Abstract
The objective of this study was to evaluate a novel thermoresponsive polyisocyanopeptide (PIC)–based hydrogel as an injectable carrier for local drug delivery for periodontal applications. Three formulations of PIC gels, 0.2%, 0.5%, and 1% w/w, were prepared. As controls, commercially available poloxamer 407 (P407) gels of 20% and 26% w/w were used. Lipoxin A4 (LXA4), a proresolving drug, was suspended into the gel solutions. The systems were evaluated regarding dynamic mechanical properties, injectability and stability, release and bioactivity of LXA4, and cytocompatibility. Results showed that the gelation temperatures of PIC and P407 gels were around 13°C to 23°C. PIC gels were less viscous and mechanically weaker than P407 gels due to the low polymer concentrations. However, PIC gels kept gel integrity for at least 2 wk when incubated with phosphate-buffered saline, whereas P407 gels were disintegrated totally within 1 wk. LXA4 was chemically stable in both neutral and alkaline medium for over 1 mo. The release of LXA4 from either 1% PIC or 26% P407 gels depicted an initial burst release followed by a sustained release for around 4 d. The extent of burst release was negatively correlated to the polymer concentration. LXA4 remained bioactive after release from PIC gels. No cytotoxicity was observed for 1% PIC gel. However, 26% P407 inhibited periodontal ligament cell and gingival epithelial cell growth. In conclusion, the thermoresponsive PIC gel is a potential candidate for periodontal drug delivery.
Keywords: periodontitis, hydrogel, poloxamer, Anti-inflammatory agents, control release, phagocytosis
Introduction
Periodontitis is initiated by microbial biofilms, which in turn elicit a host response and result in the destruction of the periodontal tissue (Van Dyke and Serhan 2003). Uncontrolled inflammation and failed resolution of the inflammation are considered the underlying mechanism of periodontitis (Van Dyke and Serhan 2003; Serhan et al. 2007; Medzhitov 2010; Serhan 2010). In literature, robust evidence has shown that an endogenous anti-inflammatory and proresolving process can depress chronic inflammation and activate wound healing with tissue regeneration (Serhan et al. 2007; Serhan et al. 2008; Ortega-Gómez et al. 2013). This active process is orchestrated, in part, by specialized endogenous proresolving lipid mediators (SPMs), including lipoxins, resolvins, protectins, and maresins (Levy et al. 2012; Kamaly et al. 2013; Ortega-Gómez et al. 2013). SPMs can actively reduce neutrophil infiltration into inflamed tissues, enhance efferocytosis and bacterial phagocytosis by monocytes and macrophages, and simultaneously inhibit inflammatory cytokine production (Lee et al. 2016).
Topical usage of resolvin E1 and lipoxin A4 (LXA4) has already been proven to prevent periodontal bone loss and activate the periodontal regeneration in rabbits and swine, without the multiple side effects ascribed to traditional anti-inflammatory antagonist-based treatments (Hasturk et al. 2006; Hasturk et al. 2007; Van Dyke et al. 2015; Lee et al. 2016). We hypothesize that topical application of SPMs without an appropriate carrier may not be able to achieve an effective retention in the periodontal pocket and a drug carrier system would be advantageous. For instance, Van Dyke et al. (2015) delivered membrane-shed vesicles incorporating LXA4 in swine, resulting in significant regeneration of bone and connective tissues and reestablishment of the periodontal organ. The commonly used carriers for sustained drug release include microspheres, polymer chips, fibers, and hydrogels (Joshi et al. 2016). Among all the carriers, thermoresponsive hydrogels are particularly attractive, because they are liquid at low temperature and thus can be easily injected into deep pockets, and then, they can form gels under body temperature to facilitate sustained drug release (Vyas et al. 2000; Maheshwari et al. 2006; Ji et al. 2010; Nasra et al. 2017). The commercially available poloxamer 407 (P407) has been studied for this purpose. P407 exhibits a thermoresponsive manner at a concentration above 20% w/w, but it is easy to be eroded after gelation under the influence of surrounding liquid (Zhang et al. 2002). Recently, another thermoresponsive hydrogel based on polyisocyanopeptide (PIC) has been developed, which can form a gel at extremely low polymer concentrations (~0.5%) and displays strain-stiffening properties comparable to the natural extracellular matrix (Kouwer et al. 2013; Das et al. 2016). This gel system may have the advantages of 1) low viscosity, allowing the drug solution to perfuse periodontal pockets fully, and 2) low toxicity induced by the polymers.
This study aimed at evaluating the thermoresponsive PIC hydrogel loaded with LXA4 as a representative SPM for the periodontal application. To this end, 0.2% to 1% w/w PICs were prepared. Then, the physical stability and dynamic mechanical properties of the gels were tested, in comparison with 20% and 26% w/w P407 controls. LXA4 release profiles from PICs or P407s were further investigated. The efficacy of the released LXA4 was tested by a phagocytic test. Finally, the cytocompatibility of the gel systems was tested using periodontal ligament cells (PDLCs) and gingival epithelial cells (GECs).
