Abstract
Objectives:
This study aimed to assess the overall health-related quality of life in type 2 diabetes mellitus patients with diabetic foot disease compared to diabetic patients without diabetic foot and to identify the clinical utility of this assessment.
Methods:
A total of 250 consecutive patients with type 2 diabetes mellitus (100/150 with/without diabetic foot, respectively) were interviewed. The questionnaires of the 36-item short-form survey and region-specific foot and ankle ability measure were applied. Wagner–Meggitt wound classification was used for foot-ulcer evaluation. Follow-up of patients for 3–6 weeks was done to identify the potential clinical short outcomes of diabetic foot ulcers.
Results:
Type 2 diabetes mellitus patients with diabetic foot exhibited poor mental and physical health consequences. Females had more prevalence of forefoot lesions, larger ulcer size, advanced Wagner grade, and higher frequency of unhealed ulcers. Receiver operating characteristic curve analysis demonstrated high value of foot and ankle ability measure and 36-item short-form questionnaires to discriminate type 2 diabetes mellitus patients with and without diabetic foot at cutoff values of 66 and 49.6, respectively. Foot and ankle ability measure questionnaire also showed high performance for differentiating the clinical outcome of foot ulcer. Total foot and ankle ability measure subscale score above the cutoff value of 65.5 could discriminate patients with complete healing and unhealed ulcer lesions at a high sensitivity and specificity.
Conclusion:
The current findings confirm the impact of diabetic foot disease on type 2 diabetes mellitus overall health-related quality of life reflected in 36-item short-form questionnaire and foot and ankle ability measure questionnaire which showed high discriminative values for type 2 diabetes mellitus patient sub-grouping. Their application in routine clinical health assessment with continuous medical education programs is highly recommended to achieve a better health-related quality of life.
Keywords: Health-related quality of life, diabetic foot, 36-item short-form score, foot and ankle ability measure score, Saudi Arabia
Introduction
Diabetes mellitus (DM), a common metabolic disease worldwide,1 causes an array of dysfunction in multiple body systems.2 Diabetic foot (DF) is one of the most significant complications of diabetes, imposing major medical, social, and financial burden upon patients and leading frequently to disability and leg amputation.3 It occurs in 5%–10% of diabetic patients.4,5 Uncontrolled hyperglycemia, diabetic neuropathy, peripheral artery disease (PAD), repeated minor traumas, and infection are the main risk factors for the DF ulceration.6 Early diagnosis and efficient treatment strategies are essential to avoid lower extremity amputation and preserve the quality of life for such patients.
Health-related quality of life (HRQoL) assessment can provide a landscape of global health of diabetic patients and lower limb function in particular, which in turn raises patients’ awareness of health care and possible outcomes.7 Common surveys used for DF patients are the 36-item short form (SF-36)8 and foot and ankle ability measure (FAAM).9 Previous studies demonstrated that diabetics with a lower HRQoL score have higher frequency of hospital admission and mortality.10 Since foot ulceration is a highly preventable complication,11 identifying the role and the predictive value of the risk factors influencing this condition will enable health providers to set up a better management plan to improve their health care. As much of the research has been only descriptive in nature, to the author’s knowledge, no studies have been published showing the use of both a general and another specific HRQoL assessment to predict the occurrence and/or the fate of DF disease in the researcher’s area. Hence, in this study, the impact of DF disease on different parameters of patients’ HRQoL assessed by SF-36 and FAAM questionnaires will be explored in the local region of the researcher.
Methods
Study population
A case-control study was conducted to collect data from a total of 250 consecutive Saudi patients with type 2 diabetes mellitus (T2DM; 100 patients presented with a DF lesion and 150 patients without DF). They were recruited from six primary health care (PHC) centers in Khobar, Eastern Province of Saudi Arabia in the period between 2 June and 30 August 2018. A two-stage random sampling technique was used as reported by previous researchers in the same region.12 In the first stage, three of the six PHC centers were selected using a simple random sampling technique. In the second stage, a stratified systematic random sampling was used to select T2DM patients using their records in each PHC center. The selection was stratified by sex. T2DM without DF diseases were also selected from the registered patients in the PHC centers. The inclusion criteria included T2DM with any age, sex, and duration of DM. Type 1 DM, secondary diabetes induced by other causes or due to history of taking any medications, patients who had cognitive or mental illness and pregnant patients were excluded. In addition, patients with missing data that did not allow for scoring of the applied questionnaires were excluded. A DF ulcer was defined as a breach of the normal full thickness of skin manifesting as an induration, ulceration, or change in foot color lasting for 2 weeks or more in a known DM or an individual recently diagnosed with DM.13
The study was done according to the Declaration of Helsinki and approved by the local ethical committee “the Medical and Bioethics local committee of Northern Border University” (no. 49/40/7). The study procedures were explained to all participants and written consent was obtained.
HRQoL assessment
A structured interview was designed and applied to diabetic patients by the author to collect information on demographic data (age, gender, nationality, residence, marital status, personal income, educational level, smoking status, and comorbidities) and disease characteristics (duration of diabetes and current medication use). The generic measure of quality of life SF-36 questionnaire which has been reported and validated on a diverse group of medical conditions, including diabetes,14,15 was used to assess the overall HRQoL. It provides information on overall physical and mental quality of life by covering eight health concepts: general health perceptions (5 items), physical functioning (10 items), role limitations due to physical health problems (4 items), bodily pain (2 items), role limitations due to personal or emotional problems (3 items), energy/fatigue (4 items), emotional well-being (5 items), and social functioning (2 items).10 Each scale is directly transformed into a 0–100 scale, with the lower the score, the more disability. In addition, region-specific FAAM questionnaire which has been found previously9,16,17 to be reliable and responsive in assessing DF disease was applied to evaluate lower extremity function and assess the relative burden of DF ulcers on patients’ health. It includes 21 questions covering activities of daily living (ADL) subscale score and 8 questions that constitute a sports subscale score.13 The participant had a score of 4 if being “no difficulty at all” to 0 being “unable to do,” with a total score of 84 and 32 in ADL and sports subscale, respectively.16 A higher score for each represents a better physical function.
Clinical assessment of diabetic patients
Type, onset, and duration of diabetes were reported by the patients and confirmed from their medical reports. Weight and height were measured, and body mass index (BMI) was estimated as weight divided by height in m2 and categorized according to the World Health Organization classification.18 Treatment strategies were undertaken, and follow-up plans were documented. Features suggesting the presence of long-term diabetic complications as diabetic retinopathy, nephropathy, neuropathy, and so on were recorded.
