Abstract
Periodontitis is twice as prevalent in diabetics as in nondiabetics, and type 2 diabetes (T2D)–associated periodontitis is severe in many cases due to the altered and aberrant functions of bone cells in hyperglycemic conditions. Therefore, developing an effective method to halt the disease process, as well as restore and regenerate lost alveolar bone to reserve the natural teeth in diabetics, is critically important. In the current study, we applied a newly discovered adiponectin receptor agonist AdipoRon (APR) in experimental periodontitis in diabetic animal models and demonstrated the underlying molecular mechanisms. We found that when APR systemically quenched the blood sugar level in diet-induced obesity (DIO) diabetic mice, it reduced osteoclast numbers and alveolar bone loss significantly due to APR’s inhibition on osteoclast differentiation shown in our in vitro studies. APR also decreased the production of proinflammatory molecules CC chemokine ligand 2 and interleukin 6 in diseased gingival tissues. On the other hand, APR promoted alveolar bone regeneration through enhancing osteogenic differentiation and decreasing stromal cell–derived factor 1 in the bone marrow that facilitates stem cell migration. Same results were achieved by APR treatment of periodontitis induced in adiponectin (APN) knockout mice, indicating the ability of APR to activate the endogenous APN receptors to exert osteoanabolic effects. In summary, our study supports the notion that APR could be used as an effective multipronged approach to target T2D-associated periodontitis.
Keywords: AdipoRon, alveolar bone loss, type 2 diabetes mellitus, osteoclast, osteogenesis, animal disease model
Introduction
Patients with type 1 and type 2 diabetes mellitus (T1DM, T2DM) have been reported to suffer a range of bone-related complications. Twice as prevalent in diabetics as nondiabetics, periodontitis is the sixth most common complication of diabetes (Preshaw et al. 2012; Sanz et al. 2018). T2DM-associated periodontitis is particularly severe in many cases (Sima and Van Dyke 2016). The consequence of periodontitis is excessive alveolar bone resorption leading to tooth loss, with severe negative impacts on chewing, swallowing, nutrient intake, speaking, facial expression, and aesthetics (Eke et al. 2012). Furthermore, it can trigger general inflammation, impairing cardiovascular, endocrine, and reproductive systems. The local periodontal inflammation also leads to inadequate glycemic control and worsens diabetic conditions (Preshaw et al. 2012; Polak and Shapira 2018). The diabetes-associated process of periodontitis and delayed bone wound healing both are associated with supernormal osteoclastogenesis (Liu et al. 2006; Kayal et al. 2007). Diabetes-associated periodontitis shows more proinflammatory mediators including tumor necrosis factor α (TNF-α), interleukin (IL)–6, and CC chemokine ligand 2 (CCL2) in periodontal tissues (Wu et al. 2017), promoting osteoclast formation through receptor activator of nuclear factor κΒ ligand (RANKL), which leads to increased osteoclast numbers and its activity. Diabetes also affects bone-healing phases via increased osteoclastogenesis, leading to a prolonged bone turnover and reformation (Kasahara et al. 2010).
Most T2DM patients also exhibit dysfunction of osteoblastic differentiation and a failure to mobilize stromal cells from the bone marrow space to the circulation, referred to as diabetic stem cell mobilopathy (DiPersio 2011; Fadini et al. 2013). Stromal cell–derived factor 1 (SDF-1) is one of the mobilizing factors (Otsuru et al. 2008), normally produced by bone marrow mesenchymal cells (BMSCs), cells of the osteoblastic lineage, and endothelial cells (Lapid et al. 2012). Binding of SDF-1 to its receptor CXCR4 results in the mobilization of stem cells from the bone marrow to the circulation (DiPersio 2011) and recruitment of BMSCs from bone marrow to bone wound area (Otsuru et al. 2008).
Adiponectin (APN) signaling is emerging as a promising target to treat diabetes and its bone complications resulting from excessive osteoclastic activity or deficient bone wound healing. Several studies have reported a direct correlation between low APN levels and severe periodontal disease in humans (Teles et al. 2012; Bharti et al. 2013; Zimmermann et al. 2013), and APN was shown to ameliorate inflammation and experimental periodontitis in diet-induced obesity (DIO) mice (Zhang et al. 2014). Our laboratory also reported APN inhibited osteoclastogenesis (Tu et al. 2011; Wu et al. 2014), enhanced BMSCs’ migration from bone marrow to peripheral blood, and promoted calvarial bone regeneration in DIO mice (Yu et al. 2015). However, clinical use of APN as a treatment has significant disadvantages, including the high potential for adverse immunoreactions, the need for constant intravenous (IV) injection of high doses to elicit effects, and the challenge of producing APN protein on a large scale.
AdipoRon (APR) is an orally active small molecule that binds and activates the APN receptors AdipoR1 and AdipoR2, leading to similar antidiabetic effects through APN signaling (Okada-Iwabu et al. 2013). APR ameliorated insulin resistance and glucose intolerance and prolonged the shortened life span of diabetic mice. In this study, we investigated whether APR could attenuate diabetic periodontitis by establishing experimental periodontitis mouse models in APN knockout (KO) and DIO mice. We also characterized APR direct effects in osteoclasts and osteoblasts in vitro.
Materials and Methods
Mice, Experimental Periodontitis Model, and APR Treatment
Wild-type (WT; C57BL/6J, Jax#000664), DIO (Jax#380050), and APN KO (Jax#008195) mice were purchased from the Jackson Laboratory. The DIO mice were fed with a high-fat diet (containing 60% kcal from fat; Jackson Laboratory) at 6 wk of age and later. The WT mice were fed with an ordinary diet as normal chow (NC)–fed mice, and APN KO mice were maintained and fed with a NC diet as previously described (Tu et al. 2011). Experimental periodontitis was established on 20-wk-old mice and induced by the 5-0 silk suture (Zhang et al. 2014). Briefly, animal surgeries were performed under ketamine/xylazine and buprenorphine, and the suture was placed around the maxillary second molar and tied on the palatal side and changed every 2 wk if the suture was loose.
