Abstract
An action potential is typically described as a purely electrical change that propagates along the membrane of excitable cells. However, recent experiments have demonstrated that non-linear acoustic pulses that propagate along lipid interfaces and traverse the melting transition, share many similar properties with action potentials. Despite the striking experimental similarities, a comprehensive theoretical study of acoustic pulses in lipid systems is still lacking. Here we demonstrate that an idealized description of an interface near phase transition captures many properties of acoustic pulses in lipid monolayers, as well as action potentials in living cells. The possibility that action potentials may better be described as acoustic pulses in soft interfaces near phase transition is illustrated by the following similar properties: correspondence of time and velocity scales, qualitative pulse shape, sigmoidal response to stimulation amplitude (an ‘all-or-none’ behavior), appearance in multiple observables (particularly, an adiabatic change of temperature), excitation by many types of stimulations, as well as annihilation upon collision. An implication of this work is that crucial functional information of the cell may be overlooked by focusing only on electrical measurements.
Introduction
Excitable cells generate a characteristic transient change in transmembrane voltage that propagates along the cell membrane in response to suitable stimuli. The phenomenon was first identified in a frog nerve by Emil du Bois-Reymond in 1843, who called it “negative variation”, later to be termed “action current” (Ludimar Hermann, 1868) and subsequently action potential (Blair, Hill and Rushton, among others, 1930s)1. Cellular excitability was recognized in a large category of cells, including neurons, myocytes, epithelial cells, fibroblasts, glia cells, enteroendocrine cells, pancreas beta cells, as well as non-specialized cells in corals, plants, fungi, Protozoa and possibly even bacteria1–11. Action potentials (APs) are principally associated with behavioral activities of many organisms. Therefore, an understanding of the mechanism of APs, as well as their actions and interactions, constitutes one of the fundamental aspects of biology.
Classically APs are described as a purely electrical phenomenon induced by the flow of specific ions across the cell membrane and down their concentration gradient (sodium and potassium in the original model)12,13. The non-linear electric response is believed to be associated with specialized protein components that coordinate these ionic fluxes. An unavoidable implication of this view is that the phenomenon should only exist in living cells, and could not be identified in non-living systems. However, pulses with similar properties have been observed in non-living soft systems such as lipids and gels14,15. In addition, many experimental facts are neither readily explained nor predicted by the electrical theory of APs. A partial list includes (1) non-electric aspects that co-propagate with the electric pulse16; (2) the existence of APs in absence of sodium and potassium in the intra- and extracellular solutions17, and in absence of ion-concentration gradient18; and (3) ion-channel like current fluctuations in the absence of ion channel proteins19. Therefore, the classical picture not only is not derived from fundamental physical principles, it also does not satisfactory describe the observed phenomenology. Alternative approaches, that treat the AP on a more physicochemical basis, have been proposed16,20–22. A common thread of these approaches is that electricity is merely one aspect of the pulse, and it is, therefore, very likely that valuable information is overlooked due to misunderstanding of its mechanism.
One conjecture, which is the focus of this manuscript, is that APs are acoustic pulses that propagate along the lipid interface and cross the phase-transition from the so called liquid-expanded to the liquid-condensed phase20,21. Indeed, experimental observations in lipid monolayers have demonstrated that solitary acoustic pulses can be excited, and that they share many properties with APs. A comparison of key experimental observations between APs and acoustic pulses in lipid interfaces is as follows. (1) APs are not purely electrical waves. Rather, they were identified in multiple observables–electrical, magnetic, mechanical, optical, as well as thermal16,23,24. Acoustic pulses in lipids were also identified in multiple observables (density, pressure, electrical, optical and pH)14,25–28. Particularly, the electric potential difference between the liquid-expanded and the liquid-condensed phase is ∼100 mV, similar to the amplitude of APs25. (2) The production and absorption of heat during an AP is adiabatic29, and an acoustic description is the natural physical approach for an adiabatic propagating pulse. (3) The time and velocity scales of APs vary by 5–6 orders of magnitude between cells, s and m/s, respectively7,8,12. These values are comparable to sound pulses in lipid systems that traverse the phase transition ( s and ~0.1–1 m/s, respectively)14,27. (4) Excitation of an AP is obtained by many types of stimulations: electrical, mechanical (pressure, touch, ultrasound), optical (by shining light), as well as a change of temperature (heating or cooling)1,30. Acoustic pulses in lipids can also be excited by various types of stimulations (mechanical, electrical and chemical (acid as well as solvent))14,28,31,32. (5) Even the less intuitive properties of APs, namely threshold behavior (a sigmoidal response, the so called all-or-none)33 and annihilation upon collision34,35, can be demonstrated as an acoustic behavior near phase transition. Specifically, stimulation was shown to prompt a sigmoidal response of density pulses in lipid interfaces near phase transition27, and these pulses were demonstrated to annihilate upon collision36.
Despite the striking experimental similarities, a comprehensive theoretical study of acoustic pulses that traverse the phase transition in lipid systems is still lacking. Theoretical modeling was initiated by Heimburg and Jackson, and was focused on solitonic solutions in a small-amplitude analysis of an acoustic model of the membrane interface20. Two main criticisms of the model have been an overestimation of the propagation velocity and the lack of annihilation of pulses. Kappler et al. later demonstrated that the viscous coupling between the interface and a bulk fluid attenuates the propagation velocity of interfacial longitudinal waves, and results in a corrected velocity that agrees with experimental evidence37. Still, some observations are not captured by these previous works, including the annihilation of pulses upon collision, as well as a thorough analysis of different aspects of the pulses under adiabatic conditions (e.g., density, pressure, temperature and electrical properties).
