Abstract
Coccolithophores are unicellular marine phytoplankton and important contributors to global carbon cycling. Most work on coccolithophore sensitivity to climate change has been on the small, abundant bloom-forming species Emiliania huxleyi and Gephyrocapsa oceanica. However, large coccolithophore species can be major contributors to coccolithophore community production even in low abundances. Here we fit an analytical equation, accounting for simultaneous changes in CO2 and light intensity, to rates of photosynthesis, calcification and growth in Scyphosphaera apsteinii. Comparison of responses to G. oceanica and E. huxleyi revealed S. apsteinii is a low-light adapted species and, in contrast, becomes more sensitive to changing environmental conditions when exposed to unfavourable CO2 or light. Additionally, all three species decreased their light requirement for optimal growth as CO2 levels increased. Our analysis suggests that this is driven by a drop in maximum rates and, in G. oceanica, increased substrate uptake efficiency. Increasing light intensity resulted in a higher proportion of muroliths (plate-shaped) to lopadoliths (vase shaped) and liths became richer in calcium carbonate as calcification rates increased. Light and CO2 driven changes in response sensitivity and maximum rates are likely to considerably alter coccolithophore community structure and productivity under future climate conditions.
Introduction
Coccolithophores are a group of phytoplankton (division Haptophyta, class Prymnesiophyceae), which produce both particulate organic carbon through photosynthesis and particulate inorganic carbon through calcification1,2. They are found in almost all ocean ecosystems from the equator to sub-polar latitudes, from nutrient rich upwelling zones to nutrient poor oligotrophic regions and from surface waters to over 100 m in depth3–7. Within ocean ecosystems coccolithophores play an important role in the production and cycling of carbon contributing up to 20% to total organic carbon fixation8,9 and up to 50% of calcium carbonate flux to marine sediments10–12. Because of this, coccolithophores have become amongst the most intensively studied organisms in terms of the effects of ocean acidification in particular and climate change in general (e.g.13–16). Recent work, suggests that coccolithophores have a common over-arching response to changes in carbonate chemistry16–18. However, these responses vary between species, strains and additionally can be modulated by changes in light intensity and temperature16,19,20.
Of approximately 200 extant species of coccolithophores (e.g.21), the majority of research into coccolithophore responses to climate change come from only a few species such as Emiliania huxleyi, Gephyrocapsa oceanica, Calcidiscus leptoporus, and Coccolithus pelagicus spp. braarudii. Of these most studies have been on E. huxleyi which is considered a keystone species due to its ability to form massive blooms22, its dominance within the coccolithophore assemblage3,6,23, and its almost global distribution3 (North and South Atlantic),22 (North Atlantic),6 (Equatorial and sub-equatorial Pacific),24 (East Mediterranean),25,26 (Southern Ocean),27 (North Sea). While potentially dominant in terms of abundance E. huxleyi s contribution to both the organic and inorganic carbon cycle is likely less of importance. Among the coccolithophores E. huxleyi is quite small (cell size ∼5 μm) and has a fairly low cellular calcium carbonate (CaCO3) content (2.88 to 12.12 pg C cell−1)16,28–30 (Fig. 1). Meanwhile larger and less abundant species such as C. pelagicus (∼16 μm), C. leptoporus (∼13.8 μm), Helicosphaera carteri or Scyphosphaera apsteinii (∼17.6 μm) produce more than 20 times this amount29,31,32. Recent research suggests that these larger and more CaCO3 rich species may be the dominant contributors to carbon production and export in the coccolithophore assemblage10,29,33–35. Despite this, little is known about the effects of climate change on these larger species. As such the aim of this study was to examine the effects of changing carbonate chemistry and light intensity on the large coccolithophore S. apsteinii.
Figure 1.

Scanning electron microscope images of a E. huxleyi, S. apsteinii and a Coccolithus species showcasing size and cellular calcium carbonate quota differences between species. Species shown were either isolated or cultured by the authors.
Results
Growth of S. apsteinii cultures was observed between starting conditions of 50–5000 μatm, but not at 7000 μatm. The fit to combined light and carbonate chemistry conditions (Eq. 2) explained up to 75% of the variability in measured response rates across a range of carbonate chemistry (47–2570 μatm) and light (50–515 μmol photons m−2 s−1) conditions (Table 1). The equation was able to capture the limiting, stimulating and inhibiting effects of changing carbonate chemistry (Fig. 2) and light intensity (Fig. 3) on all physiological rates.
Table 1.
Fit coefficients (k1, k2, k3, k4, k5, k6), R2, p-values, F-values and degrees of freedom obtained from fit equation (2) for calcification (pg C cell−1 d−1), photosynthetic carbon fixation (pg C cell−1 d−1) and growth rate (d−1) fits to all data. Note that for calcification and photosynthetic carbon fixation rates the unit of k1 is pg C cell−1 day−1 and for growth rates day−1.
| Calcification | Photosynthesis | Growth rate | |
|---|---|---|---|
| k1 (pg C cell−1 day−1 or day−1) | 5.77E + 13 | −1.18E + 05 | −7.57E + 04 |
| k2 (μmol photons m−2 s−1) | 2.56E + 18 | −1.79E + 10 | −1.97E + 12 |
| k3 (kg mol−1 μmol photons m−2 s−1) | −1.00E + 03 | 6.21E + 12 | 4.78E + 14 |
| k4 (mol kg−1) | 2.29E + 09 | −2.81 | −569.84 |
| k5 (dimensionless) | −1.15E + 12 | 1.35E + 03 | 2.12E + 05 |
| k6 (kg mol−1 μmol photons−1 m2 s) | 7.95E + 16 | −1.09E + 08 | −2.69E + 10 |
| R2 | 0.7501 | 0.7012 | 0.5727 |
| (p-value) | (4.43E-12) | (3.00E-11) | (2.41E-8) |
| F-value | 105.05 | 86.91 | 49.74 |
| Degrees of freedom | 35 | 37 | 37 |
Figure 2.