APR was orally gavaged (50 mg/kg body weight) (Okada-Iwabu et al. 2013) concurrently to the establishment of experimental periodontitis for 2 wk. In alveolar bone regeneration studies, APR postperiodontitis treatment was given in mice that were previously subjected to experimental periodontitis for 4 wk, after removing the suture for the 3 following weeks.
Tissue Sampling, Micro–Computed Tomography, and Histology Staining Protocols
Bone marrow macrophages (BMMs) were isolated from the DIO and NC mice to evaluate their differentiation toward the osteoclastic lineage. Gingival tissues on the palatal side and bone marrow flushed from tibia and femur were sampled for expression analysis. All tissue samples were immediately stored at −80°C until further use.
The skulls were scanned by Bruker Skyscan micro–computed tomography (CT) system. The alveolar bone loss was defined as the distance from the cement-enamel junction (CEJ) to the alveolar bone crest (ABC) measured at 6 sites (mesiobuccal, midbuccal, distobuccal, mesiopalatal, midpalatal, and distopalatal) using Ctan software (Bruker microCT). For some experiments, mice were subjected to micro-CT detection in vivo under isoflurane anesthesia to establish the baseline of alveolar bone loss after 4-wk suture placement. After micro-CT analysis, the bone samples were processed for histology as previously reported (Zhang, Tang, et al. 2017). Tissue sections were stained with tartrate-resistant acid phosphatase (TRAP).
Cell Culture, Osteoclastogenesis, and Osteogenesis Induction
For osteoclastogenesis induction, RAW 264.7 (ATCC) cells and BMMs were routinely cultured as we described previously (Zhang et al. 2014). TRAP staining was performed with the Acid Phosphatase, Leukocyte (TRAP) Kit (Sigma) according to the manufacturer’s instructions. For mineralization induction, primary calvarial bone osteoblasts were isolated and cultured as we previously described (Tu et al. 2008). The cells were induced in osteogenic medium for 6 d and serum starved overnight, then they were treated with or without APR for 3 d to conduct alkaline phosphatase (ALP) staining and expression studies or for 6 days to perform Alizarin red (AR) staining.
RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction Analysis
Total RNA from gingival soft tissues and bone marrow were prepared with TRIzol reagent (Life Technologies) according to the manufacturer’s instructions. Reverse transcription and quantitative reverse transcription polymerase chain reaction (qRT-PCR) assays were performed as described previously (Zhang et al. 2014). Primers used for PCR amplification are listed in Appendix Table 1.
Western Blot Analysis
Western blot analyses were performed as previously described (Zhang, Valverde, et al. 2017). Antibodies for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:5,000) were purchased from Cell Signaling Technology, and antibodies for AdipoR1 (1:1,000), osteocalcin (1:1,000), NFATc1 (1:1,000), β-actin (1:5,000), and Lamin B1 (1:5,000) were purchased from Abcam and Santa Cruz companies. Blots were visualized using ECL chemiluminescence reagents from Thermo Fisher.
Statistical analysis
Data are presented as mean ± SD. Statistical significance was evaluated using the t test between 2 groups and 1-way analysis of variance (ANOVA) among 3 or more groups following Dunnett’s test if other groups comparing the mean of each column with the mean of a control column. All statistical analysis was performed using SPSS software 14.0 (SPSS, Inc.) and Prism GraphPad 6.0 (GraphPad Software). Values of P < 0.05 were considered statistically different.
Results
APR Inhibited Osteoclastogenesis In Vitro
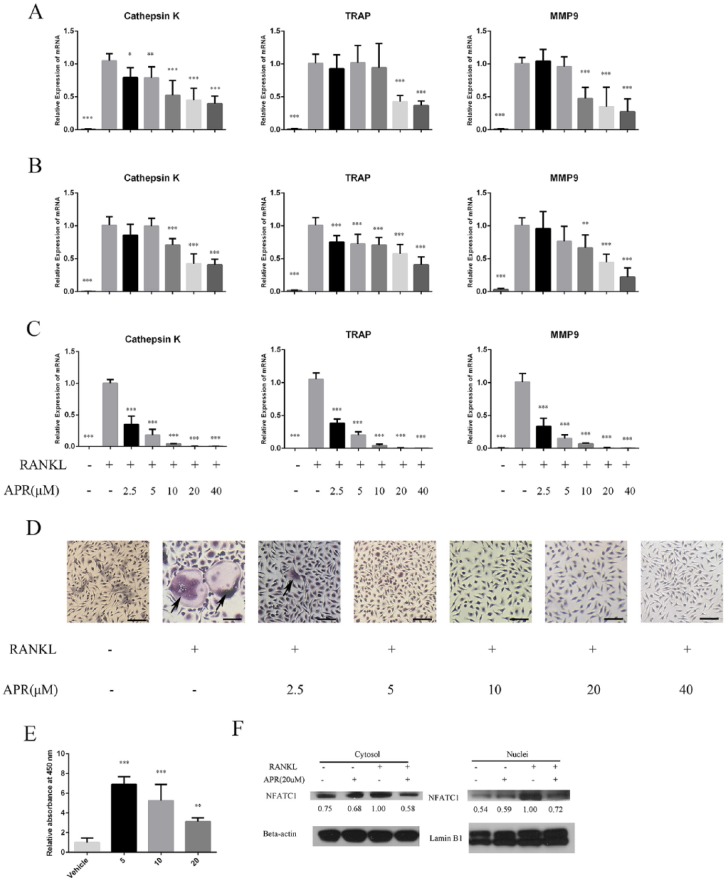
We first evaluated the ability of APR to inhibit osteoclast differentiation in RAW 264.7 and BMM cultures induced to differentiate toward the osteoclastic lineage. qRT-PCR analysis revealed that osteoclastogenesis markers, including cathepsin K, TRAP, and matrix metalloproteinase 9 (MMP-9), were significantly upregulated in RAW 264.7 cells treated with RANKL for 3 and 5 d, but cotreatment with APR significantly inhibited expression of the 3 osteoclastogenesis markers in a dose-dependent manner (Fig. 1A, B). When similar APR treatments were conducted on primary BMM cultures induced to differentiate with macrophage colony-stimulating factor (M-CSF) and RANKL for 7 d, we also found a significant dose-dependent inhibition of osteoclastogenesis marker expression (Fig. 1C). TRAP stainings of differentiated BMM cultures also revealed a dose-dependent decrease in the number of osteoclasts by APR treatment (Fig. 1D).