Herein, we demonstrate that non-linear acoustic pulses, which annihilate upon collision, arise naturally from a minimum set of well accepted physical assumptions: mass, momentum and energy conservation laws near a phase transition. Our description relies on relatively few and physically measurable parameters and variables. Particularly, no assumptions about the molecular constituents and structure, nor any parameter fit, are required. In spite of its minimalism, the model provides a rich behavior of non-linear acoustic pulses that correspond to experimental results in lipid monolayers as well as to APs in living cells.
Model Description
Our purpose is to investigate the properties of isentropic traveling waves which exist near a phase transition. We therefore consider an idealized ansatz of a one-dimensional compressible fluid with a van der Waals constitutive equation, following the works of Felderhof, Slemrod and Grinfeld38–40. While classically a van der Waals phase transition is between a gas and a liquid, the familiar pressure-versus-volume curves (isotherms) qualitatively resemble the melting transition between the liquid-expanded and the liquid-condensed state in lipids41. The conservation laws of mass, momentum and energy are written in the Lagrange frame,
| 1 |
Here w, v and E denote the specific volume, velocity and specific total energy of the fluid, respectively. The fluid density is inversely proportional to the specific volume, ρ = w−1. The spatial coordinate h (in the Lagrange frame) is related to the x-axis (in the Euler frame) by the cumulative mass of fluid particles42
| 2 |
with a normalization factor that defines the scale of h. In addition, τ1 and τ2 represent the stresses in the fluid (see below), k is the coefficient of thermal conductivity and θ is the fluid temperature.
To allow for a continuous transition between the two phases, we follow van der Waals’ hypothesis that the energy of a system depends on the gradient of density (the so called capillarity term)43,44. A similar definition appears in the Ginzburg-Landau mean field theory of phase transition as well as the Cahn-Hilliard model of coexistence of two phases45,46. The capillarity coefficient is also known as the gradient-energy coefficient, and is related to the line tension that arises between two coexisting phases (further clarification is given in the Supplemental Materials). The corresponding stress correction to the dynamics of fluids was developed by Korteweg43,47. Therefore, the expression for stress includes a capillarity term in addition to pressure and viscosity. It is defined as
| 3 |
with p the fluid pressure, ζ the dilatational viscosity and C the capillarity coefficient, treated here as constant for simplicity.
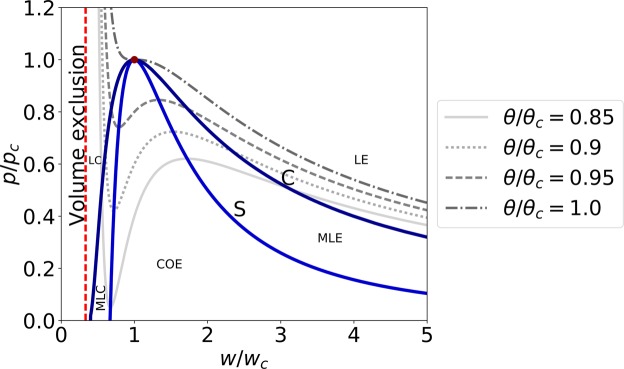
The system of equations is completed with the van der Waals constitutive equations, that qualitatively resemble the transition between a liquid-expanded and a liquid-condensed state in lipids (Fig. 1 and ref.41)
| 4 |
with kB the Boltzmann constant, m the mass of a fluid particle, a the average attraction between particles, b the volume exclusion by a fluid particle, and cv the specific heat capacity48. The phase space contains an unstable region, ∂p/∂w > 0. This region is bounded by the spinodal curve (marked by S in Fig. 1), which satisfies ∂p/∂w = 0. A slightly larger area, governed by the Maxwell equal area rule, describes the region where a coexistence of phases has a lower free energy as compared to a single phase solution (marked by C in Fig. 1). Both curves meet at the critical point (wc, pc, θc), where the distinction between the two phases disappears. The critical point can be expressed by three parameters: m, a and b (Eq. (S1) in the Supplemental Materials). A detailed list of the 6 variables and 7 parameters of the model is provided in the Supplemental Materials (Tables S1–S2).
Figure 1.
Schematic phase diagram in the w–p plane of the van der Waals model. Four different isotherms, along with the Spinodal (S) and coexistence (C) curves are plotted. The critical point is denoted by a filled circle. LE: liquid-expanded phase, MLE: metastable liquid-expanded phase, COE: coexistence phase, MLC: metastable liquid-condensed phase, and LC: liquid-condensed phase.
The critical point and the fluid viscosity were used to define proper scales (time, length and velocity, respectively)
| 5 |
These scales were used to derive a dimensionless form of the model equations (Supplemental Materials, Eqs (S3)–(S5)), that depends on only three parameters: the (dimensionless) heat capacity, thermal conductivity and capillarity coefficient
| 6 |
Dimensionless variables and parameters are hereafter marked with tilde (e.g., ).
A complete set of all of the material constants is not known for any single lipid system, nor for a composite soft interface, for example in biological cells. Therefore, typical values were estimated from experiments with DPPC lipids. The critical point of a two-dimensional DPPC monolayer was identified from isothermal state diagrams 14,25,30,41. The order of magnitude of the membrane viscosity was estimated from measurements of shear viscosity, ζ ~ 10−3 Pa ⋅ s⋅m49. The order of magnitude of the heat capacity was evaluated from experiments at constant pressure, cv ~ cp ~ 103–104 J/kg ⋅ K20,25. Unfortunately, no direct measurements of the thermal conductivity and capillarity coefficient of lipid monolayers exist. Thermal conductivity was approximated from the evaluation of heat conduction in interfacial water in a lipid system, k ~ 5 J/s ⋅ K50. The capillarity coefficient was estimated from measurements of line tension at the phase boundary, C ~ 10−27–10−24 kg2/s2 (Supplemental Materials and ref.51). For typical values of a lipid system, the proper scales are T ≈ 30 ms, L ≈ 4 m and U ≈ 120 m/s, and the dimensionless parameters are 101–103, 102, and 10−21–10−18. Although the capillarity coefficient is insignificantly small, a non-negligible value was used for numerical reasons () – to avoid sharp gradients in the density field. The use of a non-negligible capillarity coefficient did not show a major effect on our results (Fig. S1).