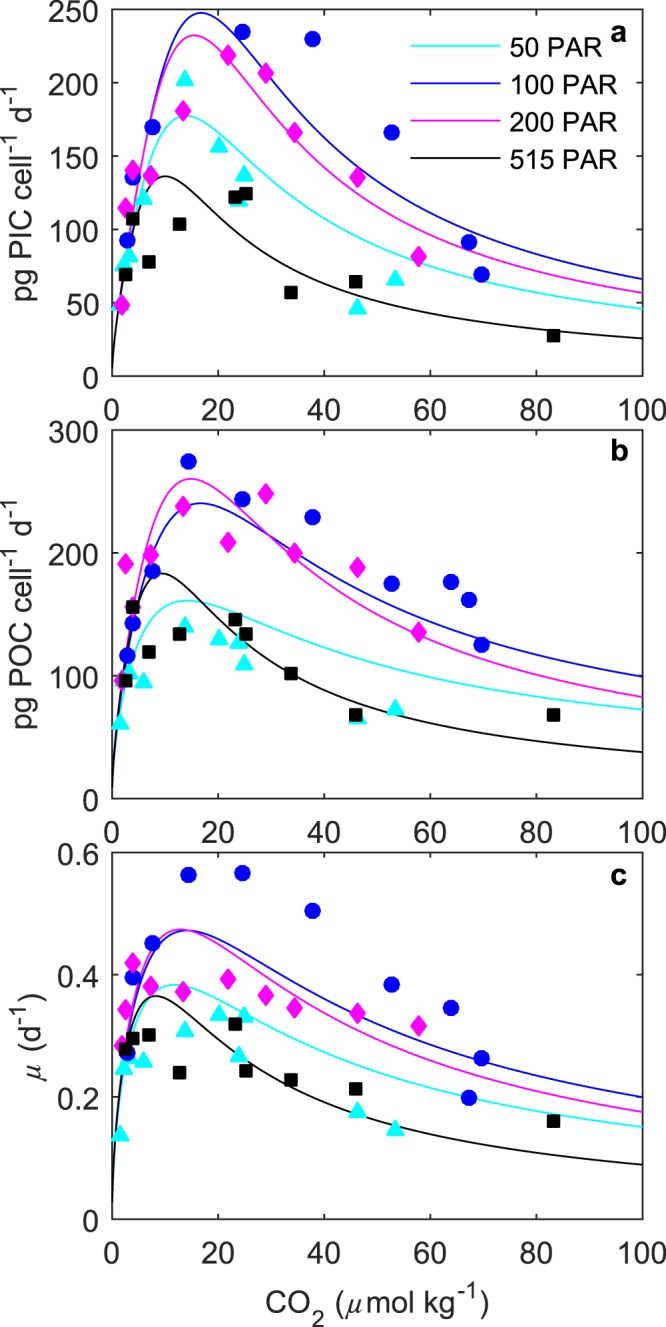
Fitted (solid lines) and measured (symbols) (a) particulate inorganic carbon (PIC), (b) particulate organic carbon (POC) production, and (c) growth rates in response to changes in CO2 concentration at six different light intensities using equation 2 and fit coefficients from Table 1. Symbols represent rate measurements at a constant temperature (20 °C) and 50 (Δ), 100 (◯), 200 (◊) and 515 (□) μmol photons m−2 s−1.
Figure 3.
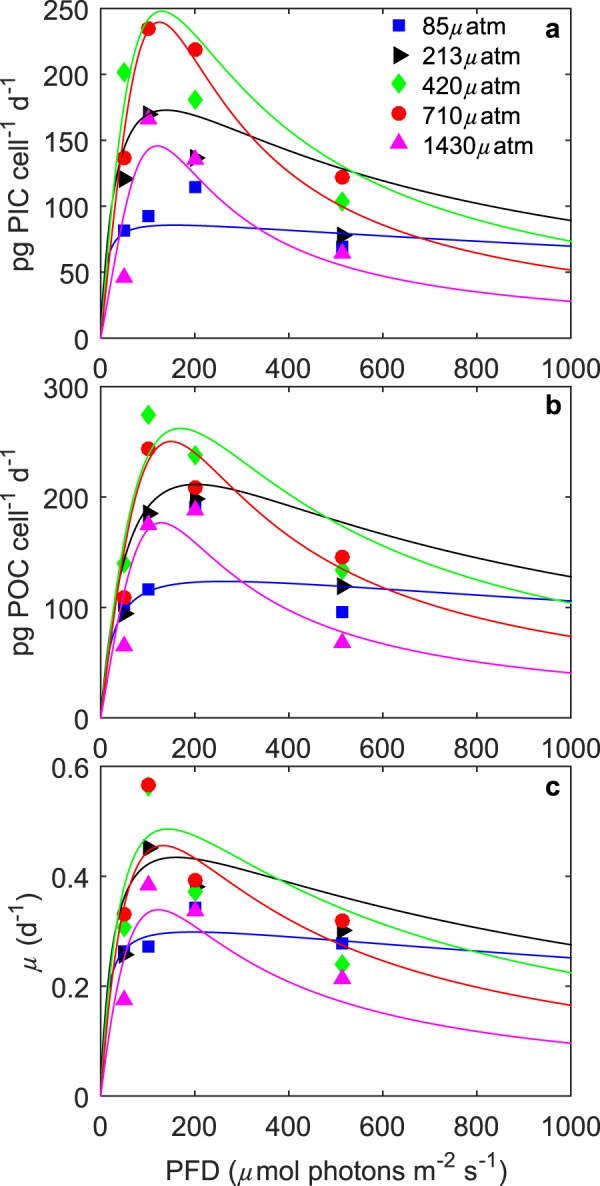
(a) Particulate inorganic carbon (PIC) and (b) particulate organic carbon (POC) production, and (c) growth rates of S. apsteinii in response to changes in light intensity at five different f CO2 levels at a constant temperature (20 °C). Symbols denote measured data at f CO2 levels of ∼85 (□), ∼213 (⊳), ∼420 (◊), and ∼710 (◯) and ∼1430 (Δ) μatm and lines represent calculated rates using equation 2 and fit coefficients in Table 1.
Responses to changing carbonate chemistry
Growth, photosynthetic carbon fixation and calcification rates all had an optimum curve response to increasing CO2 (Fig. 2, Table S1) at all light intensities. Depending upon CO2 level and light intensity, rates varied between 28–234 pg C cell−1 d−1 for calcification, 60–243 pg C cell−1 d−1 for photosynthesis and 0.14–0.57 d−1 for growth (Table S1). At all light intensities, particulate inorganic to organic carbon ratios PIC:POC ratios increased with CO2 to an optimal point before declining with further increases in CO2 (Table S1). PIC:POC ratios varied from 0.42 to 1.44 depending upon CO2 level and light intensity (Table S1).
Calcification, photosynthetic carbon fixation and growth rates had similar CO2 requirements to achieve half saturation and optimal rates (Table 2). Calcification rates were more sensitive to increasing [H+] than photosynthetic carbon fixation or growth rates (Table 2, Fig. 2). Optimum CO2 and CO2 half-saturation concentrations changed little (in comparison to the overall CO2 response range and uncertainties in the model fit) with increasing light (Table 2). CO2 half inhibition concentrations changed little with increasing light for calcification, followed a slight optimum curve response for growth, and decreased with increasing light for photosynthesis (Table 2). Differences in sensitivity to [H+] between the three rates decreased with increasing light intensity.
Table 2.
Optimum CO2 concentrations, concentrations and maximum rates (Vmax) of S. apsteinii from fit equation (2) at 50–515 μmol photons m−2 s−1 and 20 °C using fit coefficients from Table 1.
| CO2 | 50 PAR | 100 PAR | 200 PAR | 515 PAR |
|---|---|---|---|---|
| CO 2 optima ( μ mol kg −1 ) | ||||
| Calcification | 13.9 | 16.8 | 15.5 | 10.0 |
| Photosynthesis | 14.2 | 16.7 | 14.8 | 9.4 |
| Growth rate | 11.8 | 14.1 | 12.9 | 8.2 |
| V max (pg C cell −1 d −1 or d −1 ) | ||||
| Calcification | 177.4 | 247.6 | 232.3 | 136.2 |
| Photosynthesis | 161.1 | 240.3 | 260.2 | 183.2 |
| Growth rate | 0.38 | 0.47 | 0.47 | 0.37 |
| ( μ mol kg −1 ) | ||||
| Calcification | 49.9 | 53.8 | 49.7 | 36.7 |
| Photosynthesis | 84.1 | 77.2 | 59.5 | 38.3 |
| Growth rate | 71.3 | 79.4 | 68.0 | 42.6 |
| ( μ mol kg −1 ) | ||||
| Calcification | 3.2 | 4.5 | 4.1 | 2.2 |
| Photosynthesis | 1.9 | 3.0 | 3.0 | 1.8 |
| Growth rate | 1.4 | 1.8 | 1.8 | 1.1 |
Responses to changing light intensity
Maximum rates for growth, calcification and photosynthetic carbon fixation were observed to increase up to an optimum light intensity before declining with further increases in light (Fig. 3). Calcification rates increased 39% between 50 and 100 μmol photons m−2 s−1 before declining by 45% at 515 μmol photons m−2 s−1. Photosynthetic carbon fixation rates increased 62% between 50 and 200 μmol photons m−2 s−1 and declined by 30% at 515 μmol photons m−2 s−1. Growth rates increased 24% between 50 and 200 μmol photons m−2 s−1 and declined by 23% at 515 μmol photons m−2 s−1 (Table 2). The effect of carbonate chemistry (i.e. the combined change in concentrations of CO2, , and H+ as a result of rising f CO2) on metabolic rates was greatest between 100–400 μmol photons m−2 s−1 and decreased towards more extreme light intensities (Fig. 3).