Figure 1.
AdipoRon (APR) inhibits osteoclastogenesis in bone marrow macrophages (BMMs) and RAW 264.7 cells in a dose-dependent manner. RAW 264.7 cells were treated with 50 ng/mL receptor activator of nuclear factor κΒ ligand (RANKL) with or without APR ranging from 2.5 to 40 µM in 3 d (A) and in 5 d (B). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of cathepsin K, tartrate-resistant acid phosphatase (TRAP), and matrix metalloproteinase 9 (MMP-9) messenger RNA (mRNA) expression were calculated (n = 5 to 7). Isolated from 6-wk mice femur and tibia, BMMs were treated with 10 ng/mL macrophage colony-stimulating factor (M-CSF) and 50 ng/mL RANKL with or without APR ranging from 2.5 to 40 µM in 7 d (C). qRT-PCR analysis of cathepsin K, TRAP, and MMP-9 mRNA expression were calculated (n = 5) as well as TRAP-stained osteoclast-like cells, as highlighted with arrows in (D) (scale bar = 100 µm). (E) The CCK-8 analysis of RAW 264.7 cells’ proliferation at different doses of APR (0, 5, 10, or 20 µM) in 48 h. (F) Nuclear and cytosolic extracts were prepared from BMMs treated with RANKL and M-CSF with or without APR for 5 d (n = 3). NFATC1 expression was evaluated in cytoplasmic and nuclear fractions and then relative expression calculated using β-actin or Lamin B1 as loading controls for cytoplasmic and nuclear expression, respectively (n = 3). Data are shown as mean ± SD. *P < 0.05. **P < 0.01. ***P < 0.001.
To determine whether APR could partly inhibit osteoclast differentiation by decreasing proliferation, we conducted a CCK-8 cell proliferation assay on RAW 264.7 osteoclast-precursor cell cultures treated with APR for 2 d. In these studies, we found the CCK-8 readings were higher for APR-treated cells than for untreated cells (Fig. 1E). We then analyzed the expression levels of NFATc1 in nuclear and cytosolic extracts and found APR treatment decreased RANKL upregulation of NFATc1 in cytoplasm and nuclei (Fig. 1F).
APR Promoted Calvarial Bone Osteoblast Osteogenesis and Mineralization
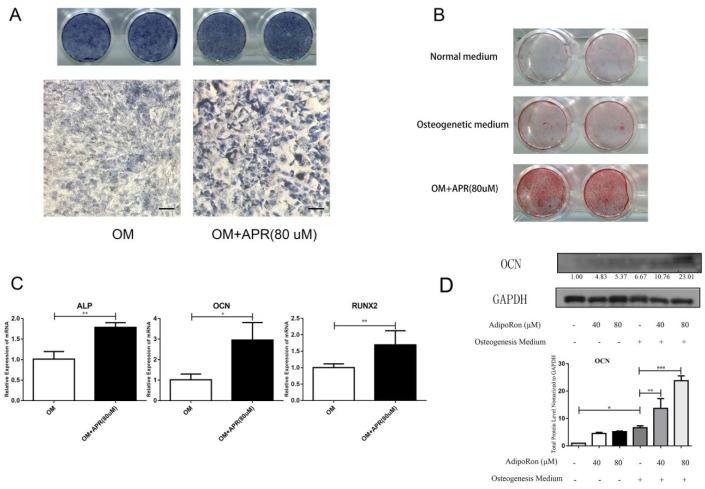
We next evaluated the ability of APR to directly induce osteogenesis and mineralization in calvarial bone osteoblasts in vitro. Higher ALP precipitation was found in APR-treated osteoblast cultures than in control cultures (Fig. 2A). Similarly, AR staining demonstrated increased production of mineralized nodules in APR-treated osteoblast cultures than in controls (Fig. 2B). In agreement with APR osteogenic induction capabilities, the messenger RNA (mRNA) expression of osteogenic markers ALP, OCN, and RUNX2 was significantly higher in the APR-treated group than in control cells (Fig. 2C), and OCN protein expression was also increased by APR (Fig. 2D). We conducted a parallel immunohistochemistry (IHC) experiment that confirmed the specificity of the OCN antibody (data not shown).
Figure 2.
AdipoRon (APR) promotes osteogenesis and mineralization in vitro. Calvarial bone osteoblasts were isolated and induced to undergo osteogenesis. Alkaline phosphatase (ALP) staining (A) and Alizarin red (AR) staining (B) of osteogenesis induced calvarial bone osteoblasts treated with or without APR at 80 µM (scale bar = 100 µm). (C) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) of ALP, OCN, and RUNX2 messenger RNA expression was examined in osteogenesis-induced calvarial bone osteoblasts treated with or without APR at 80 µM (n = 3). (D) Western blot analysis of OCN protein levels normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The numbers below represented the mean of protein/GAPDH ratios normalized to 1.0 (OM-APR-group) quantified by densitometry. Data are shown as mean ± SD. *P < 0.05. **P < 0.01. ***P < 0.001.
APR Reduced Alveolar Bone Loss in Experimental Periodontitis
To investigate whether APR could ameliorate alveolar bone loss, experimental periodontitis was induced, and simultaneously, APR was orally gavaged in NC mice for 2 wk (Fig. 3A). Control NC mice were orally gavaged with vehicle for comparison purposes. We then evaluated alveolar bone loss at 6 sites in the APR group and vehicle group and found 12% less bone loss in NC-APR mice than in NC-vehicle mice (Fig. 3B, D), with average values of 388.4 ± 34.4 µm for the vehicle group and 348.2 ± 41.2 µm for the APR group. TRAP-stained alveolar bone samples from NC-APR mice also showed a significantly lower number of osteoclasts than in NC-vehicle mice alveolar bone samples (Fig. 3F, G). Expression of inflammatory factors, including CCL2 and IL-6, was also lower in palatal gingival tissue of APR-treated mice than in the vehicle group, whereas AdipoR1 expression was upregulated by APR (Fig. 3J).