The system of equations (1)–(4) was numerically solved with the Dedalus open-source code52, which is based on a pseudo-spectral method. The model was solved using periodic boundary conditions, and with homogeneous initial conditions, (w0, p0, θ0) in the liquid-expanded phase. Excitation of a pulse was obtained by ‘injecting’ a localized stress (around h0) with an amplitude pexc for a brief time (t0) into the momentum flux; i.e., adding the following term into the right-hand-side of the middle Eq. 1
| 7 |
Here, Θ is the Heaviside function, and λ is the width of excitation.
Results
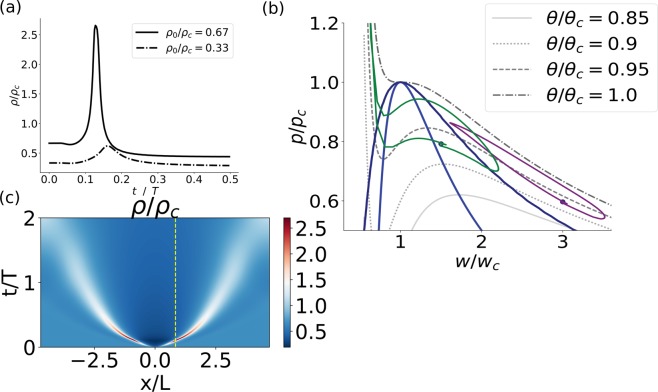
We now turn to analyze sound pulses that traverse the phase transition. Upon excitation, distinct pulses were obtained when the initial state of the fluid was near phase transition. Figure 2a (solid line) depicts the shape of a density pulse as measured at distance x/L = 1 from the excitation point. The shape of the density pulse was qualitatively very similar to experimental measurements in lipid monolayers14, as well as to the voltage signal of an AP33. Because this issue is of particular relevance to the open debate regarding the physical nature of cellular excitability, we further discuss the relation between the interface density and a transmembrane voltage measurement in the Discussion section. In contrast, pulse amplitude was much lower when the initial state was away from the phase transition (Fig. 2a, dotted-dashed line). A projection of the closed trajectory in the w–p plane is shown in Fig. 2b, for the two pulses depicted in Fig. 2a (green and purple curves, respectively). If a trajectory does not cross the phase boundary (dotted-dashed line in Fig. 2a and purple curve in Fig. 2b) the pulse undergoes only a little amplification in density (). The change in density is accompanied by a parallel increase in pressure () and temperature. Subsequently, the local state decreases in all three fields (density, pressure and temperature) into a rarefaction state before relaxing back into the initial state. In contrast, once a pulse traverses the phase transition region, the density and pressure evolve non-linearly (solid line in Fig. 2a and green curve in Fig. 2b). At first, a significant increase in density is obtained (), with almost no change in pressure. Subsequently, as the local state approaches the volume exclusion region, the pressure sharply increases (), with only a slight increase in density. A parallel small increase in temperature also occurs. Relaxation follows in a reverse order–a sharp decrease in pressure is followed by a sharp decrease in density into a rarefaction that relaxes back into the initial state.
Figure 2.
(a) Density pulse as a function of time, as measured at distance x/L = 1 from the excitation point, with an initial density of = 0.67 (solid line) and 0.33 (dotted-dashed line). (b) A projection of phase space into the w–p plane (w = ρ−1). Several isotherms are plotted for reference (shades of grey lines) as well as the coexistence and spinodal curves (dark blue and blue lines, respectively). The trajectory of the two pulses shown in (a) is plotted in green and purple respectively. (c) Density field of the entire fluid as a function of space (x-axis) and time (y-axis) with initial density = 0.67. Dashed yellow line marks the solid line solution depicted in (a). Parameters of the model were = (600, 100, 1), additional initial conditions were = (0, 0.93), and excitation parameters were = (0, 0.1, 300, 0.088). Numerical calculation was conducted with 4096 grid points, x-domain [−3π/2, 3π/2] and dt = 5 ⋅ 10−4.
The solution of the density field across the entire fluid is plotted in Fig. 2c. Following an excitation around x = 0 that lasted 0.1 T, two localized pulses were generated, propagating in opposite directions. Their length, time and velocity scales were ≈0.5 L, ≈0.1 T, and ≈5 U, respectively. An important observation is that these pulses did not maintain a constant velocity and shape, and eventually dispersed; i.e., these solutions are neither solitons, nor homoclinic orbits. Nonetheless, the pulses had a distinct shape for a distance of 3–5 times the pulse width. Variation of the duration or width of the stimulation (t0 and λ in Eq. (7), respectively) did not have much influence on the time and length scales of the pulse (Fig. S2). However, upon increasing the duration of the stimulation (t0), the pulses maintained a distinct shape for a longer distance (more than 10 times the pulse width, Fig. S2b). The dashed yellow line in Fig. 2c marks the solution that was plotted in Fig. 2a (solid line).