Increasing f CO2 resulted in an increase in light half-saturation intensities , a decrease in optimal light requirements, and an increase in sensitivity to high light for all rates (Table 3). Calcification reached optimal rates at lower light intensities (120–157 μmol photons m−2 s−1) than growth (122–196 μmol photons m−2 s−1) or photosynthesis (128–263 μmol photons m−2 s−1), though differences between the rates decreased with increasing f CO2 (Table 3). Inhibiting light levels were lowest for calcification (385–1050 μmol photons m−2 s−1), higher for photosynthesis (447–1346 μmol photons m−2 s−1) and highest for growth (521–1524 μmol photons m−2 s−1).
Table 3.
Calculated optimum light intensities, maximum rates (Vmax) and light values of S. apsteinii at 20 °C and 85, 213, 420, 710 and 1430 μatm using equation 2 and fit coefficients from Table 1.
| Light | 85 μatm | 213 μatm | 420 μatm | 710 μatm | 1430 μatm |
|---|---|---|---|---|---|
| Optima ( μ mol photons m − 2 s − 1 ) | |||||
| Calcification | 157 | 139 | 130 | 125 | 120 |
| Photosynthesis | 263 | 203 | 169 | 149 | 129 |
| Growth rate | 196 | 161 | 143 | 133 | 123 |
| V max (pg C cell −1 d −1 or d −1 ) | |||||
| Calcification | 85.6 | 172.9 | 247.9 | 239.6 | 145.7 |
| Photosynthesis | 123.6 | 211.4 | 262.1 | 250.3 | 176.6 |
| Growth rate | 0.30 | 0.43 | 0.49 | 0.46 | 0.34 |
| ( μ mol photons m − 2 s − 1 ) | |||||
| Calcification | >2500 | 1050 | 548 | 423 | 386 |
| Photosynthesis | >2500 | 1346 | 754 | 561 | 448 |
| Growth rate | >2500 | 1524 | 894 | 667 | 521 |
| ( μ mol photons m − 2 s − 1 ) | |||||
| Calcification | 7.2 | 18.3 | 30.6 | 36.8 | 37.4 |
| Photosynthesis | 18.4 | 30.6 | 38.0 | 39.7 | 37.1 |
| Growth rate | 9.9 | 17.0 | 22.8 | 26.4 | 28.8 |
Coccolith morphology
Between the different light and CO2 treatments, coccosphere size ranged from 12.0–22.4 μm (average 17.2, Table S2). The number of muroliths per cell varied between five and 23 (average 12.4), while the number of lopadoliths per cell varied between zero and six (average 2.3). The ratio of muroliths to lopadoliths on a cell varied from zero to 22 (average 6.4). The length of muroliths was 6.1–10.9 μm (average 8.3) while the width was 4.0–9.1 μm (average 6.1). The length of lopadoliths varied between 2.8–13.9 μm (average 8.1) and the width 6.1–16.3 μm (average 10.5). The ratio of muroliths to lopadoliths was observed to increase with increasing light at 100 (n = 479 df = 2, Chi-sq = 14.34, p = 7.69E-4) and 400 μatm CO2 (n = 357 df = 2, Chi-sq = 11.44, p = 3.28E-3). There was also a significant effect of calcification rate on PIC per lith, with liths containing more PIC in treatments with higher calcification rates (n = 8 R2 = 0.80, F = 23.45 p = 2.87E-3, Fig. 4). Other than this, no environmentally significant patterns were observed in any morphological features with changing CO2 or light (see supplementary information for discussion on these results).
Figure 4.
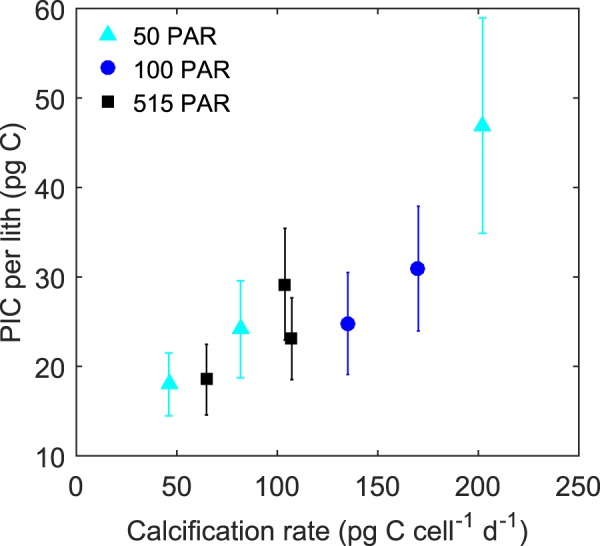
PIC content, by weight, of average S. apsteinii coccolith (mix of muroliths and lopadoliths) versus calcification rate under different light and CO2 conditions. Error bars represent standard deviation for each treatment condition.
Discussion
Increasing CO2 concentrations resulted in an optimum curve response in all physiological rates for S. apsteinii (Fig. 2). This response pattern has now been observed for multiple coccolithophore species16,18, and is most likely driven by the combined effects of physiological rate stimulation by increasing substrate (CO2 and ) availability and physiological rate inhibition by increasing [H+]14,17,18,36.
Larger species generally require more substrate than smaller species to sustain growth. However, larger species have less surface area, relative to their volume, over which to take up essential materials and nutrients37. As such, it is expected that larger species, with a lower surface area to volume ratio, would require either a higher substrate concentration or a faster uptake rate in order to support their higher cellular requirements37. If higher substrate concentrations were needed then it could be expected that S. apsteinii might have higher CO2 half-saturation and optimum requirements than E. huxleyi and G. oceanica for all rates. However, S. apsteinii had a similar and CO2 optimum as the two smaller species for growth and photosynthetic carbon fixation rates and a slightly higher CO2 optimum requirement for calcification rates (Table 2,20,30). This suggests that S. apsteinii would need to support its greater substrate demand by fixing more carbon per unit surface area than smaller species. To see if this was the case, daily carbon fixation per unit surface area was calculated using carbon fixation rates (POC, PIC and TPC from16,19,20 and this paper at 20 °C) and average cell diameters of 5.59, 9.33, and 17.59 μm for E. huxleyi, G. oceanica and S. apsteinii, respectively.