Figure 3.
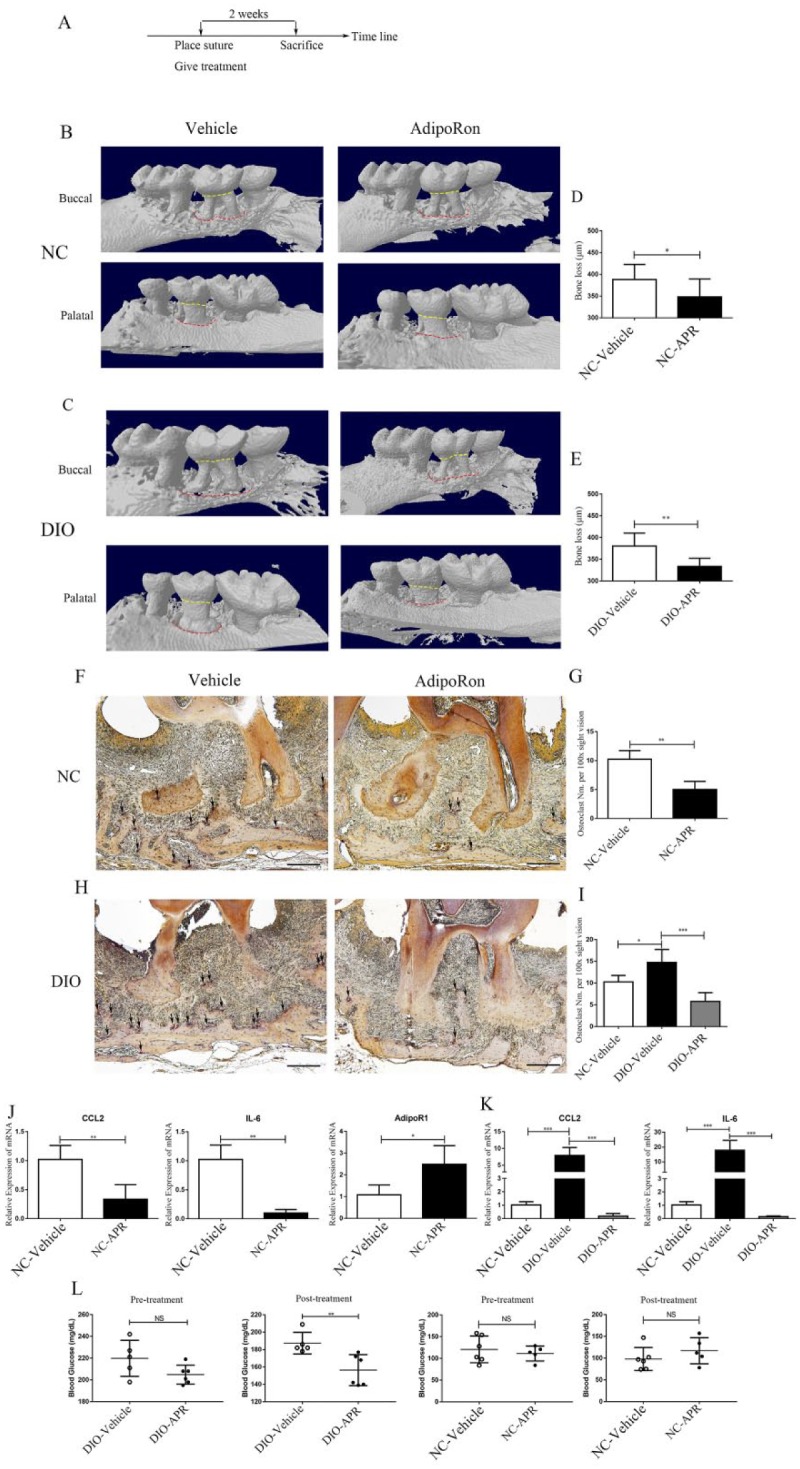
AdipoRon (APR) reduced alveolar bone loss in experimental periodontitis. (A) Timeline experimental design to evaluate APR effects in alveolar bone loss. Representative 3-dimensional model reconstruction of the experimental periodontitis of normal chow (NC) mice (B) and diet-induced obesity (DIO) mice (C). Periodontal tissue in buccal and palatal sides and alveolar bone crests are shown in red dotted lines; the CEJ was shown in yellow dotted lines; the distance between the cementoenamel junction (CEJ) and alveolar bone crest (ABC) in 6 sites is shown in (D) (n = 7) and (E) (n = 9). (F) Representative tartrate-resistant acid phosphatase (TRAP) staining of the periodontal tissue in NC mice. Black arrow, osteoclast (scale bar = 200 µm). (G) Osteoclast number in periodontal tissue slice was 10.3 ± 1.5 (vehicle) and 5.0 ± 1.4 (APR) (n = 4). (H) Representative TRAP staining of periodontal tissue from DIO mice. Black arrow, osteoclast (scale bar = 200 µm). (I) Osteoclast number in periodontal tissue slice was 14.8 ± 3.0 (vehicle) and 5.8 ± 2.1 (APR) (n = 4). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) of CC chemokine ligand 2 (CCL2), interleukin 6 (IL-6), and AdipoR1 messenger RNA expression was examined in gingival tissue from NC mice (J) (n = 4) and DIO mice (K) (n = 4), normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are shown as mean ± SD. *P < 0.05. **P < 0.01. ***P < 0.001. (L) Pre- and posttreatment fasting blood glucose concentration of DIO and NC mice with or without APR treatment. **P < 0.01.
