Abstract
The aim of this study was to determine how the addition of gold and silver nanoparticles to culture media affects the composition of essential oils extracted from Lavandula angustifolia propagated on MS media with the addition of 10 and 50 mg·dm−3 of gold (24.2 ± 2.4 nm) and silver (27.5 ± 4.8 nm) nanocolloids. The oil extracted from the lavender tissues propagated on the medium with 10 mg·dm−3 AgNPs (silver nanoparticles) differed the most with respect to the control; oil-10 compounds were not found at all, and 13 others were detected which were not present in the control oil. The addition of AuNPs (gold nanoparticles) and AgNPs to the media resulted in a decrease of lower molecular weight compounds (e.g., α- and β-pinene, camphene, δ-3-carene, p-cymene, 1,8-cineole, trans-pinocarveol, camphoriborneol), which were replaced by those of a higher molecular weight (τ- and α-cadinol 9-cedranone, cadalene, α-bisabolol, cis-14-nor-muurol-5-en-4-one, (E,E)-farnesol).
Keywords: nanoparticles, secondary metabolites, shoot cultures, micropropagation, elicitor
1. Introduction
Nanotechnology has become one of the fastest growing interdisciplinary fields of science today. Nanoparticles, i.e., compounds or elements reduced to the size of less than 100 nanometers, differ in terms of their atomic structure compared to the material they are derived from, and also differ in terms of their physical, chemical, and biological properties. The most important advantage of nanoparticles is a high surface-to-volume ratio, which tends to increase with the reduction in their diameter, whereby nanoparticles demonstrate very high chemical activity [1]. Their highly developed active surface area significantly affects their adsorption properties, material reactivity, and antimicrobial properties [2].
The most widely used and known nanoparticles are those of precious metals: Gold and silver. They undergo various processes which are not observed in macroscopic environments. Nanosilver has antimicrobial (antifungal and antibacterial) properties. Gold in its nanoform offers therapeutic effects due to its ease to penetrate body cells where it strongly stimulates their regeneration [3,4]. Nanoparticles of precious metals form stable colloidal solutions, which can be applied to plant in vitro cultures [5].
Nanoparticles are easily absorbed and accumulated by plants. The processes of nanopenetration into the cells of living organisms are still to be explored in detail. However, it has been confirmed they enter certain cells through endocytosis or through pass through surface pores in plant cell walls [6,7]. The selective properties of cell walls enable the transport of particles measuring from 5 to 20 nm, allowing nanoparticles to easily penetrate cells and spread throughout the entire organism, ultimately affecting biological processes occurring in the cells [8].
With their unique nanostructural properties, these materials are used in many key industries, such as pharmaceuticals, electronics, cosmetology, medicine, environmental protection, textiles, and packaging. They are also applied in biotechnology, and recently, in plant in vitro cultures [9]. Nanosilver is used in plant in vitro cultures at the culture initiation stage to prevent contaminations, offering a viable alternative to antibiotics used in plant micropropagation [10].
Ongoing studies are attempting to determine the suitability of nanoparticles as elicitors in the in vitro cultures. Currently used elicitors are either biotic agents derived from biological sources, such as components of fungal and bacterial cell wall structures (polysaccharides, glycoproteins, inactivated enzymes, curdlan, chitosan), or abiotic factors of chemical or physical origin (heavy metal salts, osmotic stress, mechanical damage, ultraviolet radiation) [11]. Nanometal particles have shown a high capacity for attaching to plant tissues and activate enzymatic pathways responsible for the production of secondary metabolites [12]. They also contribute to the peroxidation of cellular membranes in plant cells and influence the expression of genes responsible for the production of biologically active compounds [13].
So far, attempts to use gold and silver nanocolloids as elicitors in plant in vitro cultures have been limited. The addition of these substances increased the production of secondary metabolites in the cultures of Salvia miltiorrhiza [14], Artemisia annua [15], Brugmensia candida [16], Corylus avellana [17], Prunella vulgaris [18], and Aloe vera [13]. The influence of nanoparticles on plants depends on several factors, such as plant species, its age, growing conditions, culture medium, exposure time of the plant to nanomaterial, and administration method.
Essential oils constitute mixtures of volatile compounds, sesquiterpenes, and primarily monoterpenes [19]. The main components of the essential oils isolated from L. angustifolia tissue are, among others, linalool, borneol, geraniol, and linalool acetate [20,21]. The composition of an essential oil depends mainly on the plant genotype, yet its composition may differ under the influence of developmental and environmental factors, i.e., sun exposure, plant age, seedling collection method or essential oil isolation method [22,23].
There have been no literature reports so far regarding the influence of nanoparticles on the production of essential oils by plants propagated in in vitro cultures. The studies by Hatami et al. [24] and Ghanati and Bakhtarian [25] show that the application of metal nanoparticles to plants growing under natural conditions results in a change in the essential oil content extracted from their tissues. The aim of this study was to verify how gold and silver nanocolloids influence the composition of essential oils in narrow-leaved lavender (Lavandula angustifolia) propagated in in vitro cultures.
2. Results and Discussion
Hydrodistillation of the dried leafy shoots of Lavandula angustifolia generated pale yellow liquids with a yield from 0.81% (10 mg·dm−3 AuNPs) to 1.27% (10 Ag mg·dm−3 NPs) (Table 1). Interestingly, the results of this study are comparable with those obtained from lavender flowers. Kara and Baydar [26] studied four lavender cultivars and indicated that the oil content varied from 0.35 to 2.0%. Zheljazkov et al. [27] reported the content of volatile oil in dried flowers to range from 0.71 to 1.30%. However, the content of volatile oil in the leaves of lavender cultivated in Northwest Iran (0.64%) was lower when compared with the results discussed herein [28].
Table 1.
Essential oil content in lavender plants as a function of medium used.
| Medium [mg·dm−3] | Essential Oil Content (%w/w) |
|---|---|
| 0—control | 1.15 |
| 50 Au | 0.95 |
| 10 Au | 0.81 |
| 50 Ag | 0.82 |
| 10 Ag | 1.27 |
The chemical composition of L. angustifolia essential oils is shown in Table 2 and Table 3, where the percentage composition and retention indices of the constituents are given. A total of 97 different compounds representing 99.29–99.95% of the oils were identified. The main volatile constituents were: Borneol (12.14–16.46%), τ-cadinol (12.96–16.63%), caryophyllene oxide (8.79–12.23%), γ-cadinene (4.54–6.08%), and 1,8-cineole (2.80–4.58%). Other important constituents were: Cis-14-nor-muurol-5-en-4-one (2.68–4.45%), β-pinene (1.93–3.14%), camphor (2.05–2.79%), and α-santalene (1.42–2.64%). The extracted oils were the most abundant in oxygenated sesquiterpenes (36.34–43.36%), followed by oxygenated monoterpenes (27.77–38.23%), sesquiterpene hydrocarbons (10.27–14.35%), and monoterpene hydrocarbons (5.57–10.40%).
Table 2.
