivaud M., Chevalley S., Deharo E., Jullian V., Valentin A. Biological activities of nitidine, a potential antimalarial lead compound. Malaria J. 2012;11:2–8. doi: 10.1186/1475-2875-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman C.D., Austarheim I., Mollard V., Mikolo B., Malterud K.E., McFadden G.I., Wangensteen H. Natural products from Zanthoxylum heitzii with potent activity against the malaria parasite. Malaria J. 2016;15:481–488. doi: 10.1186/s12936-016-1533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cesari I., Grisoli P., Paolillo M., Milanese C., Massolini G., Brusotti G. Isolation and characterization of the alkaloid Nitidine responsible for the traditional use of Phyllanthus muellerianus (Kuntze) Excell stem bark against bacterial infections. J. Pharm. Biomed. Anal. 2015;105:115–120. doi: 10.1016/j.jpba.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 22.Tayebwa D.S., Tuvshintulga B., Guswanto A., Nugraha A.B., Batiha G.E., Gantuya S., Rizk M.A., Vudriko P., Sivakumar T., Yokoyama N., et al. The effects of nitidine chloride and camptothecin on the growth of Babesia and Theileria parasites. Ticks Tick Borne Dis. 2018;9:1192–1201. doi: 10.1016/j.ttbdis.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Patel R.D., Kumar S.P., Patel C.N., Shankar S.S., Pandya H.A., Solanki H.A. Parallel screening of drug-like natural compounds using Caco-2 cell permeability QSAR model with applicability domain, lipophilic ligand efficiency index and shape property: A case study of HIV-1 reverse transcriptase inhibitors. J. Mol. Struct. 2017;1146:80–95. doi: 10.1016/j.molstruc.2017.05.019. [DOI] [Google Scholar]
- 24.Phillips S.D., Castle R.N. A Review of the Chemistry of the Antitumor Benzo[c]phenanthridine Alkaloids Nitidine and Fagaronine and of the Related Antitumor Alkaloid Coralyne. J. Heterocycl. Chem. 1981;18:223–232. doi: 10.1002/jhet.5570180202. [DOI] [Google Scholar]
- 25.Chen S., Yang L., Feng J. Nitidine chloride inhibits proliferation and induces apoptosis in ovarian cancer cells by activating the Fas signalling pathway. J. Pharm. Pharmacol. 2018;70:778–786. doi: 10.1111/jphp.12901. [DOI] [PubMed] [Google Scholar]
- 26.Fang Z., Tang Y., Jiao W., Xing Z., Guo Z., Wang W. Nitidine chloride induces apoptosis and inhibits tumor cell proliferation via suppressing ERK signaling pathway in renal cancer. Food Chem. Toxicol. 2011;66:210–216. doi: 10.1016/j.fct.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 27.Pan X., Han H., Wang L., Yang L., Li R., Li Z., Liu J., Zhao Q., Qian M., Liu M., Du B. Nitidine Chloride inhibits breast cancer cells migration and invasion by suppressing c-Src/FAK associated signaling pathway. Cancer Lett. 2011;313:181–191. doi: 10.1016/j.canlet.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Sun M., Zhang N., Wang X., Cai C., Cun J., Li Y., Lv S., Yang Q. Nitidine chloride induces apoptosis, cell cycle arrest, and synergistic cytotoxicity with doxorubicin in breast cancer cells. Tumor Biol. 2014;35:10201–10212. doi: 10.1007/s13277-014-2327-9. [DOI] [PubMed] [Google Scholar]
- 29.Li P., Yan S., Dong X., Li Z., Qiu Y., Ji C., Zhang J., Ji M., Li W., Wang H., et al. Cell Cycle Arrest and Apoptosis Induction Activity of Nitidine Chloride on Acute Myeloid Leukemia Cells. Med. Chem. 2018;14:60–66. doi: 10.2174/1573406413666170620091543. [DOI] [PubMed] [Google Scholar]
- 30.Parenty A.D.C., Smith L.V., Guthrie K.M., Long D.-L., Plumb J., Brown R., Cronin L. Highly Stable Phenanthridinium Frameworks as a New Class of Tunable DNA Binding Agents with Cytotoxic Properties. J. Med. Chem. 2005;48:4504–4506. doi: 10.1021/jm050320z. [DOI] [PubMed] [Google Scholar]
- 31.Whittaker J., McFadyen W.D., Wickham G., Wakelin L.P.G., Murray V. The interaction of DNA-targeted platinum phenanthridinium complexes with DNA. Nucleic Acids Res. 1998;26:3933–3939. doi: 10.1093/nar/26.17.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whittaker J., McFadyen W.D., Baguley B.C., Murray V. The interaction of DNA-targeted platinum phenanthridinium complexes with DNA in human cells. Anti-Cancer Drug Des. 2001;16:81–89. [PubMed] [Google Scholar]
- 33.Thai K.-M., Bui Q.-H., Tran T.-D., Huynh T.-N.-P. QSAR Modeling on Benzo[c]phenanthridine Analogues as Topoisomerase I Inhibitors and Anti-cancer Agents. Molecules. 2012;17:5690–5712. doi: 10.3390/molecules17055690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.