Materials and Methods
Preparation of Thermoresponsive Gels
PIC polymer (molecular weight ~ 635 kDa) was provided by Dr. Paul H. J. Kouwer (Institute for Molecules and Materials, Radboud University, the Netherlands). PIC or P407 (Sigma-Aldrich) polymer was dissolved in cold phosphate-buffered saline (PBS). Three PIC solutions at 0.2, 0.5, and 1% w/w and 2 P407 solutions at 20% and 26% w/w were prepared. The pH of the prepared solutions was determined at 4°C.
Physical Characterization of the Gels
Dynamic mechanical properties of gels were tested by a rheometer with a temperature sweep program (see Appendix for method). The sol-gel transition temperature (Tsol-gel) was determined as the crossing point of storage (G′) and loss (G′′) moduli during temperature sweep measurements. The values of G′ and G′′ of each gel at 37°C were recorded after a 2-min equilibration. Injectability of gels was tested with Milli-Q water as a control (see Appendix for method).
Physical stability of hydrogels, in an aqueous solution, was tested by a gravimetric method (Zhang et al. 2002). Briefly, 0.5 mg gel solution was injected into the bottom of an Eppendorf tube with a known weight (W0/mg) and gelled at 37°C. Three aqueous buffers at different pH were prepared: 1) PBS (pH 7.4), 2) 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 8.5), and 3) citrate acid (pH 5). All buffers had an ionic strength of 154 mM. Afterward, 1 mL buffer was carefully laid over the surface of the gel. All samples were incubated at 37°C with gentle agitation at 120 rpm/min. At each predetermined time point, all the supernatant was carefully removed, and the Eppendorf tubes were weighted again (Wt/mg). Then, 1 mL fresh buffer was refilled, and samples were incubated until the next time point. The erosion profiles were obtained by plotting the percentage remaining for each gel formulation ([Wt – W0]/0.5 × 100%) against the incubation time (n = 3).
In Vitro Release of LXA4
To detect the stability of LXA4 in aqueous solutions, 100 µg/mL 15(R)-LXA4 in ethanol (Cayman Chemical) was diluted to 1 µg/mL using the 3 above aqueous buffers at different pH. After dilution, the samples (n = 3) were incubated at 37°C and the amount of LXA4 in the buffers at predetermined time points was detected by reverse-phase high-performance liquid chromatography (RP-HPLC; Hitachi) (for details, see Appendix).
Release studies were performed at 37°C by a membraneless dissolution method (Zhang et al. 2002). Briefly, 200 µL of a PIC or P407 solution was mixed with 1 µg LXA4 on ice and then gelled in an Eppendorf at 37°C (n = 4). Subsequently, 1 mL PBS prewarmed at 37°C was laid over the gels. Samples were then incubated at 37°C with agitation at 200 rpm/min. At each predetermined time point, 900 µL supernatant was withdrawn carefully and 900 µL fresh PBS was refilled. The amount of released LXA4 in the supernatant was detected by RP-HPLC.
To further investigate the interactions between LXA4 and hydrogel carriers and influential factors of LXA4 release, the release study was also performed in media with detergent of 0.1% v/v Tween 20 in PBS (n = 4) and in buffers with different pH (n = 4): 1) PBS (pH 7.4), 2) HEPES (pH 8.5), and 3) citrate acid (pH 5) (Song et al. 2015).
In Vitro Efficacy Test
The efficacy of the released LXA4 was assessed by the ability to stimulate macrophage phagocytosis. The phagocytic activity of RAW264.7 macrophages was examined using a fluorescent bead internalization assay (Prescott and McKay 2011). Cells were seeded in 96-well plates at a density of 2 × 104 cells/cm2 in α–minimum essential medium eagle containing 1% penicillin-streptomycin (P/S) and 10% fetal bovine serum (FBS) and left for 24 h for attachment prior to experimentation. Thereafter, each macrophage was exposed to 100 latex beads labeled with Nile red fluorescence (D = 2 µm; Invitrogen). Then, 50 µL LXA4 releasate from gels (concentration calculated by HPLC results) or freshly prepared LXA4 (positive control) was added into the culture to reach a final LXA4 concentration of 100 nM. The same volume of ethanol, the drug vehicle, was used as a negative control. After incubation for 4 h, the fluorescence of unphagocytic beads was quenched with 0.4% trypan blue. The fluorescence intensity of each well was read in a Synergy HTX multimode reader (BioTek Instruments), with the excitation/emission wavelength at 535/575 nm. The wells without beads were assessed to determine background fluorescence. Data were expressed as a percent change in fluorescence in treated versus control wells. Phagocytosis results were also visualized by fluorescent microscopy (see Appendix for method).
In Vitro Cytocompatibility Test
Cytocompatibility of LXA4/PIC and LXA4/P407 was tested using human PDLCs and GECs. Cells were harvested from extracted third molars with the informed consent of the patients (all ~20 y old). Cell culture methods are shown in the Appendix. PDLCs or GECs were seeded on 24-well plates or Thermanox coverslips at a density of 1 × 104 cells/cm2 for 24 h before the assays.
Then, 200 µL cold gel solution with or without LXA4 was injected in Corning Transwell cell culture inserts (pore size = 8 µm) and gelled at 37°C. Next, the transwells were inserted separately into the wells with PDLCs or GECs. The final concentration of LXA4 for the LXA4-loaded groups was 100 nM calculated according to the whole liquid volume in each well. The cytocompatibility of gels was tested by alamarBlue, DNA assay, and live/dead staining at days 2 and 4 (see Appendix for methods).