Statistical analysis of main compounds.
| Compound | RI | Control | 50 mg·dm−3 Au | 10 mg·dm−3 Au | 50 mg·dm−3 Ag | 10 mg·dm−3 Ag | |
|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 933 | 1.46a | 0.99b | 1.03b | 1.11b | 0.64c |
| 2 | β-Pinene | 977 | 3.14a | 2.56b | 2.25b | 2.53b | 1.93b |
| 3 | p-Cymene | 1025 | 1.39a | 1.18b | 1.17b | 0.91c | 0.92c |
| 4 | 1,8-Cineole | 1031 | 4.49a | 4.58a | 2.80b | 2.95b | 2.95b |
| 5 | trans-Pinocarveol | 1140 | 1.61a | 1.65a | 1.54b | 1.42c | 1.14d |
| 6 | Camphor | 1145 | 2.75a | 2.79a | 2.41b | 2.06c | 2.05c |
| 7 | Pinocarvone | 1164 | 1.32a | 1.36a | 1.32a | 1.16b | 0.90c |
| 8 | Borneol | 1170 | 16.00a | 16.46a | 12.78b | 12.14b | 12.99b |
| 9 | Myrtenol | 1198 | 2.25a | 2.35a | 1.94a | 2.13a | 1.85a |
| 10 | Geranylacetate | 1385 | 1.20a | 1.38a | 1.14a | 0.59b | 1.41a |
| 11 | α-Santalene | 1422 | 1.90bc | 1.42d | 2.16b | 2.64a | 1.74c |
| 12 | γ-Cadinene | 1518 | 4.97c | 4.54d | 5.09c | 6.08a | 5.36b |
| 13 | Caryophylleneoxide | 1589 | 9.12c | 8.54c | 11.06b | 12.23a | 8.79c |
| 14 | τ-Cadinol | 1648 | 12.96c | 14.35b | 13.65bc | 14.17b | 16.63a |
| 16 | α-Cadinol | 1662 | 1.33a | 1.13b | 1.35a | 1.36a | 1.08c |
| 17 | Cadalene | 1675 | 1.87d | 1.58e | 2.34b | 2.53a | 2.03c |
| 18 | cis-14-nor-Muurol-5-en-4-one | 1693 | 2.68c | 3.72b | 3.72b | 3.37c | 4.45a |
| 19 | (E,E)-Farnesol | 1720 | 1.20d | 1.43b | 1.32c | 1.45b | 1.57a |
| 20 | Bisabolol oxide A | 1750 | 1.97c | 2.26b | 2.23b | 2.13b | 2.60a |
 compounds with a significantly lower content as compared with the control oil;
compounds with a significantly lower content as compared with the control oil;  compounds with a significantly higher content as compared with the control oil; a, b, c—values followed by the same letter are not significantly different at p ≤ 0.05 according to the LSD (least significant differences) Tukey test.
compounds with a significantly higher content as compared with the control oil; a, b, c—values followed by the same letter are not significantly different at p ≤ 0.05 according to the LSD (least significant differences) Tukey test.
Table 3.
Relative percentage composition of lavender essential oils depending on the medium ±SD (n = 3).
| No. | Compound | RI | Control | 50 mg·dm−3 Au | 10 mg·dm−3 Au | 50 mg·dm−3 Ag | 10 mg·dm−3 Ag | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | MH | α-Thujene | 927 | 0.09 | ±0.01 | ||||||||
| 2. | MH | α-Pinene | 933 | 1.46 | ±0.16 | 0.99 | ±0.10 | 1.03 | ±0.01 | 1.11 | ±0.18 | 0.64 | ±0.04 |
| 3. | MH | Camphene | 948 | 1.08 | ±0.16 | 0.78 | ±0.07 | 0.85 | ±0.04 | 0.77 | ±0.11 | 0.48 | ±0.03 |
| 4. | MH | Thuja-2,4(10)-diene | 954 | 0.09 | ±0.01 | ||||||||
| 5. | MH | β-Thujene | 971 | 0.33 | ±0.02 | 0.24 | ±0.01 | 0.28 | ±0.01 | 0.20 | ±0.00 | 0.21 | ±0.01 |
| 6. | MH | Sabinene | 974 | 0.31 | ±0.01 | 0.25 | ±0.03 | 0.23 | ±0.01 | 0.23 | ±0.03 | 0.21 | ±0.01 |
| 7. | MH | β-Pinene | 977 | 3.14 | ±0.26 | 2.56 | ±0.26 | 2.25 | ±0.08 | 2.53 | ±0.46 | 1.93 | ±0.09 |
| 8. | MH | δ-3-Carene | 1010 | 0.93 | ±0.08 | 0.72 | ±0.06 | 0.77 | ±0.03 | 0.78 | ±0.10 | 0.59 | ±0.03 |
| 9. | MH | m-Cymene | 1022 | 0.52 | ±0.05 | 0.45 | ±0.04 | 0.49 | ±0.02 | 0.33 | ±0.01 | 0.32b | ±0.01 |
| 10. | MH | p-Cymene | 1025 | 1.39 | ±0.13 | 1.18 | ±0.10 | 1.17 | ±0.07 | 0.91 | ±0.06 | 0.92c | ±0.04 |
| 11. | MH | D-Limonene | 1029 | 0.81 | ±0.11 | 0.33 | ±0.47 | 0.64 | ±0.02 | 0.58 | ±0.04 | 0.27a | ±0.38 |
| 12. | OM | 1,8-Cineole | 1031 | 4.49 | ±0.26 | 4.58 | ±0.01 | 2.80 | ±0.12 | 2.95 | ±0.43 | 2.95b | ±0.33 |
| 13. | MH | γ-Terpinene | 1060 | 0.10 | ±0.01 | 0.08 | ±0.01 | ||||||
| 14. | OM | cis-Sabinenehydrate | 1068 | 0.070 | ±0.00 | 0.09 | ±0.00 | ||||||
| 15. | MH | α-Terpinolene | 1091 | 0.24 | ±0.01 | 0.23 | ±0.00 | 0.27 | ±0.02 | 0.15 | ±0.06 | ||
| 16. | OM | Linalool | 1101 | 0.65 | ±0.03 | 0.77 | ±0.05 | 0.55 | ±0.04 | 0.30 | ±0.06 | 0.51b | ±0.01 |
| 17. | O | α-Pineneoxide | 1110 | 0.210 | ±0.01 | ||||||||
| 18. | OM | Fenchol | 1114 | 0.21 | ±0.01 | 0.26 | ±0.01 | 0.16 | ±0.01 | 0.19 | ±0.00 | ||
| 19. | O | 3-Octanol acetate | 1122 | 0.15 | ±0.00 | 0.15 | ±0.01 | 0.20 | ±0.01 | 0.13 | ±0.01 | ||
| 20. | OM | α-Campholenal | 1127 | 0.23 | ±0.00 | 0.22 | ±0.01 | 0.24 | ±0.01 | 0.21 | ±0.01 | 0.20 | ±0.01 |