To emulate T2DM-associated periodontitis and alveolar bone destruction, we induced experimental periodontitis in DIO mice for 2 wk and compared DIO-vehicle and DIO-APR groups orally gavaged with vehicle or APR, respectively. Routine characterization of weight and pretreatment blood glucose level for DIO mice is shown in Appendix Figure 1 and Figure 3L. The average alveolar bone loss measurement at 6 sites was 380.5 ± 29.8 µm for DIO-vehicle and 333.6 ± 18.9 µm for DIO-APR mice. Whereas there was 13% lower bone loss in the APR-treated group (Fig. 3C, E), differences between DIO-vehicle and NC-vehicle groups were not significantly different (data not shown). TRAP-stained alveolar bone samples from DIO-vehicle mice exhibited a higher number of osteoclasts than NC-vehicle mice, but APR reduced the osteoclast number in the DIO-APR group (Fig. 3H, I). Whereas inflammatory factors, including CCL2 and IL-6, were enhanced in DIO-vehicle gingival tissue compared with the NC-vehicle group, APR significantly mitigated their upregulation (Fig. 3K). In addition, after systemic APR treatment, fasting blood glucose concentration in the DIO-APR group showed a significant improvement compared to the DIO-vehicle group while the pre- and posttreatment glucose levels from the NC mice were no different (Fig. 3L).
APR Promoted Postperiodontitis Bone Regeneration in DIO and APN KO Mice
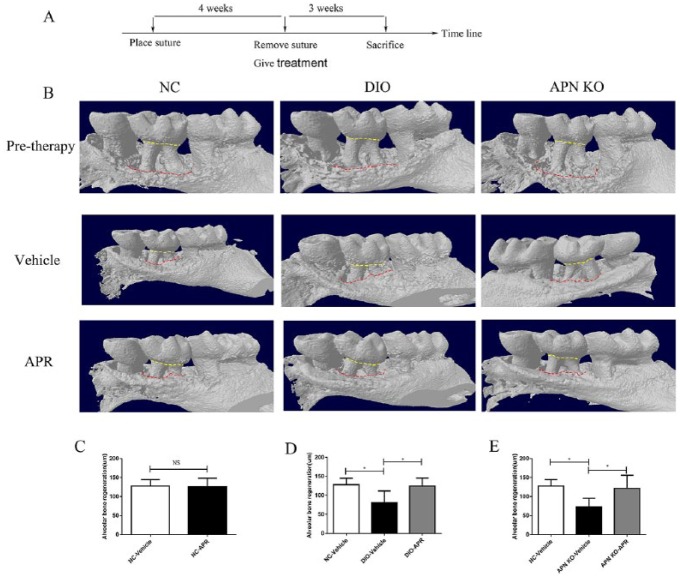
We then investigated the putative effects of APR in promoting regeneration of alveolar bone loss postperiodontitis. To that end, APR was administered after removing the suture for 3 wk in mice previously subjected to experimental periodontitis for 4 wk (Fig. 4A). We first found that differences in bone loss baseline among the NC, DIO, and APN KO groups after 4-wk suture placement were not significant (Appendix Fig. 2). We then defined alveolar bone regeneration as the difference between CEJ-ABC distance in both post- and pretreatment time points. Using this methodology, we did not identify significant differences in alveolar bone regeneration between the APR-treated and vehicle-treated NC mouse groups (Fig. 4B, C).
Figure 4.
AdipoRon (APR) promotes postperiodontitis bone regeneration in diet-induced obesity (DIO) and adiponectin (APN) knockout (KO) mice. (A) Timeline experimental design to evaluate APR effects in promoting postperiodontitis alveolar bone regeneration. (B) Representative buccal side of the 3-dimensional model reconstruction of the alveolar bone in normal chow (NC), DIO, and APN KO mice. The distance between CEJ and ABC in 6 sites was measured. The bone regeneration was defined as the difference of the distance between the pretreatment time point and the post-3-wk time point shown in (C), (D), and (E) for NC, DIO, and APN KO mice, respectively (n = 5). Data are shown as mean ± SD. *P < 0.05.
The same postperiodontitis alveolar bone regeneration experiment was conducted in DIO mice to mimic the T2DM pathological environment. As expected, the alveolar bone regeneration in the DIO-vehicle group was significantly lower than in the NC-vehicle group. Most importantly, APR treatment of DIO mice significantly enhanced the alveolar bone regeneration measurements (Fig. 4B, D). Similarly, APR treatment of APN KO mice led to higher bone regeneration parameters than in the APN KO-vehicle group (Fig. 4B, E).
Declined Osteoclastogenesis of BMMs and Decreased Expression of SDF-1 in Bone Marrow in APR-Treated DIO and APN KO Mice
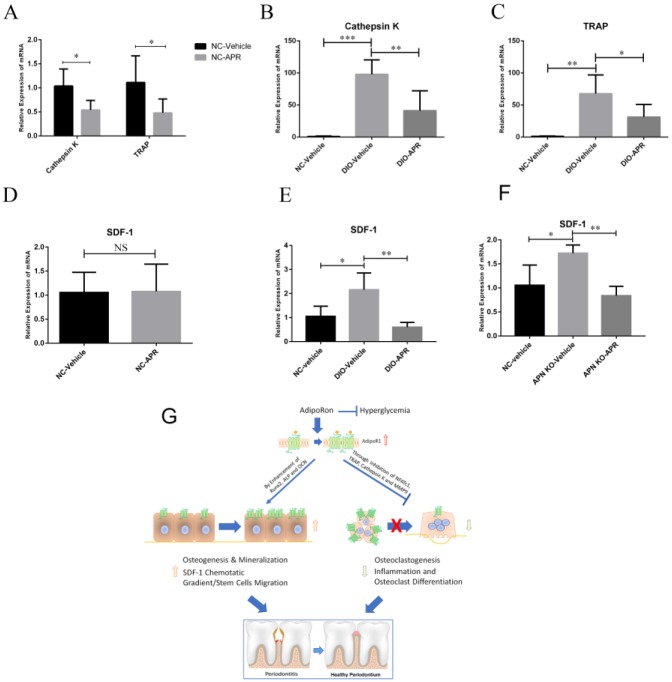
We then conducted a series of ex vivo experiments to corroborate the antiosteoclastogenic effects of APR treatment. To that end, NC and DIO mice were treated with APR or vehicle for 2 wk, and BMMs were isolated from tibia and femur and osteoclastogenesis induced with M-CSF and RANKL for 5 d. The mRNA expression levels of cathepsin K and TRAP were significantly lower in the APR-treated NC group than in vehicle control (Fig. 5A). While both osteoclastogenesis markers were significantly higher in the DIO-vehicle group than in the NC-vehicle group, APR decreased cathepsin K and TRAP expression in the DIO-APR group (Fig. 5B, C).