Foot ulcer assessment and classification
At presentation, the site of the ulcer was noted. After wound debridement, the area of the lesion was graded and staged. The Wagner–Meggitt wound classification was used and validated for foot-ulcer evaluation.19 It is based mainly on wound depth and consists of six grades. These include the following: grade 0 (intact skin), grade 1 (superficial ulcer), grade 2 (deep ulcer to tendon, bone, or joint), grade 3 (deep ulcer with abscess or osteomyelitis), grade 4 (forefoot gangrene), and grade 5 (whole foot gangrene).19 According to the new University of Texas (UT) diabetic wound classification system, ulcer depth, the presence of wound infection, and the presence of clinical signs of lower extremity ischemia were assessed using a matrix of grade on the horizontal axis and staged on the vertical axis.20 The wound grade was evaluated as follows: grade 0 (pre- or post-ulcerative site that has healed), grade 1 (superficial wound not involving tendon, capsule, or bone), grade 2 (wound penetrating to tendon or capsule), and grade 3 (wound penetrating bone or joint). Within each grade, there are four stages: clean wounds (stage A), non-ischemic infected wounds (stage B), ischemic non-infected wounds (stage C), and ischemic infected wounds (stage D). Finally, clinically DF ulcers were categorized clinically as neuropathic, ischemic, or neuroischemic, depending on how complications such as peripheral neuropathy (PN) and arterial disease affect the ulcer’s etiology.5
Ulcers were labeled infected if oozing a purulent discharge and presented with one of the following local signs: warmth, erythema, lymphangitis, lymphadenopathy, oedema, or pain.4 Wound depths were assessed using a blunt probe. Osteomyelitis was suspected if the probe penetrated to bone with features of local or systemic infection.21 The diagnosis of lower extremity vascular insufficiency was made clinically on the basis of absence of both pedal pulses of the involved foot and/or an ankle-brachial pressure (ABI) index of <0.9.22,23 The presence of clinically significant PN was defined if revised neuropathy disability score (NDS) in the lower limbs was six points or more.24–26 It includes the ankle reflex, vibration perception (128 Hz tuning fork), pin-prick, and temperature (cold tuning fork) sensation at both sides of the great toes with a maximum score of 10 points.23 Fissures, callus, joint deformity (subluxation, claw toe, hammer toe, or charcot foot), gangrene, and lower limb amputation were also reported. Prescribed treatment for glycemic control and medical care for DF was documented. Follow-up of patients for 3–6 weeks was done to identify the short clinical outcomes of DF ulcers in at least three consecutive visits.
Statistical analysis
Statistical analysis was performed using SPSS v.23 23 (IBM SPSS, Inc., Chicago, IL, USA). Study sample size was calculated using G power software (http://www.gpower.hhu.de). Calculations showed that with the specified study design (unmatched case-control study), and allowable error rates, an alpha error of 0.5 with sample size 100/150 for patient group/controls, respectively, can give 87% power with an effect size of 0.4. Categorical data are presented as percentage and compared by chi-square and Fisher’s exact tests. Quantitative variables were represented as mean and standard deviation and tested by Student’s t-test. Pearson’s test was performed for correlation analysis. Receiver operating characteristic (ROC) curve analysis was applied to test the questionnaires’ ability to predict the occurrence of foot disease and to determine the prognostic performance for differentiating the clinical outcome of foot ulcer disease for the study participants. Significance was set at p < 0.05.
Results
Baseline characteristics of the study population
The study included a total of 250 T2DM patients (100 patients with DF ulcer compared to another 150 without DF disease). Socio-demographic and clinical characteristics of diabetic patients with and without foot ulcer are shown in Table 1. No significant differences between the two groups were observed in all parameters. Mean age of the whole diabetic patients was 56.8 ± 12.4 years, with about 90% of them aged above 40 years. Nearly, three quarters of patients were females. About 72% of the study populations were Saudi patients. Two-thirds (62.4%) were married, 66% were employed with no career shift after the onset of DF, and most of them were educated (84.8%). Their mean body weight was 76.1 ± 14.5 kg, height was 159.7 ± 8.2 cm, and BMI was 29.9 ± 6.2 kg/m2. One-fifth of patients had normal weight, 22% had a positive family history of diabetes, 40% had hypertension, and 32.4% had other comorbidities such as cardiac and renal diseases. Nearly half of them had the disease within the last 10 years. Mean duration of diabetes was 10.1 ± 9.9 years. Most of them (82.8%) are having their medications regularly. They are taking oral hypoglycemic drugs (84%) and/or insulin (20.4%).
Table 1.
Socio-demographic and clinical data of diabetic patients with and without foot ulcer.