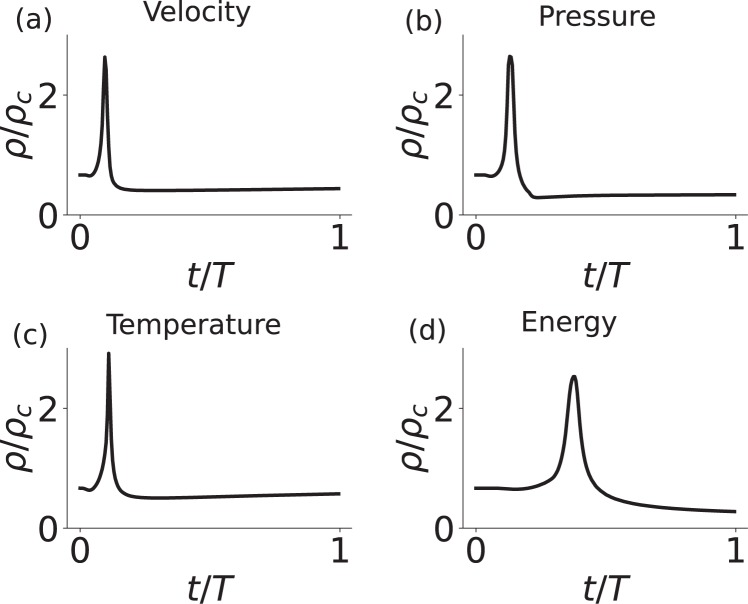
Stimulation by different model variables (velocity, pressure, temperature or energy) was obtained by adding the excitation term to other model equations (Eqs (1) or (4)). Figure 3 shows the density pulses that were generated by different ‘types’ of stimulations. The resulting pulses were qualitatively similar.
Figure 3.
Excitation by a local injection of (a) velocity (middle of Eq. (1)), (b) pressure (upper Eq. (4)), (c) temperature (lower Eq. (4)), and (d) energy (lower Eq. (1)). Excitation parameters were = (300, 0.1), (300, 0.2), (3000, 0.1) and (2 · 104, 0.2), respectively. A is the normalized amplitude of excitation (pexc/pc, pexc/pc, θexc/θc and Eexc/U2, respectively). Density as a function of time was plotted at x/L = 0.8, 1.0, 0.8 and 1.4, respectively.
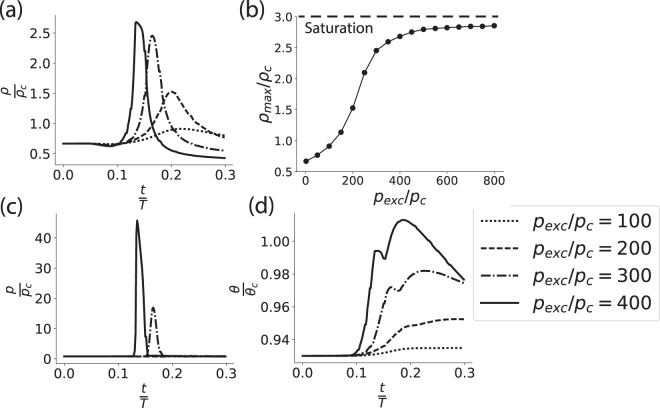
By increasing the amplitude of excitation, at a given initial state, a non-linear (sigmoidal) response of the density pulse was obtained (Fig. 4a,b). At low amplitudes of excitation the pulse response was qualitatively similar to previous theoretical results obtained in a small-amplitude analysis37. However, at larger amplitudes of excitation the amplitude of the density pulse saturated at ρ = 3 ρc. The saturation of density is a direct result of the exclusion of volume given by the van der Waals equation (upper Eqs (4) or (S5)). The response resembles experimental observations in lipid monolayers27 as well as voltage measurements of APs in living cells33.
Figure 4.
(a) Pulse shape at four amplitudes of the excitation, as reflected by the change of density. (b) Non-linear response of the amplitude of the density pulse (ρmax) to stimulation amplitude (pexc). (c) Pressure and (d) temperature aspects that co-appear with the density pulse. The first two pressure curves are indistinguishable, as the state did not reach the liquid-condensed phase. Initial density was = 0.67, and pulse was measured at distance x/L = 1.2 from the excitation point. Other parameters appear in the caption of Fig. 2.
Figure 4c shows the simultaneous pressure aspect of the pulse. Pressure increases significantly when the system is driven into the liquid-condensed phase. In addition, during the adiabatic compression and decompression, the temperature increases and subsequently decreases (Fig. 4d). Not surprisingly, the temporal width of the temperature aspect crucially depends on the thermal conductivity (data not shown).
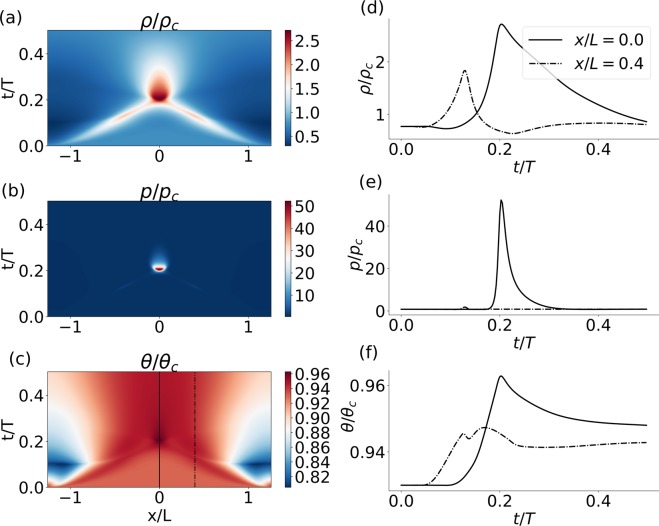
We proceed now to investigate the question of collision between longitudinal pulses. While linear (small amplitude) pulses generally superimpose, these non-linear waves displayed a rich behavior of interaction, annihilation and in some cases even repulsion. The type of interaction between pulses strongly depends on the value of the heat capacity and thermal conductivity of the fluid. Here we provide an example of annihilation of two pulses that were excited in a small sized domain. Figure 5 and Movie S1 show the dynamics of the density, pressure and temperature fields before, during and after a collision. The propagation of the pulses is evident in all three fields, and the collision is characterized by a localized increase in amplitude, most dramatically observed in the pressure field.