Based on these calculations, S. apsteinii fixes more carbon per unit surface area per day than either E. huxleyi or G. oceanica under most CO2 conditions at low to moderate light intensities (Fig. 5a and b). However, at higher light intensities it fixes approximately the same amount of carbon per unit surface area per day (Fig. 5c). The relative decrease in carbon fixation at these higher light intensities in comparison to E. huxleyi and G. oceanica is likely due to a higher light sensitivity (see below). Meanwhile below this threshold, the generally higher fixation of carbon per unit surface area per day by S. apsteinii can be achieved by higher inorganic carbon transporter density (higher substrate uptake), or by less diffusive CO2 leakage from the cell. It may be that by having a proportionally lower amount of the cells internal volume interacting with the surrounding media (due to a much lower surface area to volume ratio) S. apsteinii loses proportionally less CO2 through leakage. While decreased leakage in larger coccolithophore species has been suggested previously37, this difference in leakage was thought to be driven by the uptake of different proportions of CO2 and between the species rather than size alone. While evidence suggests that carbon uptake efficiency is higher in some species and phytoplankton groups (i.e.38,39), it is not yet clear if cell size has a consistent effect on carbon uptake efficiencies.
Figure 5.
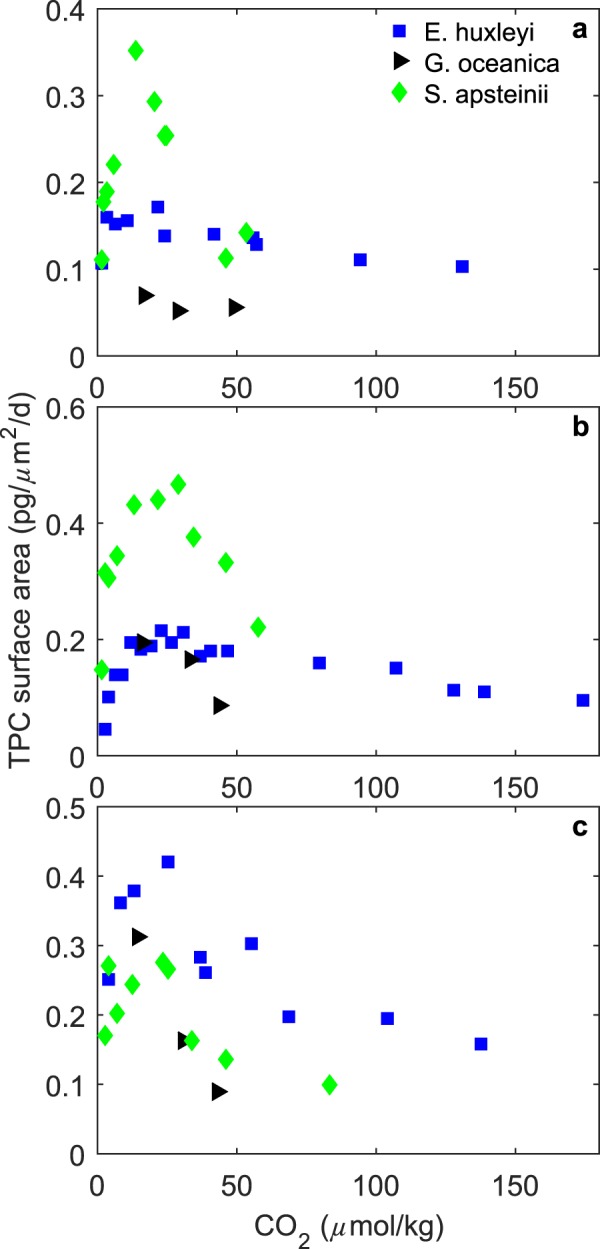
Total particulate carbon production rate per unit surface area (μm2) for E. huxleyi, G. oceanica and S. apsteinii across a range of CO2 concentrations and at (a) 50, (b) 150–200, and (c) 515–600 μmol photons m−2 s−1.
Sensitivity to high CO2/low pH varied between the three rates with calcification being more sensitive than photosynthesis and growth (Table 2). This agrees with previous work on G. oceanica, E. huxleyi and C. pelagicus, and provides additional evidence for the general notion that calcification by coccolithophores will be negatively impacted by future ocean changes16,18–20. Sensitivity of calcification rates to high CO2 are similar between S. apsteinii and E. huxleyi, with G. oceanica being much more sensitive20,30. The steeper decline of calcification rates, beyond optimum CO2, of G. oceanica compared to E. huxleyi has previously been speculated to be connected to a higher degree of inorganic versus organic carbon production (PIC:POC) in G. oceanica18,20. The relatively greater production of intracellular H+, via calcification, makes it more difficult for species species with higher PIC content to maintain intracellular pH homeostasis17. In this regard, S. apsteinii with a similar PIC:POC as E. huxleyi would fit into this concept.
Significant inhibition of all rates by light was observed in S. apsteinii when CO2 levels exceeded 100 μatm (Fig. 3, Table 3). That is in sharp contrast to rates in E. huxleyi and G. oceanica for which little light inhibition is observed regardless of f CO2 levels19,40–45. Optimal light intensities for growth, calcification and photosynthesis of S. apsteinii also differ from those in E. huxleyi and G. oceanica. While optimal physiological rates for S. apsteinii are observed at light intensities between ∼120–263 μmol photons m−2 s−1, depending upon f CO2 level, optimal physiological rates for G. oceanica and E. huxleyi, under comparable experimental conditions, have been observed at light intensities of 400–800 μmol photons m−2 s−1 and 500–1200 μmol photons m−2 s−1, respectively (Table 2,19,20). S. apsteinii also tends to half-saturate its rates at lower light intensities than either E. huxleyi or G. oceanica under most CO2 conditions (Table 2,20,30, unpublished E. huxleyi data). This suggests that S. apsteinii may be a lower light adapted species which is also supported by the fact that it has been observed to grow at light intensities as low as 5 μmol photons m−2 s−1 46. Oceanic abundance data for S. apsteinii is sparse, as a result it is currently not possible to confirm if similar low light preferences are observed in the natural environment.
As f CO2 levels increased S. apsteinii needed lower light intensities to sustain optimal rates. These results agree with those for G. oceanica, which also required less light to support optimum calcification and photosynthetic carbon fixation rates as f CO2 increased30. The results also agree with those for E. huxleyi growth rates, which required less light with rising f CO2, but not calcification or photosynthetic carbon fixation rates which were insensitive to changing light intensity20 (Table S3). In all three-above species, the increase in f CO2 levels also resulted in an overall decrease in maximum rates20,30 (Table 2). Most phytoplankton acquire inorganic carbon through a combination of passive CO2 diffusion into the cell, and CO2 concentrating mechanisms (CCMs) which actively transport inorganic carbon into the cell47,48. As CO2 concentrating mechanisms are an active process, they require energy to function39,48. The efficiency of passive uptake depends upon the cell to seawater CO2 concentration gradient (uptake versus leakage rates), membrane permeability and area39. So, assuming that cell size and membrane permeability stay constant, increasing seawater CO2 concentrations increase the diffusive influx and reduce loss through leakage. This would reduce the need for an active CCM thus lowering the cell’s energy requirements. As a result, higher substrate uptake efficiency could be indicated by a decrease in light levels needed to reach half-saturated and optimal rates (see Fig. 6 dotted line). Another explanation, for reduced light requirements could be the inhibiting effects of high CO2/H+. As CO2/H+ concentrations increase to above optimum levels it suppresses maximum rates to lower and lower absolute rates. Lower rates result in decreased demand for resources such as carbon. This lower substrate demand could result in decreased CCM activity thereby decreasing optimum light requirements under elevated CO2/H+ (Fig. 6 dashed line). All three species show decreased maximum rates under elevated CO2/H+. As a result, decreased energy requirements because of inhibited maximum rates could explain decreased optimum light requirements in E. huxleyi, G. oceanica and S. apsteinii. However, G. oceanica also shows indications of increased substrate uptake efficiency, through decreasing and optimal light requirements30. So, for G. oceanica it appears that increasing CO2/H+ may decrease light optimal requirements through a combination of increased substrate uptake efficiency and through inhibition of maximum rates (Fig. 6 dot-dashed line).