| Total | DM | FU | p-value | ||
|---|---|---|---|---|---|
| Total number | 250 | 150 | 100 | ||
| Age (years) | 56.8 ± 12.4 | 56.9 ± 12.6 | 56.5 ± 12.3 | 0.798 | |
| Age categories (years) | ⩽40 | 27 (10.8) | 16 (10.7) | 11 (11.0) | 0.911 |
| ⩽60 | 134 (53.6) | 79 (52.7) | 55 (55.0) | ||
| ⩽80 | 89 (35.6) | 55 (36.7) | 34 (34.0) | ||
| Sex | Female | 181 (72.4) | 109 (72.7) | 72 (72.0) | 0.908 |
| Male | 69 (27.6) | 41 (27.3) | 28 (28.0) | ||
| Nationality | Saudi | 187 (74.8) | 111 (74.0) | 76 (76.0) | 0.721 |
| Non-Saudi | 53 (21.2) | 39 (26.0) | 24 (24.0) | ||
| Residence | Rural | 192 (76.8) | 121 (80.7) | 71 (71.0) | 0.076 |
| Urban | 58 (23.2) | 29 (19.3) | 29 (29.0) | ||
| Marital status | Single | 17 (6.8) | 11 (7.3) | 6 (6.0) | 0.251 |
| Married | 156 (62.4) | 89 (59.3) | 67 (67.0) | ||
| Divorced | 62 (24.8) | 43 (28.7) | 19 (19.0) | ||
| Widowed | 15 (6.0) | 7 (4.7) | 8 (8.0) | ||
| Occupation | Unemployed | 27 (10.8) | 16 (10.7) | 11 (11.0) | 0.102 |
| Employed | 165 (66.0) | 106 (70.7) | 59 (59.0) | ||
| Retired | 58 (23.2) | 28 (18.7) | 30 (30.0) | ||
| Personal income level (SR/month) | <2000 | 31 (12.4 | 21 (14.0) | 10 (10.0) | 0.300 |
| 2000–10,000 | 198 (79.2) | 114 (76.0) | 84 (84.0) | ||
| >1000 | 21 (8.4) | 15 (10.0) | 6 (6.0) | ||
| Educational level | Illiterate | 38 (15.2) | 23 (15.3) | 15 (15.0) | 0.801 |
| High school | 138 (53.6) | 78 (52.0) | 56 (56.0) | ||
| University | 78 (31.2) | 49 (32.7) | 29 (29.0) | ||
| Weight (kg) | 76.1 ± 14.5 | 75.8 ± 14.5 | 76.4 ± 14.6 | 0.747 | |
| Height (cm) | 159.7 ± 8.2 | 159.5 ± 8.1 | 160.1 ± 8.4 | 0.662 | |
| Body mass index (kg/m2) | 29.9 ± 6.2 | 28.3 ± 6.2 | 30.2 ± 6.2 | 0.931 | |
| BMI categories | Normal weight | 55 (22.0) | 33 (22.0) | 22 (22.0) | 0.998 |
| Overweight | 93 (37.2) | 56 (37.3) | 37 (37.0) | ||
| Class I obesity | 59 (23.6) | 35 (23.3) | 24 (24.0) | ||
| Class II obesity | 19 (7.6) | 12 (8.0) | 7 (7.0) | ||
| Class III obesity | 24 (9.6) | 14 (9.3) | 10 (10.0) | ||
| Family history of DM | Negative | 195 (78.0) | 118 (78.7) | 77 (77.0) | 0.758 |
| Positive | 55 (22.0) | 32 (21.3) | 23 (23.0) | ||
| Smoking | Non-smoker | 17 (6.8) | 11 (7.3) | 6 (6.0) | 0.800 |
| Smoker | 233 (93.2) | 139 (92.7) | 94 (94.0) | ||
| Hypertension | Negative | 150 (60.0) | 90 (60.0) | 60 (60.0) | 1.000 |
| Positive | 100 (40.0) | 60 (40.0) | 40 (40.0) | ||
| Other comorbidities | Negative | 169 (67.6) | 96 (64.0) | 73 (73.0) | 0.136 |
| Positive | 81 (32.4) | 54 (36.0) | 27 (27.0) | ||
| Duration of diabetes | Newly diagnosed | 17 (6.8) | 11 (7.3) | 6 (6.0) | 0.986 |
| ⩽10 years | 122 (48.8) | 73 (48.7) | 49 (49.0) | ||
| ⩽20 years | 74 (29.6) | 43 (28.7) | 31 (31.0) | ||
| ⩽30 years | 32 (12.8) | 20 (13.3) | 12 (12.0) | ||
| >30 years | 5 (2.0) | 3 (2.0) | 2 (2.0) | ||
| Glycemic control | Diet | 9 (3.6) | 6 (4.0) | 3 (3.0) | 0.869 |
| Oral | 190 (76.0) | 114 (76.0) | 76 (76.0) | ||
| Insulin | 31 (12.4) | 17 (11.3) | 14 (14.0) | ||
| Oral + insulin | 20 (8.0) | 13 (8.7) | 7 (7.0) | ||
| Medication regularity | Regular | 207 (82.8) | 128 (85.3) | 79 (79.0) | 0.193 |
| Irregular | 43 (17.2) | 22 (14.7) | 21 (21.0) | ||
| Autonomic neuropathy | Negative | 232 (92.8) | 143 (95.3) | 89 (89.0) | 0.057 |
| Positive | 28 (11.2) | 7 (4.7) | 11 (21.0) |
DM: diabetes mellitus; FU: diabetic patients with foot ulcer; comorbidities: heart and renal diseases; SR: Saudi Riyal.
Irregular drug intake = compliance <90% in last month. Student’s t and chi-square/Fisher’s exact tests were used for comparison.
Assessment of DF ulcer
As shown in Table 2, all patients had a single ulcer disease. Lesions in less than three quarters (71%) were in the left foot, and most ulcers were in the forefoot region. Local examination showed dryness and fissured skin with only three cases with callus, one case with fungal infection, two of them with claw toe deformity, and one patient with hammer toe. Most ulcers (77%) were of Wagner grade 2. Mean duration of diabetes was 13.6 ± 2.54 years, duration of ulcer was 8.1 ± 1.75 weeks, and the size of the ulcers was 3.0 ± 0.54 cm2. All patients had intact ankle reflex and most of them had intact sensation. DF patients received mainly treatment for the infection and pressure offloading. In the DF disease patients, 28% had complete resolution of the lesions, 72% had unhealed ulcer, one female patient had below knee amputation, and one male patient died during the follow-up period. Stratification analysis by gender revealed more prevalence of forefoot lesions (p < 0.001), larger ulcer size (p = 0.004), advanced Wagner grade (p < 0.001), and higher frequency of unhealed ulcer (p < 0.001) among female participants.
Table 2.
Clinical characteristics of diabetic foot lesions.