| 21. | OM | 1,2-Dihydrolinalool | 1136 | 0.24 | ±0.01 | 0.21 | ±0.01 | 0.26 | ±0.01 | 0.20 | ±0.01 | 0.18 | ±0.00 |
| 22. | OM | trans-Pinocarveol | 1140 | 1.61 | ±0.04 | 1.65 | ±0.10 | 1.54 | ±0.08 | 1.42 | ±0.05 | 1.14 | ±0.02 |
| 23. | OM | Camphor | 1145 | 2.75 | ±0.08 | 2.79 | ±0.21 | 2.41 | ±0.13 | 2.06 | ±0.13 | 2.05 | ±0.03 |
| 24. | OM | Pinocarvone | 1164 | 1.32 | ±0.01 | 1.36 | ±0.08 | 1.32 | ±0.08 | 1.16 | ±0.01 | 0.90 | ±0.00 |
| 25. | OM | Borneol | 1170 | 16.00 | ±0.58 | 16.46 | ±1.51 | 12.78 | ±0.44 | 12.14 | ±1.20 | 12.99 | ±0.16 |
| 26. | OM | Terpinen-4-ol | 1179 | 0.69 | ±0.04 | 0.64 | ±0.04 | 0.62 | ±0.01 | 0.60 | ±0.06 | 0.48 | ±0.00 |
| 27. | OM | p-Cymen-8-ol | 1184 | 0.80 | ±0.04 | 0.84 | ±0.01 | 0.99 | ±0.06 | 0.57 | ±0.07 | 0.40 | ±0.02 |
| 28. | OM | Cryptone | 1187 | 0.55 | ±0.25 | 0.71 | ±0.03 | 0.77 | ±0.04 | 0.52 | ±0.06 | 0.35 | ±0.01 |
| 29. | OM | α-Terpineol | 1193 | 0.66 | ±0.01 | 0.65 | ±0.04 | 0.51 | ±0.00 | 0.52 | ±0.06 | 0.41 | ±0.01 |
| 30. | OM | Myrtenol | 1198 | 2.25 | ±0.06 | 2.35 | ±0.11 | 1.94 | ±0.06 | 2.13 | ±0.22 | 1.85 | ±0.03 |
| 31. | OM | Verbenone | 1210 | 0.78 | ±0.06 | 0.69 | ±0.08 | 0.61 | ±0.02 | 0.51 | ±0.07 | 0.45 | ±0.01 |
| 32. | OM | cis-Carveol | 1221 | 0.18 | ±0.01 | 0.18 | ±0.00 | 0.24 | ±0.01 | 0.08 | ±0.11 | ||
| 33. | OM | trans-Carveol | 1224 | 0.20 | ±0.00 | 0.23 | ±0.01 | 0.23 | ±0.00 | 0.09 | ±0.12 | ||
| 34. | OM | Bornylformate | 1229 | 0.86 | ±0.01 | 0.85 | ±0.04 | 0.63 | ±0.00 | 0.62 | ±0.02 | 0.91 | ±0.01 |
| 35. | OM | d-Carvone | 1247 | 0.24 | ±0.00 | 0.24 | ±0.01 | 0.32 | ±0.01 | 0.10 | ±0.14 | 0.20 | ±0.02 |
| 36. | OM | Geraniol | 1254 | 0.46 | ±0.01 | 0.43 | ±0.01 | 0.40 | ±0.01 | 0.33 | ±0.02 | 0.31 | ±0.01 |
| 37. | OM | α-Citral | 1272 | 0.12 | ±0.01 | 0.12 | ±0.00 | 0.11 | ±0.01 | ||||
| 38. | OM | Bornylacetate | 1287 | 0.32 | ±0.01 | 0.29 | ±0.00 | 0.32 | ±0.04 | 0.27 | ±0.00 | 0.38 | ±0.03 |
| 39. | OM | Lavandulylacetate | 1292 | 0.20 | ±0.00 | 0.16 | ±0.00 | 0.21 | ±0.01 | 0.19 | ±0.01 | 0.18 | ±0.01 |
| 40. | OM | Piperitenone | 1341 | 0.11 | ±0.01 | 0.12 | ±0.01 | 0.14 | ±0.00 | 0.05 | ±0.06 | 0.12 | ±0.01 |
| 41. | OM | Nerylacetate | 1367 | 0.10 | ±0.01 | ||||||||
| 42. | OM | Geranylacetate | 1385 | 1.20 | ±0.06 | 1.38 | ±0.07 | 1.14 | ±0.06 | 0.59 | ±0.28 | 1.41 | ±0.27 |
| 43. | SH | α-Cedrene | 1416 | 0.40 | ±0.06 | 0.39 | ±0.04 | 0.45 | ±0.04 | 0.47 | ±0.01 | 0.45 | ±0.04 |
| 44. | SH | α-Santalene | 1422 | 1.90 | ±0.04 | 1.42 | ±0.02 | 2.16 | ±0.01 | 2.64 | ±0.00 | 1.74 | ±0.04 |
| 45. | SH | α-Bergamotene | 1438 | 0.28 | ±0.01 | 0.25 | ±0.01 | 0.33 | ±0.01 | 0.38 | ±0.02 | 0.29 | ±0.00 |
| 46. | SH | Aromadendrene | 1448 | 0.11 | ±0.01 | 0.08 | ±0.00 | 0.14 | ±0.00 | 0.14 | ±0.01 | 0.12 | ±0.01 |
| 47. | SH | β-Santalene | 1450 | 0.11 | ±0.01 | 0.12 | ±0.01 | 0.15 | ±0.01 | 0.10 | ±0.00 | ||
| 48. | SH | trans-β-Bergamotene | 1460 | 0.15 | ±0.01 | 0.09 | ±0.04 | 0.16 | ±0.06 | ||||
| 49. | SH | β-Chamigrene | 1463 | 0.11 | ±0.01 | 0.09 | ±0.00 | 0.11 | ±0.00 | 0.14 | ±0.01 | 0.12 | ±0.00 |
| 50. | SH | Di-epi-α-Cedrene | 1470 | 0.13 | ±0.00 | 0.14 | ±0.01 | 0.15 | ±0.00 | 0.15 | ±0.01 | 0.15 | ±0.01 |
| 51. | SH | cis-β-Farnesene | 1488 | 0.10 | ±0.00 | 0.12 | ±0.06 | 0.19 | ±0.05 | 0.15 | ±0.04 | 0.16 | ±0.01 |
| 52. | SH | β-Bisabolene | 1511 | 0.07 | ±0.09 | ||||||||
| 53. | SH | γ-Cadinene | 1518 | 4.97 | ±0.06 | 4.54 | ±0.11 | 5.09 | ±0.03 | 6.08 | ±0.03 | 5.36 | ±0.05 |
| 54. | SH | β-Sesquiphellandrene | 1522 | 0.39 | ±0.01 | 0.44 | ±0.04 | 0.56 | ±0.01 | 0.50 | ±0.01 | 0.52 | ±0.01 |
| 55. | SH | δ-Cadinene | 1526 | 0.45 | ±0.01 | 0.42 | ±0.03 | 0.49 | ±0.01 | 0.54 | ±0.01 | 0.50 | ±0.02 |
| 56. | SH | trans-Calamenene | 1533 | 0.24 | ±0.00 | 0.24 | ±0.03 | 0.33 | ±0.01 | 0.32 | ±0.03 | 0.35 | ±0.01 |
| 57. | SH | Cadina-1,4-diene | 1536 | 0.53 | ±0.01 | 0.60 | ±0.06 | 0.59 | ±0.03 | 0.53 | ±0.02 | 0.60 | ±0.01 |
| 58. | SH | α-Cadinene | 1543 | 0.19 | ±0.01 | 0.13 | ±0.18 | ||||||
| 59. | SH | α-Calacorene | 1547 | 0.36 | ±0.01 | 0.52 | ±0.06 | 0.51 | ±0.03 | 0.45 | ±0.05 | 0.48 | ±0.01 |
| 60. | SH | Germacrene B | 1557 | 0.89 | ±0.02 | 0.82 | ±0.07 | 1.14 | ±0.03 | 1.23 | ±0.05 | 0.86 | ±0.01 |
| 61. | SH | β-Calacorene | 1563 | 0.10 | ±0.01 | 0.11 | ±0.03 | 0.06 | ±0.08 | 0.13 | ±0.01 | ||