Figure 5.
Decreased osteoclastogenesis and expression of stromal cell–derived factor 1 (SDF-1) in bone marrow in AdipoRon (APR)–treated diet-induced obesity (DIO) and adiponectin (APN) knockout (KO) mice. After 2 wk of treatment with vehicle or APR, bone marrow macrophages (BMMs) were isolated from the normal chow (NC) and DIO mice and induced to differentiate through the osteoclastogenic lineage by ex vivo treatment with 10 ng/mL macrophage colony-stimulating factor (M-CSF) and 50 ng/mL receptor activator of nuclear factor κΒ ligand (RANKL) for 5 d. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) of cathepsin K and tartrate-resistant acid phosphatase (TRAP) messenger RNA (mRNA) expression in NC mice treated with vehicle or APR (A) (n = 4–6), as well as cathepsin K (B) (n = 4–6) and TRAP (C) (n = 4–6) in DIO mice treated with vehicle or APR. SDF-1 mRNA expression was examined in a postperiodontitis regeneration mice model in bone marrow (n > 4) sampled from NC mice (D), DIO mice (E), and APN KO mice (F) treated with APR or vehicle. Data are shown as mean ± SD. *P < 0.05. **P < 0.01. ***P < 0.001. (G) Proposed model of APR mechanisms to ameliorate diabetic bone disorders. APR, an orally active synthetic APN receptor agonist, inhibited osteoclastogenesis and nuclear expression of NFATc1. In addition to alleviating diabetic alveolar bone loss in our periodontitis model, APR increased osteogenesis and mineralization, as well as promoted alveolar bone regeneration postperiodontitis in DIO mice. APR also decreased expression of SDF-1 in bone marrow, which might create a favorable SDF-1–mediated chemotactic gradient to facilitate stem cell migration from the bone marrow to the circulation for further use in alveolar bone repair and homeostasis in DIO mice.
We then evaluated the impact of APR on SDF-1 expression in bone marrow subjected to the postperiodontitis alveolar bone regeneration experimental model. We found that differences in SDF-1 expression between the APR-treated and vehicle-treated NC mouse groups were not significant (Fig. 5D), while the SDF-1 expression level was significantly higher in the vehicle-treated DIO and APN KO group compared to the NC group (Fig. 5E, F), and APR lowered the SDF-1 expression level in the DIO-APR group (Fig. 5E) and APN KO-APR group (Fig. 5F). On the other hand, the mRNA expression of the receptor of SDF-1, CXCR4, was not significantly different between vehicle and APR treatment groups in NC, DIO, and APN KO mice (Appendix Fig. 3).
Discussion
In addition to mediating antidiabetic properties, APR (Okada-Iwabu et al. 2013) has been described to exhibit efficacy to treat diabetic complications in the cardiovascular and other organs such as the kidney in animal models (Zhang et al. 2015; Kim et al. 2018). We therefore investigated whether APR could ameliorate type 2 diabetic bone complications like periodontitis in mouse models of experimental periodontitis established in DIO, APN KO, and NC mice. We first demonstrated APR inhibited osteoclastogenesis in vitro, and inhibition of osteoclastogenesis by APR did not seem to result from an inhibition of osteoclast-precursor cell proliferation. These results were in agreement with the ability of APR to emulate APN signaling in osteoclast-precursor cells to inhibit osteoclastogenesis and NFATc1 expression (Oshima et al. 2005; Tu et al. 2011; Zhang et al. 2014).
We then established experimental periodontitis in NC and DIO mice and evaluated APR impact in ameliorating alveolar bone loss. We found lower alveolar bone loss in APR-treated mice than in vehicle-treated mice. These results were consistent with lower osteoclast numbers in alveolar bone samples isolated from APR-treated mice and decreased mRNA expression of inflammatory cytokines in palatal gingival tissue of APR-treated groups. Indeed, recent studies using lineage-specific approaches indicate that RANKL provided by periodontal ligament and bone-lining cells participates in periodontal bone resorption (Yang et al. 2018). Our previous study also found that APN inhibits RANKL-induced osteoclastogenesis and bone resorption via suppression of Akt1 (Tu et al. 2011). In the experimental model of periodontitis that we used in this study, the differences in alveolar bone loss between DIO-vehicle and NC-vehicle groups were not significantly different, but the diabetic condition in the DIO-vehicle group was associated with increased osteoclast numbers in alveolar bone and higher mRNA expression of inflammation cytokines in palatal gingival tissue. Using type 2 Zucker diabetic fatty (ZDF) rats, Liu et al. (2006) demonstrated that diabetes further enhances alveolar bone loss through increased resorption and diminished bone formation.
It is well known that periodontal inflammation not only stimulates osteoclastogenesis and alveolar bone loss but also interferes with the uncoupling of bone formation and bone resorption (Graves et al. 2011). Patients with diabetes mellitus have a higher risk of delayed bone fracture healing (Hamann et al. 2012). We conducted a postperiodontitis alveolar bone regeneration series of experiments and found that the alveolar bone recovery was shorter in the DIO group than in the NC group. Increased osteoclast numbers and inflammatory cytokine expression in the bone tissue and delayed bone wound recovery in the DIO group corroborated that diabetes progression negatively affected bone metabolism by promoting bone loss and inhibiting bone regeneration.
APN has been reported to promote osteogenesis on BMSCs and preosteoblast cultures (Chen et al. 2015; Neumann et al. 2016; Pu et al. 2016). Furthermore, BMSCs overexpressing APN were previously shown to promote calvarial bone defect healing (Wang et al. 2017). In this work, we conducted in vitro studies that revealed APR could mimic APN actions in inducing osteogenesis of primary osteoblasts. We found that the maximal concentrations of APR affecting osteogenesis or osteoclast differentiation were different (80 vs. 40 µM), which can be useful for in vivo applications. We further evaluated whether APR could regenerate alveolar bone loss associated with experimental periodontitis in DIO mice. We also conducted these experiments in APN KO to exclude the potential interference of endogenous APN in APR osteogenic effects in vivo. The results demonstrated that APR could promote bone formation, even in the absence of APN. Therefore, APR can be used to emulate previously reported effects of APN administration in promoting bone formation and calvarial bone regeneration (Yu et al. 2015; Wang et al. 2017).