| Total | Female | Male | p-value | ||
|---|---|---|---|---|---|
| Total number | 100 | 72 | 28 | ||
| Side of affected foot | Right | 29 (29.0) | 21 (29.2) | 8 (28.6) | 0.992 |
| Left | 71 (71.0) | 51 (70.8) | 20 (71.4) | ||
| Site of ulcer | Forefoot | 78 (78.0) | 64 (88.9) | 14 (50.0) | <0.001 |
| Midfoot | 12 (12.0) | 6 (8.3) | 6 (21.4) | ||
| Hindfoot | 10 (10.0) | 2 (2.8) | 8 (28.6) | ||
| Number of lesions | Single | 100 (100) | 72 (100) | 28 (100) | |
| Ulcer size (cm2) | 3.0 ± 0.54 | 3.1 ± 0.6 | 2.8 ± 0.4 | 0.004 | |
| Local examination | Dryness | 97 (97.0) | 69 (95.8) | 28 (100) | 0.273 |
| Fissure | 43 (43.0) | 35 (948.6) | 8 (28.6) | 0.078 | |
| Callus | 3 (3.0) | 3 (4.2) | 0 (0.0) | 0.273 | |
| Fungal infection | 1 (1.0) | 1 (1.4) | 0 (0.0) | 0.530 | |
| Deformity | 3 (3.0) | 0 (0.0) | 3 (10.7) | 0.004 | |
| Type of ulcer | NN-NI | 70 (70.0) | 54 (75.0) | 16 (57.1) | 0.063 |
| Neuroischemic | 26 (26.0) | 15 (20.8) | 11 (39.3) | ||
| Neuropathic | 3 (3.0) | 3 (4.2) | 0 (0.0) | ||
| Ischemic | 1 (1.0) | 0 (0.0) | 1 (3.6) | ||
| Wagner grade | Grade 0 | 8 (8.0) | 0 (0.0) | 8 (28.6) | <0.001 |
| Grade 1 | 15 (15.0) | 7 (9.7) | 8 (28.6) | ||
| Grade 2 | 77 (77.0) | 65 (90.3) | 12 (42.9) | ||
| Duration of ulcer (weeks) | 8.1 ± 1.75 | 8.0 ± 1.7 | 8.2 ± 1.9 | 0.789 | |
| Intact sensation | Vibration | 96 (96.0) | 68 (94.4) | 28 (100) | 0.574 |
| Touch pressure | 95 (95.0) | 67 (93.1) | 28 (100) | 0.359 | |
| Proprioception | 94 (94.0) | 67 (93.1) | 27 (96.4) | 0.751 | |
| Pain sensation | 92 (92.0) | 65 (90.3) | 27 (96.4) | 0.575 | |
| Intact ankle reflex | 100 (100) | 72 (100) | 28 (100) | 1.000 | |
| Treatment of ulcer | Local antibiotic | 100 (100) | 72 (100) | 28 (100) | 1.000 |
| Systemic antibiotic | 95 (95.0) | 67 (93.1) | 28 (100) | 0.359 | |
| Debridement | 1 (1.0) | 0 (0.0) | 1 (1.4) | 0.530 | |
| Offloading | 10 (10.0) | 8 (28.6) | 2 (2.8) | 0.522 | |
| NPWT | 3 (3.0) | 3 (4.2) | 0 (0.0) | 0.273 | |
| HBOT | 3 (3.0) | 3 (4.2) | 0 (0.0) | 0.273 | |
| Clinical outcomes | Complete healing | 28 (28.0) | 12 (16.7) | 16 (57.1) | <0.001 |
| Not healed | 72 (72.0) | 60 (83.3) | 12 (42.9) | ||
| Amputation | 1 (1.0) | 1 (1.4) | 0 (0.0) | ||
| Patient death | 1 (1.0) | 0 (0.0) | 1 (3.6) |
NN-NI: non-neuropathic, non-ischemic; debridement: surgical, autolytic, chemical, mechanical, and biologic; NPWT: negative pressure wound therapy; HBOT: hyperbaric oxygen therapy; offloading: pressure offloading.
Student’s t and chi-square tests were used for comparison. Bold values indicate significance at p < 0.05.
HRQoL in patients with DF disease
Two HRQoL assessment questionnaires, SF-36 and FAAM, were applied to assess the overall health and the specific lower limb function, respectively. The measured HRQoL total score suggested that, on average, the subjects without DF ulcers considered that their general quality of life to be more satisfactory than those experienced DF disease (49.4 ± 12.0 vs 40.5 ± 10.6, p < 0.001). Diabetic patients with foot ulcers exhibited poor mental and physical health consequences compared to patients without DF disease. However, there was no significant difference between the two study groups regarding body pain (p = 0.654), emotional health problems (p = 0.931), and mental health (p = 0.154). Consistently, physical assessment of lower limbs by FAAM questionnaire showed better ADL and sports subscale scores in patients without DF disease (80.4 ± 13.0 vs 61.7 ± 13.3, p < 0.001) (Table 3). Pearson’s analysis showed significant positive correlations between various health domains of the two questionnaires as depicted in Table 4. Physical component summary (PCS) of SF-36 score was directly correlated with total FAAM score (r = 0.235, p < 0.001), sports (r = 0.295, p < 0.001), and mental component summary (MCS; r = 0.348, p < 0.001).
Table 3.
Health-related quality of life of diabetic patients with and without foot ulcer.
| No. of items | DM (n = 150) |
FU (n = 100) |
p-value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| SF-36 scales | |||||||
| PCS score | General health | 6 | 54.0 | 14.6 | 45.12 | 18.4 | <0.001 |
| Physical health problems | 10 | 76.7 | 8.6 | 71.0 | 8.3 | <0.001 | |
| Limitations activities | 4 | 75.0 | 32.7 | 39.5 | 36.9 | <0.001 | |
| Bodily pain | 2 | 35.7 | 25.8 | 34.2 | 24.5 | 0.654 | |
| Total PCS | 22 | 60.3 | 12.1 | 47.5 | 13.3 | <0.001 | |
| MCS score | Emotional health problems | 3 | 47.1 | 40.9 | 46.6 | 37.6 | 0.931 |
| Vitality | 4 | 35.8 | 26.6 | 27.6 | 22.6 | 0.012 | |
| Social activities | 2 | 39.0 | 20.1 | 32.0 | 15.6 | 0.004 | |
| Mental health | 5 | 32.3 | 23.5 | 28.2 | 19.7 | 0.154 | |
| Total MCS | 14 | 38.5 | 17.1 | 33.6 | 12.9 | 0.015 | |
| Total SF-36 | 49.4 | 12.0 | 40.5 | 10.6 | <0.001 | ||
| FAAM scoring system | |||||||
| ADL subscale | 21 | 59.3 | 13.0 | 41.7 | 13.3 | <0.001 | |
| Sports subscale | 8 | 21.1 | 2.5 | 20.0 | 1.07 | <0.001 | |
| Total FAAM | 29 | 80.4 | 13.0 | 61.7 | 13.3 | <0.001 | |
DM: diabetes mellitus; FU: diabetic patients with foot ulcer; SD: standard deviation; SF-36: 36-item short-form questionnaire; PCS: physical component summary; MCS: mental component summary; FAAM: foot and ankle ability measure; ADL: activities of daily living.
Student’s t-test was used for comparison. Bold values indicate significance at p < 0.05.
Table 4.