| 62. | OS | Nerolidol | 1569 | 0.51 | ±0.01 | 0.64 | ±0.07 | 0.62 | ±0.02 | 0.60 | ±0.02 | 0.66 | ±0.01 |
| 63. | O | (Z)-3-Hexenyl benzoate | 1579 | 0.63 | ±0.01 | 0.76 | ±0.08 | 0.74 | ±0.04 | 0.73 | ±0.01 | 0.76 | ±0.01 |
| 64. | OS | Caryophylleneoxide | 1589 | 9.12 | ±0.16 | 8.54 | ±0.31 | 11.06 | ±0.13 | 12.23 | ±0.21 | 8.79 | ±0.07 |
| 65. | O | Hexadecane | 1600 | 0.26 | ±0.01 | 0.30 | ±0.05 | 0.32 | ±0.01 | 0.29 | ±0.01 | 0.36 | ±0.01 |
| 66. | OS | Humuleneepoxide | 1605 | 0.21 | ±0.00 | 0.22 | ±0.04 | 0.30 | ±0.01 | 0.28 | ±0.01 | 0.26 | ±0.01 |
| 67. | OS | Humuleneepoxide II | 1613 | 0.65 | ±0.02 | 0.69 | ±0.06 | 0.87 | ±0.01 | 0.89 | ±0.04 | 0.80 | ±0.01 |
| 68. | OS | epi-Cubenol | 1619 | 1.55 | ±0.04 | 1.73 | ±0.12 | 1.70 | ±0.04 | 1.77 | ±0.08 | 2.01 | ±0.01 |
| 69. | OS | γ-Eudesmol | 1628 | 0.25 | ±0.01 | 0.28 | ±0.04 | 0.32 | ±0.03 | 0.29 | ±0.01 | 0.20 | ±0.02 |
| 70. | OS | Isospathulenol | 1638 | 0.17 | ±0.04 | 0.24 | ±0.02 | 0.25 | ±0.03 | 0.18 | ±0.01 | ||
| 71. | OS | Caryophylla-4(12),8(13)-dien-5β-ol | 1642 | 0.30 | ±0.00 | 0.47 | ±0.04 | 0.26 | ±0.37 | 0.62 | ±0.12 | ||
| 72. | OS | τ-Cadinol | 1648 | 12.96 | ±0.69 | 14.35 | ±0.64 | 13.65 | ±0.14 | 14.17 | ±0.69 | 16.63 | ±0.06 |
| 73. | OS | α-Muurolol | 1655 | 0.44 | ±0.02 | 0.47 | ±0.06 | 0.54 | ±0.01 | 0.53 | ±0.00 | 0.59 | ±0.00 |
| 74. | OS | α-Eudesmol | 1659 | 0.46 | ±0.03 | 0.51 | ±0.06 | 0.55 | ±0.01 | 0.53 | ±0.07 | 0.69 | ±0.01 |
| 75. | OS | α-Cadinol | 1662 | 1.33 | ±0.04 | 1.13 | ±0.06 | 1.35 | ±0.06 | 1.36 | ±0.02 | 1.08 | ±0.04 |
| 76. | OS | 9-Cedranone | 1667 | 1.13 | ±0.04 | 1.29 | ±0.16 | 1.33 | ±0.01 | 1.37 | ±0.06 | 1.41 | ±0.07 |
| 77. | O | Cadalene | 1675 | 1.87 | ±0.05 | 1.58 | ±0.16 | 2.34 | ±0.04 | 2.53 | ±0.18 | 2.03 | ±0.01 |
| 78. | OS | α-Bisabolol | 1681 | 0.77 | ±0.04 | 0.87 | ±0.09 | 0.96 | ±0.03 | 0.92 | ±0.04 | 0.91 | ±0.00 |
| 79. | OS | epi-α-Bisabolol | 1691 | 0.69 | ±0.03 | 0.38 | ±0.53 | ||||||
| 80. | OS | cis-14-nor-Muurol-5-en-4-one | 1693 | 2.68 | ±0.08 | 3.72 | ±0.22 | 3.72 | ±0.05 | 3.37 | ±0.7 | 4.45 | ±0.03 |
| 81. | O | Heptadecane | 1703 | 0.28 | ±0.05 | 0.30 | ±0.01 | 0.13 | ±0.18 | 0.31 | ±0.00 | ||
| 82. | O | 5-Ethyl-5-methylpentadecane | 1709 | 0.27 | ±0.01 | 0.31 | ±0.06 | 0.38 | ±0.01 | 0.33 | ±0.03 | 0.41 | ±0.01 |
| 83. | O | Pentadecanal | 1714 | 0.46 | ±0.04 | 0.53 | ±0.07 | 0.57 | ±0.01 | 0.52 | ±0.01 | 0.68 | ±0.01 |
| 84. | OS | (E,E)-Farnesol | 1720 | 1.20 | ±0.07 | 1.43 | ±0.14 | 1.32 | ±0.04 | 1.45 | ±0.04 | 1.57 | ±0.02 |
| 85. | O | 5-Phenyldodecane | 1733 | 0.50 | ±0.05 | 0.55 | ±0.13 | 0.68 | ±0.02 | 0.62 | ±0.03 | 0.81 | ±0.01 |
| 86. | OS | Bisabolol oxide A | 1750 | 1.97 | ±0.12 | 2.26 | ±0.27 | 2.23 | ±0.03 | 2.13 | ±0.12 | 2.60 | ±0.03 |
| 87. | OS | (E)-α-Atlantone | 1777 | 0.12 | ±0.02 | 0.19 | ±0.03 | 0.19 | ±0.00 | 0.22 | ±0.03 | 0.23 | ±0.05 |
| 88. | O | Octadecane | 1805 | 0.13 | ±0.02 | 0.20 | ±0.03 | 0.27 | ±0.02 | ||||
| 89. | DT | Phytane | 1811 | 0.21 | ±0.00 | ||||||||
| 90. | O | Diisobutylphthalate | 1872 | 0.24 | ±0.01 | 0.25 | ±0.08 | 0.33 | ±0.01 | ||||
| 91. | DT | m-Camphorene | 1957 | 0.14 | ±0.01 | 0.26 | ±0.02 | ||||||
| 92. | O | Eicosane | 2003 | 0.12 | ±0.08 | ||||||||
| 93. | O | Octadecanal | 2021 | 0.20 | ±0.03 | ||||||||
| 94. | O | 1-Octadecanol | 2088 | 0.29 | ±0.03 | 0.66 | ±0.08 | ||||||
| 95. | O | 1-Tricosene | 2296 | 0.31 | ±0.16 | ||||||||
| 96. | O | Tricosane | 2300 | 0.25 | ±0.13 | 0.44 | ±0.09 | ||||||
| 97. | O | 2-Heneicosanone | 2307 | 0.66 | ±0.20 | 0.84 | ±0.21 | 2.22 | ±0.35 | ||||
| Total identified [No.] | 82 | 81 | 81 | 83 | 83 | ||||||||
| Total identified [%] | 99.29 | 99.95 | 99.95 | 99.69 | 99.72 | ||||||||
| Monoterpene hydrocarbnons (MH) | 10.40 | 7.90 | 7.98 | 7.59 | 5.57 | ||||||||
| Oxygenated monoterpenes (OM) | 37.19 | 38.23 | 31.34 | 27.77 | 28.65 | ||||||||
| Sesquiterpene hydrocarbons (SH) | 11.22 | 10.27 | 12.36 | 14.35 | 12.06 | ||||||||
| Oxygenated sesquiterpenes (OS) | 36.34 | 38.96 | 41.21 | 43.36 | 43.06 | ||||||||
| Diterpenes (DT) | - | - | 0.14 | - | 0.47 | ||||||||
| Other (O) | 4.14 | 4.59 | 6.92 | 6.62 | 9.91 | ||||||||
RI: Retention indices relative to n-alkanes (C7-C40) on HP-5MS capillary column; -: Not detected.