Figure 5.
Collision between two pulses as appeared in the (a) density, (b) pressure and (c) temperature fields. x- and y-axis represent space and time, respectively. A local description of the (d) density, (e) pressure and (f) temperature is plotted as a function of time at the collision point (x/L = 0, solid line) and at some distance away from it (x/L = 0.4, dashed-dotted line). The location of these solutions in space is marked in (c) by solid and dashed-dotted line, respectively. Fluid initial density was = 0.77. Excitation was conducted at = ±0.4 π. Numerical calculation was performed with 8192 grid points, and the x-domain was [−0.4π, 0.4π]. Other parameters are similar to those given in caption of Fig. 2.
Discussion
The lipid-based interface is a ubiquitous component of biological cells, and plays a critical role in many cellular functions. Particularly, the discovery of solitary longitudinal pulses that travel along lipid interfaces have stimulated a discussion on the functional role of acoustics in biological systems14,20,21,26–28,30,31. In this paper we continued this line of research and demonstrated that an idealized fluid model near phase transition captures many properties of experimentally measured acoustic pulses in lipid monolayers as well as APs in living cells.
Time, length and velocity scales of density pulses in lipid interfaces
In a small amplitude analysis, the propagation velocity of acoustic pulses is inversely related to the square root of the compressibility20,31. In contrast, the local compressibility of a van der Waals fluid is ill-defined within the coexistence region. Instead, we have shown that the scales of the system are associated with the critical point and the interfacial viscosity (Eq. (5). Specifically, density pulses that traverse the phase transition region were demonstrated to scale as ≈0.1 T, ≈0.5 L, and ≈5U, respectively (Fig. 2). For typical lipid values, these corresponded to ≈3 ms, ≈ 2 m, and ≈600 m/s. While the theoretical time scale agrees with measurements (≈5–10 ms), the length and velocity scales are overestimated by 2–3 orders of magnitude (≈1–10 mm and ≈0.1–1 m/s)14,27. Similar estimation of velocity was previously obtained by others20,31. The discrepancy results from our attempt to keep the model simple, and would disappear by extending the model to include the non-negligible coupling to the bulk26. It was previously demonstrated that the viscous coupling between a compressible interface and the bulk attenuates the velocity and length of acoustic pulses by a factor of 37. Here, ρb is the bulk density, ηb the bulk viscosity, tp the pulse duration, and ρi the density of the interface. For a typical lipid interface coupled to bulk water, this results in attenuation by 2–3 orders of magnitudes, and agrees with experimental observations in lipids31,37, as well as of APs in living cells7,8,12.
Dispersion of shape and propagation velocity
According to the classical electrical description, APs are pulses that maintain a stable shape and a constant propagation velocity along an ‘infinitely’ long cell (a homoclinic orbit)12. Similar characteristics were also suggested by the acoustic soliton model20. Surprisingly, a validation of this hypothesis, by physiological measurements of velocity and shape at more than two sites, was hardly investigated, presumably due to the small cellular size. However, recent experiments have showed, using a multi-electrode array, that the propagation velocity as well as the shape of an AP are not constants during propagation. Rather, a clear trend of decrease in velocity and amplitude, as well as an increase in width, was observed53,54. These findings are in accord with our results, that do not preserve a constant velocity and shape, and eventually disperse. We have, nonetheless, demonstrated that distinct pulses were maintained at distances up to 1–10 times the length of a pulse (Figs 2c and S2b). Interestingly, the length of many excitable cells is of similar order. For example, in Loligo Pealei squids the length of a giant axon is 3–5 times the length of an AP (the axonal length is 10–20 cm, and the estimated length of an AP is ≈4 cm (the pulse duration is ≈2 ms and the propagation velocity is ≈20 m/s)12,55). Because the characteristics of APs over distances of the order of the pulse size were scarcely investigated, it may be worth to examine these properties in a future work.
Voltage signal of acoustic pulses
We now turn to examine the effect of density changes on a transmembrane voltage measurement. Changes in electric properties, such as capacitance and resistance, are unavoidable during propagation of acoustic pulses in soft materials32,56. Furthermore, the surface potential of some lipid monolayers differ by ~100 mV between the liquid-expanded and the liquid-condensed phases, the same order of magnitude as the electric aspect of an AP25. To quantify these changes, we explore a simple extension to the model; a standard electrophysiological measurement across a lipid interface. For the moment we ignore any transmembrane current of ions, and focus on currents following solely from voltage changes generated by the layer itself. The conservation of charge requires
| 8 |
with the local capacitance of the material, and V the local transmembrane voltage. The equation implies an inverse relation between the voltage and the material capacitance
| 9 |
with the initial voltage and capacitance, respectively. The capacitance of the system can be associated with the area, thickness and relative permittivity of the material (A, d and ε, respectively)
| 10 |
where all variables (including ε) depend on the local thermodynamic state of the membrane56. Replacing the area term with the density of the membrane, we obtain a linear relation between the voltage and the local density
| 11 |
Thus, a propagating density pulse (Fig. 1), if detected by a voltage sensor, should display a distinct shape that includes a depolarization, repolarization and hyperpolarization phase, similar to measurements of APs. However, a voltage pulse is expected to display a sharper non-linear response (a threshold) as compared with the density pulse (Fig. 4a,b). This is because the relative permittivity and thickness are also state dependent, the former decreases and the latter increases as the pulse traverses into the condensed phase25,57,58. On top of these results, one could include the resistance of the soft system to transmembrane ionic currents, which is governed by a state dependent permeability19,59. In conclusion, the electric potential difference across a lipid interface responds non-linearly when acoustic pulses travel along the interface.