Figure 6.
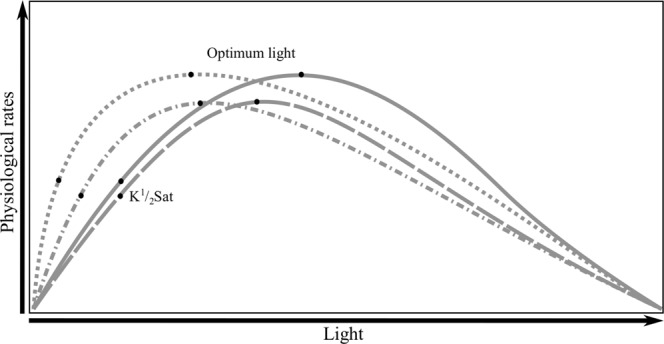
Conceptual diagram depicting how the light intensities required to support half-saturated and optimum rates change with changing CO2. While the solid line represents the default response to rising light levels, the dotted line represents an increase in substrate uptake efficiency with rising CO2, the dashed line represents an increase in H+ inhibition with rising CO2 and the dot-dashed line represents an increase in both substrate uptake efficiency and H+ inhibition with rising CO2.
As well as decreasing light intensities for optimal rates, increasing f CO2 also resulted in a narrowing of the light tolerances (light niche) of S. apsteinii. This was driven by a combined increase in light intensities required for half saturation of rates, and a decrease in light intensities required to half inhibit rates with rising f CO2 (Table 3). The CO2 niche of this species was also observed to narrow with increasing light intensity, with an increased sensitivity to high CO2 and a decrease in CO2 requirements for optimal physiological rates (Table 2). So S. apsteinii, when already under stress from one environmental condition, becomes less tolerant to other extreme environmental conditions, similar to E. huxleyi and G. oceanica for high CO2 sensitivity under rising temperature20,30. However, when exposed to the same stress of rising light intensities, E. huxleyi becomes less sensitive to high CO2 conditions, while the sensitivity of G. oceanica to high CO2 depends upon the rate considered20,30. Similarly, when exposed to the stress of rising CO2 conditions, neither E. huxleyi or G. oceanica show any change in sensitivity to high light conditions20,30 (Table S3). So, it would appear that the response to multiple environmental stressors is species-specific. This adds weight to the suggestion that coccolithophore assemblages may undergo shifts in species composition, under future ocean change.
Depending on the emission scenario, p CO2 levels are projected to reach between 420 to 985 μatm by 2100 resulting in ocean temperature increases of 2.6 to 4.8 °C (RCP 2.6–8.5)49,50. Ocean warming is expected to strengthen stratification of the water column, resulting in a shallower mixed layer and thus a higher average light availability within the mixed layer2,51. The dependence of light responses on f CO2 levels, and vice-versa, could have important implications for S. apsteinii under future ocean conditions where both light and f CO2 availabilities are expected to change. S. apsteinii already appears to be a low light adapted species with rates saturating at relatively low light intensities under current day f CO2 conditions (<170 μmol photons m−2 s−1). Considering that light levels to saturate and inhibit rates for this species increase and decrease, respectively, with rising f CO2 (Table 3), this species could become restricted to a narrower light range under predicted future ocean conditions.
Conclusion
S. apsteinii appears to be a low light adapted species, with a similar optimum curve response to rising CO2 as E. huxleyi and G. oceanica, C. pelagicus and C. leptoporus. Calculations suggest that for S. apsteinii, a single unfavourable growth condition (CO2, or light) will result in increased sensitivity to changes in other environmental variables. This contrasts with E. huxleyi, which either becomes less sensitive or shows no change in sensitivity to changes in other environmental variables when at extreme CO2 or light conditions, and G. oceanica whose change in sensitivity varies between different physiological rates. With light and CO2 levels both set to increase over the coming century2,51 S. apsteinii could become restricted to narrower light and CO2 ranges while under rising temperature, E. huxleyi and G. oceanica could become more sensitive to high CO2. These species-specific changes in temperature/light/CO2 sensitivity are likely to affect coccolithophore community composition under future ocean conditions.
Data from E. huxleyi, G. oceanica and S. apsteinii indicate that rising CO2 levels will also result in decreased optimum light requirements of all species as a result of reduced maximum rates and, in the case of G. oceanica, increased substrate uptake efficiency. This adds further evidence to the idea that rising CO2 will not only result in changes in species composition, but also in community-wide shifts in total organic and inorganic carbon production by coccolithophores, with consequent effects on local carbon cycling and sequestration.
Methods
Experimental set-up
Mono-specific cultures of S. apsteinii (strain RCC1456 isolated from the Mediterranean Sea, Spain) were grown in artificial seawater (ASW)52 at a salinity of 35 and temperature of 20 °C across a p CO2 gradient of ∼50–7000 μatm. Cultures were incubated at 50, 100, 200 and 515 μmol photons m−2 s−1 of photosynthetically active radiation (PAR) on a 16:8 h light-dark cycle in a Panasonic Versatile Environmental Test Chamber (MLR-352-PE). Light intensities, for bottle placement, within the chamber were measured using a LI-193 spherical sensor (LI-COR). Cells were pre-acclimated to experimental conditions for 7–13 days depending upon cell division rates. Initial cell densities for each bottle varied between 35–50 cells ml−1 depending upon the treatment conditions.
Media preparation
Artificial seawater (ASW), salinity 35, was prepared according to52, minus the initial addition of bicarbonate. The ASW was enriched with f/8 trace metals and vitamins53, 64 μmol kg−1 nitrate (), 4 μmol kg−1 phosphate (), 10 nmol kg−1 SeO2 and 1 ml kg−1 of sterile filtered seawater collected from Shelly beach, Ballina. ASW medium was sterile-filtered (0.2 μm pore size, WhatmanTM Polycap 75 AS) into autoclaved polycarbonate bottles, Nalgene for acclimation (0.5 L) or experimentation (2 L), with a small head-space left for the adjustment of carbonate chemistry conditions.
Carbonate chemistry manipulation
Total alkalinity (TA) and dissolved inorganic carbon (DIC) for each treatment was adjusted through calculated additions of hydrochloric acid (certified 3.571 mol L−1 HCl, Merck) and 1.4 mol L−1 sodium carbonate (Na2 CO3 Sigma-Aldrich, TraceSELECT quality dried for 2 hours at 240 °C). At the end of the experiment samples for TA and DIC were taken, stored and measured following methods used in20. Carbonate chemistry speciation (, , CO2, pH) for each treatment was calculated from measured TA, DIC, temperature, salinity and [] using the program CO2SYS54, the stoichiometric equilibrium constants (K1 and K2) for carbonic acid determined by55 and refitted by56, KS for sulphuric acid determined by57 and KB for boric acid following58.