The growing medium applied affected the percentage composition of each essential oil constituent. The highest concentrations of borneol (16.46%) and 1,8-cineole (4.58%) were noticed in the volatile oil isolated from plants cultivated on the medium supplemented with gold nanoparticles (50 mg·dm−3 AuNPs). Addition of silver nanoparticles (50 mg·dm−3 AgNPs) to the growing medium increased the content of γ-cadinene (6.08%) and caryophyllene oxide (12.23%) in the oil (Figure 1).
Figure 1.
Plants of Lavandula angustifolia Mill. propagated on medium with 50 mg·dm−3 AuNPs.
However, the percentage content of camphor was lower in the plants cultivated on the medium supplemented with AgNPs (10 and 50 mg·dm−3). Moreover, volatile oil derived from lavender cultivated on MS medium was richer in β-pinene (3.14%), α-pinene (1.46%), p-cymene (1.39%), camphene (1.08%), and δ-3-carene (0.93%).
Phytochemical studies revealed that linalool (9.3–68.8%) and linalyl acetates (1.2–59.4%) were the main components of the aerial parts and flowers of Lavandula angustifolia [29,30]. However, the essential oil obtained from plants cultivated in North Africa [31] had 1,8-cineole (29.4%) and camphor (24.6%) as the major constituents. 1,8-cineole (65.4%) and borneol (11.5%) dominated in the essential oils isolated from the leaves of L. angustifolia collected near Isfahan, Iran [32]. Borneol was the main compound in the essential oils isolated from leafy stems of three lavender cultivars propagated in in vitro cultures: ‘Blue River’ (25.75%), ‘Elegance Purple’ (32.17%), and Munstead (13.38%) [33].
The percentage contents of linalool (0.30–0.77%), 1,8-cineole (2.80–4.58%), and camphor (2.05–2.79%) found in volatile oils in this study were much lower than the results reported in the referenced literature. Essential oils isolated from plants grown on control medium and 50 mg·dm−3 AuNPs medium were the only ones with higher borneol content (16.00–16.46%) compared with the results obtained by Andrys and Kulpa [29]. Linalool, lavandulol, and their esters (linalyl acetate and lavandulyl acetate) are responsible for the fresh and floral smell of lavender oil. Moreover, the quality of oil depends on both a high content of linalool and linalyl acetate and their mutual proportions (preferably higher than 1) [34].
Contrary to the results obtained by other researches, lavandulol and linalyl acetate were not detected in the oils in this study, and the content of lavandulyl acetate did not exceed 0.21%. The data reported in the literature indicated that many terpenoids are biologically active and are used medicinally [35]. Camphor, with its specific camphoraceous odor, is used commercially as a moth repellent and as a preservative in pharmaceuticals and cosmetics [36]. Borneol, a widely-used food and cosmetic additive, possesses analgesic, anti-inflammatory, and antibacterial properties [37,38]. It is well known that 1,8-cineole and camphor are responsible for the insecticidal activity of the plants from Lavandula genus [39]. Based on these facts, it can be stated that the volatile oils extracted from leafy shoots of L. angustifolia may have commercial applications.
The oil extracted from the tissues of lavender propagated on the culture medium which was supplemented with 10 mg·dm−3 of AgNPs differed the most with respect to the control culture (plants propagated on the culture medium with no nanoparticles) in terms of the number of compounds: While 10 compounds were not found in it at all, 13 others were detected which were not observed in the control oil. The addition of AuNPs and AgNPs to the media resulted in a decrease in compounds with lower molecular weight (e.g., α- and β-pinene, camphene, δ-3-carene, p-cymene, 1,8-cineole (eucalyptol), trans-pinocarveol, camphor, and borneol), which were replaced by those of higher molecular weight (τ- and α-cadinol 9-cedranone, cadalene, α-bisabolol, cis-14-nor-Muurol-5-en-4-one, (E,E)-farnesol).
Heavy metal nanocolloids that have been recently used in plant in vitro cultures, as elicitors provoke the production of secondary metabolites. There are research reports confirming that these particles are capable of eliciting responses in plants consistent with those generated when typical elicitors are used [40,41]. It is commonly believed that the production of secondary metabolites in plants is significantly affected by environmental stress. Biotic and abiotic stresses delay cellular differentiation through the production of reactive oxygen species (ROS), which directly destroy cells by producing secondary metabolites [42,43]. The researchers suggest that oxidative stress induced by nanoparticles is correlated with the production of secondary metabolites in plants. Due to their small size, nanocolloids can easily attach to plant cell walls, destroy them, change their permeability, and thus significantly affect cellular metabolism [13]. Zhang et al. [14] confirmed the effectiveness of silver as an elicitor using silver ions in the production of diterpenoids in the cultures of root hairs of Salvia miltiorrhiza genus. The addition of silver to the culture media of root hairs resulted in an increase in the production of reactive oxygen species. Activation of ROS-based mechanisms following exposure of plants from Calendula officinalis L. genus to nanoparticles was also confirmed by Ghanati and Bakhtiarian [20] in the production of secondary metabolites. Fazal et al. [18] demonstrated that a callus of Prunella vulgaris genus treated with silver and gold nanocolloids produced significant quantities of antioxidant enzymes, such as POD and SOD, as well as phenolic and flavonoid compounds that are directly related to the protection of plants against environmental stress. Silver nanocolloids were used to produce capsaicin from Capsicum sp. and resulted in a significant increase in the production of this compound [44]. Hemm et al. [45] and Liu et al. [46] showed that growth regulators combined with elicitors resulted in a larger organogenic potential of plants and increased the production of primary and secondary metabolites.
The study showed that the addition of gold and silver nanocolloids to the culture media significantly affected the composition of essential oil derived from narrow-leaved lavender cultivated in in vitro cultures. In the oils extracted from plants propagated in vitro on culture media with the addition of nanoparticles, a variety of compounds were identified that were not present in the oil derived from plants grown on the control medium. The above suggests that gold and silver nanoparticles can be successfully used to obtain essential oils of different composition which may result in different properties: Fragrance and, above all, antioxidant and antimicrobial activity, but the latter requires further studies. It is also necessary to determine the toxicity of nanoparticles in relation to plant tissues.