The realization that APs may be acoustic pulses, that carry electrical changes as they travel, should have significant implications for the field of neuroscience. For decades the field has placed much focus on measurements and analysis of electric signals in nervous-systems of many organisms. However, it is possible that crucial computational information goes unnoticed when the state of a neuron is simplified into a raster plot data (a digital-like uniformity of zeros and ones that represent events of APs). Let us describe one plausible scenario. In Fig. 4 we have demonstrated that at large excitation amplitudes very similar density pulses can be excited with a significantly different pressure signature. Thus, a voltage sensor, sensitive to density but not to pressure, would not resolve between different pressure signatures that could induce different cellular responses, and result in a considerably different computational scheme as compared to the classical electrical picture.
Model extensions
The evidence that the reported pulses do not emerge from a fine-tuned model, but rather result from an idealized description of a soft system, emphasizes the generality of the phenomenon. This, however, should only be viewed as a first step. To treat pulses in soft systems more accurately, further extensions to the model are required. (1) The parameters of the system; specifically, viscosity, heat capacity and thermal conductivity, are state dependent and not constants20,60,61. This is particularly evident near a phase transition and should modify the detailed properties of the nonlinear pulses. (2) The van der Waals constitutive equation is not an accurate representation of the state diagram of soft materials, lipids in particular. For example, in DPPC the volume exclusion appears at ≈0.8 wc25,30,41. This is quantitatively different from the van der Waals equation, where the volume exclusion occurs at wc/3. These differences clearly influence the properties of the pulse. For example, the saturation amplitude of the density pulse would be ρsat ≈ 1.2 ρc instead of ≈3 ρc. (3) The viscoelastic properties of lipids are more complicated than simply considering a capillarity term. Particularly, effects of state dependent rigidity and distortion of the lipids as well as embedded proteins clearly modify the dynamic response to lateral compression49,62. (4) No effect of geometry was considered in this work (boundary conditions, extension to two- or three-dimensions, coupling to bulk). (5) The parameters that describe the material (critical point, thermal conductivity, etc.) are very likely influenced by additional components that were not considered here. For example, pH, ions, as well as embedded proteins. (6) In order to provide a detailed analysis of electrophysiological measurements, a more accurate description of the electro-mechanical coupling is necessary. For example, we did not address space- and voltage-clamp experiments in this paper12. (7) Our description is based on a mean field approximation. Therefore, thermodynamic fluctuations were not accounted. In particular, the model does not describe current fluctuations that were experimentally measured in non-living soft systems as well as in living cells19. To accommodate for state fluctuations, an analysis of the thermodynamic susceptibilities is necessary. These aspects should be considered in a future work.
Supplementary information
Acknowledgements
The authors thank Christian Fillafer, Kevin Kang, Julian Kappler and Konrad Kaufmann for fruitful discussions and valuable comments, and to Daniel Lecoanet for support with the Dedalus open-source code. MM thanks Uri Nevo for introducing him to the subject of non-electric aspects of action potentials, and to Jay Fineberg for useful comments. We acknowledge financial support by Deutsche Forschungsgemeinschaft and TU-Dortmund University within the funding program Open Access Publishing. MM further acknowledges funds from SHENC-research unit FOR 1543.
Author Contributions
M.M. and M.F.S. devised the project and the main conceptual ideas. M.M. performed the computations. M.M. and M.F.S. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-38826-x.
References
- 1.Tasaki, I. Physiology and electrochemistry of nerve fibers. (Academic press, New York, 1982).
- 2.Mackie GO. Epithelial conduction: Recent findings, old questions, and where do we go from here? Hydrobiologia. 2004;530–531:73–80. [Google Scholar]
- 3.Parak WJ, et al. Electrically excitable normal rat kidney fibroblasts: A new model system for cell-semiconductor hybrids. Biophysical journal. 1999;76(3):1659–1667. doi: 10.1016/S0006-3495(99)77325-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fields RD. Oligodendrocytes changing the rules: action potentials in glia and oligodendrocytes controlling action potentials. The Neuroscientist. 2008;14(6):540–543. doi: 10.1177/1073858408320294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strege PR, et al. Sodium channel NaV1.3 is important for enterochromaffin cell excitability and serotonin release. Scientific Re-ports. 2017;7(1):1–12. doi: 10.1038/s41598-017-15834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic β-cell. Progress in Biophysics and Molecu-lar Biology. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 7.Leys SP. Elements of a ‘nervous system’ in sponges. The Journal of experimental biology. 2015;218(4):581–91. doi: 10.1242/jeb.110817. [DOI] [PubMed] [Google Scholar]
- 8.Beilby MJ. Action potential in charophytes. International review of cytology. 2007;257:43–82. doi: 10.1016/S0074-7696(07)57002-6. [DOI] [PubMed] [Google Scholar]
- 9.Slayman CL, Long WS, Gradmann D. Action potentials in Neurospora crassa, a mycelial fungus. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1976;426(4):732–744. doi: 10.1016/0005-2736(76)90138-3. [DOI] [PubMed] [Google Scholar]
- 10.Wood DC. Membrane permeabilities determining resting, action and mechanoreceptor potentials in Stentor coeruleus. Journal of Comparative Physiology. 1982;146(4):537–550. doi: 10.1007/BF00609450. [DOI] [Google Scholar]
- 11.Kralj JM, Hochbaum DR, Douglass AD, Cohen AE. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science. 2011;333(6040):345–348. doi: 10.1126/science.1204763. [DOI] [PubMed] [Google Scholar]
- 12.Hodgkin AL, Huxley AF. A Quantitative Description of Membrane Current and Its Application to Conduction and Excitation in Nerve. Journal of Physiology. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aidley, D. J. & Ashley, D. J. The physiology of excitable cells, volume 4. (Cambridge University Press, 1998).