Statistical Analysis
All data were reported as a mean ± standard deviation and analyzed using 1-way analysis of variance by GraphPad Prism 7.0 (GraphPad Software). Differences were considered statistically significant at P < 0.05.
Results
Physical Characterization of the Gels
As shown in the Table, the pH of all gel groups was 7.4 and same with PBS. Tsol-gel of all formulations was between 13°C and 23°C. With the increase of PIC or P407 concentration, Tsol-gel slightly dropped, whereas, G′ and G′′ increased. Both G′ and G′′ of PICs were lower than those of P407s. Overall, PIC solutions were less viscous than P407 solutions. All the ice-chilled solutions were easily pushed through a 22-gauge needle comparable to water (Appendix Fig. 1).
Table.
In Vitro Characterization of PIC or P407 Gels with Different Polymer Concentrations (n = 3): pH, Tsol-gel Sol-Gel Transition Temperature, and Storage (G′) and Loss (G′′) Moduli at 37°C.
| Gel | Concentration (w/w) |
pH | Tsol-gel (°C), Mean ± SD | G′ (Pa), Mean ± SD | G′′ (Pa), Mean ± SD |
|---|---|---|---|---|---|
| PIC | 0.2% | 7.4 | 16.5 ± 0.8a | 334 ± 62a | 33 ± 3a |
| 0.5% | 7.4 | 16.2 ± 0.4a | 1,050 ± 77a | 45 ± 11a | |
| 1% | 7.4 | 12.9 ± 1.0b | 4,964 ± 353b | 195 ± 42a | |
| P407 | 20% | 7.4 | 22.9 ± 0.4c | 16,101 ± 289c | 787 ± 121b |
| 26% | 7.4 | 18.2 ± 0.4a | 28,422 ± 1,585d | 859 ± 121b |
Different superscript letters in each column indicate significant difference between different groups.
All PIC and P407 gels stayed at the bottom of the Eppendorf without detachment. Regardless of buffers, P407, 20% and 26% w/w, disintegrated completely within 5 and 7 d, respectively (Fig. 1). In contrast, even the weakest 0.2% PIC remained ~70% after 15 d. As expected, the rate of gel disintegration decreased as the concentration of gel increased. There was almost no weight loss for 1% PIC gel up to 15 d. pH did not show any significant influence on gel integrity for both PIC and P407.
Figure 1.
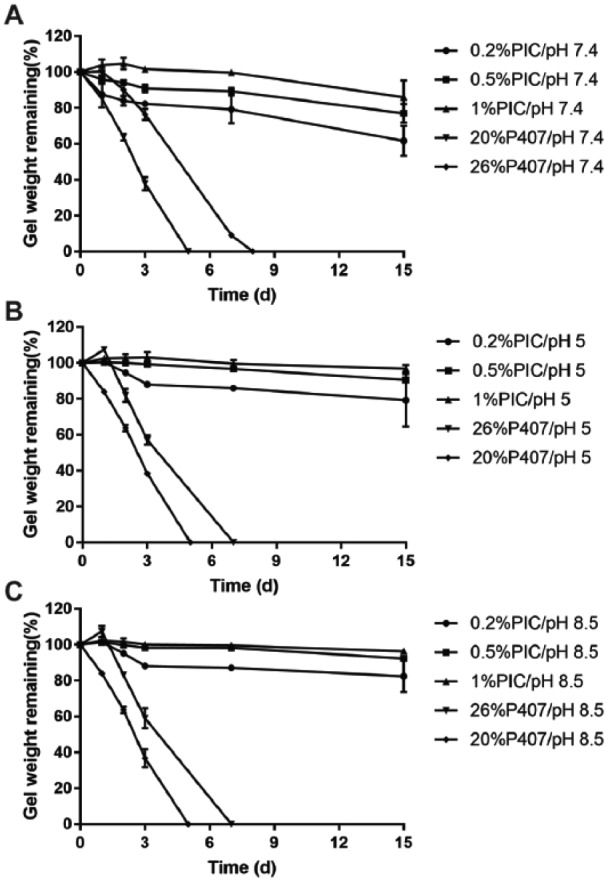
The erosion profile of polyisocyanopeptide (PIC) or poloxamer 407 (P407) gels with different polymer concentrations in phosphate-buffered saline (A), citrate buffer (B), and HEPES (C) at 37°C versus time. Results are shown as the percentage of gel-remaining weight. Data are expressed as mean ± standard deviation (n = 3). The sign of standard deviation may not visible when it is smaller than the data symbol.