SDF-1 and its receptor form a signaling axis with pivotal roles in mediating the mobilization of stem cells from the bone marrow to the peripheral circulation (DiPersio 2011) and the recruitment of BMSCs to fracture sites to promote bone repair (Otsuru et al. 2008; Kitaori et al. 2009). Our lab previously reported that systemic APN infusion ameliorated hyperglycemia and the failure to mobilize hematopoietic stem cells (HSCs) from the bone marrow to circulation while promoting calvarial bone regeneration in DIO mice (Yu et al. 2015) by creating a chemotactic gradient of SDF-1 between bone marrow and peripheral blood. In the current study, our results revealed that APR treatment led to lower SDF-1 expression in bone marrow in both APN KO-APR and DIO-APR groups compared with vehicle-treated mice. Our results supported the potential ability of APR to amplify the SDF-1 chemotactic gradient between bone marrow and circulation by downregulating SDF-1 in bone marrow to facilitate stem cell migration to the circulation for alveolar bone repair.
Our study, however, has some limitations. First, the micro-CT analysis and bone measurements were conducted by only 1 well-trained pathologist, who was blind to group classification. Second, mice were fed with the NC diet, which contains 6% fat, while low-fat diets are considered a better choice for diet studies (Dalby et al. 2017) . Third, although DIO mice used in this study are an appropriate model for studying prediabetes and diabetes-related metabolic syndrome, APR treatment of other periodontitis models of diabetes such as a polygenic diabetic model should be investigated in future studies to support the potential of APR to treat T2DM-associated periodontitis.
Current pharmacotherapies for bone metabolic diseases include antiresorptive drugs and anabolic bone-forming drugs (Valverde 2008; Weinstein et al. 2010; Henriksen et al. 2013; Tsuchie et al. 2013), without mediating known antidiabetic properties in T2DM patients. Our study supports the notion that APR could be a promising approach to target diabetes and associated periodontitis by controlling hyperglycemia, suppressing inflammation, inhibiting excessive osteoclastogenesis-induced bone loss, and improving bone-regenerating capabilities (Fig. 5G).
Author Contributions
X. Wu, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; W. Qiu, Z. Hu, J. Lian, Y. Liu, X. Zhu, M. Tu, F. Fang, Y. Yu, contributed to data acquisition, critically revised the manuscript; P. Valverde, contributed to analysis, interpretation, drafted and critically revised the manuscript; Q. Tu, J. Chen, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; Y. Yu, contributed to conception, data analysis and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034518818449 for An Adiponectin Receptor Agonist Reduces Type 2 Diabetic Periodontitis by X. Wu, W. Qiu, Z. Hu, J. Lian, Y. Liu, X. Zhu, M. Tu, F. Fang, Y. Yu, P. Valverde, Q. Tu, Y. Yu and J. Chen in Journal of Dental Research
Footnotes
This work was supported by National Institutes of Health (NIH) grants R01DE21464, RO1DE25681, and RO1DE26507; an Innovation in Oral Care Award through the International Association for Dental Research and GlaxoSmithKline Consumer Healthcare; and an award through the International Team of Implantology to J.C. and by the National Natural Science Foundation of China (#81670956) and Shanghai Cooperative International Project (#16520710400) of Shanghai Committee of Science and Technology to Y.Y.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is available online.
References
- Bharti P, Katagiri S, Nitta H, Nagasawa T, Kobayashi H, Takeuchi Y, Izumiyama H, Uchimura I, Inoue S, Izumi Y. 2013. Periodontal treatment with topical antibiotics improves glycemic control in association with elevated serum adiponectin in patients with type 2 diabetes mellitus. Obes Res Clin Pract. 7(2):e129–e138. [DOI] [PubMed] [Google Scholar]
- Chen T, Wu YW, Lu H, Guo Y, Tang ZH. 2015. Adiponectin enhances osteogenic differentiation in human adipose-derived stem cells by activating the APPL1-AMPK signaling pathway. Biochem Biophys Res Commun. 461(2):237–242. [DOI] [PubMed] [Google Scholar]
- Dalby MJ, Ross AW, Walker AW, Morgan PJ. 2017. Dietary uncoupling of gut microbiota and energy harvesting from obesity and glucose tolerance in mice. Cell Rep. 21(6):1521–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio JF. 2011. Diabetic stem-cell “mobilopathy.” N Engl J Med. 365(26):2536–2538. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ; CDC Periodontal Disease Surveillance Workgroup. 2012. Prevalence of periodontitis in adults in the united states: 2009 and 2010. J Dent Res. 91(10):914–920. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Albiero M, De Kreutzenberg SV, Boscaro E, Cappellari R, Marescotti M, Poncina N, Agostini C, Avogaro A. 2013. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care. 36(4):943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D, Li J, Cochran D. 2011. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res. 90(2):143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann C, Kirschner S, Günther K-P, Hofbauer LC. 2012. Bone, sweet bone—osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 8(5):297–305. [DOI] [PubMed] [Google Scholar]
- Henriksen K, Andersen JR, Riis BJ, Mehta N, Tavakkol R, Alexandersen P, Byrjalsen I, Valter I, Nedergaard BS, Teglbjaerg CS, et al. 2013. Evaluation of the efficacy, safety and pharmacokinetic profile of oral recombinant human parathyroid hormone [rhPTH(1-31)NH(2)] in postmenopausal women with osteoporosis. Bone. 53(1):160–166. [DOI] [PubMed] [Google Scholar]
- Kasahara T, Imai S, Kojima H, Katagi M, Kimura H, Chan L, Matsusue Y. 2010. Malfunction of bone marrow–derived osteoclasts and the delay of bone fracture healing in diabetic mice. Bone. 47(3):617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, Leone CW, Morgan EF, Gerstenfeld LC, Einhorn TA, et al. 2007. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 22(4):560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Lim JH, Kim MY, Kim EN, Yoon HE, Shin SJ, Choi BS, Kim YS, Chang YS, Park CW. 2018. The adiponectin receptor agonist adiporon ameliorates diabetic nephropathy in a model of type 2 diabetes. J Am Soc Nephrol. 29(4):1108–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. 2009. Stromal cell–derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 60(3):813–823. [DOI] [PubMed] [Google Scholar]