Correlation between SF-36 and FAAM subscales.
| ADL | Sports | FAAM | PCS | MCS | SF-36 | |
|---|---|---|---|---|---|---|
| ADL | 1.00 | 0.072 (0.257) | 0.991 (<0.001) | 0.199 (0.002) | 0.063 (0.320) | 0.156 (0.014) |
| Sports | 1.00 | 0.204 (0.001) | 0.295 (<0.001) | 0.284 (<0.001) | 0.353 (<0.001) | |
| FAAM | 1.00 | 0.235 (<0.001) | 0.098 (0.124) | 0.198 (0.002) | ||
| PCS | 1.00 | 0.348 (<0.001) | 0.798 (<0.001) | |||
| MCS | 1.00 | 0.842 (<0.001) | ||||
| SF-36 | 1.00 |
SF-36: 36-item short-form questionnaire; FAAM: foot and ankle ability measure; ADL: activities of daily living; PCS: physical component summary; MCS: mental component summary.
Pearson’s correlation coefficient (p-values) is shown for each of the two variables. Bold values indicate significance at p < 0.05.
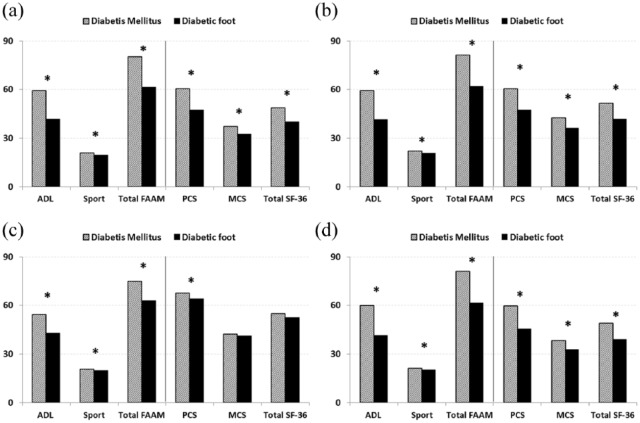
Similar to the overall analysis, stratification of DM and DF patients by age and sex also revealed significant differences in HRQoL domains between patients with and without foot disease in various subgroups except for the MCS and total SF-36 score in young patients which were not significantly deteriorated as other health domains (Figure 1).
Figure 1.
Mean scores obtained from the FAAM and SF-36 questionnaires for quality-of-life assessment of the study population: (a) male, (b) female, (c) younger, and (d) elder.
SF-36: 36-item short-form questionnaire; PCS: physical component summary; MCS: mental component summary; FAAM: foot and ankle ability measure; ADL: activities of daily living.
Student’s t-test was used for comparison. *Significance at p < 0.05.
ROC curve analysis demonstrated high discrimination value of FAAM and SF-36 questionnaires to discriminate DM patients with and without foot disease at cutoff values of 66 and 49.6, respectively (Figure 2(a)). In addition, total FAAM subscale score above the cutoff value of 65.5 could discriminate patients with/without healed ulcer at a high sensitivity and specificity (Figure 2(b)).
Figure 2.
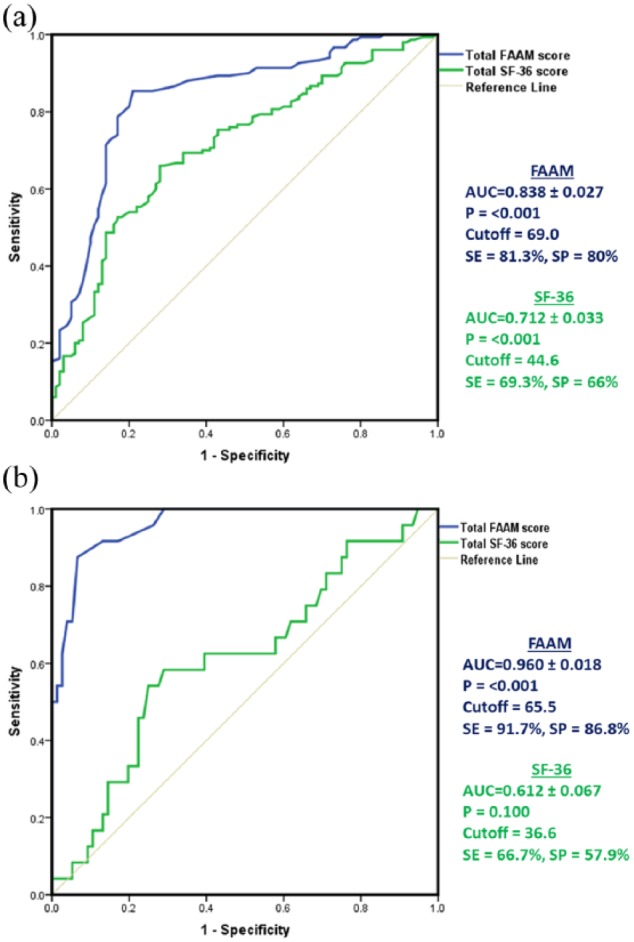
Discriminating values of FAAM and SF-36 questionnaires. ROC curve analysis of total scores of FAAM and SF-36 were employed to (a) discrimination of T2DM patients and patients with diabetic foot disease and (b) discrimination of T2DM patients with complete healing of DF ulcers and with unhealed ones.
AUC: area under the curve; SE: sensitivity; SP: specificity; SF-36: 36-item short-form questionnaire; FAAM: foot and ankle ability measure.
Association between quality-of-life scores and clinical parameters
Association analysis of HRQoL subscales with demographic and clinical characteristics is demonstrated in Table 5. In the whole study population, female patients showed significant lower scores in sports subscale of FAAM questionnaire (20.3 ± 2.0 in females vs 21.5 ± 2.3 in males, p < 0.001), while young-age diabetic patients (⩽40 years) reported better physical impact represented as higher PCS scores (66.1 ± 11.2 in young patients vs 53.9 ± 13.8 in elders, p < 0.001) and total SF-36 scores (53.9 ± 9.2 vs 44.9 ± 12.2). Similar findings were encountered when clustering patients according to DF lesions (Supplementary Figure S1). Moreover, smoking imposed a significant factor that was associated with poor quality of life for both physical and mental health domains (PCS: 55.2 ± 12.4 in non-smokers vs 45.11 ± 11.4 in smokers, p < 0.001; MCS: 47.0 ± 18.6 vs 35.8 ± 15.2, p = 0.004; and SF-36: 51.1 ± 12.9 vs 45.5 ± 12.1, p = 0.007). Comparing the questionnaire scores in DF patients with unhealed and healed ulcers showed better values of ADL and FAAM in patients with complete resolution (ADL: 36.5 ± 9.2 in unhealed ulcers vs 58.1 ± 11.1 in complete healing, p < 0.001 and FAAM: 56.5 ± 9.2 vs 78.2 ± 10.5, p < 0.001).