In Vitro Release of LXA4
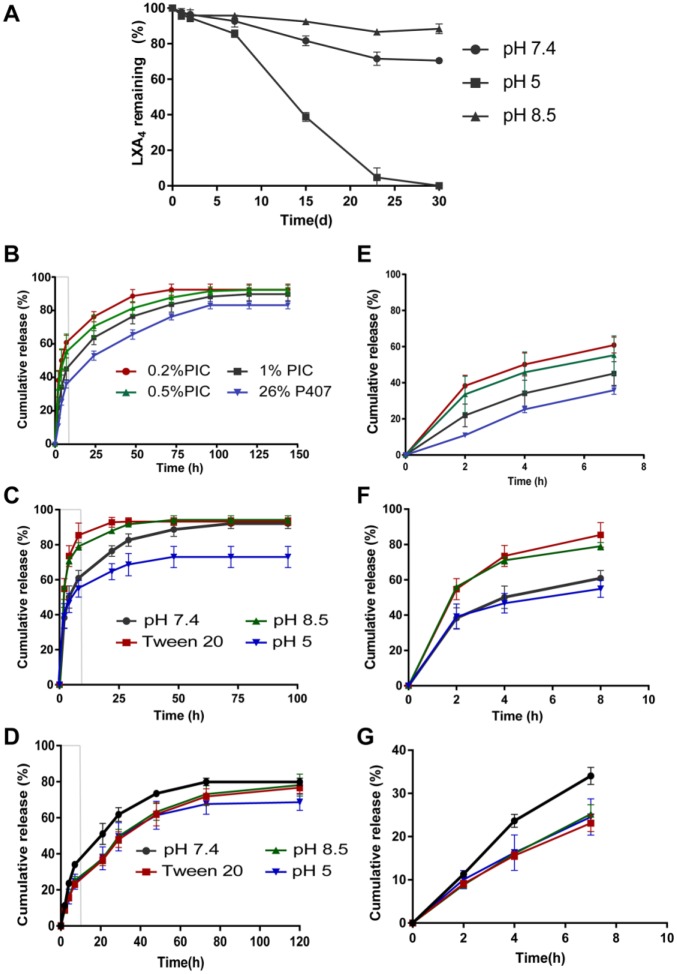
Chemical stability of LXA4 results (Fig. 2A) revealed that over 80% of LXA4 remained intact in 1 wk under various pH conditions. After 1 mo, LXA4 was relatively stable in neutral and alkaline solution but fully degraded in acidic buffer.
Figure 2.
Lipoxin A4 (LXA4) stability and release curves. (A) LXA4 chemical stability in media with different pH and (B–D) the cumulative release profiles of LXA4 from different gels with different release media. (A) Eighty percent of LXA4 remained intact after 1 wk under various pH conditions. After 1 mo, around 70% of LXA4 was left in the neutral solution and more than 90% in the alkaline buffer. However, no LXA4 could be detected in the acidic buffer. (B) LXA4 release from 0.2%, 0.5%, 1% w/w polyisocyanopeptide (PIC) and 26% w/w poloxamer 407 (P407) gels in phosphate-buffered saline release medium at 37°C. (C) Detergent and pH influence of LXA4 release from 0.2% PIC gel. (D) Detergent and pH influence of LXA4 release from 26% P407 gel. The partial enlargement of release pattern of first 8 h in B, C, and D are shown in E, F, and G, respectively. The sign of standard deviation may not visible when it is smaller than the data symbol.
The release profile displayed a burst release of 40% to 60% LXA4 in the first 8 h (Fig. 2E–G) and a sustained release thereafter for around 4 d for all groups (Fig. 2B–G). Polymer concentration had a minor effect on the duration of LXA4 release but considerably decreased the initial burst release. The 26% P407 formulation possessed a similar release pattern to 1% PIC, but the gel dissolved fast during the release test. Finally, the cumulative release amounts reached more than 90% for PICs, in contrast to around 80% for the P407s.
The addition of Tween 20 significantly increased the release rate of LXA4 with 90% of cumulative release within 1 d in PIC groups (Fig. 2C). Especially, in the first 8 h, around 20% more LXA4 was released compared to that in the PBS. The LXA4 release in alkaline medium showed the same pattern as that in Tween 20. In acidic medium, the release rate was low, but in total, only around 70% of LXA4 was released. For P407 groups, the LXA4 release was not greatly affected by detergent and alkaline medium in velocity, duration, and cumulative amount (Fig. 2D). Similarly, less LXA4 amount was finally released from the P407 in the acidic buffer.
In Vitro Efficacy and Cytocompatibility Test
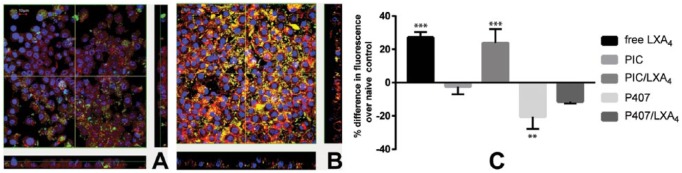
Phagocytotic activity of RAW264.7 is shown in Figure 3. Both LXA4 released from PIC and fresh LXA4 enhanced the phagocytosis activity of RAW264.7. However, no phagocytic enhancement was detected for the LXA4 released from P407 or in the groups without LXA4 (Fig. 3C). Phagocytic images illustrated that more fluorescent particles were internalized in PIC/LXA4 groups compared to PIC without LXA4 groups (Fig. 3A and B).
Figure 3.