3. Material and Methods
3.1. Nanoparticles
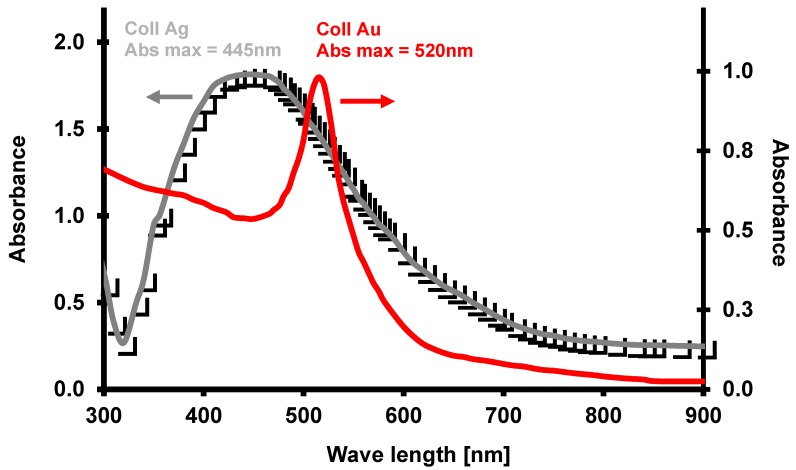
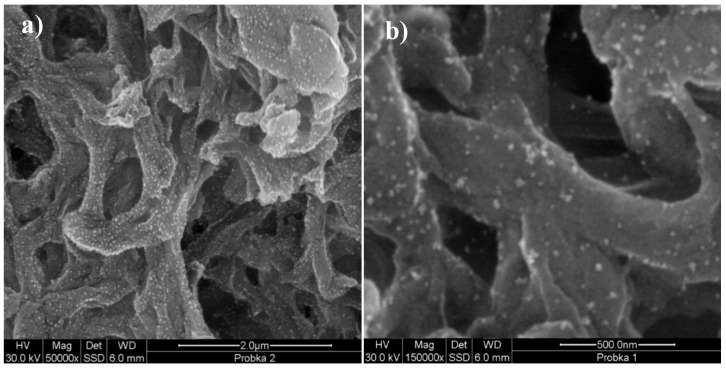
Aqueous suspensions of gold and silver nanoparticles were synthesized using Turkevich et al. [47] and Liu et al.’s [48] methods with modified synthesis conditions and a two-stage microwave-convection heating method. For this purpose, aqueous mixtures of 0.903 g·dm−3 of sodium citrate with 2.378 g·dm−3 of tetrachloroauric acid (HAuCl4), and 1.189 g·dm−3 of silver nitrate (AgNO3), respectively, were prepared. After their purification with a small (0.2 μm) pore antibacterial filter (Sartorius, Goettingen, Germany), they were placed in a microwave (MX 245), where they were stirred and heated to 100 °C at 800 W, which allowed for reaching a heating rate of approx. 1.6 °C/s. Once the preset temperature was reached, the mixture was kept in the microwave for an additional 20 s, and then placed in HBR 4 digital IKAMAG heating bath (IKA, Staufen, Germany), where it was stirred with a magnetic stirrer and incubated at 95 °C for an additional 15 min. It was then gradually cooled to room temperature (0.8 °C/min). To obtain a similar distribution of nanoparticle diameters, the resulting mixtures were homogenized in a centrifugal force field in Beckmann JA-20 centrifuge to obtain similar nanoparticle concentrations in both mixtures. Once their spectra were plotted with UV-VIS EPOCH microplate spectrophotometer (BioTek, Bad Friedrichshall, Germany), the optical density of the fractions obtained was adjusted to a common DEV value, using the following spectra absorbance maxima λmax = 520 nm and λmax = 445 nm for gold and silver colloids, respectively (Figure 2). The similarities in morphology, shape, and size of the synthesized and prepared gold (24.2 ± 2.4 nm) and silver (27.5 ± 4.8 nm) nanoparticles were assessed after their application to the surface of a nylon membrane (Supelco, Park Bellefonte, PA, USA) and through an analysis of images from a scanning electron microscope (SEM, FEI Quanta 200 FEG model) (FEI Company, Tokyo, Japan, Figure 3).
Figure 2.
UV-VIS spectral spectra of the fraction of 4-5000 x g of gold and colloidal silver.
Figure 3.
View of nanoparticles of gold colloids (a) and silver (b) after fractionation and placement on a nylon diaphragm (Supelco, Park Bellefonte, PA, USA) made with a help of FEI Quanta 200 FEG scanning electron microscope. The magnification applied to the observed colloidal gold and silver 50,000 (a) and 150,000 (b) times, respectively.
3.2. In Vitro Cultures
The materials examined in this study were plants of narrow-leaved lavender (Lavendula angustifolia), ‘Munstead’ cultivar. Single-node shoot fragments with a length of 1–1.5 cm were put in glass jars with a capacity of 300 mL, filled with 30 mL of the medium. The media, with a mineral composition developed by Murashige and Skoog [49] (MS media), were supplemented with 2 mg·dm−3 kinetin (KIN) and 0.2 mg·dm−3 indole-3-acetic acid (IAA) [50] with the addition of gold (AuNPs) with a diameter of 24.2 ± 2.4 nm and silver (AgNPs) with a diameter of 27.5 ± 4.8 nm nanocolloids with the concentrations of: 10 and 50 mg·dm−3, respectively. Furthermore, the media were supplemented with: 30 g·dm−3 of sucrose, 100 mg·dm−3 of inositol, and solidified with agar at 7 g·dm−3. Medium pH was set at 5.7 using 0.1 M solutions of HCl and NaOH. The jars were sterilized at 121 °C for 20 min. The jars with cultures were placed in a phytotron, with a humidity of 70–80% and temperature of 24 °C. The cultures were illuminated for 16 h a day, and the illuminance was kept at 35 µEM−2s−1 PAR.
3.3. Extraction of Essential Oils
Fifteen grams of the entire dried aerial parts of lavender were placed in 1000 mL round-bottomed flasks along with 400 mL of distilled water and subjected to hydrodistillation (3 replicates) for two hours using a Clevenger apparatus as recommended by the European Pharmacopoeia 5.0 [51]. The essential oil extracts were dried over anhydrous sodium sulfate, filtered, weighed and stored in dark sealed vials at 4 °C until gas chromatography/mass spectrometry (GC-MS) analysis was performed. Essential oil percentage was calculated based on the dry weight of plant material and expressed as (% w/w) in Table 1.
3.4. Gas Chromatography/Mass Spectrometry (GC-MS) Analyses of Essential Oils
The qualitative GC-MS analysis of the extracted essential oils was carried out using HP 6890 gas chromatograph coupled with HP 5973 Mass Selective Detector (Agilent Technologies, Foster City, CA, USA) operating in 70 eV mode. Samples of 2 μL (40 mg of oil dissolved in 1.5 mL of dichloromethane) were injected in a split mode at a ratio of 5:1. The compounds were separated on a 30 m long capillary column (HP-5MS), 0.25 mm in diameter and with 0.25 µm thick stationary phase film ((5% phenyl)-methylpolysiloxane).
The flow rate of helium through the column was kept at 1.2 mL·min−1. The initial temperature of the column was 45 °C, then it was increased to 200 °C at a rate of 5 °C·min−1 (kept constant for 10 min), and then heated up to a final temperature of 250 °C at a rate of 5 °C min−1. The oven was kept at this temperature for 20 min. The injector temperature was 250 °C, the transfer line temperature was 280 °C, and the ion source temperature was 230 °C. The solvent delay was 4 min. The scan range of the MSD was set at 40 to 550 mm/z. The total running time for a sample was about 71 min. The relative percentage of the essential oil constituents was evaluated from the total peak area (TIC) by apparatus software [52,53]. Essential oil constituents were identified by comparison of their mass spectra with those stored in the Wiley NBS75K.L and NIST/EPA/NIH (2002 version, National Institute of Standards and Technology, Gaithersburg, MD, USA) mass spectral libraries using various search engines (PBM, Nist02). The identity of compounds was also confirmed by comparison of their calculated retention indices with those reported in NIST Chemistry WebBook (http://webbook.nist.gov/chemistry/). For retention indices (RI) calculation [54,55], a mixture of homologus series of n-alkanes C7-C40 (Supelco, Bellefonte, PA, USA) was used, under the same chromatographic conditions which were applied for the analysis of the lavender essential oils.