- Lapid K, Glait-Santar C, Gur-Cohen S, Canaani J, Kollet O, Lapidot T. 2012. Egress and mobilization of hematopoietic stem and progenitor cells: a dynamic multi-facet process. Cambridge, MA: Harvard Stem Cell Institute. [PubMed] [Google Scholar]
- Liu R, Bal HS, Desta T, Krothapalli N, Alyassi M, Luan Q, Graves DT. 2006. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J Dent Res. 85(6):510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E, Junker S, Schett G, Frommer K, Müller-Ladner U. 2016. Adipokines in bone disease. Nat Rev Rheumatol. 12(5):296–302. [DOI] [PubMed] [Google Scholar]
- Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, et al. 2013. A small-molecule adipor agonist for type 2 diabetes and short life in obesity. Nature. 503(7477):493–499. [DOI] [PubMed] [Google Scholar]
- Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. 2005. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 331(2):520–526. [DOI] [PubMed] [Google Scholar]
- Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. 2008. Circulating bone marrow–derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 26(1):223–234. [DOI] [PubMed] [Google Scholar]
- Polak D, Shapira L. 2018. An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 45(2):150–166. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, Taylor R. 2012. Periodontitis and diabetes: a two-way relationship. Diabetologia. 55(1):21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Wu H, Lu S, Hu H, Li D, Wu Y, Tang Z. 2016. Adiponectin promotes human jaw bone marrow stem cell osteogenesis. J Dent Res. 95(7):769–775. [DOI] [PubMed] [Google Scholar]
- Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, et al. 2018. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 45(2):138–149. [DOI] [PubMed] [Google Scholar]
- Sima C, Van Dyke TE. 2016. Therapeutic targets for management of periodontitis and diabetes. Curr Pharm Des. 22(15):2216–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles FR, Teles RP, Martin L, Socransky SS, Haffajee AD. 2012. Relationships among interleukin-6, tumor necrosis factor-α, adipokines, vitamin D, and chronic periodontitis. J Periodontol. 83(9):1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchie H, Miyakoshi N, Kasukawa Y, Aonuma H, Shimada Y. 2013. Intermittent administration of human parathyroid hormone before osteosynthesis stimulates cancellous bone union in ovariectomized rats. Tohoku J Exp Med. 229(1):19–28. [DOI] [PubMed] [Google Scholar]
- Tu Q, Zhang J, Dong LQ, Saunders E, Luo E, Tang J, Chen J. 2011. Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. J Biol Chem. 286(14):12542–12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Q, Zhang J, Paz J, Wade K, Yang P, Chen J. 2008. Haploinsufficiency of Runx2 results in bone formation decrease and different BSP expression pattern changes in two transgenic mouse models. J Cell Physiol. 217(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde P. 2008. Pharmacotherapies to manage bone loss-associated diseases: a quest for the perfect benefit-to-risk ratio. Curr Med Chem. 15(3):284–304. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang X, Shao J, Liu H, Liu X, Luo E. 2017. Adiponectin regulates BMSC osteogenic differentiation and osteogenesis through the Wnt/beta-catenin pathway. Sci Rep. 7(1):3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. 2010. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 151(6):2641–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Song LT, Li JS, Zhu DW, Jiang SY, Deng JY. 2017. Microrna-126 regulates inflammatory cytokine secretion in human gingival fibroblasts under high glucose via targeting tumor necrosis factor receptor associated factor 6. J Periodontol. 88(11):e179–e187. [DOI] [PubMed] [Google Scholar]
- Wu Y, Tu Q, Valverde P, Zhang J, Murray D, Dong LQ, Cheng J, Jiang H, Rios M, Morgan E. 2014. Central adiponectin administration reveals new regulatory mechanisms of bone metabolism in mice. Am J Physiol Endocrinol Metab. 306(12):E1418–E1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Jeon HH, Alshabab A, Lee YJ, Chung CH, Graves DT. 2018. Rankl deletion in periodontal ligament and bone lining cells blocks orthodontic tooth movement. Int J Oral Sci. 10(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Tu Q, Han Q, Zhang L, Sui L, Zheng L, Meng S, Tang Y, Xuan D, Zhang J. 2015. Adiponectin regulates bone marrow mesenchymal stem cell niche through a unique signal transduction pathway: an approach for treating bone disease in diabetes. Stem Cells. 33(1):240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Valverde P, Zhu X, Murray D, Wu Y, Yu L, Jiang H, Dard MM, Huang J, Xu Z. 2017. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 5:16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Meng S, Tu Q, Yu L, Tang Y, Dard MM, Kim S-H, Valverde P, Zhou X, Chen J. 2014. Adiponectin ameliorates experimental periodontitis in diet-induced obesity mice. PLoS One. 9(5):e97824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tang Y, Zhu X, Tu T, Sui L, Han Q, Yu L, Meng S, Zheng L, Valverde P, et al. 2017. Overexpression of MiR-335-5p promotes bone formation and regeneration in mice. J Bone Miner Res. 32(12):2466–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhao J, Li R, Lau WB, Yuan YX, Liang B, Li R, Gao EH, Koch WJ, Ma XL, et al. 2015. Adiporon, the first orally active adiponectin receptor activator, attenuates postischemic myocardial apoptosis through both AMPK-mediated and AMPK-independent signalings. Am J Physiol Endocrinol Metab. 309(3):E275–E282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann GS, Bastos MF, Dias Gonçalves TE, Chambrone L, Duarte PM. 2013. Local and circulating levels of adipocytokines in obese and normal weight individuals with chronic periodontitis. J Periodontol. 84(5):624–633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034518818449 for An Adiponectin Receptor Agonist Reduces Type 2 Diabetic Periodontitis by X. Wu, W. Qiu, Z. Hu, J. Lian, Y. Liu, X. Zhu, M. Tu, F. Fang, Y. Yu, P. Valverde, Q. Tu, Y. Yu and J. Chen in Journal of Dental Research