Lipoxin A4 (LXA4) released from polyisocyanopeptide (PIC)/LXA4 upregulated phagocytosis by RAW264.7 macrophages, as visualized by fluorescence microscopy and quantified. (A) RAW264.7 cells incubated with released medium from PIC gel without LXA4; (B) RAW264.7 cells incubated with 100 nM LXA4 released from PIC/LXA4 (nuclei were stained by 4’,6-diamidino-2-phenylindole (DAPI) to blue; actin was stained by phalloidin 568 to red; particles were yellow-green). (C) Differences of fluorescence in groups over naive control (n = 4, results are expressed as mean ± SD, **P < 0.01, ***P < 0.001 compared with naive controls). In positive control (free LXA4), a 27.18% ± 3.2% increase in RAW264.7 phagocytosis; LXA4 released from PIC gel enhanced the phagocytosis activity by 23.75% ± 8.4%. No positive effect on phagocytosis could be found for the LXA4 released from poloxamer 407 (P407). As expected, no enhancement of phagocytosis was found for the groups without LXA4 (1% PIC and 26% P407) as well.
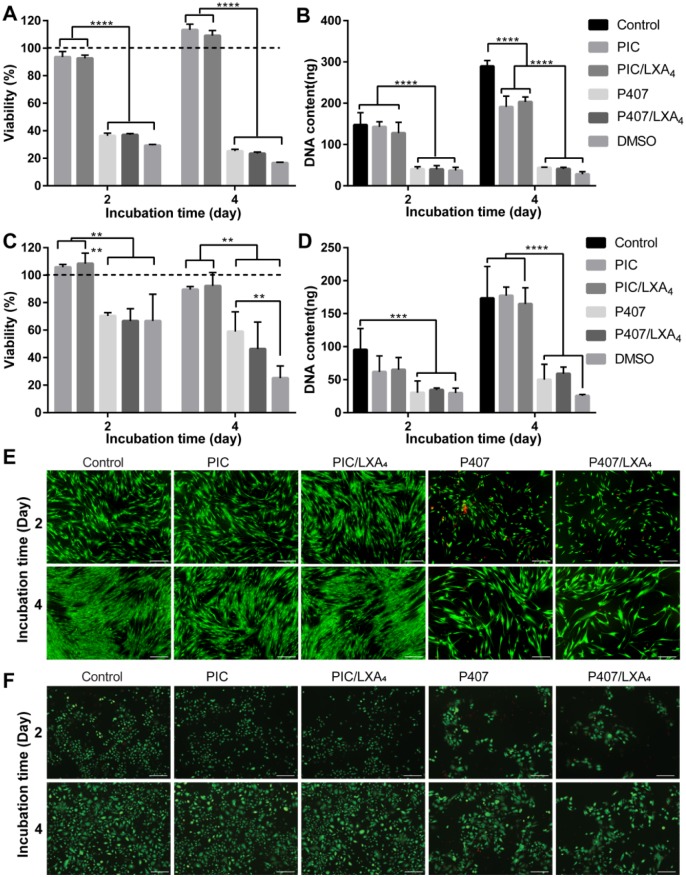
Results of cytocompatibility test are shown in Figure 4. The response of PDLCs and GECs to different gel systems was similar. One percent PIC and PIC/LXA4 showed good cell viability, while 26% P407 and P407/LXA4 hindered cell growth, similar to 5% dimethyl sulfoxide (DMSO) (Fig. 4A and C). The DNA assay results corroborated that cells cultured with PICs had significantly higher amounts of DNA than those with P407s (Fig. 4B and D). The live/dead staining (Fig. 4E and F) displayed that most cells were viable in PIC groups, and dead cells were seldom found. However, a considerable number of dead cells and less live cells could be seen in the P407 groups.
Figure 4.
Cytocompatibility of 1% polyisocyanopeptides (PICs) and 26% poloxamer 407 (P407s) with or without lipoxin A4 (LXA4) on periodontal ligament cells (PDLCs) and gingival epithelial cells (GECs). (A) PDLC viability test on day 2 and day 4 by alamarBlue. PIC and PIC/LXA4 showed good cell viability comparable to the positive control, while P407 and P407/LXA4 had only around 36% cell viability at day 2 and 25% at day 4, same as 5% dimethyl sulfoxide (DMSO). Cell viability is normalized by control group (cells cultured on cell culture plate without gel); the black dashed line represents the control group with 100% viability. (B) Total PDLC DNA content on day 2 and day 4. Cells cultured with PIC gels had significantly higher amounts of DNA than those with P407 gels. ****P < 0.0001. (C) GEC viability test on day 2 and day 4 by alamarBlue. PIC and PIC/LXA4 showed good viability on GECs comparable to that on PDLCs. P407 and P407/LXA4 were less toxic to GECs than to PDLCs, which is around 70% cell viability at day 2 and 50% at day 4. (D) Total GEC DNA content. In the PIC and PIC/LXA4 group, DNA content is comparable to control on days 2 and 4. A significantly lower DNA amount was shown for P407 and P407/LXA4 than that for the control group. Live/dead staining of PDLCs (E) and GECs (F) culturing with 1% PICs and 26% P407s with or without LXA4 of 2 d and 4 d. A few fibroblasts from the gingival tissues could also be found in GEC culture. Green, live cells; red, dead cells. Scale bar is 200 µm.