Cell densities and particulate and dissolved carbon
Cell densities for each treatment were checked every 2–3 days using a flow cytometer (Becton Dickinson FACSCalibur). Living cells were detected by scatter plots of red autofluorescence in relation to orange fluorescence of the cells (FL3 vs. FL2). Specific growth rate (μ) was determined using cell density counts from the beginning and at the end of the experiment. Growth rate was calculated as:
| 1 |
where Ct and C0 are the cell densities at the end and the beginning of the experiment respectively and d is incubation length in days. Calcification and photosynthetic rates were obtained by multiplying cellular quotas of particulate inorganic (calcification) or particulate organic (photosynthesis) carbon with growth rates. End of experiment sampling started approximately two hours after the onset of the light period and lasted no longer than 3 hours. Duplicate samples for total and organic particulate carbon (TPC and POC) were filtered (−200 mbar) onto pre-combusted (500 °C for 4 hours) Whatmann GF/F filters and stored in pre-combusted (500 °C for 4 hours) glass petri-dishes at −20 °C. Prior to analysis, POC filters were treated with 37% fuming HCl in a desiccator for 2 hours to remove all particulate inorganic carbon (PIC). TPC and POC filters were dried and analysed for carbon content and carbon isotopic signatures on an elemental analyser (Flash EA, Thermo Fisher) coupled to an isotope ratio mass spectrometer (IRMS, Delta V plus, Thermo Fisher) according to59. PIC content was calculated by subtracting measured values of particulate organic carbon (POC) from total particulate carbon (TPC).
Data fitting
Cellular metabolic rates (n = 39 for calcification and photosynthesis and n = 37 for growth) were fitted to a non-linear equation derived in30 as
| 2 |
where the metabolic rate (MR) of photosynthesis, calcification or growth is dependent on substrate (S = CO2 and ), [H+] (H) and light (I) conditions, and fit coefficients k1, k2, k3, k4, k5 and k6. For the experiment, a higher number of treatment levels was used at the expense of replication within treatments. This approach provides more information on the functional relationship, and tipping points, between carbonate chemistry and cellular rates without a significant loss of statistical power (see60). Statistical results (R2, fit coefficients, p-values, F-values and degrees of freedom), as well as Vmax (maximum production rates), (conditions under which rates are reduced to half of maximum by either high light or high CO2 concentrations), (conditions where low light or low CO2 availability reduces rates to half of maximum) and optima (CO2 or light conditions where Vmax is reached), are presented in Tables 1, 2 and 3, respectively. Please note changes in CO2 optima and half-saturation of less than 10 μmol kg−1 were considered to be within the uncertainties of the model fit.
Coccolith morphology
S. apsteinii is unusual in that it bears two types of coccoliths; the plate-like muroliths and the vase-shaped lopadoliths61. To assess potential changes in coccolith morphology under different treatments, samples for scanning electron microscopy (SEM) were filtered onto NucleoporeTM polycarbonate membrane filters (25 mm diameter, 0.8 μm pore size) and air-dried at room temperature over 12 hours. Samples were then stored in a desiccator until analysis. Samples were mounted onto metallic stubs using sticky tabs and sputter-coated with gold before being visualized using a ZEISS EVO/LS15 scanning electron microscope. For each treatment 80–100 cells were examined and the number of muroliths and lopadoliths per cell, average of murolith and lopadolith length and width per cell, and the ratio of muroliths to lopadoliths were recorded. Due to time constraints, only a sub-set of treatments could be examined. These were; 100, 400 and 2000 μatm f CO2 at 50 and 515 μmol photons m−2 s−1 and 100, 200, 400 and 2000 μatm at 100 μmol photons m−2 s−1 of PAR. Comparisons between treatments were made using a Kruskal-Wallis ANOVA with an alpha level of significance of 0.05. The Kruskall-Wallis ANOVA was chosen firstly to avoid an inflated Type I error rate which can occur if making multiple comparisons between groups, and secondly to account for the slight non-normal distribution of the SEM data. The mass of PIC per coccolith was calculated by dividing PIC quota per cell by the total number of liths (muroliths + lopadoliths) for each treatment. Patterns in PIC per coccolith were tested using a linear regression with an alpha of significance of 0.05. Linear regressions were chosen for the reasons explained in Section ‘Cell densities and particulate and dissolved carbon’ above.
Supplementary information
Supplementary material for: The innfluence of light and carbonate chemistry on metabolic rates in Scyphosphaera apsteinii: A discussion of species-specific sensitivities and requirements
Acknowledgements
This study was funded by the Australian Research Council (ARC) FT120100384 awarded to KGS and DP150102092 awarded to BDE and KGS. We also thank Dr. Matheus Carvalho for analysing particulate carbon samples. We also acknowledge Ian Probert and the Roscoff culture collection who provided us with the original S. apsteinii cultures.
Author Contributions
All authors conceived and designed the experiments, N.G. conducted the experiments, K.S. and N.G. analysed the data. All authors reviewed the manuscript.
Data Availability
Datasets on physiological rate responses generated and compared during the current study are available in this published article (and its Supplementary Information files) and in20,30. Datasets containing the raw scanning electron microscopy values are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-38661-0.
References
- 1.Katz ME, Finkel ZV, Grzebyk D, Knoll AH, Falkowski PG. Evolutionary trajectories and biogeochemical impacts of marine eukaryotic phytoplankton. Annual Review of Ecology, Evolution, and Systematics. 2004;35:523–556. doi: 10.1146/annurev.ecolsys.35.112202.130137. [DOI] [Google Scholar]
- 2.Rost, B. & Riebesell, U. Coccolithophores and the biological pump: Responses to environmental changes in Coccolithophores: From Molecular Processes to Global Impacts (eds Thierstein, H. R. & Young, J. R.) 99–125, 10.1007/978-3-662-06278-4_5 (Springer, Berlin, Heidelberg, 2004).
- 3.McIntyre A, Bé AWH. Modern Coccolithophoridae of Atlantic Ocean -I. Placoliths and cyrtoliths. Deep-Sea Research and Oceanographic Abstracts. 1967;14:561–597. doi: 10.1016/0011-7471(67)90065-4. [DOI] [Google Scholar]
- 4.Winter, A., Jordan, R. W. & Roth, P. H. Biogeography of living coccolithophores in ocean waters in Coccolithophores (eds Winter, A. & Siesser, W. G.) 161–177 (Cambridge University Press, Cambridge, United Kingdom, 1994).