Author Contributions
A.W. isolated the essential oil from plant tissue and performed the analysis of GC-MS as well as analyzed the obtained data; P.J. performed the experiments in in vitro cultures, analyzed the data, and wrote the manuscript; D.K. contributed to the revisions of the manuscript; W.P. prepared the solution of nanoparticles. All authors were responsible for processing information and manuscript writing. All authors read and approved the final manuscript.
Funding
The study was supported by the Polish Ministry of Science and Higher Education (Project BMN 517-07-017-5799/17 ZUT).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Song J.Y., Kim B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009;32:79–84. doi: 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- 2.Penyala N.R., Pena-Mendez E.M., Havel J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? Review. J. Appl. Biomed. 2008;6:117–129. [Google Scholar]
- 3.Kim Y.K., Lee Y.S., Jeong D.H., Cho M.H. Antimicrobial effect of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Choi O., Deng K.K., Kim N., Jr., Ross L., Rao Y.S., Hu Z. The inhibitory effects of silver nanoparticles, silver ionsand silver chloride colloids on microbial growth. Water Res. 2008;42:3066–3074. doi: 10.1016/j.watres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Hackenberg S., Scherzed A., Kessler M., Hummel S., Technau A., Froelich K., Ginzkey C., Koehler C., Hagen R., Kleinsasser N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011;25:27–33. doi: 10.1016/j.toxlet.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Jamshidi M., Ghanti F. Taxanes content and cytotoxity of hazel cells extract after elicitation with silver nanoparticles. Plant Psychol. Chem. 2016;110:178–184. doi: 10.1016/j.plaphy.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J., Hu Q., Guo Y.Q., Zhu W.H. Elicitor-induced indole alkaloid biosynthesis in Catharanthus roseus cell cultures is related to Ca? Influx and the oxidative burst. Plant Sci. 2001;161:423–431. doi: 10.1016/S0168-9452(01)00422-8. [DOI] [Google Scholar]
- 8.Nair R., Varghese S.H., Nair B.G., Maekaa T., Yoshida Y., Kumar D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010;179:154–163. doi: 10.1016/j.plantsci.2010.04.012. [DOI] [Google Scholar]
- 9.Navarro E., Baun A., Behra R., Hartmann N.B., Filser J., Miao A., Quigg A., Santschi P.H., Sigg I. Environmental behaviour and ecotoxicity of engineered nanoparticles to algae, plants and fungi. Ecotoxicol. 2008;17:372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- 10.Spinoso-Castillo J.L., Chavez-Santoscoy R.A., Bogdanchikova N., Pérez-Sato J.A., Morales-Ramos V., Bello-Bello J.J. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tiss. Organ Cult. 2017;129:195–207. [Google Scholar]
- 11.Ramachandra R.S., Ravishankar G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002;20:101–153. doi: 10.1016/S0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 12.Shakeran Z., Keyhanfari M., Asghari G., Ghanadian M. Improvement of atropine production by diferent biotic and abiotic elicitors in hairy root cultures of Datura metel. Turk. J. Biol. 2015;39:111–118. doi: 10.3906/biy-1405-25. [DOI] [Google Scholar]
- 13.Raei M., Angaji A.A., Omidi M., Khodayari M. Effect of abiotic elicitors on tissue culture of Aloe vera. Inter. J. Biosc. 2014;5:74–81. [Google Scholar]
- 14.Zhang C., Yan Q., Cheuk W., Wu J. Enhancement of Tanshinone Production in Salvia miltiorrhiza hairy root culture by Ag elicitation and nutrient feeding. Planta Med. 2004;70:147–151. doi: 10.1055/s-2004-815492. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B., Zheng L.P., Wan Wen W.J. Stimulation of Artemisinin Production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr. Nanosc. 2013;9:363–370. doi: 10.2174/1573413711309030012. [DOI] [Google Scholar]
- 16.Pitta-Alvarez S.I., Spollansky T.C., Giulietti A.M. The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enz. Microb. Technol. 2000;26:252–258. doi: 10.1016/S0141-0229(99)00137-4. [DOI] [PubMed] [Google Scholar]
- 17.Jamshidi M., Ghanati F., Rezaei A., Bemani E. Change of antioxidant enzymes activity of hazel (Corylus avellana L.) cells by AgNPs. Cytotech. 2016;68:525–530. doi: 10.1007/s10616-014-9808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazal H., Abbasi B.H., Ahmad N. Optimization of adventitious root culture for production of biomass and secondary metabolites in Prunella vulgaris L. Appl. Biochem Biotechnol. 2014;174:2086–2096. doi: 10.1007/s12010-014-1190-x. [DOI] [PubMed] [Google Scholar]
- 19.De Falco E., Mancini E., Roscigno G., Mignola E., Taglialatela-Scafati O., Senatore F. Chemical composition and biological activity of essential oils of Origanum vulgare subsp. vulgare L. under different growth conditions. Molecules. 2013;18:14948–14960. doi: 10.3390/molecules181214948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancini E., Camele I., Elshafie H.S., De Martino L., Pellegrino C., Grulova D. Chemical Composition and Biological Activity of the Essential Oil of Origanum vulgare ssp. hirtum from Different Areas in the Southern Apennines (Italy) Chem. Biodiv. 2014;11:639–651. doi: 10.1002/cbdv.201300326. [DOI] [PubMed] [Google Scholar]
- 21.Smigielski K., Prusinowska R., Stobiecka A., Kunicka-Styczyñska A., Gruska R. Biological Properties and Chemical Composition of Essential Oils from Flowers and Aerial Parts of Lavender (Lavandula angustifolia) J. Essent. Oil Bear. Pl. 2018;21:1303–1314. doi: 10.1080/0972060X.2018.1503068. [DOI] [Google Scholar]
- 22.Wesolowska A., Grzeszczuk M., Kulpa D. GC-MS analysis of the essential oil from flowers of Chrysanthemum coronarium L. propagated conventionally and derived from in vitro cultures. Acta Chromat. 2015;27:525–539. doi: 10.1556/AChrom.27.2015.3.9. [DOI] [Google Scholar]
- 23.Wesolowska A., Grzeszczuk M., Wilas J., Kulpa D. Gas Chromatography-Mass Spectrometry (GC-MS) analysis of indole alkaloids isolated from Catharanthus roseus (L.) G. don cultivated conventionally and derived from in vitro cultures. Not. Bot. Hort. Agrobot. Cluj-Napoca. 2016;44:100–106. doi: 10.15835/nbha44110127. [DOI] [Google Scholar]
- 24.Hatami M., Hatamzadeh A., Ghasemnezhad M., Sajidi R.H. Variations of the Phytochemical Compounds in Rosescented Geranium Plant Exposed to Nanosilver Particles. J. Essent. Oil Bear. Pl. 2016. 19:1747–1753. [Google Scholar]