Discussion
This study aimed to explore the feasibility of thermoresponsive PIC gel for controlled release of LXA4 and to evaluate their potential for clinical periodontal applications. Three important parameters were considered: 1) the placement and retention of the dosage form at the site of application, 2) the ability to offer controlled drug release, and 3) the cytocompatibility of the gel (Jones et al. 2009). P407 was adopted as the control (Bruschi et al. 2007; Kumari and Pathak 2012). The results revealed that PIC possessed good clinical potential featured by its convenient handling ability and long-term stability, facilitating sustained release of LXA4 and cytocompatibility.
When developing a topically applied drug, the first consideration would be the local physiological conditions. The pH within the periodontal pocket was reported as ranging from 2 to 9, depending on the physiological or pathological conditions (Galgut 2001). The stability results of LXA4 stated its integrity for at least 1 wk regardless of the pH. However, LXA4 quickly degraded or isomerized in 1 mo in the acidic solution, indicating that the drug formulation needs to be carefully designed if a longer treatment period in a low pH environment is desired.
To obtain a sustained release of the intended drug in the periodontal pockets, thermoresponsive gels have unique advantages to easily perfuse the deep or irregular pockets upon injection at low temperature and then form gel immediately to facilitate drug release in the pockets. The average pocket temperature has been reported to be 36.6°C ± 0.4°C (Haffajee et al. 1992). Both PIC and P407 gels had Tsol-gel between 13°C and 23°C; thus, they can be easily injected through thin needles in a second and gel in situ, which contributes to their good handling ability. The process of thermally induced PIC gel formation is attributed to hydrophobic effects caused by the ethylene glycol tails grafted onto its polyisocyanide backbone (Kouwer et al. 2013). For P407 gel, micellar desolvation and swelling give rise to a large cross-linked micelle network when the temperature rises (Veyries et al. 1999). The PIC gels showed a much lower G′′ than P407, indicating a superior flowability to fully irrigate deep, irregular pockets. After injection, the long-term retention of the gel in the pockets is the premise of a sustained release. P407 gels disintegrated much faster than PIC gels. This result corroborates the previous report that the orderly packed micelles of P407 dissociated gradually with the influence of surrounding liquid (Dumortier et al. 2006). In contrast, PIC has a stiff helical polyisocyanide backbone stabilized with the hydrogen-bonded dialanyl groups, which enables PIC to form a semiflexible network in water and thus tolerate the microflow. However, this setting of the gel stability test in this study is not comparable to the in vivo complicated condition, for example, a) continuous epithelial turnover at the interface, b) flow of gingival crevicular fluid, c) efflux of immune cells, and d) gravity. Although PIC gel is not biodegradable, it can be removed by cold water irrigation. Several thermosensitive hydrogel products, mostly based on poloxamers, have been developed for periodontal application (Maheshwari et al. 2006; Pandit et al. 2016; Nasra et al. 2017). Poloxamers degrade very slowly (Erlandsson 2002) and preferably should be cleaned by cold rinsing. At the same time, such a procedure would also prevent the (unlikely) possibility that remaining gel would hinder the reattachment of periodontal tissue. To our knowledge, there have been no reports showing that periodontal gels prevented the reattachment; instead, gels incorporated with therapeutic agents displayed a favorable effect on periodontal regeneration (Maheshwari et al. 2006; Nasra et al. 2017). After disintegration, PIC gels may enter the digestive tract before irrigation and may be excreted out of body with feces like PEG gel (Sternebring et al. 2016) or with urine, as shown in the other study of our group (unpublished data). Based on the results of stability and drug release in this study, PIC gel is suggested to be changed in weekly cycles.
Besides handling ability and physical stability, a more important aspect is the release of the targeted drugs. Several aspects may affect the release of LXA4 from gels: the solubility and diffusion rate of the drug, as well as the erosion of the gels. LXA4 theoretically possesses 3 hydroxyl residues, 1 carboxyl residue, and 4 double bonds (Serhan et al. 1984), which facilitate binding to the polyisocyanide backbone and ethylene glycol tails of PIC by hydrogen bonds and hydrophobic interactions. It can be inferred that the higher the polymer concentration, the more hydrogen bonds are formed, resulting in a slower and prolonged release, as shown in this study. The addition of detergent significantly increased the release rate, which confirms the hydrophobic interaction (Song et al. 2015). For practical concerns, if enough surfactant in the dentifrices goes inside the pockets, it may accelerate LXA4 release. Similarly, LXA4 release accelerates when pH increases, possibly because the carboxyl residues become more ionized in the base, which increases the drug solubility in water (Savjani et al. 2012). Correspondingly, the release rate was slower in acid probably due to decreased drug solubility. Regarding P407, LXA4 can be trapped inside of its micelles, which act as a barrier to weaken the effect of detergent and base (Dumortier et al. 2006). Because P407 is easily eroded, LXA4 release relied on the gel erosion besides diffusion (Zhang et al. 2002; Maheshwari et al. 2006). The LXA4 total release amount diminished in the acidic medium for both PIC and P407 likely due to the degradation induced by acid. Finally, it is mentionable that a cumulative release amount reached around 90% in PICs and 80% in P407s. Not fully releasing may also partially be attributed to the slow degradation of LXA4.