- 5.Hagino K, Okada H, Matsuoka H. Spatial dynamics of coccolithophore assemblages in the Equatorial Western-Central Pacific Ocean. Marine Micropaleontology. 2000;39:53–72. doi: 10.1016/S0377-8398(00)00014-1. [DOI] [Google Scholar]
- 6.Hagino K, Okada H. Intra-and infra-specific morphological variation in selected coccolithophore species in the equatorial and subequatorial Pacific Ocean. Marine Micropaleontology. 2006;58:184–206. doi: 10.1016/j.marmicro.2005.11.001. [DOI] [Google Scholar]
- 7.Boeckel B, Baumann K-H. Vertical and lateral variations in coccolithophore community structure across the subtropical frontal zone in the South Atlantic Ocean. Marine Micropaleontology. 2008;67:255–273. doi: 10.1016/j.marmicro.2008.01.014. [DOI] [Google Scholar]
- 8.Poulton AJ, Adey TR, Balch WM, Holligan PM. Relating coccolithophore calcification rates to phytoplankton community dynamics: Regional differences and implications for carbon export. Deep Sea Research Part II: Topical Studies in Oceanography. 2007;54:538–557. doi: 10.1016/j.dsr2.2006.12.003. [DOI] [Google Scholar]
- 9.Poulton AJ, et al. Coccolithophore dynamics in non-bloom conditions during late summer in the central Iceland Basin (July-August 2007) Limnology and Oceanography. 2010;55:1601–1613. doi: 10.4319/lo.2010.55.4.1601. [DOI] [Google Scholar]
- 10.Ziveri P, de Bernardi B, Baumann K-H, Stoll HM, Mortyn PG. Sinking of coccolith carbonate and potential contribution to organic carbon ballasting in the deep ocean. Deep Sea Research Part II: Topical Studies in Oceanography. 2007;54:659–675. doi: 10.1016/j.dsr2.2007.01.006. [DOI] [Google Scholar]
- 11.Broecker W, Clark E. Ratio of coccolith CaCO3 to foraminifera CaCO3 in late Holocene deep sea sediments. Paleoceanography. 2009;24:PA3205. doi: 10.1029/2009PA001731. [DOI] [Google Scholar]
- 12.Mertens KNJM, Lynn M, Aycard M, Lin H-L, Louwye S. Coccolithophores as palaeoecological indicators for shifts of the ITCZ in the Cariaco Basin during the late Quaternary. Journal of Quaternary Science. 2009;24:159–174. doi: 10.1002/jqs.1194. [DOI] [Google Scholar]
- 13.Riebesell U, et al. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature. 2000;407:364–367. doi: 10.1038/35030078. [DOI] [PubMed] [Google Scholar]
- 14.Langer G, et al. Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochemistry, Geophysics, Geosystems. 2006;7:1–12. doi: 10.1029/2005GC001227. [DOI] [Google Scholar]
- 15.Langer G, Nehrke G, Probert I, Ly J, Ziveri P. Strain-specific responses of Emiliania huxleyi to changing seawater carbonate chemistry. Biogeosciences. 2009;6:2637–2646. doi: 10.5194/bg-6-2637-2009. [DOI] [Google Scholar]
- 16.Sett S, et al. Temperature modulates coccolithophorid sensitivity of growth, photosynthesis and calcification to increasing seawater pCO2. PloS one. 2014;9:e88308. doi: 10.1371/journal.pone.0088308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bach LT, Riebesell U, Schulz KG. Distinguishing between the effects of ocean acidification and ocean carbonation in the coccolithophore Emiliania huxleyi. Limnology and Oceanography. 2011;56:2040–2050. doi: 10.4319/lo.2011.56.6.2040. [DOI] [Google Scholar]
- 18.Bach LT, Riebesell U, Gutowska MA, Federwisch L, Schulz KG. A unifying concept of coccolithophore sensitivity to changing carbonate chemistry embedded in an ecological framework. Progress in Oceanography. 2015;135:125–138. doi: 10.1016/j.pocean.2015.04.012. [DOI] [Google Scholar]
- 19.Zhang Y, Bach LT, Schulz KG, Riebesell U. The modulating influence of light intensity on the response of the coccolithophore Gephyrocapsa oceanica to ocean acidification. Limnology and Oceanography. 2015;60:2145–2157. doi: 10.1002/lno.10161. [DOI] [Google Scholar]
- 20.Gafar NA, Schulz KG, Eyre BD. A three-dimensional niche comparison of Emiliania huxleyi and Gephyrocapsa oceanica: Reconciling observations with projections. Biogeosciences. 2018;15:3541–3560. doi: 10.5194/bg-15-3541-2018. [DOI] [Google Scholar]
- 21.Winter, A. & Siesser, W. G. Atlas of living coccolithophores in Coccolithophores (eds Winter, A. & Siesser, W. G.) 107–159 (Cambridge University Press, Cambridge, United Kingdom, 1994).
- 22.Holligan PM, et al. A biogeochemical study of the coccolithophore, Emiliania huxleyi, in the North Atlantic. Global Biogeochemical Cycles. 1993;7:879–900. doi: 10.1029/93GB01731. [DOI] [Google Scholar]
- 23.Samtleben C, et al. Plankton in the Norwegian-Greenland Sea: From living communities to sediment assemblages -an actualistic approach. Geologische Rundschau. 1995;84:108–136. doi: 10.1007/BF00192245. [DOI] [Google Scholar]
- 24.Triantaphyllou M, et al. Seasonal variation in Emiliania huxleyi coccolith morphology and calcification in the Aegean Sea (Eastern Mediterranean) Geobios. 2010;43:99–110. doi: 10.1016/j.geobios.2009.09.002. [DOI] [Google Scholar]
- 25.Charalampopoulou A, et al. Environmental drivers of coccolithophore abundance and calcification across Drake Passage (Southern Ocean) Biogeosciences. 2016;13:5917–5935. doi: 10.5194/bg-13-5917-2016. [DOI] [Google Scholar]
- 26.Saavedra-Pellitero M, Baumann K-H, Flores J-A, Gersonde R. Biogeographic distribution of living coccolithophores in the Pacific Sector of the Southern Ocean. Marine Micropaleontology. 2014;109:1–20. doi: 10.1016/j.marmicro.2014.03.003. [DOI] [Google Scholar]
- 27.Krueger-Hadfield SA, et al. Genotyping an Emiliania huxleyi (Prymnesiophyceae) bloom event in the North Sea reveals evidence of asexual reproduction. Biogeosciences. 2014;11:5215–5234. doi: 10.5194/bg-11-5215-2014. [DOI] [Google Scholar]
- 28.Paasche E. A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions. Phycologia. 2001;40:503–529. doi: 10.2216/i0031-8884-40-6-503.1. [DOI] [Google Scholar]
- 29.Daniels CJ, Sheward RM, Poulton AJ. Biogeochemical implications of comparative growth rates of Emiliania huxleyi and Coccolithus species. Biogeosciences. 2014;11:6915–6925. doi: 10.5194/bg-11-6915-2014. [DOI] [Google Scholar]
- 30.Gafar NA, Schulz KG, Eyre BD. A conceptual model for projecting coccolithophorid growth, calcification and photosynthetic carbon fixation rates in response to global ocean change. Frontiers in Marine Science. 2018;4:1–18. doi: 10.3389/fmars.2017.00433. [DOI] [Google Scholar]
- 31.Beaufort L, Heussner S. Coccolithophorids on the continental slope of the Bay of Biscay–production, transport and contribution to mass fluxes. Deep Sea Research Part II: Topical Studies in Oceanography. 1999;46:2147–2174. doi: 10.1016/S0967-0645(99)00058-2. [DOI] [Google Scholar]
- 32.Young JR, Ziveri P. Calculation of coccolith volume and it use in calibration of carbonate flux estimates. Deep Sea Research Part II: Topical Studies in Oceanography. 2000;47:1679–1700. doi: 10.1016/S0967-0645(00)00003-5. [DOI] [Google Scholar]
- 33.Ziveri P, Broerse ATC, Van Hinte JE, Westbroek P, Honjo S. The fate of coccoliths at 48 N 21 W, northeastern Atlantic. Deep Sea Research Part II: Topical Studies in Oceanography. 2000;47:1853–1875. doi: 10.1016/S0967-0645(00)00009-6. [DOI] [Google Scholar]
- 34.Baumann, K.-H., Böckel, B. & Frenz, M. Coccolith contribution to South Atlantic carbonate sedimentation in Coccolithophores 367–402 (Springer, 2004).