- 14.Shrivastava S, Schneider MF. Evidence for 2D Solitary SoundWaves in a Lipid Controlled Interface and its Biological Implications for Biological Signaling. Journal of The Royal Society Interface. 2014;11:1–8. doi: 10.1098/rsif.2014.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przybylski AT, Stratten WP, Syren RM, Fox SW. Membrane, action, and oscillatory potentials in simulated protocells. Naturwissenschaften. 1982;69(12):561–563. doi: 10.1007/BF00396351. [DOI] [PubMed] [Google Scholar]
- 16.Tasaki I. Rapid Structural Changes in Nerve Fibers and Cells Associated With Their Excitation Processes. Japanese Journal of Physiology. 1999;49:125–138. doi: 10.2170/jjphysiol.49.125. [DOI] [PubMed] [Google Scholar]
- 17.Tasaki I, Watanabe A, Singer I. Excitability of Squid Giant Axon in the Absence of Univalent Cations in the External Medium. Proceedings of the National Academy of Sci-ences. 1966;56:1116–1122. doi: 10.1073/pnas.56.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terakawa S. Periodic responses in squid axon membrane exposed intracellularly and extracellularly to solutions containing a single species of salt. The Journal of Membrane Biology. 1981;63(1-2):51–59. doi: 10.1007/BF01969445. [DOI] [PubMed] [Google Scholar]
- 19.Wunderlich B, et al. Phase-state dependent current fluctuations in pure lipid membranes. Biophysical Journal. 2009;96(11):4592–4597. doi: 10.1016/j.bpj.2009.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimburg T, Jackson AD. On Soliton Propagation in Biomembranes and Nerves. Proceedings of the National Academy of Sciences. 2005;102(28):9790–9795. doi: 10.1073/pnas.0503823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann, K. Action Potentials and Electromechanical Coupling in the Macroscopic Chiral Phospholipid Bilayer. (Caruaru, Brazil, 1989).
- 22.Gilbert, N. & Ling, A. Revolution in the Physiology of the Living Cell. (1992).
- 23.Hodgkin AL, Huxley AF. Resting and Action Potentials in Single Nerve Fibers. J. Physiol. 1945;104:176–195. doi: 10.1113/jphysiol.1945.sp004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wikswo JP, Barach JP, Freeman JA. Magnetic field of a nerve impulse: first measurements. Science (New York, N.Y.) 1980;208(4439):53–55. doi: 10.1126/science.7361105. [DOI] [PubMed] [Google Scholar]
- 25.Steppich D, et al. Thermomechanic-electrical coupling in phospholipid monolayers near the critical point. Physical Review E. 2010;81(6 Pt 1):061123. doi: 10.1103/PhysRevE.81.061123. [DOI] [PubMed] [Google Scholar]
- 26.Griesbauer J, Bössinger S, Wixforth A, Schneider MF. Propagation of 2D Pressure Pulses in Lipid Monolayers and Its Possible Implications for Biology. Physical Review Letters. 2012;108(19):198103. doi: 10.1103/PhysRevLett.108.198103. [DOI] [PubMed] [Google Scholar]
- 27.Shrivastava S, Kang KH, Schneider MF. Solitary shock waves and adiabatic phase transition in lipid interfaces and nerves. Physical Review E. 2015;91(1):1–7. doi: 10.1103/PhysRevE.91.012715. [DOI] [PubMed] [Google Scholar]
- 28.Fichtl B, Shrivastava S, Schneider MF. Protons at the speed of sound: Predicting specific biological signaling from physics. Scientific Reports. 2016;6(1):22874. doi: 10.1038/srep22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchie JM, Keynes RD. The production and absorption of heat associated with electrical activity in nerve and electric organ. Quarterly Reviews of Biophysics. 1985;18(4):451–476. doi: 10.1017/S0033583500005382. [DOI] [PubMed] [Google Scholar]
- 30.Heimburg, T. Thermal Biophysics of Membranes. (Wiley, 2008).
- 31.Griesbauer J, Wixforth A, Schneider MF. Wave propagation in lipid monolayers. Biophysical Journal. 2009;97(10):2710–2716. doi: 10.1016/j.bpj.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griesbauer J, Bössinger S, Wixforth A, Schneider MF. Simultaneously Propagating Voltage and Pressure Pulses in Lipid Monolayers of Pork Brain and Synthetic Lipids. Physical Review E. 2012;86(6):1–5. doi: 10.1103/PhysRevE.86.061909. [DOI] [PubMed] [Google Scholar]
- 33.Hodgkin AL, Huxley AF, Katz B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. The Journal of Physiology. 1952;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tasaki I. Collision of two nerve impulses in the nerve fibre. Biochimica et Biophysica Acta. 1949;3:494–497. doi: 10.1016/0006-3002(49)90121-3. [DOI] [Google Scholar]
- 35.Follmann R, Rosa E, Stein W. Dynamics of signal propagation and collision in axons. Physical Review E. 2015;92(3):1–11. doi: 10.1103/PhysRevE.92.032707. [DOI] [PubMed] [Google Scholar]
- 36.Shrivastava, S., Kang, K. H., and Schneider, M. F. Annihilation upon Collision of Solitary Shock Waves propagating in a Lipid Interface. Journal of The Royal Society Interface15(143), 20170803 (2018). [DOI] [PMC free article] [PubMed]
- 37.Kappler J, Shrivastava S, Schneider MF, Netz RR. Nonlinear fractional waves at elastic interfaces. Physical Review Fluids. 2017;2(11):1–18. doi: 10.1103/PhysRevFluids.2.114804. [DOI] [Google Scholar]
- 38.Slemrod M. Dynamic phase transitions in a van der Waals uid. Journal of differential equations. 1984;52(1):1–23. doi: 10.1016/0022-0396(84)90130-X. [DOI] [Google Scholar]
- 39.Grinfeld M. Nonisothermal dynamic phase transitions. Quarterly of Applied Mathematics. 1989;47(1):71–84. doi: 10.1090/qam/987896. [DOI] [Google Scholar]
- 40.Felderhof BU. Dynamics of the diffuse gas-liquid interface near the critical point. Physica. 1970;48(4):541–560. doi: 10.1016/0031-8914(70)90184-9. [DOI] [Google Scholar]
- 41.Albrecht O, Gruler H, Sackmann E. Polymorphism of phospholipid monolayers. Journal de Physique. 1978;39(3):301–313. doi: 10.1051/jphys:01978003903030100. [DOI] [Google Scholar]
- 42.Courant, R. & Friedrichs, K. O. Supersonic ow and shock waves, volume 21 (Springer, 1948).