Table 5.
Association between quality-of-life scores and clinical parameters of diabetic patients with and without diabetic foot.
| ADL | Sports | FAAM | PCS | MCS | SF-36 | |
|---|---|---|---|---|---|---|
| Gender | 0.927 | <0.001 | 0.687 | 0.966 | 0.032 | 0.181 |
| Age | 0.391 | 0.177 | 0.315 | <0.001 | 0.073 | <0.001 |
| Obesity | 0.509 | 0.834 | 0.283 | 0.200 | 0.885 | 0.990 |
| Smoking | 0.620 | 0.971 | 0.298 | <0.001 | 0.004 | 0.007 |
| Hypertension | 0.338 | 0.413 | 0.293 | 0.752 | 0.657 | 0.653 |
| FH of DM | 0.252 | 0.368 | 0.200 | 0.553 | 0.389 | 0.842 |
| Duration DM | 0.573 | 0.224 | 0.542 | 0.209 | 0.397 | 0.180 |
| Duration of ulcer | 0.949 | 0.769 | 0.973 | 0.496 | 0.808 | 0.334 |
| Side of ulcer | 0.160 | 0.064 | 0.209 | 0.824 | 0.362 | 0.688 |
| Site of ulcer | 0.087 | 0.596 | 0.058 | 0.839 | 0.162 | 0.206 |
| Type of ulcer | 0.729 | 0.430 | 0.776 | 0.480 | 0.220 | 0.579 |
| Wagner grade | 0.148 | 0.903 | 0.285 | 0.594 | 0.863 | 0.200 |
| Clinical outcome | <0.001 | 0.299 | <0.001 | 0.255 | 0.136 | 0.108 |
FH: family history; DM: diabetes mellitus; SF-36: 36-item short-form questionnaire; PCS: physical component summary; MCS: mental component summary; FAAM: foot and ankle ability measure; ADL: activities of daily living; ANOVA: analysis of variance.
p-values of student’s t and ANOVA tests are shown. Bold values indicate significance at p < 0.05.
Discussion
In light of the previous studies about DF along with its great burden in Saudi Arabia where its prevalence ranges from 11.4% to 29.7%,27,28 the current study was conducted to identify the clinical utility of the two selected HRQoL assessment questionnaires: the SF-36 and the region-specific FAAM assessment in prediction of DF outcome in a sample of Saudi T2DM patients with and without DF.
The mean age of the study participants was 56.8 (±12.4) years with about 90% of them aged above 40 years. In the same vein, Al-Rubeaan et al.11 in their retrospective cohort study have reported that “age ⩾45 years is a risk factor for developing diabetic foot ulcers in a Saudi population.” Similarly, more recently, Musa et al.29 in their study of the factors associated with amputation among patients with DF ulcers in the same population highlighted that the “worldwide metabolic syndrome epidemic,” which is particularly affecting the Arabian Gulf area, could be responsible for the younger age of presentation.
Unlike the finding of some studies that male gender was predominant in DF patients,7,23 female patients in the current cohort and DF disease patients were more prevalent. The influence of gender on foot ulcers has been controversial.30 Dinh and Veves31 in their multicenter, prospective study which included 248 patients with diabetes found that gender may impose a significant risk factor for the DF ulcer development. They argued that females could have a lower risk than males for DF ulcers in part due to less severe neuropathy, increased joint mobility, and lower foot pressures. However, once neuropathy or other risk factors for foot ulceration are present, females have equal risk as males for developing DF and its complications. After ruling out any source of selection bias in the current work (as female patients at the local area of the researcher tend to seek medical advice and have high response rate for female doctors in contrary to male patients), the author cannot exclude the impact of gender norms on women’s health in the local region. According to Aldosari:32
women may find health services inaccessible, unavailable, or conditioned on certain cultural justifications or gender norms. Limitations on women’s autonomy, such as those imposed by the male guardianship system, women’s driving ban, gender segregation, and religious norms, influence access, quality, and outcomes of health care for women in Saudi Arabia.
These factors could also contribute to the more prevalence of larger ulcer size, advanced Wagner grade, and higher frequency of unhealed ulcers among female participants on the current stratification analysis by gender. More large-scale research in this area is warranted.
In this study, the duration of T2DM in patients with DF was relatively short and most patients had normal sensation. Although DF most commonly occurs in patients with longer duration of diabetes and greater number of diabetic complications, however, the current finding was in line with a variety of previous studies.33,34 New et al.33 reported that patients even in the first year after diagnosis of diabetes presented high risk of DF disorders and amputation. One prospective cohort study did not observe a significant association between diabetes duration and foot ulcers.35 In addition, the high prevalence of diabetes reported from previous studies at the local region6,23,29 has suggested that DF disorders might be more common among diabetic populations in the Middle East. Considering both high prevalence of diabetes (23.9%) and potential vulnerability of Saudi population to DF diseases, Saudi Arabia ranks number four among the top five Middle Eastern and North African countries for the number of people with diabetes with 3.6 million diabetes cases.27 DF disorders threaten a heavy burden on the health care system and an intractable public health challenges in Saudi Arabia,23 as well as cause substantial psychological burden to the patients and society.27
As expected, the currently measured HRQoL total score by SF-36 assessment and the physical assessment of lower limbs by FAAM questionnaire showed better subscale scores in T2DM patients without DF disease. Sothornwit et al.36 in their recent study have confirmed that DF has the greatest negative impact on HRQoL as compared with other diabetic-related complications such as diabetic retinopathy, end-stage renal disease, or coronary artery disease.