- 25.Ghanati F., Bakhtiarian S. Changes of natural compounds of Artemisia annua L. by methyl jasmonate and silver nanoparticles. Adv. Envir. Biol. 2013;7:2251–2258. [Google Scholar]
- 26.Kara N., Baydar H. Determination of lavender and lavandin cultivars (Lavandula sp.) containing high quality essential oil in Isparta, Turkey. Turk. J. Field Crops. 2013;18:58–65. [Google Scholar]
- 27.Zheljazkov V., Astatkie T., Hristov A. Lavender and hyssop productivity, oil content and bioactivity as function of harvest time and drying. Ind. Crops Prod. 2012;36:222–228. doi: 10.1016/j.indcrop.2011.09.010. [DOI] [Google Scholar]
- 28.Hassanpouraghdam M.B., Hassani A., Vojodi L., Asl B.H., Rostami A. Essential oil constituents of Lavandula officinalis Chaix. from Northwest Iran. Chemija. 2011;22:167–171. [Google Scholar]
- 29.Verma R.S., Rahman L.U., Chanotiya C.S., Verma R.K., Chauhan A., Yadav A., Singh A., Yadav A.K. Essential oil composition of Lavandula angustifolia Mill. cultivated in the mid hills of Uttarakhand, India. J. Serb. Chem. Soc. 2010;75:343–348. doi: 10.2298/JSC090616015V. [DOI] [Google Scholar]
- 30.Śmigielski K., Prusinowska R., Raj A., Sikora M., Wolińska K., Gruska R. Effect of drying on the composition of essential oil from Lavandula angustifolia. J. Ess. Oil Bearing Plants. 2011;14:532–542. doi: 10.1080/0972060X.2011.10643970. [DOI] [Google Scholar]
- 31.Mostefa M.B., Kabouche A., Abaza I., Aburjai T., Touzani R., Kabouche Z. Chemotypes investigation of Lavandula essential oils growing at different North African soils. J. Mater. Environ. Sci. 2014;5:1896–1901. [Google Scholar]
- 32.Hajhashemi V., Ghannadi A., Sharif B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 2003;89:67–71. doi: 10.1016/S0378-8741(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 33.Andrys D., Adaszyńska-Skwirzyńska M., Kulpa D. Jasmonic acid changes the composition of essential oil isolated from narrow-leaved lavender propagated in in vitro cultures. Nat. Prod. Res. 2018;32:834–839. doi: 10.1080/14786419.2017.1309533. [DOI] [PubMed] [Google Scholar]
- 34.Andrys D., Kulpa D. In Vitro Propagation Affects the Composition of Narrow-Leaved Lavender Essential Oils. Acta Chrom. 2018;30:225–230. doi: 10.1556/1326.2017.00317. [DOI] [Google Scholar]
- 35.Prusinowska R., Śmigielski K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L.). A review. Herba Pol. 2014;60:56–66. doi: 10.2478/hepo-2014-0010. [DOI] [Google Scholar]
- 36.Cherneva E., Pavlovic V., Smelcerovic A., Yancheva D. The effect of camphor and borneol on rat thymocyte viability and oxidative stress. Molecules. 2012;17:10258–10266. doi: 10.3390/molecules170910258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elshafie H.S., Sakr S., Mang S.M., Belviso S., De Feo V., Camele I. Antimicrobial activity and chemical composition of three essential oils extracted from Mediterranean aromatic plants. J. Med. Food. 2016;19:1096–1103. doi: 10.1089/jmf.2016.0066. [DOI] [PubMed] [Google Scholar]
- 38.Elshafie H.S., Camele I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017;2017:9268468. doi: 10.1155/2017/9268468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Love J.N., Sammon M., Smereck J. Are one or two dangerous? Camphor exposure in toddlers. J. Emerg. Med. 2004;27:49–54. doi: 10.1016/j.jemermed.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Nakahashi H., Miyazawa M. Biotransformation of (−)-camphor by Salmonella typhimurium OY1002/2A6 expressing human CYP2A6 and NADPH-P450 reductase. J. Oleo Sci. 2011;60:545–548. doi: 10.5650/jos.60.545. [DOI] [PubMed] [Google Scholar]
- 41.Dai J.P., Chen J., Bei Y.F., Han B.X., Wang S. Influence of borneol on primary mice oral fibroblasts: A penetration enhancer may be used in oral submucous fibrosis. J. Oral. Pathol. Med. 2009;38:276–281. doi: 10.1111/j.1600-0714.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan K. Black pepper and its pungent principle-piperine:a review of diverse physiological effects. Critical Rev. Food Sci. Nutr. 2007;47:735–748. doi: 10.1080/10408390601062054. [DOI] [PubMed] [Google Scholar]
- 43.Abbasi B.H., Khan M., Guo B., Bokhari S.A., Khan M.A. Efficient regeneration and antioxidative enzyme activities in Brassica rapa var. turnip. Plant Cell Tiss. Organ Cult. 2011;105:337–344. doi: 10.1007/s11240-010-9872-8. [DOI] [Google Scholar]
- 44.Bhat P., Bhat A. Silver nanoparticles for enhancement of accumulation of capsaicin in suspension culture of Capsicum sp. J. Exp. Scien. 2016;7:1–6. [Google Scholar]
- 45.Hemm M.R., Rider S.D., Ogas J., Murry D.J., Chapple C. Light induces phenylpropanoid metabolism in Arabidopsis roots. Plant J. 2004;38:765–778. doi: 10.1111/j.1365-313X.2004.02089.x. [DOI] [PubMed] [Google Scholar]
- 46.Liu C.Z., Guo C., Wang Y., Ouyang F. Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua. Proc. Biochem. 2002;38:581–585. doi: 10.1016/S0032-9592(02)00165-6. [DOI] [Google Scholar]
- 47.Turkevich J., Stevenson P.C., Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951;11:55–75. doi: 10.1039/df9511100055. [DOI] [Google Scholar]
- 48.Liu F.K., Ker C.J., Chang Y.C., Ko F.H., Chu T.C., Dai B.T. Microwave heating for the preparation of nanometer gold particles. Jpn. J Appl. Physic. 2003;42:4152–4158. doi: 10.1143/JJAP.42.4152. [DOI] [Google Scholar]
- 49.Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 50.Andrys D., Kulpa D., Grzeszczuk M., Bihun M., Dobrowolska A. Antioxidant and antimicrobial activities of Lavandula angustifolia Mill. field-grown and propagated in vitro. Folia Hort. 2017;29:161–180. doi: 10.1515/fhort-2017-0016. [DOI] [Google Scholar]
- 51.European Pharmacopoeia 5.0. EDQM; Strasbourg, France: 2005. p. 1894. [Google Scholar]
- 52.Hassanpouraghdam M.B., Hassani A., Shalamzari M.S. Menthone-and estragole-rich essential oil of cultivated Ocimum basilicum L. from Northwest Iran. Chemija. 2010;21:59–62. [Google Scholar]
- 53.Rosas J.F., Zoghbi M.G.B., Andrade E.H.A., van den Berg M.E. Chemical composition of a methyl-(E)-cinnamate Ocimum micranthum Willd. from the Amazon. Flavour Fragr. J. 2005;20:161–163. doi: 10.1002/ffj.1374. [DOI] [Google Scholar]
- 54.Van Den Dool H., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 55.Babushok V.I., Linstrom P.J., Zenkevich I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data. 2011;40:043101. doi: 10.1063/1.3653552. [DOI] [Google Scholar]