- 43.Gorban AN, Karlin IV. Beyond Navier-Stokes equations: capillarity of ideal gas. Contemporary Physics. 2016;7514(November):1–21. [Google Scholar]
- 44.van der Waals, J. D. The thermodynamic theory of capillarity ow under the hypothesis of a continuous variation of density (Verhandel/Konink. Akad. Weten., vol. 1, English Translation). Journal of Statistical Physics, 20 (1893).
- 45.Landau, L. D. & Lifshitz, E. M. Statistical Physics, Part 1: Volume 5. (Pergamon, 1980).
- 46.Cahn JW, Hilliard JE. Free energy of a nonuniform system. I. Interfacial free energy. The Journal of chem-ical physics. 1958;28(2):258–267. doi: 10.1063/1.1744102. [DOI] [Google Scholar]
- 47.Korteweg DJ. Sur la forme que prennent les équations du mouvement des fluides si l’on tient compte des forces capillaires causées par des variations de densité considérables mais continues et sur la théorie de la capillarité dans l’hypothese d’une variatio. Archives Néerlandaises des Sciences exactes et naturelles. 1901;6(1):6. [Google Scholar]
- 48.Johnston, D. C. Thermodynamic properties of the van der Waals fluid. arXiv:1402.1205 (2014).
- 49.Espinosa G, López-Montero I, Monroy F, Langevin D. Shear rheology of lipid monolayers and insights on membrane fluidity. Proceedings of the National Academy of Sciences. 2011;108(15):6008–6013. doi: 10.1073/pnas.1018572108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clegg, J. S. & Drost-Hansen, W. On the biochemistry and cell physiology of water, Chapter 1. (Elsevier, 1991).
- 51.Benvegnu DJ, Mcconnell HM. Line Tension between Lipid Domains in Lipid Monolayers. Journal of Physical Chemistry. 1992;96:6820–6824. doi: 10.1021/j100195a053. [DOI] [Google Scholar]
- 52.Burns, K. J. et al. A Flexible Framework for Spectrally Solving Differential Equations. Astrophysics Source Code Library (2016).
- 53.Bakkum DJ, et al. Tracking axonal action potential propagation on a high-density microelectrode array across hundreds of sites. Nature communications. 2013;4:2181. doi: 10.1038/ncomms3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patolsky F, et al. Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science. 2006;313(5790):1100–1104. doi: 10.1126/science.1128640. [DOI] [PubMed] [Google Scholar]
- 55.Young JZ. The structure of nerve fibres in Cephalopods and Crustacea. Proceedings of the Royal Society B: Biological Sciences. 1936;121(823):319–337. [Google Scholar]
- 56.Heimburg T. The capacitance and electromechanical coupling of lipid membranes close to transitions: The effect of electrostriction. Biophysical Journal. 2012;103(5):918–929. doi: 10.1016/j.bpj.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heimburg T. Mechanical aspects of membrane thermodynamics. Estimation of the mechanical properties of lipid membranes close to the chain melting transition from calorimetry. Biochimica et Biophysica Acta - Biomembranes. 1998;1415(1):147–162. doi: 10.1016/S0005-2736(98)00189-8. [DOI] [PubMed] [Google Scholar]
- 58.Kimura Y, Ikegami A. Local dielectric properties around polar region of lipid bilayer membranes. The Journal of mem- brane biology. 1985;85(3):225–231. doi: 10.1007/BF01871517. [DOI] [PubMed] [Google Scholar]
- 59.El-Mashak EM, Tsong TY. Ion selectivity of temperature - induced and electric field induced pore in dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1985;24:2884–2888. doi: 10.1021/bi00333a010. [DOI] [PubMed] [Google Scholar]
- 60.Hermans E, Vermant J. Interfacial shear rheology of DPPC under physiologically relevant conditions. Soft matter. 2014;10:175–186. doi: 10.1039/C3SM52091A. [DOI] [PubMed] [Google Scholar]
- 61.Youssefian S, Rahbar N, Lambert CR, Van Dessel S. Variation of thermal conductivity of DPPC lipid bilayer membranes around the phase transition temperature. J. R. Soc. Interface. 2017;14:20170127. doi: 10.1098/rsif.2017.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arriaga LR, Löpez-Montero I, Ignés-Mullol J, Monroy F. Domain-growth kinetic origin of nonhorizontal phase coexistence plateaux in langmuir monolayers: Compression rigidity of a raft-like lipid distribution. Journal of Physical Chemistry B. 2010;114(13):4509–4520. doi: 10.1021/jp9118953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.