- 35.Daniels CJ, et al. Species-specific calcite production reveals Coccolithus pelagicus as the key calcifier in the Arctic Ocean. Marine Ecology Progress Series. 2016;555:29–47. doi: 10.3354/meps11820. [DOI] [Google Scholar]
- 36.Krug S, Schulz K, Riebesell U. Effects of changes in carbonate chemistry speciation on Coccolithus braarudii: A discussion of coccolithophorid sensitivities. Biogeosciences. 2011;8:771–777. doi: 10.5194/bg-8-771-2011. [DOI] [Google Scholar]
- 37.Rickaby REM, Henderiks J, Young JN. Perturbing phytoplankton: response and isotopic fractionation with changing carbonate chemistry in two coccolithophore species. Climate of the Past. 2010;6:771–785. doi: 10.5194/cp-6-771-2010. [DOI] [Google Scholar]
- 38.Rost B, Riebesell U, Burkhardt S, Sültemeyer D. Carbon acquisition of bloom-forming marine phytoplankton. Limnology and Oceanography. 2003;48:55–67. doi: 10.4319/lo.2003.48.1.0055. [DOI] [Google Scholar]
- 39.Schulz KG, et al. The effect of iron availability on the regulation of inorganic carbon acquisition in the coccolithophore Emiliania huxleyi and the significance of cellular compartmentation for stable carbon isotope fractionation. Geochimica et Cosmochimica Acta. 2007;71:5301–5312. doi: 10.1016/j.gca.2007.09.012. [DOI] [Google Scholar]
- 40.Balch WM, Holligan PM, Kilpatrick KA. Calcification, photosynthesis and growth of the bloom-forming coccolithophore. Emiliania huxleyi. Continental Shelf Research. 1992;12:1353–1374. doi: 10.1016/0278-4343(92)90059-S. [DOI] [Google Scholar]
- 41.van Bleijswijk JDL, Kempers RS, Velhuis MJ. Cell and growth characteristics of types A and B of Emiliania huxleyi (Prymnesiophyceae) as determined by flow cytometry and chemical analysis. Journal of Phycology. 1994;30:230–241. doi: 10.1111/j.0022-3646.1994.00230.x. [DOI] [Google Scholar]
- 42.Nielsen MV. Photosynthetic characteristics of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae) exposed to elevated concentrations of dissolved inorganic carbon. Journal of Phycology. 1995;31:715–719. doi: 10.1111/j.0022-3646.1995.00715.x. [DOI] [Google Scholar]
- 43.Nanninga HJ, Tyrrell T. Importance of light for the formation of algal blooms by Emiliania huxleyi. Marine Ecology Progress Series. 1996;136:195–203. doi: 10.3354/meps136195. [DOI] [Google Scholar]
- 44.van Rijssel M, Gieskes WWC. Temperature, light, and the dimethylsulfoniopropionate (DMSP) content of Emiliania huxleyi (Prymnesiophyceae) Journal of Sea Research. 2002;48:17–27. doi: 10.1016/S1385-1101(02)00134-X. [DOI] [Google Scholar]
- 45.Larsen, S. H. Dimethylsulphoniopropionate (DMSP) production of Gephyrocapsa oceanica in response to environmental forcing. Doctor of Philosophy, 10.4225/03/58a24e0331904 (School of Biological Sciences, Melbourne, 2012).
- 46.Drescher B, Dillaman RM, Taylor AR. Coccolithogenesis in Scyphosphaera apsteinii (Prymnesiophyceae) Journal of Phycology. 2012;48:1343–1361. doi: 10.1111/j.1529-8817.2012.01227.x. [DOI] [PubMed] [Google Scholar]
- 47.Badger MR, et al. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Canadian Journal of Botany. 1998;76:1052–1071. doi: 10.1139/b98-074. [DOI] [Google Scholar]
- 48.Thoms S, Pahlow M, Wolf-Gladrow DA. Model of the carbon concentrating mechanism in chloroplasts of eukaryotic algae. Journal of Theoretical Biology. 2001;208:295–313. doi: 10.1006/jtbi.2000.2219. [DOI] [PubMed] [Google Scholar]
- 49.IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the FifthAssessment Report of the Intergovernmental Panel on Climate Change (eds Stocker, T. F. et al.) pp.1535 (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2013).
- 50.IPCC. Summary for policy makers in Climate Change2013: The Physical Science Basis. Contribution of Working Group I to the FifthAssessment Report of the Intergovernmental Panel on Climate Change (eds Stocker, T. F. et al.) 1–27 (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2013).
- 51.Bopp L, et al. Potential impact of climate change on marine export production. Global Biogeochemical Cycles. 2001;15:81–99. doi: 10.1029/1999GB001256. [DOI] [Google Scholar]
- 52.Kester DR, Duedall IW, Connors DN, Pytkowicz RM. Preparation of artificial seawater. Limnology and Oceanography. 1967;12:176–179. doi: 10.4319/lo.1967.12.1.0176. [DOI] [Google Scholar]
- 53.Guillard, R. R. L. Culture of phytoplankton for feeding marine invertebrates in Culture of Marine Invertebrate Animals (eds Smith, W. L. & Chanley, M. H.) 26–60 (Plenum Press, New York, USA, 1975).
- 54.Lewis, E., Wallace, D. & Allison, L. J. Program Developed for CO2System Calculations, 10.3334/CDIAC/otg.CO2SYS_DOS_CDIAC105 (Carbon Dioxide Information Analysis Center, managed by Lockheed Martin Energy Research Corporation for the US Department of Energy Tennessee, Oak Ridge, 1998).
- 55.Mehrbach C, Culberson CH, Hawley JE, Pytkowicx RM. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnology and Oceanography. 1973;18:897–907. doi: 10.4319/lo.1973.18.6.0897. [DOI] [Google Scholar]
- 56.Lueker TJ, Dickson AG, Keeling CD. Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: Validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Marine Chemistry. 2000;70:105–119. doi: 10.1016/S0304-4203(00)00022-0. [DOI] [Google Scholar]
- 57.Dickson AG, Wesolowski DJ, Palmer DA, Mesmer RE. Dissociation constant of bisulfate ion in aqueous sodium chloride solutions to 250 °C. Journal of Physical Chemistry. 1990;94:7978–7985. doi: 10.1021/j100383a042. [DOI] [Google Scholar]
- 58.Uppström, L. R. The boron/chlorinity ratio of deep-sea water from the Pacific Ocean in Deep Sea Research and Oceanographic Abstracts21, 161–162, 10.1016/0011-7471(74)90074-6 (Elsevier, 1974).
- 59.Sharp JH. Improved analysis for “particulate” organic carbon and nitrogen from seawater. Limnology and Oceanography. 1974;19:984–989. doi: 10.4319/lo.1974.19.6.0984. [DOI] [Google Scholar]
- 60.Riebesell, U., Fabry, V. J., Hansson, L. & Gattuso, J.-P. Guide to Best Practices for Ocean Acidification Research and Data Reporting pp 260 (Publications Office of the European Union Luxembourg, 2010).
- 61.Siesser WG. Calcareous nannofossil genus Scyphosphaera: Structure, taxonomy, biostratigraphy, and phylogeny. Micropaleontology. 1998;44:351–384. doi: 10.2307/1486040. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for: The innfluence of light and carbonate chemistry on metabolic rates in Scyphosphaera apsteinii: A discussion of species-specific sensitivities and requirements
Data Availability Statement
Datasets on physiological rate responses generated and compared during the current study are available in this published article (and its Supplementary Information files) and in20,30. Datasets containing the raw scanning electron microscopy values are available from the corresponding author on reasonable request.