A phagocytic activity test was used to evaluate the efficacy of LXA4 released from gels (Prescott and McKay 2011). Only LXA4 from PIC gels promoted phagocytosis of macrophages. Since there is no obvious chemical reaction between LXA4 and P407, it is highly unlikely that the presence of P407 leads to deactivation of LXA4. Therefore, other substances in the P407 releasates may hinder the phagocytosis process, which counteracts the LXA4 effect. Further cytocompatibility experimentation revealed that P407 could be toxic to PDL and GEC cells, which could explain the low phagocytosis in the P407/LXA4 group. In practice, w/w ≥20% P407 is widely used (e.g., for oral, rectal, ophthalmic usage) in vivo (Ji et al. 2010). Some studies reported that low concentrations of P407 had no inherent cytotoxicity (Hokett et al. 2000; Exner et al. 2005), but the cytotoxicity at 20% or higher concentration has not been reported yet. Based on our study, 26% P407 has unexpected growth inhibition to PDL cells in transwell culture, which may be due to its easy dissolution to the medium through the transwell pores. Such hindrance of cell growth should be further investigated. In contrast to P407, PIC did not erode to such an extent but remained more stable and cytocompatible. Because metabolic assays (e.g., alamarBlue) could not accurately reflect cellular proliferation rates, a discrepancy was observed between the viability and proliferation results in PIC groups and the control group (Fig. 4A and B) (Quent et al. 2010). A recent study demonstrated that fibroblasts, endothelial cells, adipose-derived stem cells, and melanoma cells did survive, thrive, and differentiate in PIC gels, further confirming its cytocompatibility (Zimoch et al. 2018).
Conclusion
In this study, PIC/LXA4 gel featured by convenient handling ability was stable for over 2 wk. It was capable of releasing LXA4 in a sustained manner while preserving biological activity and was cytocompatible. In conclusion, PIC hydrogel was appropriate for the intended periodontal applications, and its clinical potential for treating periodontitis should be further developed.
Author Contributions
B. Wang, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; J. Shao, J.A. Jansen, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; X. F. Walboomers, F. Yang, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034518810213 for A Novel Thermoresponsive Gel as a Potential Delivery System for Lipoxin by B. Wang, J. Shao, J.A. Jansen, X.F. Walboomers and F. Yang in Journal of Dental Research
Acknowledgments
The authors thank Dr. Paul H. J. Kouwer and Dr. Onno I. van den Boomen for providing the PIC polymers.
Footnotes
This work was financially supported by the NWO Domain Applied and Engineering Sciences (project number 13844) and the Chinese Scholarship Council (project number 201509370020).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is available online.
References
- Bruschi ML, Jones DS, Panzeri H, Gremião MP, De Freitas O, Lara EH. 2007. Semisolid systems containing propolis for the treatment of periodontal disease: in vitro release kinetics, syringeability, rheological, textural, and mucoadhesive properties. J Pharm Sci. 96(8):2074–2089. [DOI] [PubMed] [Google Scholar]
- Das RK, Gocheva V, Hammink R, Zouani OF, Rowan AE. 2016. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat Mater. 15(3):318–325. [DOI] [PubMed] [Google Scholar]
- Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. 2006. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 23(12):2709–2728. [DOI] [PubMed] [Google Scholar]
- Erlandsson B. 2002. Stability-indicating changes in poloxamers: the degradation of ethylene oxide-propylene oxide block copolymers at 25 and 40°C. Polym Degradation Stab. 78(3):571–575. [Google Scholar]
- Exner AA, Krupka TM, Scherrer K, Teets JM. 2005. Enhancement of carboplatin toxicity by pluronic block copolymers. J Control Release. 106(1):188–197. [DOI] [PubMed] [Google Scholar]
- Galgut P. 2001. The relevance of pH to gingivitis and periodontitis. J Int Acad Periodontol. 3(3):61–67. [PubMed] [Google Scholar]
- Haffajee A, Socransky S, Goodson J. 1992. Subgingival temperature (i). Relation to baseline clinical parameters. J Clin Periodontol. 19(6):401–408. [DOI] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. 2007. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 179(10):7021–7029. [DOI] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis N, Levy B, Serhan C, Van Dyke T. 2006. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J. 20(2):401–403. [DOI] [PubMed] [Google Scholar]
- Hokett SD, Cuenin MF, O’Neal RB, Brennan WA, Strong SL, Runner RR, McPherson JC, Van Dyke TE. 2000. Pluronic polyol effects on human gingival fibroblast attachment and growth. J Periodontol. 71(5):803–809. [DOI] [PubMed] [Google Scholar]
- Ji QX, Zhao QS, Deng J, Lü R. 2010. A novel injectable chlorhexidine thermosensitive hydrogel for periodontal application: preparation, antibacterial activity and toxicity evaluation. J Mater Sci Mater Med. 21(8):2435–2442. [DOI] [PubMed] [Google Scholar]
- Jones DS, Bruschi ML, de Freitas O, Gremião MPD, Lara EHG, Andrews GP. 2009. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int J Pharm. 372(1):49–58. [DOI] [PubMed] [Google Scholar]