The current data also suggest that the included T2DM patients with foot ulcer may represent a group with less severe or earlier stage disease because 15% and 77% of the patients had the first- and second grade of Wagner, respectively. It is reasonable to question whether the differences between the study groups are actually related to the foot ulcer severity or to other factors. However, the current data are reasonable with respect to the SF-36 and the FAAM domains most affected, and even small and hidden ulcers may bother patients consistent with that found by others.7,37
A high discrimination value of FAAM and SF-36 questionnaires to differentiate patients with and without foot disease in study population has been proved in this work. Furthermore, FAAM questionnaire, which is a validated measure of the physical function of patients across a broad spectrum of lower extremity musculoskeletal pathologies,14 also showed high performance for differentiating patients with complete healing and unhealed DF ulcer lesions at a cutoff value of 65.5 with a high sensitivity and specificity. These findings in part are consistent with others7,11,14,36–41 who reported that lower scores on SF-36 and/or FAAM scales were associated with the severity of complications among T2DM patients. As SF-36 is a generic measure of HRQoL and might not independently fully assess lower extremity function, the researcher combined this generic measurement with a region-specific instrument, the FAAM. The SF-36 and FAAM scales in combination have been used in several studies of DF disease.9,36,42–44 On employing correlation analysis between the various health domains of the two questionnaires, significant slight positive correlations were depicted between the PCS of SF-36 score and the total FAAM score, sports, and MCS. This indicates that SF-36 could capture different outcome assessments than the FAAM. Hence, using both measurements is advantageous to improve the specificity and to provide additional and complimentary information about patient outcomes as confirmed previously,45–47 and there is no one “gold standard” or “ideal instrument” exist as revealed by Hogg et al.48 in their systematic review.
Despite the limitations of the current study, the relatively small sample size and the cross-sectional analysis design which limits the ability to establish the cause–effect relationships, with short follow up-period (3–6 weeks) for T2DM patients, and the study population might not be representative for all Saudi population because the patients were recruited from single city of Saudi Arabia and non-Saudi patients were also included, albeit the present work was the first in the local region of the researcher to use two instruments (SF-36 and the FAAM) in combination to assess the outcome of T2DM patients with DF ulcers. This work has revealed the high value of SF-36 and FAAM assessments to discriminate T2DM patients with/without foot disease at certain cutoff values. In addition, FAAM assessment also showed high performance at a specific cutoff value for differentiating T2DM with complete healing/unhealed DF ulcer lesions at a high sensitivity and specificity levels in the current study population. Taken together, DF care and foot assessment by different scales should be promoted and applied as routine measurements in the out-patient clinics.
Supplemental Material
Supplemental material, Supplementary_Figure for Impaired quality of life and diabetic foot disease in Saudi patients with type 2 diabetes: A cross-sectional analysis by Sana A AlSadrah in SAGE Open Medicine
Footnotes
Data availability: All data and materials related to the present work have been included in this article and the related supplementary materials.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the Medical and Bioethics local committee of Northern Border University (No. 49/40/7).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: All participants provided written informed consent to participate in the study after being informed with its purpose.
Trial registration: This randomized clinical trial was not registered because this was a survey study focusing on patients’ prescription information only.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Sana A AlSadrah  https://orcid.org/0000-0002-1254-3542
https://orcid.org/0000-0002-1254-3542
References
- 1. Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2104; 103: 137–149. [DOI] [PubMed] [Google Scholar]
- 2. Wang F, Zhang J, Yu J, et al. Diagnostic accuracy of monofilament tests for detecting diabetic peripheral neuropathy: a systematic review and meta-analysis. J Diabetes Res 2017; 2017: 8787261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Driver VR, Fabbi M, Lavery LA, et al. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc 2010; 100(5): 335–341. [DOI] [PubMed] [Google Scholar]
- 4. Oyibo SO, Jude EB, Tarawneh I, et al. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care 2001; 24(1): 84–88. [DOI] [PubMed] [Google Scholar]
- 5. Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther 2012; 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fawzy MS, Alshammari MA, Alruwaili AA, et al. Factors associated with diabetic foot among type 2 diabetes in Northern area of Saudi Arabia: a descriptive study. BMC Res Notes 2019; 12(1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ribu L, Hanestad BR, Moum T, et al. A comparison of the health-related quality of life in patients with diabetic foot ulcers, with a diabetes group and a nondiabetes group from the general population. Qual Life Res 2007; 16(2): 179–189. [DOI] [PubMed] [Google Scholar]
- 8. WareJE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Med Care 1992; 30(6): 473–483. [PubMed] [Google Scholar]
- 9. Martin RL, Hutt DM, Wukich DK. Validity of the foot and ankle ability measure (FAAM) in diabetes mellitus. Foot Ankle Int 2009; 30(4): 297–302. [DOI] [PubMed] [Google Scholar]
- 10. Wukich DK, Raspovic KM. Assessing health-related quality of life in patients with diabetic foot disease: why is it important and how can we improve? The 2017 Roger E. Diabetes Care 2018; 41(3): 391–397. [DOI] [PubMed] [Google Scholar]
- 11. Al-Rubeaan K, AlDerwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS ONE 2015; 10(5): e0124446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Shehri AH, Taha AZ, Bahnassy AA, et al. Health-related quality of life in type 2 diabetic patients. Ann Saudi Med 2008; 28(5): 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hunt DL. Diabetes: foot ulcers and amputations. BMJ Clin Evid 2011; 2011: 0602. [PMC free article] [PubMed] [Google Scholar]
- 14. Dhawan V, Spratt KF, Pinzur MS, et al. Reliability of AOFAS diabetic foot questionnaire in Charcot arthropathy: stability, internal consistency, and measurable difference. Foot Ankle Int 2005; 26(9): 717–731. [DOI] [PubMed] [Google Scholar]