- Joshi D, Garg T, Goyal AK, Rath G. 2016. Advanced drug delivery approaches against periodontitis. Drug Deliv. 23(2):363–377. [DOI] [PubMed] [Google Scholar]
- Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC. 2013. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci U S A. 110(16):6506–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouwer PH, Koepf M, Le Sage VA, Jaspers M, van Buul AM, Eksteen-Akeroyd ZH, Woltinge T, Schwartz E, Kitto HJ, Hoogenboom R. 2013. Responsive biomimetic networks from polyisocyanopeptide hydrogels. Nature. 493(7434):651–655. [DOI] [PubMed] [Google Scholar]
- Kumari N, Pathak K. 2012. Dual controlled release, in situ gelling periodontal sol of metronidazole benzoate and serratiopeptidase: statistical optimization and mechanistic evaluation. Curr Drug Deliv. 9(1):74–84. [DOI] [PubMed] [Google Scholar]
- Lee C-T, Teles R, Kantarci A, Chen T, McCafferty J, Starr JR, Brito LCN, Paster BJ, Van Dyke TE. 2016. Resolvin E1 reverses experimental periodontitis and dysbiosis. J Immunol. 197(7):2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Vachier I, Serhan CN. 2012. Resolution of inflammation in asthma. Clin Chest Med. 33(3):559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari M, Miglani G, Mali A, Paradkar A, Yamamura S, Kadam S. 2006. Development of tetracycline-serratiopeptidase-containing periodontal gel: formulation and preliminary clinical study. AAPS PharmSciTech. 7(3):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. 2010. Inflammation 2010: new adventures of an old flame. Cell. 140(6):771–776. [DOI] [PubMed] [Google Scholar]
- Nasra MM, Khiri HM, Hazzah HA, Abdallah OY. 2017. Formulation, in-vitro characterization and clinical evaluation of curcumin in-situ gel for treatment of periodontitis. Drug Deliv. 24(1):133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Gómez A, Perretti M, Soehnlein O. 2013. Resolution of inflammation: an integrated view. EMBO Mol Med. 5(5):661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit AP, Pol VV, Kulkarni VS. 2016. Xyloglucan based in situ gel of lidocaine HCl for the treatment of periodontosis. J Pharm. 2016:3054321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D, McKay DM. 2011. Aspirin-triggered lipoxin enhances macrophage phagocytosis of bacteria while inhibiting inflammatory cytokine production. Am J Physiol Gastrointest Liver Physiol. 301(3):G487–G497. [DOI] [PubMed] [Google Scholar]
- Quent VM, Loessner D, Friis T, Reichert JC, Hutmacher DW. 2010. Discrepancies between metabolic activity and DNA content as tool to assess cell proliferation in cancer research. J Cell Mol Med. 14(4):1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savjani KT, Gajjar AK, Savjani JK. 2012. Drug solubility: importance and enhancement techniques. ISRN Pharma. 2012:195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. 2010. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 177(4):1576–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. 2007. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21(2):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 8(5):349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B. 1984. Trihydroxytetraenes: a novel series of compounds formed from arachidonic acid in human leukocytes. Biochem Biophys Res Commun. 118(3):943–949. [DOI] [PubMed] [Google Scholar]
- Song J, Odekerken JC, Löwik DW, López-Pérez PM, Welting TJ, Yang F, Jansen JA, Leeuwenburgh SC. 2015. Influence of the molecular weight and charge of antibiotics on their release kinetics from gelatin nanospheres. Macromol Biosci. 15(7):901–911. [DOI] [PubMed] [Google Scholar]
- Sternebring O, Christensen JK, Bjørnsdottir I. 2016. Pharmacokinetics, tissue distribution, excretion, and metabolite profiling of pegylated rFIX (nonacog beta pegol, N9-GP) in rats. Eur J Pharm Sci. 92:163–172. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, Serhan CN. 2015. Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res. 94(1):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke TE, Serhan CN. 2003. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 82(2):82–90. [DOI] [PubMed] [Google Scholar]
- Veyries M, Couarraze G, Geiger S, Agnely F, Massias L, Kunzli B, Faurisson F, Rouveix B. 1999. Controlled release of vancomycin from poloxamer 407 gels. Int J Pharm. 192(2):183–193. [DOI] [PubMed] [Google Scholar]
- Vyas S, Sihorkar V, Mishra V. 2000. Controlled and targeted drug delivery strategies towards intraperiodontal pocket diseases. Journal of clinical pharmacy and therapeutics. 25(1):21–42. [DOI] [PubMed] [Google Scholar]
- Zhang L, Parsons DL, Navarre C, Kompella UB. 2002. Development and in-vitro evaluation of sustained release poloxamer 407 (P407) gel formulations of ceftiofur. J Control Release. 85(1):73–81. [DOI] [PubMed] [Google Scholar]
- Zimoch J, Padial JS, Klar AS, Vallmajo-Martin Q, Meuli M, Biedermann T, Wilson CJ, Rowan A, Reichmann E. 2018. Polyisocyanopeptide hydrogels: a novel thermo-responsive hydrogel supporting pre-vascularization and the development of organotypic structures. Acta Biomater. 70:129–139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034518810213 for A Novel Thermoresponsive Gel as a Potential Delivery System for Lipoxin by B. Wang, J. Shao, J.A. Jansen, X.F. Walboomers and F. Yang in Journal of Dental Research