- 15. Nabuurs-Franssen MH, Huijberts MS, NieuwenhuijzenKruseman AC, et al. Health-related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia 2005; 48(9): 1906–1910. [DOI] [PubMed] [Google Scholar]
- 16. Kivlan BR, Martin RL, Wukich DK. Responsiveness of the foot and ankle ability measure (FAAM) in individuals with diabetes. Foot 2011; 21(2): 84–87. [DOI] [PubMed] [Google Scholar]
- 17. Martin RL, Irrgang JJ, Burdett RG, et al. Evidence of validity for the foot and ankle ability measure (FAAM). Foot Ankle Int 2005; 26(11): 968–983. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization (WHO). Obesity: preventing and managing the global epidemic. Report of a WHO consultation, World Health Organization Technical Report Series 894, 2000, http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ [PubMed]
- 19. Wagner FW., Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981; 2(2): 64–122. [DOI] [PubMed] [Google Scholar]
- 20. Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. J Foot Ankle Surg 1996; 35(6): 528–531. [DOI] [PubMed] [Google Scholar]
- 21. Grayson ML, Gibbons GW, Balogh K, et al. Probing to bone in infected pedal ulcers: a clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995; 273(9): 721–723. [PubMed] [Google Scholar]
- 22. Apelqvist J, Castenfors J, Larsson J. Prognostic value of ankle and toe blood pressure levels in outcome of diabetic foot ulcers. Diabetes Care 1989; 12: 373–378. [DOI] [PubMed] [Google Scholar]
- 23. Alzahrani HA, Wang D, Alzahrani AH, et al. Incidence of diabetic foot disorders in patients with diabetes in Jeddah, Saudi Arabia. Int J Diab Develop Countries 2015; 35: 115–122. [Google Scholar]
- 24. Franklin GM, Kahn LB, Baxter J, et al. Sensory neuropathy in non-insulin-dependent diabetes mellitus: the San Luis Valley Diabetes Study. Am J Epidemiol 1990; 131(4): 633–643. [DOI] [PubMed] [Google Scholar]
- 25. Young MJ, Boulton AJM, Williams DRR, et al. A multi-centre study of the prevalence of diabetic neuropathy in patients attending UK diabetic clinics. Diabetologia 1993; 36: 150–154. [DOI] [PubMed] [Google Scholar]
- 26. Yang Z, Chen R, Zhang Y, et al. Scoring systems to screen for diabetic peripheral neuropathy. Cochrane Db Syst Rev 2014; 3: CD010974, https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010974/full [Google Scholar]
- 27. Hu Y, Bakhotmah BA, Alzahrani OH, et al. Predictors of diabetes foot complications among patients with diabetes in Saudi Arabia. Diabetes Res Clin Pract 2014; 106(2): 286–294. [DOI] [PubMed] [Google Scholar]
- 28. Mairghani M, Elmusharaf K, Patton D, et al. The prevalence and incidence of diabetic foot ulcers among five countries in the Arab world: a systematic review. J Wound Care 2017; 26(Suppl. 9): S27–S34. [DOI] [PubMed] [Google Scholar]
- 29. Musa IR, Ahmed MON, Sabir EI, et al. Factors associated with amputation among patients with diabetic foot ulcers in a Saudi population. BMC Res Notes 2018; 11(1): 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peek ME. Gender differences in diabetes-related lower extremity amputations. Clin Orthop Relat Res 2011; 469(7): 1951–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dinh T, Veves A. The influence of gender as a risk factor in diabetic foot ulceration. Wounds 2008; 20(5): 127–131. [PubMed] [Google Scholar]
- 32. Aldosari H. The effect of gender norms on women’s health in Saudi Arabia. The Arab Gulf States Institute in Washington, http://www.agsiw.org/effect-gender-norms-womens-health-saudi-arabia/ (2017, accessed 28 August 2018).
- 33. New J, McDowell D, Burns E, et al. Problem of amputations in patients with newly diagnosed diabetes mellitus. Diabet Med 1998; 15(9): 760–764. [DOI] [PubMed] [Google Scholar]
- 34. Schaper N, Andros G, Apelqvist J, et al. Diagnosis and treatment of peripheral arterial disease in diabetic patients with a foot ulcer. Diabetes Metab Res Rev 2012; 28(Suppl. 1): 218–224. [DOI] [PubMed] [Google Scholar]
- 35. Boyko EJ, Ahroni JH, Stensel V, et al. A prospective study of risk factors for diabetic foot ulcer. Diabetes Care 1999; 22(7): 1036–1042. [DOI] [PubMed] [Google Scholar]
- 36. Sothornwit J, Srisawasdi G, Suwannakin A, et al. Decreased health-related quality of life in patients with diabetic foot problems. Diabetes Metab Syndr Obes 2018; 11: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med 2016; 4: 5052926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jacobson AM, deGroot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care 1994; 17(4): 267–274. [DOI] [PubMed] [Google Scholar]
- 39. Evans AR, Pinzur MS. Health-related quality of life of patients with diabetes and foot ulcers. Foot Ankle Int 2005; 26(1): 32–37. [DOI] [PubMed] [Google Scholar]
- 40. Willrich A, Pinzur M, McNeil M, et al. Health related quality of life, cognitive function, and depression in diabetic patients with foot ulcer or amputation: a preliminary study. Foot Ankle Int 2005; 26(2): 128–134. [DOI] [PubMed] [Google Scholar]
- 41. Boutoille D, Feraille A, Maulaz D, et al. Quality of life with diabetes-associated foot complications: comparison between lower-limb amputation and chronic foot ulceration. Foot Ankle Int 2008; 29(11): 1074–1078. [DOI] [PubMed] [Google Scholar]
- 42. Wukich DK, Pearson KT. Self-reported outcomes of trans-tibial amputations for non-reconstructable Charcot neuroarthropathy in patients with diabetes: a preliminary report. Diabet Med 2013; 30(3): e87–e90. [DOI] [PubMed] [Google Scholar]
- 43. Raspovic KM, Wukich DK. Self-reported quality of life and diabetic foot infections. J Foot Ankle Surg 2014; 53(6): 716–719. [DOI] [PubMed] [Google Scholar]
- 44. Raspovic KM, Wukich DK. Self-reported quality of life in patients with diabetes: a comparison of patients with and without Charcot neuroarthropathy. Foot Ankle Int 2014; 35(3): 195–200. [DOI] [PubMed] [Google Scholar]
- 45. Wukich DK, Sambenedetto TL, Mota NM, et al. Correlation of SF-36 and SF-12 component scores in patients with diabetic foot disease. J Foot Ankle Surg 2016; 55(4): 693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tang TS, Yusuf FLA, Polonsky WH, et al. Assessing quality of life in diabetes: II—deconstructing measures into a simple framework. Diabetes Res Clin Pract 2017; 126: 286–302. [DOI] [PubMed] [Google Scholar]
- 47. Tang TS, Yusuf FLA, Polonsky WH, et al. Assessing quality of life in diabetes: I. Diabetes Res Clin Pract 2017; 126: 286–302. [DOI] [PubMed] [Google Scholar]
- 48. Hogg FR, Peach G, Price P, et al. Measures of health-related quality of life in diabetes-related foot disease: a systematic review. Diabetologia 2012; 55(3): 552–565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Figure for Impaired quality of life and diabetic foot disease in Saudi patients with type 2 diabetes: A cross-sectional analysis by Sana A AlSadrah in SAGE Open Medicine